Abstract
Innate immunity plays a central role in resolving infections by pathogens. Host survival during plague, caused by the Gram-negative bacterium Yersinia pestis, is favored by a robust early innate immune response initiated by IL-1β and IL-18. These cytokines are produced by a two-step mechanism involving NF-κB-mediated pro-cytokine production and inflammasome-driven maturation into bioactive inflammatory mediators. Because of the anti-microbial effects induced by IL-1β/IL-18, it may be desirable for pathogens to manipulate their production. Y. pestis type III secretion system effectors YopJ and YopM can interfere with different parts of this process. Both effectors have been reported to influence inflammasome caspase-1 activity; YopJ promotes caspase-8-dependent cell death and caspase-1 cleavage, whereas YopM inhibits caspase-1 activity via an incompletely understood mechanism. However, neither effector appears essential for full virulence in vivo. Here we report that the sum of influences by YopJ and YopM on IL-1β/IL-18 release is suppressive. In the absence of YopM, YopJ minimally affects caspase-1 cleavage but suppresses IL-1β, IL-18, and other cytokines and chemokines. Importantly, we find that Y. pestis containing combined deletions of YopJ and YopM induces elevated levels of IL-1β/IL-18 in vitro and in vivo and is significantly attenuated in a mouse model of bubonic plague. The reduced virulence of the YopJ-YopM mutant is dependent on the presence of IL-1β, IL-18, and caspase-1. Thus, we conclude that Y. pestis YopJ and YopM can both exert a tight control of host IL-1β/IL-18 production to benefit the bacteria, resulting in a redundant impact on virulence.
Keywords: caspase 1 (CASP1), cell death, inflammasome, interleukin 1 (IL-1), macrophage, type III secretion system (T3SS), Yersinia pestis, YopJ, YopM, interleukin-18 (IL-18)
Introduction
Many pathogens rely upon a strong suppression or evasion of host immune responses to cause disease. Yersinia pestis, the etiologic agent of plague, achieves high virulence in part by actively suppressing the host immune system. A key component of this strategy is the type III secretion system (T3SS),2 by which the bacterium delivers seven Yop (Yersinia outer protein) effectors into host immune cells. These Yops manipulate intracellular pathways to inhibit phagocytosis, motility, cytokine expression, and other vital immune processes (1). Two effectors, YopJ and YopM, have been extensively studied for over two decades, but key questions about their roles in disease remain unanswered.
In vitro studies have revealed YopJ to be an acetyl transferase targeting and inhibiting IKKβ (2), MAP kinase kinases (3, 4), and TAK1 (5, 6). YopJ has also been reported to behave like a deubiquitinase (7). YopJ robustly inhibits NF-κB-mediated transcription of pro-inflammatory cytokines and induces caspase-8/RIP1-mediated apoptosis, caspase-1 cleavage, and IL-1β release, effects that are dependent on its enzymatic activity (8–10). However, despite its powerful effects in vitro, YopJ appears perplexingly dispensable during Y. pestis infection in vivo (11, 12).
YopM has also been reported to limit the recruitment of monocytes, neutrophils, and NK cells to infected organs (13, 14). It was recently demonstrated that YopM inhibits caspase-1 (15, 16). Caspase-1 mediates maturation of IL-1β and IL-18, cytokines that promote a robust early immune response that enhances host resistance against Y. pestis infection (17, 18). Thus, YopM may promote virulence by inhibiting the processing of these cytokines. However, the impact of YopM on Y. pestis virulence appears minor (14, 19), and it is unclear whether IL-1β/IL-18 levels would increase in vivo during Y. pestis infection without YopM.
IL-1β and IL-18 are produced in a two-step process requiring an initial signal to trigger the transcription/translation of their pro-forms, and a second signal to trigger their processing by caspase-1 into their biologically active secreted forms (20). The first signal is NF-κB-dependent, typically downstream of stimulation via Toll-like receptors by microbially associated molecules such as LPS. The second signal is catalyzed by inflammasomes. A canonical inflammasome is formed when pathogen- or danger-associated molecular patterns are sensed by a NOD-like receptor (NLR), such as NLRP3, NLRC4, or the non-NLR protein AIM2. This leads to recruitment and oligomerization of the adaptor protein ASC, which in turn recruits pro-caspase-1 dimers to be autoproteolyzed into the catalytically active p20 form. This process is normally accompanied by an inflammatory form of cell death called pyroptosis. Importantly, noncanonical inflammasomes involving caspase-8 (21, 22) or caspase-11 (23, 24) have also been described.
Because of the potential importance of IL-1β/IL-18 in fighting infection, the interaction of Y. pestis with host inflammasomes warrants close attention. Because YopJ and YopM appear to target the IL-1β/18 maturation pathway in distinct ways, we hypothesized that these two effectors may cooperate for optimal effects on the immune system. We set out to investigate whether such an interaction exists and what role this may play during infection. Surprisingly, we found that Y. pestis lacking both YopJ and YopM induced increased IL-1β/IL-18 compared with parental bacteria or strains lacking one of the effectors. We also found a strain lacking both effectors to be significantly attenuated in a bubonic plague model in an IL-18/IL-1β/caspase-1-dependent fashion, suggesting that tuning down inflammasome activity and IL-1β/IL-18 release are key features of Y. pestis pathogenesis.
Experimental Procedures
Bacterial Strains and Growth Conditions
The fully virulent KIM1001 strain of Y. pestis, the attenuated KIM5 (Δpgm) BSL2 strain, and KIM5ΔYopJ were previously described (8, 18, 25). The ΔYopM and ΔYopM/J strains were generated both on the KIM5 and KIM1001 background as follows: an in-frame deletion removing amino acids 3–408 of 410 of the yopM gene was created via allelic exchange. PCR products made with primer sets yopM-A, yopM-B and yopM-C, yopM-D, respectfully, were used to make a fused product by overlap PCR using primers A and D (26). This product was cloned in the allelic exchange vector pRE107 (27) in Escherichia coli K12 strain β2155, a diamiopimelic acid auxotroph, and transferred to Y. pestis KIM1001 by conjugation. KIM1001 recombinants were selected on TB medium containing 100 μg/ml ampicillin but no diaminopimelic acid. Following counter selection with 5% sucrose, deletion mutants were identified by PCR. The same procedure was followed to construct an in-frame deletion mutant of yopJ and yopE in KIM1001 and KIM1001 ΔyopM using the respective gene specific A, B, C, and D primers shown in Table 1. Attenuated Δpgm derivatives of each strain, bearing the designation KIM5 to indicate their altered chromosomal genotype, were derived from their respective KIM1001 parents by selection for loss of pigmentation on HIB Congo Red agar at 26 °C. KIM5 Δpla was also generated as described (25). Loss of the pigmentation region (Δpgm)/iron acquisition was confirmed by PCR with primers pgm-F, pgm-R; psn-F, psn-R; and hmsH-F, hmsH-R. Expression of YopM and YopJ was confirmed by RT-PCR.
TABLE 1.
