Abstract
The potential antitumor activity of cannabinoid receptor agonists, such as the aminoalklylindole WIN55,212-2 (WIN2), has been studied extensively, but their potential interaction with conventional cancer therapies, such as radiation, remains unknown. In the present work, the influence of WIN2 on the antiproliferative activity of radiation in human (MCF-7 and MDA-MB231) and murine (4T1) breast cancer cells was investigated. The antiproliferative effects produced by combination of WIN2 and radiation were more effective than either agent alone. The stereoisomer of WIN2, WIN55,212-3 (WIN3), failed to inhibit growth or potentiate the growth-inhibitory effects of radiation, indicative of stereospecificity. Two other aminoalkylindoles, pravadoline and JWH-015 [(2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenyl-methanone], also enhanced the antiproliferative effects of radiation, but other synthetic cannabinoids (i.e., nabilone, CP55,940 [(+)-rel-5-(1,1-dimethylheptyl)-2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-phenol], and methanandamide) or phytocannabinoids [i.e., Δ9-tetrahydrocannabinol (THC) and cannabidiol] did not. The combination treatment of WIN2 + radiation promoted both autophagy and senescence but not apoptosis or necrosis. WIN2 also failed to alter radiation-induced DNA damage or the apparent rate of DNA repair. Although the antiproliferative actions of WIN2 were mediated through noncannabinoid receptor-mediated pathways, the observation that WIN2 interfered with growth stimulation by sphingosine-1-phosphate (S1P) implicates the potential involvement of S1P/ceramide signaling pathways. In addition to demonstrating that aminoalkylindole compounds could potentially augment the effectiveness of radiation treatment in breast cancer, the present study suggests that THC and nabilone are unlikely to interfere with the effectiveness of radiation therapy, which is of particular relevance to patients using cannabinoid-based drugs to ameliorate the toxicity of cancer therapies.
Introduction
The cannabinoids Δ9-tetrahydrocannabinol (THC; Marinol; Solvay Pharmaceuticals, Marietta, GA) and nabilone (Cesamet; Meda Pharmaceuticals, Somerset, NJ) are approved by the Food and Drug Administration for the treatment of emesis and nausea associated with cancer chemotherapy (Russo, 2008). The results of preclinical trials suggest that these agents may prove to be of use for patients experiencing nausea and vomiting due to radiation therapy (Darmani et al., 2007). Cannabinoids are also known to suppress growth or promote cell death in a variety of cancer cell lines, including glioma, pancreatic, melanoma, lymphoma, lung, and breast (Carracedo et al., 2006; Qamri et al., 2009; Salazar et al., 2009; McAllister et al., 2011; Preet et al., 2011; Scuderi et al., 2011; Wasik et al., 2011). Given that cannabinoid-based drugs are used for suppression of nausea and for appetite stimulation in patients with cancer, as well as their potential utility as adjunctive treatments along with conventional therapies such as radiation, the present studies were initiated to determine whether cannabinoids might augment the antiproliferative actions of radiation in breast tumor cells.
The aminoalkylindole derivative WIN55,212-2 (WIN2) has been extensively used to investigate the endogenous cannabinoid system (Compton et al., 1992). WIN2 binds with high affinity at both identified cannabinoid G-protein-coupled receptors, CB1 and CB2, and is considered to be a high-efficacy agonist based on agonist-stimulated [35S]guanosine 5[prime]-O-(3-thio)triphosphate binding assays (Sim et al., 1996; Breivogel et al., 1998), although it also stimulates noncannabinoid G-protein-coupled receptors (Breivogel et al., 2001; Nguyen et al., 2010) and the nuclear protein receptors peroxisome proliferator-activated receptors α-γ (PPARα-γ) (O’Sullivan, 2007). It is noteworthy that WIN2 suppresses the growth of melanoma, mantle cell lymphoma, non–small cell lung cancer, and breast cancer cell lines (Qamri et al., 2009; Preet et al., 2011; Scuderi et al., 2011; Wasik et al., 2011), as well as suppressing radiation-induced emesis in the least shrew (Darmani et al., 2007) and producing antinociceptive actions in rodent models of cancer pain (Guerrero et al., 2008). Despite its prevalent use as a cannabinoid pharmacological tool, WIN2 has yet to be examined for potential interactions with radiation in terms of tumor growth effects, despite the fact that radiation has been a mainstay of cancer therapy for decades.
The purpose of the present study was to determine the effect of WIN2 on the antiproliferative actions of radiation in breast cancer cells. Mechanistic studies were conducted on human MCF-7 cells, whereas human MDA-MB231 and murine 4T1 cells lines were used to assess whether the impact of the combined administration of WIN2 and radiation could be extended to other breast cancer lines. For the purpose of assessing selectivity, the combination treatment was also assessed in MCF-10A cells, a model of normal breast epithelial cells. Stereospecificity was determined utilizing the stereoisomer WIN55,212-3, which does not bind to either CB1 or CB2 receptors. The effect of radiation on breast tumor cell growth was also assessed in combination with a variety of structurally distinct cannabinoids, including THC, nabilone, CP55,940, methanandamide, cannabidiol (CBD), JWH-015, and pravadoline. Growth arrest and cell death were evaluated by monitoring senescence (Debacq-Chainiaux et al., 2009), autophagy (Goehe et al., 2012), apoptosis (Vermes et al., 1995), and mitotic catastrophe (Jonathan et al., 1999). Potential receptor binding sites that may mediate the antiproliferative actions of WIN2 were examined, which included selective CB1 and CB2 receptors, peroxisome proliferator-activated receptors (PPARs) (O’Sullivan 2007), and TRPV1 receptors (Pertwee et al., 2010). Finally, studies were performed evaluating the potential capacity of WIN2 to antagonize growth stimulation induced by sphingosine-1-phosphate (S1P), the S1P agonist SEW2871, and estradiol.
Materials and Methods
Cell Lines
MCF-7, MDA-MB231, and MCF-10A cells were obtained from ATCC (Manassas, VA). Luciferase transfected 4T1 cells were obtained from Caliper (Hopkinton, MA). MCF-7, MDA-MB231, and 4T1 cells were cultured in RPMI 1640 media with 1% penicillin/streptomycin solution, 5% fetal bovine serum, and 5% bovine calf serum. MCF-10A cells were cultured in Dulbecco’s modified Eagle’s medium/F12 media supplemented with 1% penicillin/streptomycin solution, 10% horse serum, 10 μg/ml insulin, 100 ng/ml cholera toxin, 20 ng/ml EGF, and 500 ng/ml hydrocortisone. For studies under low serum conditions, cells were cultured in RPMI 1640 medium with 1% penicillin/streptomycin, 0.05% fetal bovine serum, and 0.05% bovine calf serum. For studies using estradiol, MCF-7 cells were cultured in phenol red free improved minimum essential medium supplemented with 1% penicillin/streptomycin solution and 10% fetal bovine serum.