Primers used for generation of bacterial strains
| Primer name | Sequence 5′ to 3′ |
|---|---|
| yopM-A | ATAGAGCTCTTCAAAAGGGGTACTGGATAC |
| yopM-B | GAACATATTGAATGCCTTTCT |
| yopM-C | AGAAAGGCATTCAATATGTTCGAGTAGTACGCAAGAGCGTTC |
| yopM-D | GGGTCTAGATTTACCAATTTTTTGATGGGG |
| yopJ-A | ATAGAGCTCCACTACTGATTCAACTTGGACG |
| yopJ-B | ACGGCAAATGCAGAGCAGTCCGATCATTTATTTATCCTTATTCA |
| yopJ-C | CTGCTCTGCATTTGCCGTTAATGTATTTTGGAAATCTTGCT |
| yopJ-D | GGGTCTAGACTGATGTCGTTTATTTCTGGGTAT |
| yopE-A | ATAGAGCTCAGCATTACACACTCCACAGTTGGGT |
| yopE-B | ACGCAGGCAGCAAATGAGATCAAA |
| yopE-C | CTCATTTGCTGCCTGCGTATATTGATCACTTGTTTG |
| yopE-D | ATATCTAGATATCCAGGCTGTTCAATGGTTGTCGAT |
| Pgm-F | CCGCAACAACATCATCCGTATTCA |
| Pgm-R | TTCGCTACCACTGAAATCCAAGAC |
| Psn-F | ATTGCTCCCCGCCATTGCTA |
| Psn-R | CATTGCTCTTACCCTGGTCGCCA |
| hmsH-F | CGTTTCAGTTGCCTGTGTGCTAAC |
| hmsH-R | CATCACTCGGTGTAGACATCGCT |
A type III secretion effector deficient strain (ΔT3SSe) was constructed by making sequential in-frame deletions, as described above, of yopM (amino acids 3–408 of 410), yopE (amino acids 40–197 of 220), yopH (amino acids of 3–467 of 469), ypkA (amino acids of 3–731 of 733), yopJ (amino acids 4–288 of 289), yopK (amino acids 4–181 of 183), and yopT (amino acids of 3–320 of 323) using the respective gene specific A, B, C, and D primers shown in Table 1. The deletions were made in Y. pestis KIM 1001, and a KIM5 derivative was generated as described above. This strain lacks Yops M/E/J/H/T/K and YpkA (also called YopO) but expresses Yops B/D and the machinery necessary to assemble a T3SS needle with a functional pore-forming translocon complex.
All Y. pestis strains were grown using TB medium supplemented with 2.5 mm CaCl2. KIM1001 and derivative strains were plated on agar incubated at 37 °C overnight and passed once before preparing inoculum for injection. Strains on the KIM5 background were plated overnight from frozen glycerol stocks and then grown at 26 °C in liquid broth overnight; on the day of infection, cultures were diluted 1:20 and grown for 2 h at 26 °C followed by a shift to 37 °C for 2 h. This transition is important to up-regulate expression of the T3SS while minimizing expression of F1 protein capsule (28), which interferes with cell-based assays. Bacteria were then washed three times in RPMI 1640 medium (prewarmed to 37 °C), quantified by A600, and added to cells at a multiplicity of infection (MOI) of 10 bacteria per cell in a 10-μl volume within 1 h of preparation.
Cell Stimulations
Bone marrow-derived macrophages (BMDMs) were differentiated in RPMI 1640 medium supplemented with 10% FCS, 25 mm HEPES, 10 μg/ml ciprofloxacin, and 10% L929 conditioned medium containing M-CSF for 5 days. Bulk bone marrow-derived dendritic cells (BMDCs) were differentiated in R10 medium consisting of RPMI 1640 medium, 10% FCS, 20 mm HEPES, 2 mm l-glutamine, 50 μm β-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, and 20 ng/ml recombinant murine GM-CSF (Peprotech) for 9 days. BMDMs or BMDCs were harvested and seeded at a density of 100,000 cells/well in a 96-well plate format overnight and stimulated the following day. The cells were primed with 100 ng/ml LPS for 5 h (unless otherwise indicated) or allowed to rest in antibiotic-free RPMI 1640 medium with 10% FCS and 25 mm HEPES without antibiotic before addition of bacteria at an MOI of 10. End points were as follows: 2 h postinfection for RNA extraction, 3 h postinfection for caspase-8 enzymatic assays (Caspase-Glo 8 kit, Promega catalog no. G8200), 5 h postinfection for LDH assay (CytoTox 96, Promega), and 6 h for harvesting of supernatant and/or cells for analysis of cytokines by ELISA (R&D Systems) or caspase-1 activation by SDS-PAGE and Western blot (anti-caspase-1 catalog no. AG-20B-0042-C100, Adipogen; anti-IL-1β catalog no. DY401 840135, R&D Systems). For time points exceeding 3 h, 50 μg/ml gentamicin was added at 3 h postinfection Each graph represents results of two or more independent cell stimulations on separate days.
Cell Death Assays
The LDH assay was used to measure cell death at a fixed 5-h end point; for this assay, the RPMI 1640-based medium was replaced with X-vivo (Lonza) supplemented with 3.5% FCS and HEPES prior to infection. Additionally, a kinetic cell death assay using a DNA binding dye was performed as follows. The cells were incubated with 0.2 μm ethidium homodimer (EthD-1, Sigma catalog no. 46043) in the RPMI 1640 medium mentioned above 1 h before adding bacteria. Upon infection, the plate was placed in a Synergy H4 plate reader at 37 °C, and UV-induced fluorescence was measured every 10 min. Increased fluorescence correlates with DNA binding by EthD-1 upon entry through increasingly permeable cell membranes as cell death progresses.
RT-PCR
RNA was extracted using the Qiagen RNEasy kit, followed by RT-PCR with the SYBR Green DNA probe (Bio-Rad). C(t) values were normalized to GAPDH internal controls, and the means were normalized to the unstimulated negative control group. For RT-PCR on RNA extracted from bacterial lysates, the results were normalized to Y. pestis 16S rRNA internal control. The primers used for RT-PCR are listed in Table 2.
TABLE 2.
Primers used for RT-PCR
| Primer name | Sequence 5′ to 3′ |
|---|---|
| GAPDH F | TGTGTCCGTCGTGGATCTGA |
| GAPDH R | CCTGCTTCACCACCTTCTTGA |
| Pro-IL-1β F | AGGCCACAGGTATTTTGTCG |
| Pro-IL-1β R | GCCCATCCTCTGTGACTCAT |
| Pro-IL-18 F | CAGGCTGTCTTTTGTCAACGA |
| Pro-IL-18 R | GACTCTTGCGTCAACTTCAAGG |
| IFN-β F | CTGTCTGCTGGTGGAGTTCA |
| IFN-β R | ATAAGCAGCTCCAGCTCCAA |
| IL-6 F | GAGCATTGGAAATTGGGGTA |
| IL-6 R | AACGATGATGCACTTGCAGA |
| YopM F | TTACCGCAGAGCCTGAAATC |
| YopM R | GCAACTCTGGCAATTCTTCC |
| YopJ F | TAGAAGTCATGCCCGCATTG |
| YopJ R | TGTCCTTATTGCCAGCATCG |
Mice
All experiments involving mice were approved by the Institutional Animal Care and Use Committee. Mouse strains used in this study were described previously (17) and bred in-house. TCRβ−/−δ−/− (TCRβδ dKO, lacking TCRαβ and TCRγδ) were from Jackson Laboratories and provided by Ray Welsh. BMDMs and BMDCs were differentiated from bone marrow harvested from the femurs of 6–20-week-old mice. Peritoneal macrophages were harvested by injecting mice intraperitoneally with 1 ml of thioglycolate and lavaging the peritoneal cavity 72 h later with RPMI 1640 medium. The cells were seeded at 500,000/well in 12-well format for subsequent stimulation.
To investigate a system where neutrophils are likely playing a major role in inflammatory signals, we conducted infections directly in the peritoneum of mice after stimulating heavy neutrophil recruitment with thioglycolate. The mice were injected intraperitoneally with 0.7 ml of 4% thioglycolate followed 3 h later by same side injection with 1 × 108 cfu of KIM5, KIM5ΔYopM, KIM5ΔYopJ, KIM5ΔYopM/J, or mock in 0.3 ml of PBS. The peritoneal cavity was lavaged with 3 ml of Hanks' buffered saline solution 6 h postinfection. The cells were centrifuged and analyzed by flow cytometry with anti-Ly6G) and anti-CD11b (Becton Dickinson), and the cleared lavage fluid was assayed for IL-1β by ELISA. This approach gave a similar cellular composition of 90–98% Ly6G/CD11b+ cells. Flow cytometry data were analyzed by FlowJo software.
Subcutaneous inoculations were conducted by injecting mice with 50–140 cfu in 50 μl of PBS in the nape of the neck behind the right shoulder blade; the mice were then either monitored for survival for up to 40 days or sacrificed for collection of organs at 90 h postinfection. Intravenous inoculations were conducted by injecting mice with 40–50 cfu in 200 μl of PBS via the tail vein and harvesting serum, spleen, and liver at 42 h postinfection. Upon dissection (subcutaneous infection), a single large lymph node on the side of infection was harvested from each mouse. Spleens and lymph nodes were collected in 0.7 ml of PBS and homogenized in the closed system Miltenyi gentleMacs homogenizer. 50 μl of homogenate was taken for serial dilutions and quantification of bacterial loads per organ. Excess spleen homogenate was treated immediately with protease and phosphatase inhibitor (Roche) and ciprofloxacin and analyzed for cytokines by ELISA. Blood was harvested by cheek bleed and processed for collection of serum as previously described (17). Livers were fixed in 10% formalin and stained by hematoxylin and eosin. Vaccination experiments were performed by infecting mice surviving infection with KIM1001ΔM/J on day 14 or 21 with virulent KIM1001 and monitoring survival for 25 days.
Statistical Analysis
In vitro assays were analyzed by two-way ANOVA followed by Bonferroni post-test. In vivo cytokines were tested for significance by one-way ANOVA followed by Bonferroni's multiple comparison. Nonparametric bacterial load data were analyzed by the Kruskal Wallis test followed by Dunn's post-test. Differences in survival were analyzed by Kaplan-Meyer analysis and log rank test. The values where p < 0.05 were considered significant.
Results
YopM and YopJ Differentially Influence Inflammasomes and Inflammatory Cytokines and Chemokines
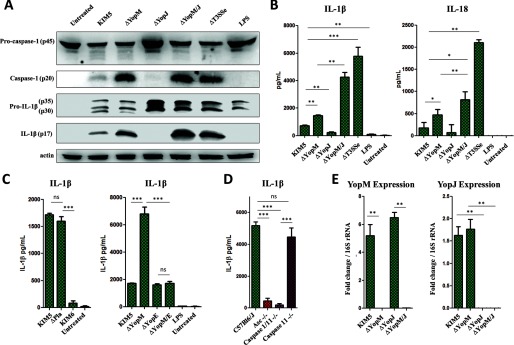
Deletion of YopM results in increased IL-1β/IL-18 secretion and caspase-1 cleavage (Fig. 1, A and B) in BMDMs compared with the parental strain. By contrast, loss of YopJ alone results in reduced IL-1β/IL-18 secretion (Fig. 1B) and caspase-1 activation (Fig. 1A), despite an increase in pro-IL-1β and pro-caspase-1. Loss of both YopM and YopJ leads to a further increase in ASC/caspase-1-dependent IL-1β and IL-18 secretion but an insignificant increase in caspase-1 activation compared with loss of YopM alone (Fig. 1, A, B, and D). We confirmed Yop expression in the ΔYopJ and ΔYopM strains (Fig. 1E).
FIGURE 1.
YopM and YopJ have opposing effects on caspase-1 cleavage, but in sum reduce IL-1β and IL-18 levels during infection. A, total protein from total BMDM samples (cell lysate and supernatant) at 6 h postinfection was separated by SDS-PAGE and analyzed by Western blot for IL-1β and caspase-1. B and C, BMDMs were infected with Y. pestis strains at MOI 10, and supernatants were harvested at 6 h postinfection for analysis of IL-1β or IL-18 for ELISA. D, supernatants from BMDMs from the indicated mouse genotypes were harvested 6 h postinfection with KIM5 ΔYopM/J (MOI 10) and assayed for IL-1β by ELISA. E, RNA was extracted from Y. pestis (grown at 37 °C to up-regulate T3SS expression), and amplified by RT-PCR using primers specific for YopM or YopJ. C(t) values for each primer pair were normalized to Y. pestis 16S rRNA internal control C(t) values. The figure is representative of three or more experiments. Shown are the means ± S.D. for triplicate wells. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ns, not significant.
We found that YopE may play a part in triggering the IL-1β release that is inhibited by YopM (Fig. 1C). YopE is an effector that regulates GTPase activity in host cells (29). We are still uncertain of how YopE influences the signaling, but YopE may impact bacterial effector translocation (30) or directly affect the host signaling pathways.
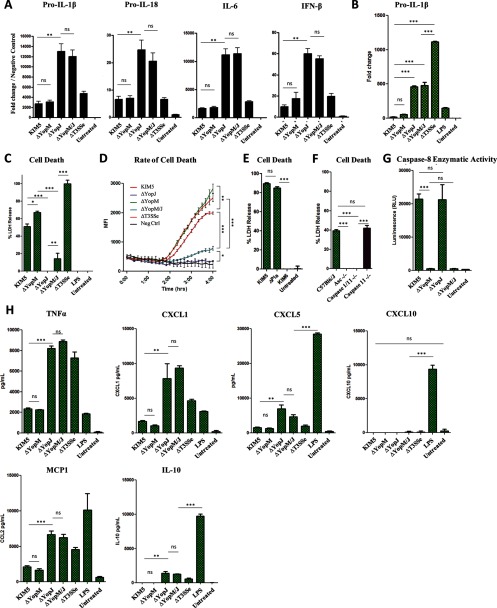
RT-PCR analysis demonstrated that YopJ, and not YopM, is primarily responsible for inhibiting the transcription of IL-1β/IL-18 precursors, as well as IL-6 and IFN-β (Fig. 2, A and B). We found that YopM confers protection from caspase-1-dependent cell death in the absence of YopJ but has only a weak cytoprotective effect when YopJ is present (Fig. 2, C, D, and F). We also confirmed that YopJ, but not YopM, drives the enzymatic activity of caspase-8 (Fig. 2G). Expression of TNFα, CXCL1, MCP1, CXCL5, and IL-10, which require no inflammasome processing, is also inhibited by YopJ but not YopM (Fig. 2H). Notably, the pCD1 plasmid containing the T3SS is essential for IL-1β induction, because the KIM6 strain lacking pCD1 failed to induce any IL-1β. (Figs. 1, A–C, and 2, C–E). The lack of another known virulence factor, the plasminogen activator protein (Pla) (25), had no effect on cell death or IL-1β release (Figs. 1C and 2E).
FIGURE 2.