Drugs and Reagents
WIN55,212-2, WIN55,212-3, chloroquine diphosphate salt, staurosporine, CP55,940, methanandamide, nabilone, pioglitazone, bezafibrate, capsaicin, adriamycin, AM251, capsazepine, GW9662, and estradiol were purchased from Sigma-Aldrich (St. Louis, MO). CBD and ΤΗC were generously provided by the National Institute on Drug Abuse (Bethesda, MD). AM630 was purchased from Enzo Life Sciences (Farmingdale, NY). Pravadoline, JWH-015, and SEW2871 were purchased from Caymen Chemical (Ann Arbor, MI). S1P was a gift from the laboratory of Dr. Sarah Spiegel (Department of Biochemistry, Virginia Commonwealth University, Richmond, VA).
Drug Treatments
All treatments with cannabinoids, capsaicin, pioglitazone, S1P, SEW2871, and estradiol were initiated with a 24-hour exposure period, after which the drug-containing media were aspirated and the cells were washed and replaced with fresh media. Radiation was administered at the same time as drug, unless otherwise indicated. Exposure to drug antagonists was coincidental with the receptor agonists. Adriamycin (doxorubicin), as a positive control for select studies, was used at 1 μM with an exposure period of 2 hours. For autophagy inhibition, chloroquine (5 μM) was administered to cells for the entirety of the experiment. In experiments under low serum conditions, drugs were added to the low serum media for the first 24 hours and then removed and replaced with regular media. In studies involving estradiol, the cells were maintained in improved minimum essential medium through the course of the experiment. All experimental results were analyzed at 96 hours, unless otherwise indicated. Cell counts for 4T1 cells were determined at 48 hours because of their rapid growth rate.
Determination of Viable Cell Number
Cells were plated into six-well plates (MCF-7 and MDA-MB231 cells, 50,000 cells/well; 4T1 cells, 100,000 cells/well). Viability was determined based on trypan blue exclusion using a hemocytometer or Invitrogen Countess automated counter (Invitrogen, Grand Island, NY).
Crystal Violet Assay
Cells were plated into 96-well plates and allowed to adhere overnight (MCF-7 and MDA-MB231 lines, 5,000 cells; 4T1 cells, 10,000 cells). After 96 hours, cells were washed with phosphate-buffered saline (PBS), fixed with methanol and stained with a 0.5% solution of crystal violet in 25% methanol. Samples were solubilized with a 0.1M sodium citrate solution in 50% ethanol before absorbance was measured at 540 nm using a microplate reader.
γH2AX Quantified by Flow Cytometry
Both adherent and nonadherent cells were collected and pelleted at indicated time points using a 4°C 5810 R Eppendorf centrifuge at 500g. Samples were fixed in formaldehyde (3.7%) in PBS for 10 minutes at 37°C before being chilled on ice and repelleted. Fixative was removed, cells were permeabilized using methanol, the methanol was removed, and cells were washed twice with 5 mg/ml bovine serum albumin (BSA) in PBS and then blocked using the BSA solution for 10 minutes at room temperature. γH2AX-fluorescein isothiocyanate conjugated antibody was added at a dilution of 1:200 in 200 µl per sample followed by incubation for 60 minutes at room temperature. Cells were washed with BSA solution twice more before being resuspended in PBS. Measurements were performed by flow cytometry at a wavelength of 520 nm.
β-Galactosidase Activity Assay
Cells were plated into six-well plates at 10,000 cells/well. At appropriate time points, samples were fixed and histochemically stained as previously described (Biggers et al., 2013) using 5-bromo-4-chloro-indolyl-β-d-galactopyranoside as a substrate. Images of representative microscopic fields were captured on an Olympus 1 ×70 inverted microscope (Olympus America, Inc., Melville, NY), and senescent cells were quantified manually and reported as a percent of the total population.
Flow Cytometry for Annexin V and Propidium Iodide Staining
Cells were harvested at the indicated time points and washed twice with PBS followed by centrifugation at 500 g in a 4°C 5810 R Eppendorf centrifuge. Annexin V and PI were obtained from BD Biosciences (San Jose, CA) and diluted in binding buffer according to the manufacturer’s instructions before being added to cells. Samples were analyzed by flow cytometry at 520 nm for fluorescein isothiocyanate-labeled annexin V and 617 nm for PI.
Cell Staining
Cells were stained using DAPI and acridine orange as previously reported (Biggers et al., 2013).
Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from cells by using TRIzol Reagent (Invitrogen, Grand Island, NY) and reverse-transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The cDNA obtained from each sample was used as template for PCR using Mouse Genotyping Kit (KAPA Biosystems, Wilmington, MA). The primer was synthesized by Invitrogen, and primer sequences were as follows: CB1 forward, 5′-GACCATAGCCATTGTGATCG-3′; CB1 reverse, 5′-GGTTTCATCAATGTGTGGGA-3′; CB2 forward, 5′-GACCGCCATTGACCGATACC-3′; CB2 reverse, 5′-GGACCCACATGATGCCCAG-3′; TRPV1 forward, 5′-CTCACCAACAAGAAGGGAATG-3′; TRPV1 reverse, 5′-AGGTCGTACAGCGAGGAGTG-3′; PPARγ forward, 5′-ATGACAGCGACTTGGCAATA-3′; PPARγ reverse, 5′-GAGGACTCAGGGTGGTTCAG-3′; β-actin forward, 5′-TGGGACGACATGGAGAAA-3′; β-actin reverse, 5′-CACAGCCTGGATAGCAACG-3′. In addition, the PCR program was as follows: 95°C for 3 minutes, 35 cycles of 95°C for 15 seconds, 58°C for 15 seconds, 72°C for 20 second, and 72°C for 2 minutes. Primer sequences for CB1 and CB2 were contributed by Dr. Mary Abood of Temple University (Department of Anatomy and Cell Biology, Philadelphia, PA).
Statistics
All experiments were performed with three to six replicates. Each experiment included vehicle, WIN2, radiation and WIN2 + radiation, unless otherwise stated. Two-way repeated-measures analysis of variance (ANOVA) was used to analyze radiation versus drug treatments. One-way repeated-measures ANOVA was used to assess overall significance for dose-response experiments. The Tukey-Kramer test was used for post hoc comparisons when appropriate (P < 0.05). Paired t test with a Bonferroni correction was used to assess comparisons of combination + drug with the individual treatments (P < 0.0156). All data are displayed as mean ± S.E.
Results
Effect of the Combination of WIN55,212-2 With Radiation in Breast Cancer Cells
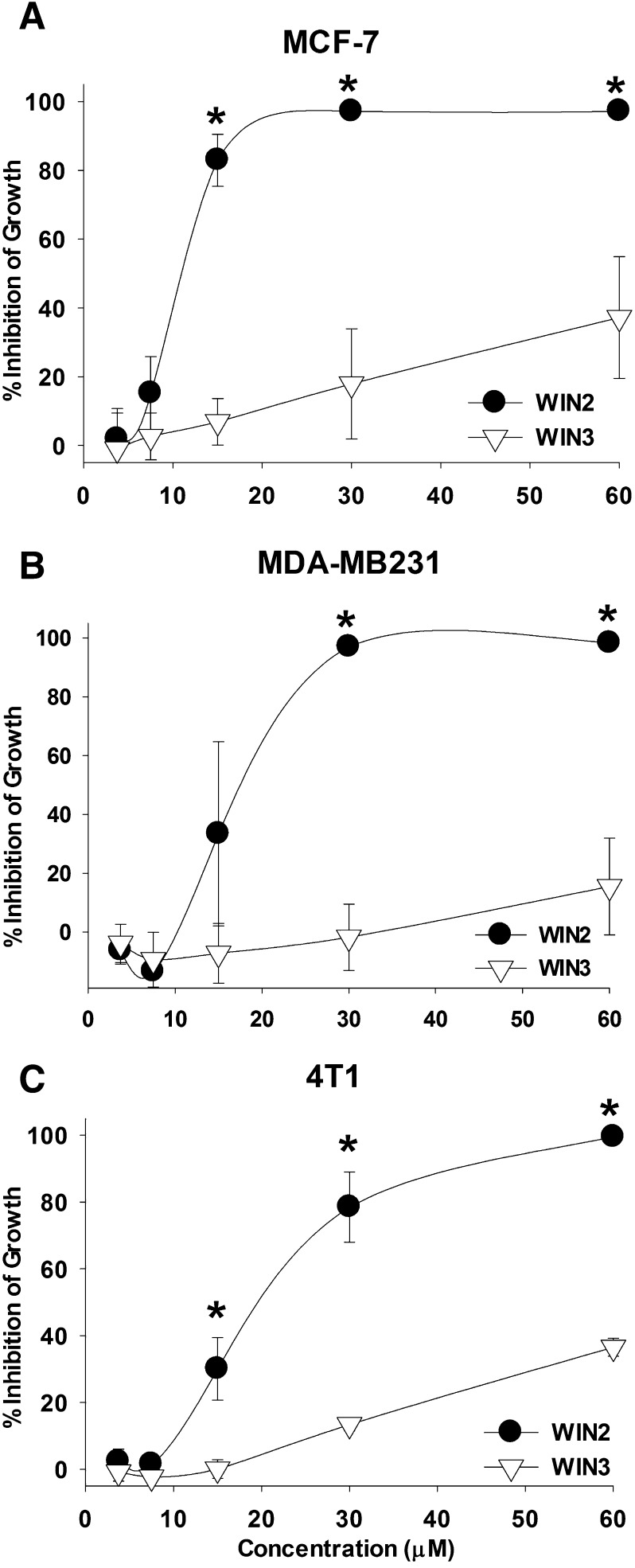
Initial studies were performed to determine sensitivity to WIN2 in two human and one murine breast tumor cell lines, specifically p53 wild-type estrogen receptor (ER)-positive MCF-7 cells, p53 mutant ER negative MDA-MB231 cells, and p53 null ER negative 4T1 cells. Figure 1, A–C, shows that WIN2 dose dependently inhibited growth of each breast cancer cell line. The ED50 values for WIN2 were 11.96 ± 3.31 μM in MCF-7 cells, 17.92 ± 6.75 μM in MDA-MB231 cells, and 18.24 ± 4.15 μM in 4T1 cells. To test whether the growth-inhibitory effects of WIN2 were stereospecific, the antiproliferative activity of its stereoisomer WIN55,212-3 (WIN3), which does not bind to cannabinoid receptors (Howlett et al., 2002), was also evaluated. As shown in Fig. 1, A–C, WIN3 lacked efficacy in all three cell lines, with no significant effects even at concentrations up to 60 µM. These findings establish stereoselectivity and support the premise that WIN2 likely interferes with breast tumor cell growth through its actions at a specific target.
Fig. 1.
WIN2 stereoselectively and dose dependently inhibits the growth of breast cancer cells. Growth inhibition by WIN2 and WIN3 was assessed at 96 hours post-treatment by the crystal violet assay in MCF-7 (A), MDA-MB231 (B), and 4T1 breast tumor cells (C). Data presented reflect the means of 4 individual experiments ± S.E.; *P < 0.05 versus WIN3 at each respective concentration of drug.
A preponderance of studies in the literature investigating the effects of cannabinoids on cancer cells have been performed under low-serum conditions (Carracedo et al., 2006; Salazar et al., 2009; McAllister et al., 2011; Wasik et al., 2011). In contrast, the experiments presented in the present work were performed using media containing 10% serum. Accordingly, to rule out the possibility that the relative activities of WIN2 and WIN3 might be a consequence of nonspecific serum binding, the capacity of WIN2 and its stereoisomer WIN3 to inhibit growth of MCF-7 cells was also assessed under serum-free conditions. Supplemental Figure 1 indicates that the absence of serum markedly increased the potency of WIN2 (a more than 3-fold reduction in the ED50 from ∼10 to ∼3 μM). However, WIN3 was entirely inactive, indicating that stereoselectivity was maintained under low-serum conditions.
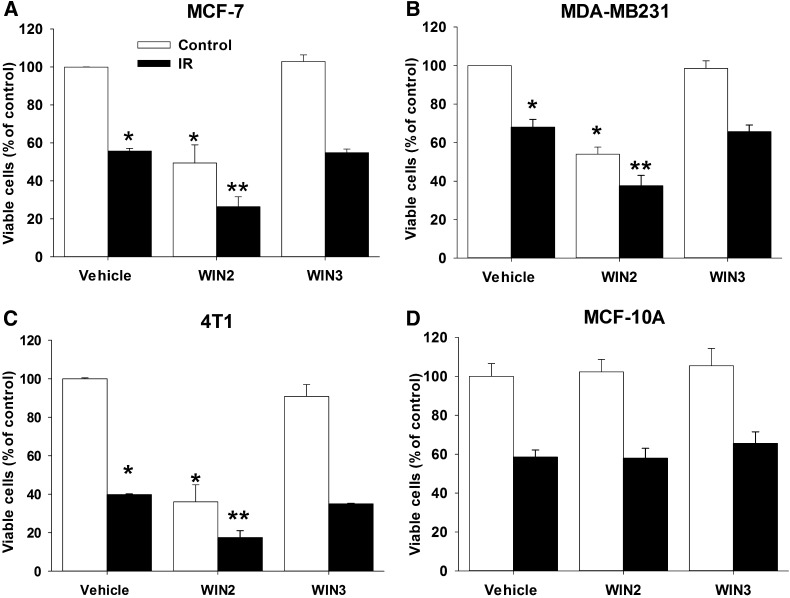
Subsequent studies were focused on determining whether WIN2 would alter the antiproliferative effects of radiation, one of the most frequently used therapies in the treatment of breast cancer. Figure 2, A–C, presents the effects of combined radiation and WIN2 (at a concentration that alone inhibits breast tumor growth by ∼50%) in each of the three breast tumor cell lines. The combination treatment was more effective than either treatment alone in all three breast tumor cell lines. WIN3 had no effect on sensitivity to radiation, again establishing the stereoselective action of WIN2.
Fig. 2.