YopJ, but not YopM, inhibits expression of pro-IL-1β and other cytokines and is a major driver of macrophage cell death. A and B, RNA from peritoneal macrophages (A, unprimed) or BMDMs (B) were harvested at 2 h postinfection and amplified by RT-PCR using primers specific for pro-IL-1β, pro-IL-18, IL-6, or IFN-β. C–E, cell death in BMDMs was assayed either at 5 h postinfection by assaying LDH release (C and E) or continuously by measuring intracellular EtHD-1 entry (D). F, cell death was assayed at 5 h postinfection with KIM5 ΔYopM/J at MOI 10 by assaying LDH release. G, caspase-8 enzymatic activity in BMDMs was assayed at 3 h postinfection. H, unprimed BMDMs were infected with Y. pestis at MOI 10, and supernatants were harvested at 6 h postinfection for quantification of cytokines by ELISA. The figure is representative of two or more independent experiments. Shown are the means ± S.D. for triplicate wells. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ns, not significant.
YopM and YopJ Have Redundant Impact on Virulence in Vivo
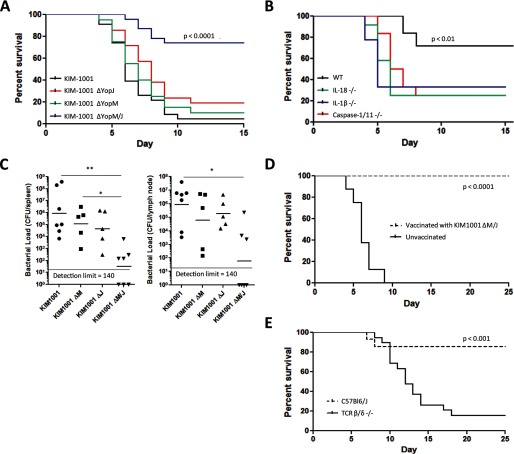
We found that in the mouse bubonic plague model, Y. pestis KIM1001 lacking both YopM and YopJ was significantly attenuated. In contrast, Y. pestis lacking either YopM or YopJ alone induced lethality comparable with the parent strain, with no significant differences from the fully virulent KIM1001 (Fig. 3A). In mice lacking caspase-1, IL-1β, or IL-18, the attenuation of the KIM1001ΔM/J strain was significantly reduced (Fig. 3B), suggesting that these components play central roles in the host defenses toward Y. pestis lacking both YopM and YopJ. We note that both WT and IL-18 KO mice die of low doses of the parental KIM1001 strain (17). On day 4 of subcutaneous infection, bacterial loads were significantly lower in mice infected with KIM1001ΔM/J both in the spleen and lymph nodes, but KIM1001ΔM and KIM1001ΔJ did not result in statistically different bacterial loads from the parental KIM1001 (Fig. 3C).
FIGURE 3.
Deletion of both YopM and YopJ attenuates Y. pestis in a manner dependent on caspase-1, IL-1β, and IL-18. A, C57Bl6/J mice were injected subcutaneously with 50–140 cfu of KIM1001 (n = 23), KIM1001ΔM (n = 21), KIM1001ΔJ (n = 20), or KIM1001ΔM/J (n = 23) and monitored for survival. The results represent pooled data from two separate experiments. B, C57Bl6/J (n = 9), IL-18 KO (n = 12), IL-1β KO (n = 9), or caspase-1/11 KO (n = 12) mice were injected with 225 cfu of KIM1001ΔM/J subcutaneously and monitored for survival. The p values reflect comparisons between KIM1001ΔM/J and other bacterial strains (A) or between wild-type mice and other mouse strains (B). C, C57Bl6/J mice were injected subcutaneously with 50–140 cfu of KIM1001 (n = 7), KIM1001ΔM (n = 5), KIM1001ΔJ (n = 5), or KIM1001ΔM/J (n = 7); spleens and lymph nodes were harvested 90 h postinfection, and bacterial load was determined. Geometric mean values are shown. D, C57Bl6/J mice that survived infection with KIM1001ΔM/J (n = 7) or naïve controls (n = 8) were injected subcutaneously with 400 cfu of KIM1001 and monitored for survival for 25 days. The p value reflects the comparison of vaccinated versus naive animals. E, C57Bl6/J mice (n = 14) or TCR β/δ −/− mice (n = 20) were injected subcutaneously with 120–180 cfu of KIM1001ΔM/J and monitored for survival for 25 days. The results represent pooled data from two separate experiments. The p value reflects comparison between wild-type and TCR β/δ −/− mice.
The increased activation of innate immunity as observed with the KIM1001ΔM/J may also impact adaptive immune responses. We found that wild-type mice that survive initial challenge with KIM1001ΔM/J were effectively protected from subsequent infection with fully virulent Y. pestis (Fig. 3D), indicating that strains containing deletions of YopM and YopJ, perhaps in combination with expression of LpxL (18), could be promising vaccine candidates. Adaptive immunity may also contribute to resistance of attenuated Y. pestis strains (18, 31), and T cells appear to play a key role (32, 33). To explore this question further, we subjected mice lacking T cell receptors (TCR β/δ −/− mice lack both αβ and γδ TCRs) (34) to the same subcutaneous challenge with KIM1001ΔM/J as in Fig. 3B. We found that the T cell receptors are very important for mice to survive the initial infection with KIM1001ΔM/J (Fig. 3E). Furthermore, the three TCR β/δ−/− mice that survived the initial challenge were not protected from KIM1001; when infected with KIM1001 on day 21, all three died by day 30, whereas 100% (total 21 of 21) of the rechallenged wild-type controls survived beyond day 40. One limitation of these studies is that we cannot rule out a role of B-cells or antibodies; nevertheless, T cells are crucial for the resistance against bacterial challenge, suggesting participation of both innate and adaptive immune responses in optimal host responses to these bacterial strains.
Inhibition of IL-1β and IL-18 Production Are Overlapping Functions of YopM and YopJ in Mice, but Other Effects Differ
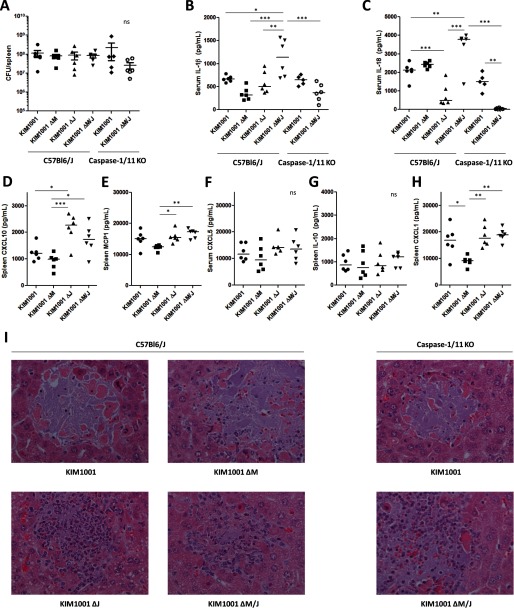
During the subcutaneous infection described above (Fig. 3), it is likely that systemic IL-1β and IL-18 levels may correlate to bacterial loads, and thus this experimental setup may not be optimal to study direct effects of YopM/J deletion on cytokine expression. For this reason, we injected the Y. pestis strains into mice intravenously and harvested tissue at 42 h to compare systemic IL-1β and IL-18 with smaller differences in host bacterial loads between the different strains (Fig. 4). We confirmed that bacterial loads in the spleen at this time were equivalent in all groups with no statistical differences, validating the comparison of cytokines between the groups (Fig. 4A). We found significantly elevated IL-1β and IL-18 in the serum of mice infected with KIM1001ΔM/J (Fig. 4, B and C). In contrast, however, we also detected a small but significant reduction in IL-18 when YopJ alone was deleted. Furthermore, although IL-1β and IL-18 expression in the KIM1001ΔM/J group was expressed in a caspase-1-dependent manner, KIM1001 induced low but significant levels of these cytokines independently of caspase-1, perhaps indicating a role of caspase-8 (8). The NF-κB-influenced chemokines CXCL1, CXCL10, CXCL5, and MCP1 were elevated in the absence of YopJ but not YopM (Fig. 4, D–F and H), similar trends as observed with macrophages in vitro (Fig. 2). Although the levels of CXCL5 were not significantly different in any strain, they trended higher in the absence of YopJ (Fig. 4F). IL-10 levels were not affected by either YopM or YopJ in vivo (Fig. 4G). On evaluation of liver histology, we noted that deletion of YopJ, but not YopM, appeared associated with the increased presence of both polymorphonuclear and mononuclear cells at foci of infection (Fig. 4I), possibly related to the regulation of chemokines (Figs. 2 and 4).