Enhanced antiproliferative effects of the combination of WIN2 and radiation. Cells were exposed to vehicle, WIN2, or WIN3 either alone or with 2 Gy radiation in MCF-7 (A), MDA-MB231 (B), 4T1 (8 Gy) (C), and MCF-10A (D) cells. Cells were treated with equieffective doses of WIN2 based on the concentration effect curves in Fig. 1 (12 µM for MCF-7 cells, 15 µM for MDA-MB231 cells, 30 µM for 4T1 cells, and 12 µM for MCF-10A cells). All experiments were analyzed for cell viability by trypan blue exclusion 96 hours after drug treatment (4T1 cells were analyzed 48 hours after treatment because of rapid growth rate). Data presented reflect the means of three or four individual experiments ± S.E.; *P < 0.05 versus vehicle and **P < 0.0156 compared with vehicle, drug treatment alone, and radiation alone. Two-way ANOVA reports: MCF-7 [F(2,12) = 12.8, P < 0.05]; MDA-MB231 [F(2,16) = 4.1, P < 0.05]; 4T1 [F(2,8) = 14.7, P < 0.01]; MCF-10A (P = 0.95).
To evaluate whether the enhanced antiproliferative effects of the WIN2-radiation combination might extend to noncancerous cells, the combination was tested in MCF-10A cells, which are considered to be a model of normal breast epithelial cells (Tait et al., 1990). Figure 2D demonstrates that a concentration of WIN2 (12 µM) that enhanced the effects of radiation in MCF-7 cells failed to alter MCF-10A cell growth or to augment the antiproliferative effects of radiation.
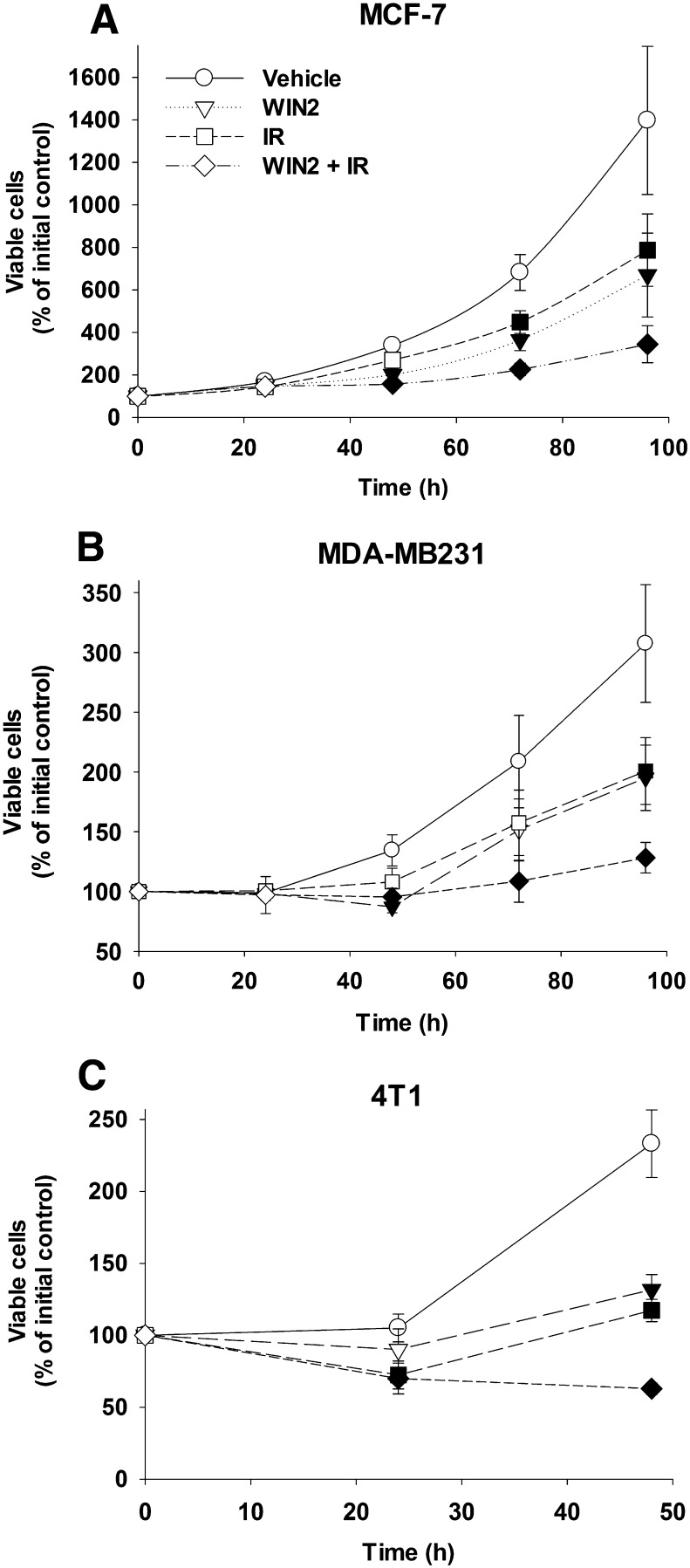
To determine whether the combination of WIN2 with radiation promoted growth arrest and/or cell death, a time course study was performed to monitor viable cell number after treatment with radiation or WIN2 alone and the combination of WIN2 and radiation. Figure 3A shows that exposure of MCF-7 cells to either radiation (2 Gy) or WIN2 (12 µM) results in growth inhibition. As in the studies presented in Fig. 2A, the combination treatment was more effective than either WIN2 or radiation alone in inhibiting breast tumor growth. Furthermore, the combination treatment of WIN2 with radiation reduced the recovery of proliferative capacity observed with either radiation alone or WIN2 alone. A similar pattern of effects (enhanced growth inhibition and suppression of proliferative recovery) was evident in the MDA-MB231 and 4T1 cells (Fig. 3, B and C).
Fig. 3.
Temporal response to the combination of WIN2 + radiation. MCF-7 (A), MDA-MB231 (B), and 4T1 (C) cells were treated as in Fig. 2. Viable cell number was monitored over a period of 96 hours using the trypan blue exclusion assay. Data presented reflect the means of 5 individual experiments ± S.E. Solid symbols = P < 0.05 versus vehicle within time points.
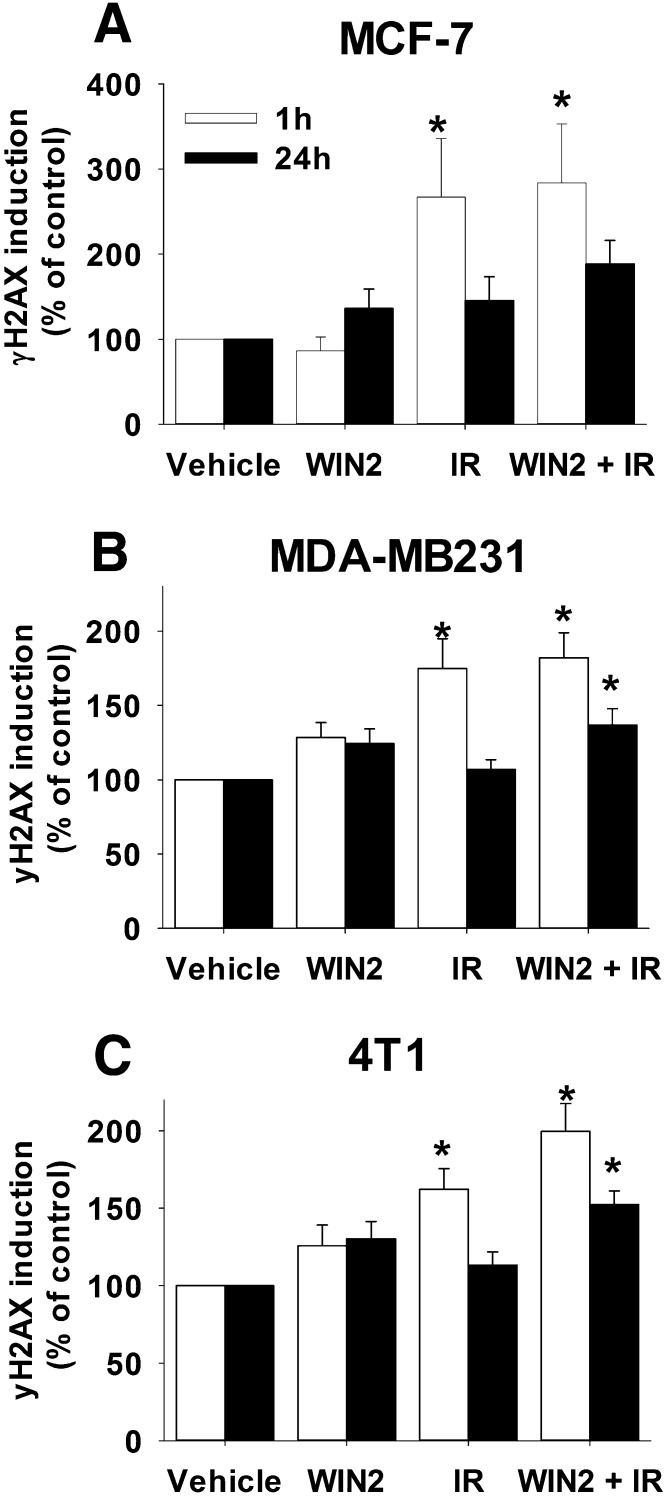
Induction of DNA Damage
It is well established that radiation acts through the induction of DNA damage, which can be monitored by γH2AX formation (Rogakou et al., 1999). The capacity of radiation alone, WIN2 alone, or the combination to affect DNA damage (1 hour) and repair (24 hour) in MCF-7 cells was evaluated based on γH2AX levels. As shown in Fig. 4A, radiation induced γH2AX foci formation was elevated at 1 hour and declined over a 24-hour period. However, WIN2 neither increased the induction of DNA damage nor interfered with the rate of repair (the latter based on the reduction of γH2AX staining). γH2AX foci formation and decline was also evaluated for radiation alone and for the WIN2 and radiation combination in MDA-MB231 and 4T1 cells (Fig. 4, B and C). As was the case with the MCF-7 cells, WIN2 did not alter the extent of DNA damage induced by radiation. However after 24 hours, residual DNA damage was slightly increased for the WIN2 + radiation combination compared with radiation alone, whereas the number of γH2AX foci had declined to background levels for radiation alone. WIN2 alone had no significant effect on γH2AX foci formation.
Fig. 4.
DNA damage induction and repair by radiation ± WIN2. MCF-7 (A), MDA-MB231 (B), and 4T1 (C) cells were treated as in Fig. 2. γH2AX formation analyzed by flow cytometry at 1 and 24 hours after drug treatment. Data were normalized to percentage of control; data presented reflect the means of 3–5 individual experiments ± S.E.; *P < 0.05 versus vehicle.
Senescent Growth Arrest and Autophagy Induced by IR or WIN2 + IR
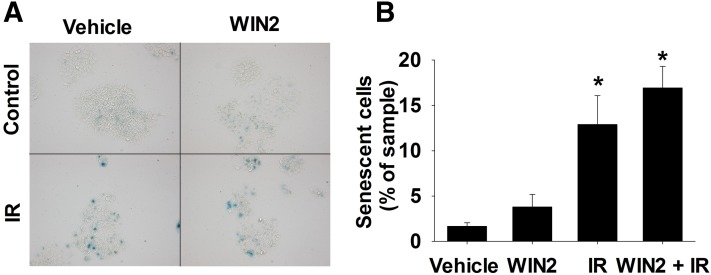
On the basis of our previous finding that senescence represents the primary antiproliferative response to radiation in p53 wild-type breast tumor cells (Jones et al., 2005), senescence induction was evaluated based on β-galactosidase staining (Debacq-Chainiaux et al., 2009). A representative image of staining for each treatment condition is shown in Fig. 5A. Quantification of senescence indicated that β-galactosidase activity was significantly elevated by radiation and WIN2 + radiation but there was no increase with WIN2 + radiation compared with radiation alone (Fig. 5B), whereas WIN2 alone did not appear to promote senescence. Adriamycin-induced senescence (Goehe et al., 2012) was used as a positive control (data not shown).
Fig. 5.
Senescence induction by radiation ± WIN2. MCF-7 cells were treated with vehicle, WIN2 (12 μM), (2 Gy) radiation or WIN2 + radiation. (A) Representative images of β-galactosidase stained cells. (B) Quantification of β-galactosidase activity 96 hours after drug treatment. Data were normalized to percentage of sample in (B); data presented reflect the means of three individual experiments ± S.E.; *P < 0.05 versus vehicle.
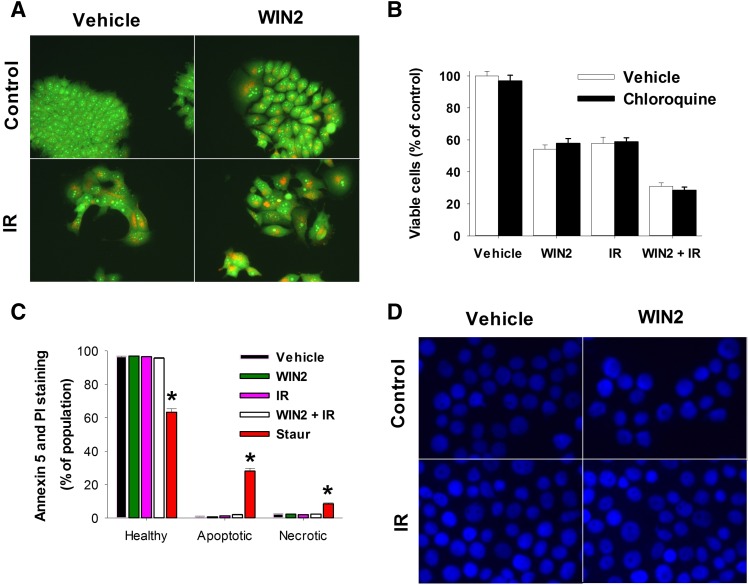
Radiation has been shown to induce a cytoprotective form of autophagy (Wilson et al., 2011; Bristol et al., 2012, 2013), whereas THC was reported to induce autophagic cell death in glioma cells (Salazar et al., 2009). Consequently, studies were designed to investigate whether WIN2 or the WIN2 and radiation combination would promote autophagy in this experimental system. Acridine orange staining clearly indicates that both WIN2 and radiation induced autophagy in MCF-7 cells (Fig. 6A). To determine whether autophagy was playing either a cytoprotective or cytotoxic function in the effects of radiation and/or WIN2, autophagy was inhibited utilizing chloroquine. However, chloroquine showed no evidence of altering the actions of either treatment alone or in combination when administered to MCF-7 cells (Fig. 6B). Inhibition of autophagy by chloroquine was validated in experiments where autophagy was induced by adriamycin, as demonstrated in Goehe et al. (2012) (data not shown).