FIGURE 4.
YopM predominantly suppresses IL-1β and IL-18, whereas YopJ may limit cell recruitment in part by reducing chemokine expression during infection in vivo. C57Bl6J or caspase-1/11 KO mice were injected intravenously with 40–50 cfu of KIM1001, KIM1001ΔM, KIM1001ΔJ, or KIM1001ΔM/J in 200 μl PBS (n = 5 caspase-1/11 KO mice infected with KIM1001ΔM/J, all other groups n = 6). Tissues were harvested at 42 h postinfection. A, bacterial load was determined from spleen homogenates. B and C, serum was used to quantify IL-1β (B) and IL-18 (C). D–H, spleen homogenates were used to quantify CXCL10, MCP1, CXCL5, IL-10, and CXCL1 by ELISA. Shown are median values. I, livers were stained with hematoxylin and eosin, and representative foci of infection were selected. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ns, not significant.
YopM and YopJ May Have Different Functions in Other Cell Types
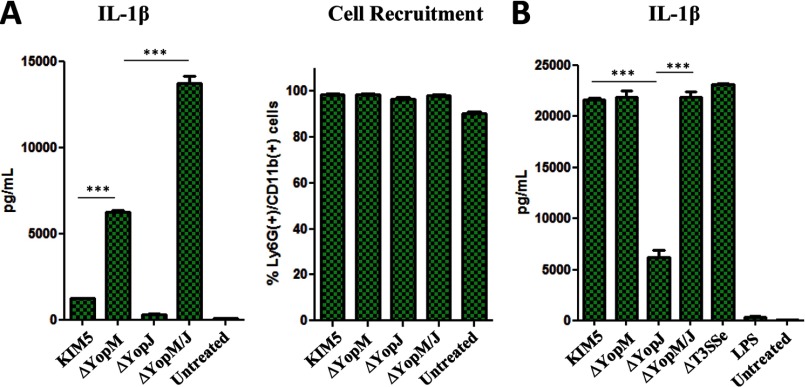
In view of some of the overlapping effects of YopM and YopJ, we investigated how they affect IL-1β production in innate immune cells other than macrophages (35). We found that the relative roles of YopM and YopJ in IL-1β production in vivo in a neutrophil-enriched (90–98% Ly6G/CD11b+) peritoneal cell population (Fig. 5A) follow a pattern similar to macrophages in vitro (Fig. 1B). In BMDCs, however, IL-1β secretion is triggered by KIM5 and significantly reduced in the absence of YopJ. By contrast, IL-1β secretion is minimally altered by the absence of YopM unless YopJ is also absent (Fig. 5B). These results suggest that the bacterial regulation of IL-1β release in dendritic cells (DCs) is markedly different from macrophages and neutrophils.
FIGURE 5.
YopJ and YopM may have different effects on IL-1β in dendritic cells compared with neutrophils and macrophages. A, mice were injected intraperitoneally with 0.7 ml of 4% thioglycolate for 3 h to trigger heavy neutrophil influx, followed by injection with 1 × 108 cfu of the indicated bacterial strains in 0.3 ml of PBS for an additional 6 h. Peritoneal lavage fluid was assayed for IL-1β ELISA (left panel). The cells from peritoneal lavage were analyzed for cell surface markers by FACS to confirm comparable and robust influx of Ly6G/CD11b positive cells (right panel, % Ly6G+/CD11b+ of all conditions). B, BMDCs were infected with Y. pestis at MOI 10, and supernatants were harvested at 6 h postinfection and assayed for IL-1β by ELISA. Shown are means of triplicates, representative of two separate experiments.
Discussion
Our findings illustrate parallel strategies of a microbial pathogen to suppress generation of pro-inflammatory cytokines as a means of resisting host defense. Because IL-1β and IL-18 are central mediators of anti-microbial host defenses, it may be particularly desirable to block their production. These cytokines are produced in a multistep process, potentially as a failsafe mechanism because excess production may also contribute to shock (36). However, this complex regulation also opens opportunities for diverse microbial approaches to dampen their production and release. The Y. pestis effectors YopJ and YopM have apparent opposite effects on caspase-1 processing; however, in the absence of both effectors, it becomes apparent that the sum of the actions of YopJ and YopM suppresses IL-1β and IL-18 release more effectively than either YopM or YopJ alone. We summarize some of our major findings about the respective roles of YopM and YopJ in the regulation of IL-1β/IL-18 production in Fig. 6. In general, the dampening of caspase-1 processing mediated by YopM is dominant, and when YopM is deleted, YopJ appears to mainly suppress pro-IL-1β and not contribute to caspase-1 cleavage in a major way. Neither of these effectors appears to enhance disease progression when the other is present, and we propose that their actions promote virulence in vivo by a redundant net effect on IL-1β and IL-18. Nevertheless, their effects may vary by cell type and reflect the heterogeneity of the pathways regulating IL-1β/IL-18 production. Although their net effects on IL-1β/IL-18 overlap, their roles in regulating other aspects of the immune response are distinct and in some ways appear to oppose one another.
FIGURE 6.
Summary of proposed YopM and YopJ effects on IL-1β/IL-18 production and cell death in host cells. In the absence of YopJ and YopM, the cells respond to Y. pestis by expressing high levels of pro-IL-1β and pro-IL-18 and activating robust caspase-1 cleavage accompanied by pyroptotic cell death. YopM can inhibit IL-1β/IL-18 release and cell death by inhibiting inflammasome-mediated caspase-1 cleavage, but it has little effect on expression of pro-IL-1β/IL-18 or other cytokines not requiring inflammasome processing. In contrast, YopJ limits IL-1β/IL-18 production by suppressing expression of precursors and possibly by inducing rapid cell death, which may overshadow the cytoprotective effect of YopM. Additionally, YopJ alone triggers modest caspase-1 cleavage with some degree of IL-1β/IL-18 production, limited by the reduced levels of precursors, including pro-caspase-1 itself.