Fig. 6.
Assessment of apoptosis, necrosis, and autophagy induction by WIN2. MCF-7 cells were treated as in Fig. 3. (A) Autophagy induction by acridine orange staining. Images were taken at 96 hours and 40× magnification. (B) Autophagy induction in the absence and presence of chloroquine. (C) Flow cytometry at 48 hours for Annexin V and propidium iodide staining. Staurosporine (1 µM, 24 hours) was used as a positive control. (D) DAPI staining for nuclear morphology at 48 hours using 40× magnification. Data were normalized to percentage of control in (B) and percentage of population in (C); data presented reflect the means of three or four individual experiments ± S.E.; *P < 0.05 versus vehicle.
The Combination of WIN2 and Radiation Fails to Induce Apoptosis or Necrosis
As shown in Fig. 3, the combination treatment of WIN2 + radiation promoted a prolonged growth arrest with limited proliferative recovery. To investigate the possibility that a low level of cell death might have contributed to the growth inhibition observed, the induction of apoptosis and/or necrosis were determined by staining with annexin V and PI (Vermes et al., 1995). However, neither apoptosis nor necrosis was detected in response to treatment (Fig. 6C). In contrast, apoptosis was clearly detected with staurosporine treatment as a positive control (Belmokhtar et al., 2001) (Fig. 6C). This observation was confirmed qualitatively using DAPI staining to assess nuclear morphology (Fig. 6D) where paclitaxel (Saunders et al., 1997) was used as positive control for apoptosis and cell death (data not shown). The lack of change in nuclear morphology was confirmed at 72 and 96 hours post-treatment (data not shown). The DAPI staining experiments also failed to indicate the induction of mitotic catastrophe, which is characterized by multinucleated cells containing micronuclei (Jonathan et al., 1999).
Interaction of Other Cannabinoids with Radiation
Although WIN2 behaves as a cannabinoid receptor agonist, other cannabinoid agonists were also tested for their capacity to interact with radiation in MCF-7 cells. As shown in Table 1, the highest concentrations of JWH-015 and pravadoline augmented the growth inhibitory effects of radiation. These two compounds belong to the class of aminoalkylindoles and share structural similarities to WIN2. In contrast, cannabinoids outside this class, including CBD, methanandamide, CP55,940, nabilone, and THC failed to enhance the antiproliferative effects of radiation alone.
TABLE 1.
Interaction of cannabinoids with radiation in MCF-7 cells
MCF-7 cells were treated with the indicated cannabinoids either alone or in combination with 2 Gy radiation, and cell viability was determined based on trypan blue exclusion at 96 hours. Drugs concentrations (in micromolars) were as follows: THC, 30, 50, 70; CBD, 10, 25, 50; nabilone, 10, 30, 50; CP55,940, 10, 20, 30; methanandamide, 10, 20, 30; provadoline, 15, 30, 45; JWH-015, 15, 30, 45. All data normalized to % of control; sample size n = 3–5 experiments/study; values expressed as mean ± S.E.
| Drug | Control |
Low Dose |
Medium Dose |
High Dose |
||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | IR, 2 Gy | Vehicle | IR, 2 Gy | Vehicle | IR, 2 Gy | Vehicle | IR, 2 Gy | |
| THC | 100 ± 0.01 | 57 ± 3.93 | 94 ± 2.93 | 55 ± 3.92 | 47 ± 4.41 | 33 ± 6.02 | 26 ± 7.80 | 21 ± 5.77 |
| CBD | 100 ± 0.01 | 60 ± 8.04 | 85 ± 7.72 | 59 ± 11.26 | 59 ± 4.47 | 45 ± 4.77 | 24 ± 7.28 | 16 ± 3.95 |
| Nabilone | 100 ± 0.01 | 56 ± 4.61 | 88 ± 5.41 | 56 ± 5.75 | 66 ± 7.56 | 50 ± 5.33 | 32 ± 14.88 | 22 ± 8.08 |
| CP-55,940 | 100 ± 0.01 | 70 ± 8.12 | 100 ± 1.41 | 77 ± 10.58 | 81 ± 4.41 | 59 ± 7.13 | 38 ± 7.18 | 37 ± 14.72 |
| Methanandamide | 100 ± 0.01 | 61 ± 8.89 | 92 ± 0.93 | 58 ± 8.33 | 66 ± 7.96 | 47 ± 7.25 | 47 ± 9.38 | 32 ± 7.98 |
| Provadoline | 100 ± 0.01 | 53 ± 5.67 | 94 ± 1.52 | 43 ± 4.95 | 60 ± 5.38 | 37 ± 5.64 | 40 ± 5.06 | 25 ± 3.85 * |
| JWH-015 | 100 ± 0.01 | 53 ± 5.67 | 79 ± 6.39 | 45 ± 4.04 | 42 ± 7.04 | 31 ± 2.61 | 24 ± 2.00 | 17 ± 1.25 * |
P < 0.0156 compared with vehicle, drug treatment alone, and radiation alone.
Assessment of Potential Cannabinoid Receptor Targets of WIN55,212-2
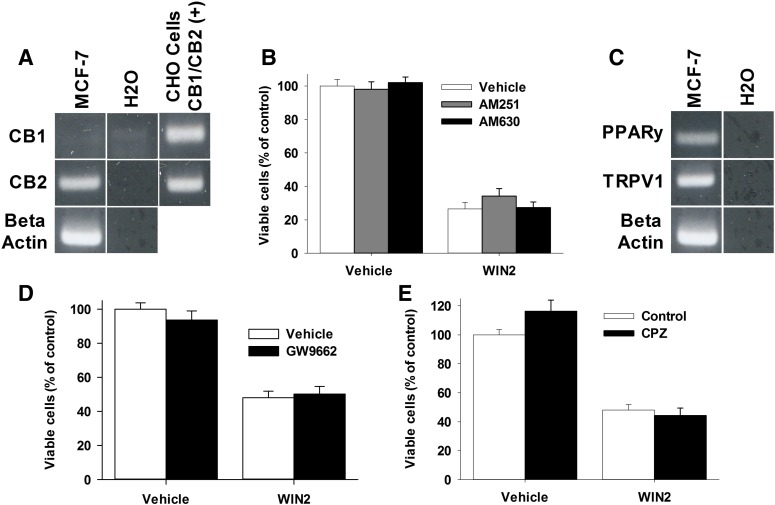
Cannabinoids have been reported to act at CB1 or CB2 receptors to inhibit the growth of tumor cells (Qamri et al., 2009; Salazar et al., 2009). RT-PCR analysis clearly showed that MCF-7 cells express CB2 receptor mRNA, whereas an extremely faint band was found for CB1 (Fig. 7A). G-protein activation studies in rat brain tissue have shown that WIN2 acts as a full agonist at CB1 and CB2 receptors (Sim et al., 1996; Breivogel et al., 1998). Therefore, the respective CB1 and CB2 receptor antagonists, AM251 (4 µM) and AM630 (4 µM), were evaluated for the ability to prevent WIN2-induced inhibition of cell growth (Lan et al., 1999; Ross et al., 1999). Neither AM251 nor AM630 significantly inhibited the growth inhibitory effects of WIN2 (Fig. 7B), suggesting that CB1 and CB2 receptor signaling may not be necessary for the antiproliferative actions of WIN2.
Fig. 7.
The antiproliferative effects of WIN2 in MCF-7 cells are mediated through a noncannabinoid receptor mechanism of action RT-PCR for the CB1 and CB2 receptor in MCF-7 cells (A). CHO cells transfected with human CB1 or CB2 receptors were used as a positive control. (B) MCF-7 cells were treated with vehicle or WIN2 (12 µM) and vehicle, AM251 (4 µM), or AM630 (4 µM) for 24 hours. (C) RT-PCR for TRPV1 and PPARγ. (D) MCF-7 cells were treated with vehicle or WIN2 (12 µM) and vehicle or GW9662 (10 µM). (E) MCF-7 cells were treated with vehicle or WIN2 (12 µM) and vehicle or capsazapine (10 µM). Cell count with trypan blue was used to assess cell viability at 96 hours. Data presented reflect the means of three individual experiments ± S.E.; no significant difference found.
Given the apparent lack of involvement of CB1 and CB2 receptors in the antiproliferative effects of WIN2, the contribution of other potential receptor targets of WIN2, including PPARγ and TRPV1 was considered. Both have been shown to be activated by various cannabinoids (O’Sullivan, 2007; Pertwee et al., 2010). RT-PCR confirmed the presence of mRNA of both TRPV1 and PPARγ in MCF-7 cells (Fig. 7C). However, neither the TRPV1 receptor antagonist capsazepine nor the PPARγ receptor antagonist GW9662 (Fig. 7, D and E) reduced the antiproliferative effects of WIN2 (Doherty et al., 2005; Willson et al., 2000). Furthermore, the observations that the PPARγ receptor agonist pioglitazone and TRPV1 agonist capsaicin failed to elicit antiproliferative activity alone (data not shown) further argues against the function of these receptors in the breast tumor cells. Similarly, the pan-PPAR agonist, bezafibrate, which is used to screen for the potential involvement of other PPAR receptors, did not inhibit the growth of MCF-7 cells or interfere with the antiproliferative activity of WIN2 (data not shown). Taken together, these experiments indicate that WIN2 does not appear to be acting through known receptor targets in MCF-7 breast tumor cells.
WIN2 Antagonizes S1P-Associated Growth Stimulation
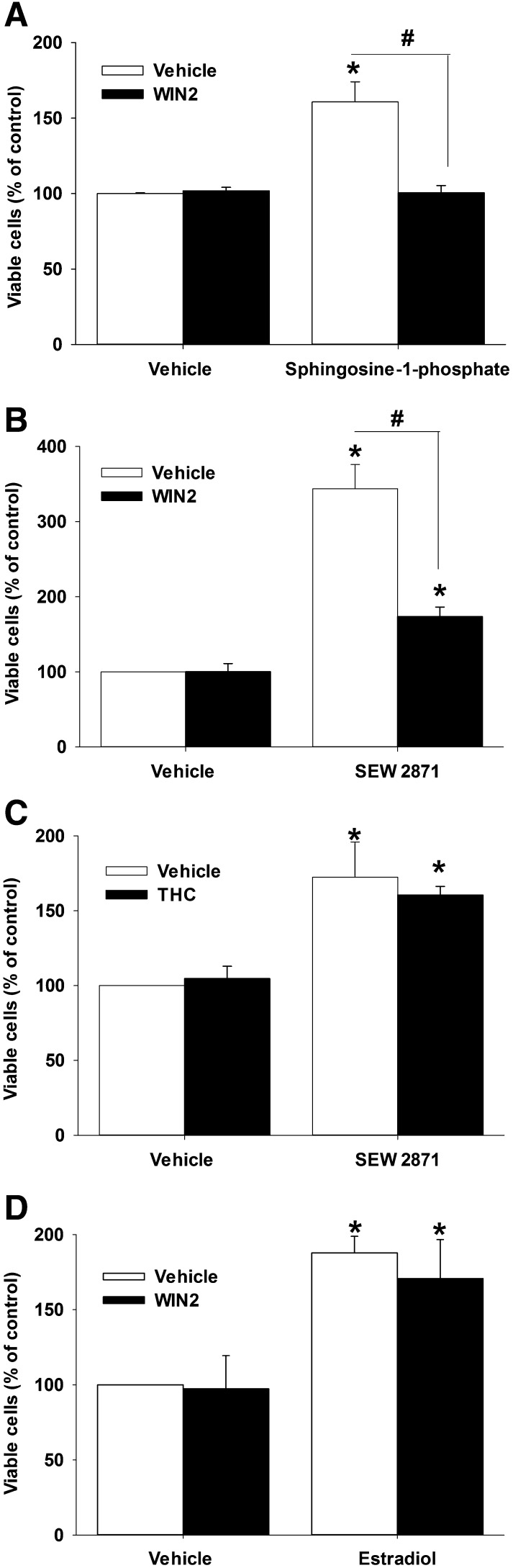
As the ceramide/S1P signaling system has been shown to stimulate the proliferation of MCF-7 cells (Sarkar et al., 2005), studies were designed to evaluate the S1P system as a potential site for the antiproliferative actions of WIN2 in MCF-7 cells. Under low-serum conditions, in which 100 nM S1P stimulated MCF-7 cell growth, a 3 µM concentration of WIN2 that did not inhibit basal cell growth effectively suppressed growth stimulation by S1P (Fig. 8A). In complementary studies under normal serum conditions, a subeffective dose of WIN2 (8 µM) also reversed the growth stimulatory effects of a 5 µM treatment with the synthetic S1P1 receptor-selective agonist SEW2871 (Fig. 8B). In contrast, 25 µM THC failed to reverse growth stimulation by SEW2871 (Fig. 8C). To explore the possibility that WIN2 might have interfered with another growth stimulatory pathway, cells were exposed to 100 nM estradiol in the absence and presence of WIN2 (8 µM); however, WIN2 failed to antagonize the growth stimulatory effects of estradiol (Fig. 8D).
Fig. 8.