Previous studies have shown several immunosuppressive functions of YopJ (3, 5–7, 37), and it has been puzzling that this effector does not appear to be needed for Y. pestis virulence (11, 12) (Fig. 3A). In contrast, immune stimulation induced by this effector molecule, such as the activation of caspase-1 and IL-1β/IL-18 release, has also been reported (9, 10). One hypothesis is that the immunosuppressive and immune stimulatory actions of YopJ could balance each other, explaining why YopJ deletion has little effect on bacterial replication in vivo. Similarly, deletion of YopM has a modest effect on virulence via intradermal delivery of the CO92 strain (14), and no significant effect upon deletion in the KIM1001 strain upon subcutaneous delivery (Fig. 3A). Notably, the CO92 strain carries a variant of YopJ that is less catalytically active than the KIM1001 variant (10, 38, 39). It is possible that this variant of YopJ in CO92 partially unmasks the contribution of YopM to virulence. Another aspect worth considering is that reduced activity or secretion of YopJ/YopP may not necessarily benefit the host (40, 41). Nevertheless, our data suggest that YopJ and YopM, rather than being dispensable for plague virulence, are effective promoters of virulence capable of replacing each other's net effect in vivo by targeting different arms of the same immune defense mechanism.
The parental KIM5 strain normally triggers only low levels of IL-1β/IL-18 release in BMDMs compared with the ΔT3SSe strain that lacks translocated Yops; this reflects the efficient suppression of IL-1β/IL-18 by the cumulative action of these Yops. We and other groups have previously observed that YopJ triggers caspase-8 activation, which triggers some caspase-1 activation and IL-1β/IL-18 release (8, 9, 40); here, this is likely reflected in Figs. 1 (A and B) and 4 (B and C). Low levels of IL-1β/IL-18 observed in response to parental Y. pestis in vivo appear less influenced by caspase-1 and could be due to direct processing of the precursor forms by caspase-8 (42). Nevertheless, these IL-1β/IL-18 levels may not alter the outcome of infection (Figs. 3 and 4). It is possible that YopJ-induced activation is offset by the significant inhibition of pro-IL-1β and pro-IL-18 or other NF-κB-dependent factors (Figs. 2 and 4). This effect on signal 1 may explain why IL-1β/IL-18 levels remain relatively low in the absence of YopM in vivo (Fig. 4, B and C).
By contrast, YopM appears not to have any effect on signal 1 (Fig. 2) but rather achieves IL-1β/IL-18 suppression by inhibiting caspase-1 activation (Fig. 1, A and D). Consistent with this role, YopM inhibits caspase-1-dependent cell death, although in the presence of YopJ, the cytoprotective role of YopM appears minor (Fig. 2, C, D, and F). It is unclear what the implications of this small effect would be in vivo. YopJ appears to be a major driver of cell death, with some inhibitory effects mediated by YopM. Very recent findings on cell death mediated by caspase-11 and caspase-1 via gasdermin D (43, 44) and by NEK7 via NLRP3/caspase-1 (45, 46) suggest that there are a number of additional players involved in regulation of inflammasome and pyroptosis pathways. Furthermore, other bacterial components, such as YopK (47), could differently impact the various death pathways. Several molecules, in addition to those studied here, are likely involved in regulating cytotoxicity induced by the different Yersinia strains. The identification of new cell death-mediating proteins could add to the understanding of why the various pathways induce different types and degrees of cell death.
Considering the seeming redundant effects of YopM and YopJ that we report here, it is notable that YopM and YopJ sequences are well conserved in the Y. pestis genome and present in all virulent strains of Y. pestis, Yersinia enterocolitica, and Yersinia pseudotuberculosis; however, it is also worth considering that the CO92 strain, which contains a less catalytically active version of YopJ, may have been more recently evolved than the KIM1001 strain used in this study. Furthermore, Y. pestis infects a wide range of animals, with significant differences in immune systems and sensitivities to infection. Because a major strategy of Y. pestis is immune evasion to assist propagation rather than to kill the organism, it is possible that YopM and YopJ evolved to suppress the IL-1β/IL-18 producing pathway in alternative ways without a significant difference in host survival.
Our results underscore some important differences between YopM and YopJ, suggesting that although their effects on IL-1β/IL-18 and survival in mice overlap, their functions should not be viewed as redundant. There may be other factors important for the fitness of Y. pestis in its unique life cycle that make both effectors indispensable, independent of host survival. YopJ inhibits the expression of multiple cytokines and chemokines both in vitro (Fig. 2H) and in vivo (Fig. 4, D–H) in addition to modulating IL-1β/IL-18, whereas YopM appears to primarily inhibit IL-1β/IL-18 maturation by affecting caspase-1. One study shows that YopM has a minor influence on the expression of immunity-related genes in vivo (48), supporting the idea of a more targeted role compared with YopJ. Furthermore, although YopM and YopJ may have similar effects on IL-1β secretion in neutrophils and macrophages (Figs. 1 and 5A), their respective roles are less clear in DCs (Fig. 5B). It appears that the absence of YopM has little bearing on IL-1β secretion in DCs, although YopM may have an effect when YopJ is absent (Fig. 5B). In any case, we propose that regulation of IL-1β release by YopJ and YopM in DCs is markedly different from in macrophages and neutrophils. Evidence for a direct role of YopM in DCs is lacking, although YopM appears to limit recruitment of DCs, as well as other cell types (13, 49); however, these observations may be explained by the role YopM plays in suppressing IL-1β and IL-18 secretion. YopJ, in contrast, has been shown to inhibit signaling and cytokine production in DCs directly (50, 51). Importantly, YopJ prevents DCs from activating natural killer cells, T cell proliferation, and IFN-γ induction. Our findings support a critical role of T cells in antibacterial defenses during Y. pestis infection (Fig. 3E), and studies in Y. enterocolitica also argue for the importance of a DC-induced T cell response (52). The ability of YopJ to inhibit TNFα and IFN pathways likely contributes to a defective CD8+ T cell response in pneumonic plague (33), potentially promoting bacterial propagation independently of YopM in some circumstances. Taken together, we think this suggests that YopJ may be particularly important for regulating immune responses coordinated by DCs and T cells.
YopJ has been proposed as a key Yersinia effector inducing caspase-1 cleavage (9, 10). Our current results suggest that YopJ does not contribute to caspase-1 cleavage in a major way when YopM is absent; rather, other components of the Y. pestis T3SS trigger the majority of caspase-1 cleavage in the absence of YopM. Unexpectedly, we found that deletion of YopE seemingly neutralized the IL-1β induction blocked by YopM (Fig. 1C). Earlier studies in other cell systems suggested that YopE could inhibit caspase-1 activation (53) and signal 1 (54). Our findings warrant further studies, evaluating the contributions of YopE both on effector protein translocation (30) and on inflammasome signals 1 and 2.
Although it is unclear which mechanisms YopM uses for inhibition of inflammasome activation, direct interaction with caspase-1 and the scaffold protein Iqgap1 have been proposed (15, 16). Interactions between YopM and kinases such as RSK1/2 and PKN1/2 (55–57), known for binding to the effector, may also play a role in caspase-1 regulation. Regardless of the method for YopM-mediated inhibition, it is possible that the pathway blocked by YopM could be a dominant pathway in Yersinia-induced inflammasome activation. Multiple candidate pathways could potentially be blocked by YopM, because NLRP3, NLRC4, and NLRP12 have all been proposed as mediators of Yersinia-induced IL-1β/IL-18 production. We cannot exclude the possible involvement of additional pathways that sense type III secretion system molecules. We also cannot disregard the idea that bacterial molecules other than those belonging to the T3SS contribute to modulation of caspase-1 cleavage and IL-1β production in vivo (58). We add to a large body of evidence (Fig. 1) suggesting minimal macrophage IL-1β release in the absence of the pCD1 T3SS-containing plasmid or the YopB/YopD components of the pore-forming translocon (59). The role of the translocon is emphasized by data suggesting that increased translocation of YopB increases inflammasome activation (10, 60). YopK also participates in regulation of influx of other molecules into the host cell, and a net effect of YopK is inhibition of caspase-1 processing by the NLRP3 and NLRC4 inflammasomes (47, 60). The picture that emerges is complex, and this suggests that many T3SS-related molecules together regulate IL-1β/IL-18 release. Our experiments indicate that their relative roles may be dependent upon the presence or absence of other specific molecules.
The fact that so many bacterial components are involved in regulating IL-1β/IL-18 suggests that it is important for the bacterium to keep these cytokines in check. Indeed, our previous studies have indicated the power of IL-18 and IL-1β in inducing host antibacterial defenses toward Y. pestis strains (17). In that respect, Y. pestis serves as an interesting model system for investigating immune evasion by pathogens and microbial manipulation of host IL-1β and IL-18 release.
Author Contributions
D. R. performed experiments, with some help from M. P. A. O., K. K. S., and R. M.-R.; M. K. P. and J. D. G. generated bacterial mutants and established the EthD-1 cytotoxic assay. E. L. conceived the study and supervised all aspects of the work. D. R. and E. L. wrote the paper with input from all authors.
Acknowledgments
We thank Dr. Christopher Sassetti and Gail Germain for help with animal experiments; Ray Welsh for TCRβ/δ−/− mice; and Neal Silverman, Kate Fitzgerald, and John Harris for discussions.
The work was supported by National Institutes of Health Grants AI07538 (to E. L.) and AI095213 (to D. R.), the Norwegian Cancer Society, and Research Council of Norway Center of Excellence Funding Scheme Project 223255/F50. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- T3SS
- type III secretion system
- NLR
- NOD-like receptor
- MOI
- multiplicity of infection
- BMDM
- bone marrow-derived macrophage
- BMDC
- bone marrow-derived dendritic cell
- DC
- dendritic cell
- TCR
- T-cell receptor.
References
- 1. Perry R. D., and Fetherston J. D. (1997) Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 10, 35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou L., Tan A., and Hershenson M. B. (2004) Yersinia YopJ inhibits pro-inflammatory molecule expression in human bronchial epithelial cells. Respir. Physiol. Neurobiol. 140, 89–97 [DOI] [PubMed] [Google Scholar]
- 3. Mittal R., Peak-Chew S. Y., and McMahon H. T. (2006) Acetylation of MEK2 and IκB kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 18574–18579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mukherjee S., and Orth K. (2008) In vitro signaling by MAPK and NFκB pathways inhibited by Yersinia YopJ. Methods Enzymol. 438, 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paquette N., Conlon J., Sweet C., Rus F., Wilson L., Pereira A., Rosadini C. V., Goutagny N., Weber A. N., Lane W. S., Shaffer S. A., Maniatis S., Fitzgerald K. A., Stuart L., and Silverman N. (2012) Serine/threonine acetylation of TGFβ-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl. Acad. Sci. U.S.A. 109, 12710–12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meinzer U., Barreau F., Esmiol-Welterlin S., Jung C., Villard C., Léger T., Ben-Mkaddem S., Berrebi D., Dussaillant M., Alnabhani Z., Roy M., Bonacorsi S., Wolf-Watz H., Perroy J., Ollendorff V., and Hugot J. P. (2012) Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe 11, 337–351 [DOI] [PubMed] [Google Scholar]
- 7. Zhou H., Monack D. M., Kayagaki N., Wertz I., Yin J., Wolf B., and Dixit V. M. (2005) Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-κB activation. J. Exp. Med. 202, 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weng D., Marty-Roix R., Ganesan S., Proulx M. K., Vladimer G. I., Kaiser W. J., Mocarski E. S., Pouliot K., Chan F. K., Kelliher M. A., Harris P. A., Bertin J., Gough P. J., Shayakhmetov D. M., Goguen J. D., Fitzgerald K. A., Silverman N., and Lien E. (2014) Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. U.S.A. 111, 7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Philip N. H., Dillon C. P., Snyder A. G., Fitzgerald P., Wynosky-Dolfi M. A., Zwack E. E., Hu B., Fitzgerald L., Mauldin E. A., Copenhaver A. M., Shin S., Wei L., Parker M., Zhang J., Oberst A., Green D. R., and Brodsky I. E. (2014) Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc. Natl. Acad. Sci. U.S.A. 111, 7385–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lilo S., Zheng Y., and Bliska J. B. (2008) Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect. Immun. 76, 3911–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lemaître N., Sebbane F., Long D., and Hinnebusch B. J. (2006) Yersinia pestis YopJ suppresses tumor necrosis factor α induction and contributes to apoptosis of immune cells in the lymph node but is not required for virulence in a rat model of bubonic plague. Infect. Immun. 74, 5126–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palace S. G., Proulx M. K., Lu S., Baker R. E., and Goguen J. D. (2014) Genome-wide mutant fitness profiling identifies nutritional requirements for optimal growth of Yersinia pestis in deep tissue. MBio 5, e01385–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kerschen E. J., Cohen D. A., Kaplan A. M., and Straley S. C. (2004) The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72, 4589–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Z., Kerschen E. J., Cohen D. A., Kaplan A. M., van Rooijen N., and Straley S. C. (2009) Gr1+ cells control growth of YopM-negative Yersinia pestis during systemic plague. Infect. Immun. 77, 3791–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LaRock C. N., and Cookson B. T. (2012) The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe 12, 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung L. K., Philip N. H., Schmidt V. A., Koller A., Strowig T., Flavell R. A., Brodsky I. E., and Bliska J. B. (2014) IQGAP1 is important for activation of caspase-1 in macrophages and is targeted by Yersinia pestis type III effector YopM. MBio 5, e01402–01414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vladimer G. I., Weng D., Paquette S. W., Vanaja S. K., Rathinam V. A., Aune M. H., Conlon J. E., Burbage J. J., Proulx M. K., Liu Q., Reed G., Mecsas J. C., Iwakura Y., Bertin J., Goguen J. D., Fitzgerald K. A., and Lien E. (2012) The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37, 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montminy S. W., Khan N., McGrath S., Walkowicz M. J., Sharp F., Conlon J. E., Fukase K., Kusumoto S., Sweet C., Miyake K., Akira S., Cotter R. J., Goguen J. D., and Lien E. (2006) Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 19. Leung K. Y., Reisner B. S., and Straley S. C. (1990) YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58, 3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Latz E., Xiao T. S., and Stutz A. (2013) Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gurung P., and Kanneganti T. D. (2015) Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am. J. Pathol. 185, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bossaller L., Chiang P. I., Schmidt-Lauber C., Ganesan S., Kaiser W. J., Rathinam V. A., Mocarski E. S., Subramanian D., Green D. R., Silverman N., Fitzgerald K. A., Marshak-Rothstein A., and Latz E. (2012) Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 189, 5508–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Broz P., and Monack D. M. (2013) Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog. 9, e1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W. P., Roose-Girma M., and Dixit V. M. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 [DOI] [PubMed] [Google Scholar]
- 25. Sodeinde O. A., Subrahmanyam Y. V., Stark K., Quan T., Bao Y., and Goguen J. D. (1992) A surface protease and the invasive character of plague. Science 258, 1004–1007 [DOI] [PubMed] [Google Scholar]
- 26. Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., and Pease L. R. (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 [DOI] [PubMed] [Google Scholar]
- 27. Edwards R. A., Keller L. H., and Schifferli D. M. (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207, 149–157 [DOI] [PubMed] [Google Scholar]
- 28. Lenz J. D., Lawrenz M. B., Cotter D. G., Lane M. C., Gonzalez R. J., Palacios M., and Miller V. L. (2011) Expression during host infection and localization of Yersinia pestis autotransporter proteins. J. Bacteriol. 193, 5936–5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Viboud G. I., and Bliska J. B. (2005) Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59, 69–89 [DOI] [PubMed] [Google Scholar]
- 30. Aili M., Isaksson E. L., Carlsson S. E., Wolf-Watz H., Rosqvist R., and Francis M. S. (2008) Regulation of Yersinia Yop-effector delivery by translocated YopE. Int. J. Med. Microbiol. 298, 183–192 [DOI] [PubMed] [Google Scholar]
- 31. Szaba F. M., Kummer L. W., Wilhelm L. B., Lin J. S., Parent M. A., Montminy-Paquette S. W., Lien E., Johnson L. L., and Smiley S. T. (2009) D27-pLpxL, an avirulent strain of Yersinia pestis, primes T cells that protect against pneumonic plague. Infect. Immun. 77, 4295–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philipovskiy A. V., and Smiley S. T. (2007) Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect. Immun. 75, 878–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Szaba F. M., Kummer L. W., Duso D. K., Koroleva E. P., Tumanov A. V., Cooper A. M., Bliska J. B., Smiley S. T., and Lin J. S. (2014) TNFα and IFNγ but not perforin are critical for CD8 T cell-mediated protection against pulmonary Yersinia pestis infection. PLoS Pathog. 10, e1004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mombaerts P. (1995) Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int. Rev. Immunol. 13, 43–63 [DOI] [PubMed] [Google Scholar]
- 35. Marketon M. M., DePaolo R. W., DeBord K. L., Jabri B., and Schneewind O. (2005) Plague bacteria target immune cells during infection. Science 309, 1739–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Broz P., Ruby T., Belhocine K., Bouley D. M., Kayagaki N., Dixit V. M., and Monack D. M. (2012) Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mukherjee S., Keitany G., Li Y., Wang Y., Ball H. L., Goldsmith E. J., and Orth K. (2006) Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 38. Zheng Y., Lilo S., Mena P., and Bliska J. B. (2012) YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense. PLoS One 7, e36019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zauberman A., Cohen S., Mamroud E., Flashner Y., Tidhar A., Ber R., Elhanany E., Shafferman A., and Velan B. (2006) Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect. Immun. 74, 3239–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brodsky I. E., and Medzhitov R. (2008) Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 4, e1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zauberman A., Tidhar A., Levy Y., Bar-Haim E., Halperin G., Flashner Y., Cohen S., Shafferman A., and Mamroud E. (2009) Yersinia pestis endowed with increased cytotoxicity is avirulent in a bubonic plague model and induces rapid protection against pneumonic plague. PLoS One 4, e5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maelfait J., Vercammen E., Janssens S., Schotte P., Haegman M., Magez S., and Beyaert R. (2008) Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J. Exp. Med. 205, 1967–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kayagaki N., Stowe I. B., Lee B. L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q. T., Liu P. S., Lill J. R., Li H., Wu J., Kummerfeld S., Zhang J., Lee W. P., Snipas S. J., Salvesen G. S., Morris L. X., Fitzgerald L., Zhang Y., Bertram E. M., Goodnow C. C., and Dixit V. M. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 [DOI] [PubMed] [Google Scholar]
- 44. Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., and Shao F. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 [DOI] [PubMed] [Google Scholar]
- 45. Schmid-Burgk J. L., Chauhan D., Schmidt T., Ebert T. S., Reinhardt J., Endl E., and Hornung V. (2016) A genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi H., Wang Y., Li X., Zhan X., Tang M., Fina M., Su L., Pratt D., Bu C. H., Hildebrand S., Lyon S., Scott L., Quan J., Sun Q., Russell J., Arnett S., Jurek P., Chen D., Kravchenko V. V., Mathison J. C., Moresco E. M., Monson N. L., Ulevitch R. J., and Beutler B. (2016) NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brodsky I. E., Palm N. W., Sadanand S., Ryndak M. B., Sutterwala F. S., Flavell R. A., Bliska J. B., and Medzhitov R. (2010) A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7, 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uittenbogaard A. M., Chelvarajan R. L., Myers-Morales T., Gorman A. A., Brickey W. J., Ye Z., Kaplan A. M., Cohen D. A., Ting J. P., and Straley S. C. (2012) Toward a molecular pathogenic pathway for Yersinia pestis YopM. Front Cell Infect. Microbiol. 2, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye Z., Uittenbogaard A. M., Cohen D. A., Kaplan A. M., Ambati J., and Straley S. C. (2011) Distinct CCR2+ Gr1+ cells control growth of the Yersinia pestis DeltayopM mutant in liver and spleen during systemic plague. Infect. Immun. 79, 674–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lindner I., Torruellas-Garcia J., Kolonias D., Carlson L. M., Tolba K. A., Plano G. V., and Lee K. P. (2007) Modulation of dendritic cell differentiation and function by YopJ of Yersinia pestis. Eur. J. Immunol. 37, 2450–2462 [DOI] [PubMed] [Google Scholar]
- 51. Rosadini C. V., Zanoni I., Odendall C., Green E. R., Paczosa M. K., Philip N. H., Brodsky I. E., Mecsas J., and Kagan J. C. (2015) A single bacterial immune evasion strategy dismantles both MyD88 and TRIF signaling pathways downstream of TLR4. Cell Host Microbe 18, 682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Autenrieth S. E., Linzer T. R., Hiller C., Keller B., Warnke P., Köberle M., Bohn E., Biedermann T., Bühring H. J., Hämmerling G. J., Rammensee H. G., and Autenrieth I. B. (2010) Immune evasion by Yersinia enterocolitica: differential targeting of dendritic cell subpopulations in vivo. PLoS Pathog. 6, e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schotte P., Denecker G., Van Den Broeke A., Vandenabeele P., Cornelis G. R., and Beyaert R. (2004) Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1β. J. Biol. Chem. 279, 25134–25142 [DOI] [PubMed] [Google Scholar]
- 54. Thinwa J., Segovia J. A., Bose S., and Dube P. H. (2014) Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells. J. Immunol. 193, 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hentschke M., Berneking L., Belmar Campos C., Buck F., Ruckdeschel K., and Aepfelbacher M. (2010) Yersinia virulence factor YopM induces sustained RSK activation by interfering with dephosphorylation. PLoS One 5, e13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McPhee J. B., Mena P., and Bliska J. B. (2010) Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect. Immun. 78, 3529–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCoy M. W., Marré M. L., Lesser C. F., and Mecsas J. (2010) The C-terminal tail of Yersinia pseudotuberculosis YopM is critical for interacting with RSK1 and for virulence. Infect. Immun. 78, 2584–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Caulfield A. J., Walker M. E., Gielda L. M., and Lathem W. W. (2014) The Pla protease of Yersinia pestis degrades fas ligand to manipulate host cell death and inflammation. Cell Host Microbe 15, 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vladimer G. I., Marty-Roix R., Ghosh S., Weng D., and Lien E. (2013) Inflammasomes and host defenses against bacterial infections. Curr. Opin. Microbiol. 16, 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zwack E. E., Snyder A. G., Wynosky-Dolfi M. A., Ruthel G., Philip N. H., Marketon M. M., Francis M. S., Bliska J. B., and Brodsky I. E. (2015) Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD. MBio 6, e02095–02014 [DOI] [PMC free article] [PubMed] [Google Scholar]