WIN2 interferes with sphingosine-1-phosphate-induced growth stimulation. (A) MCF-7 cells were incubated under low-serum conditions with 100 nM sphingosine-1-phosphate ± WIN2 (3 µM) or under normal serum with 5 µM SEW2871 ± 8 µM WIN2 (B), 5 µM SEW2871 ± 25 µM THC (C), or 100 nM estradiol ± 8 µM WIN2 (D). Trypan blue exclusion was used to assess cell viability at 96 hours post-treatment. Values are presented as % of control and represent means ± S.E. for 3 or 4 replicate experiments; *P < 0.05 versus vehicle; #P < 0.05 indicated by bars. Two-way ANOVA reports: A, F(1,4) = 10.9, P < 0.05; B, F(1,4) = 36.3, P < 0.01; C, P = 0.60; D, P = 0.73.
Discussion
The current studies indicate that the aminoalkylindole, WIN2, has the capacity to inhibit growth in two human breast cancer cell lines (MCF-7 and MDA-MB231) and a murine breast tumor cell line (4T1). In addition, WIN2 augmented the antiproliferative effects of radiation in all three breast cancer cell lines. Experiments comparing WIN2 with its stereoisomer WIN3 support the conclusion that the effects of WIN2 are stereoselective. Studies in MCF-10A cells suggest that the antiproliferative effects of WIN2 are selective to tumor cells. Time course studies in all three breast tumor cell lines indicate that WIN2, either alone or in combination with radiation, promotes growth arrest rather than tumor cell killing. This conclusion is supported by experiments in MCF-7 cells showing the absence of significant apoptosis or necrosis by WIN2 alone or in combination with radiation. Both autophagy and senescence induction are evident, but neither response appears to play a central role in the antiproliferative effects of this compound. Furthermore, the increased antiproliferative activity of the WIN2 + radiation combination does not appear to be a consequence of an increase in DNA damage or decreased DNA repair compared with radiation alone.
WIN2 is known to be an agonist with high efficacy at both CB1 and CB2 receptors (Sim et al., 1996; Breivogel et al., 1998; Govaerts et al., 2004), and the expression of CB2 receptor mRNA and possibly low levels of CB1 receptor mRNA was confirmed in MCF-7 cells. However, the antiproliferative activity of WIN2 was not inhibited by the respective CB1 or CB2 antagonists, AM251 and AM630. In efforts to identify potential receptors for WIN2 action, the involvement of TPRV1 and PPAR nuclear receptors that are known to be sensitive to cannabinoids (O’Sullivan, 2007; Pertwee et al., 2010) was also assessed. RT-PCR confirmed mRNA expression for both receptors, but selective antagonists for these receptors did not reduce the antiproliferative effects of WIN2, arguing against a role for TRPV1 and PPARγ in the activity of WIN2. Consistent with these observations, TRPV1 and PPARγ agonists failed to reduce cell growth. The failure of the pan-PPAR agonist bezafibrate to affect proliferation of MCF-7 cells argues against the involvement of other PPARs in breast tumor cell proliferation under the present experimental conditions. Taken together, these data suggest that the antiproliferative actions of WIN2 in MCF-7 breast cancer cells are not mediated by conventional receptor targets of WIN2. This conclusion is further supported by the observation that WIN2 was also active in 4T1 cells that do not express either the CB1 or CB2 receptors (McKallip et al., 2005). Likewise, in studies in melanoma cells and mantle cell lymphoma, WIN2 was shown to act through a noncannabinoid receptor mechanism (Scuderi et al., 2011; Wasik et al., 2011). Our findings are somewhat distinct from those of Qamri et al. (2009) in which CB1 and CB2 antagonists prevented the antiproliferative effects of WIN2 in MDA-MB231 and MDA-MB468 breast cancer cells. However, Qamri et al. (2009) performed their study under low serum conditions and not in MCF-7 cells, which was the focus of our experiments relating to cannabinoid receptor action.
Knockdown of sphingosine kinase has implicated the S1P system in MCF-7 breast tumor cell growth (Sarkar et al., 2005). S1P-associated growth signaling has also been demonstrated in MDA-MB231 and 4T1 cells (Wang et al., 1999; Nagahashi et al., 2012), and all three of these cell lines were shown to be sensitive to the growth inhibitory effects of WIN2. WIN2 was also shown to antagonize growth stimulation in MCF-7 cells by S1P and the synthetic S1P1 receptor agonist SEW2871, but not by estradiol, suggesting some degree of specificity relating to S1P signaling pathways. The fact that THC failed to augment the antiproliferative effects of radiation or to antagonize growth stimulation by SEW2871 indicates that WIN2’s inhibition of S1P signaling cannot be generalized to other cannabinoids, further suggesting that WIN2 interferes with the S1P pathway through a noncannabinoid mechanism.
A perhaps critical difference between the current work and other studies in the literature of cannabinoid action in tumor cells is the concentration of serum used in the media. Most studies use low-serum media, whereas in the present study, the media contained 10% serum. In this context, the breast tumor cells were markedly more sensitive to WIN2 under low-serum conditions. The decreased potency of WIN2 in high-serum is likely to be a consequence of its sequestration by serum-binding proteins. Additionally, the increased potency of WIN2 in low serum could be related to the fact that reduced serum conditions are likely to make the cells fragile and susceptible to injury by exogenous stressors (Pirkmajer and Chibalin, 2011). Regardless, an important finding in the present study was that the stereoselectivity of WIN2 was sustained under both low- and high-serum conditions.
Several other synthetic cannabinoids and plant-derived cannabinoids were evaluated for their effectiveness to augment the antiproliferative effects of radiation in breast cancer cells. JWH-015 and pravadoline are aminoalkylindole compounds that are structurally similar to WIN2. THC and nabilone were selected for their clinical relevance to cancer because these are the active ingredients of the respective U.S. Food and Drug Administration-approved cannabinoid-based medications THC (Marinol) and nabilone (Cesamet) to treat cancer chemotherapy-induced nausea and emesis. Cannabidiol is a major cannabinoid found in marijuana, which does not bind to CB1 or CB2 receptors, and is a component of nabiximol (Sativex; Bayer Healthcare, Berkshire, UK), a medication prescribed in Canada and several European countries (Oreja-Guevara, 2012) for the treatment of spasticity due to multiple sclerosis. Methanandamide, a stable analog of anandamide, was used in lieu of the endogenous cannabinoid, which is rapidly hydrolyzed. Finally, CP55,940 possesses high potency and efficacy at CB1 and CB2 receptors but is structurally distinct from WIN2 (Pertwee et al., 2010). It is significant that of all of the compounds tested, only two compounds structurally similar to WIN2 (i.e., JWH-015 and pravadoline) modestly augmented the effects of radiation. It is also noteworthy that in no case did any of the agents reduce the antiproliferative effects of radiation, indicating that cannabinoid-based medications are unlikely to interfere with the effectiveness of radiation therapy.
Nevertheless, the combination treatment of WIN2 + radiation was found to be significantly more effective than radiation alone in arresting the cells for an extended period of time and suppressing proliferative recovery. Although the profound cannabimimetic effects of WIN2 (Compton et al., 1992) have impeded its clinical development, drugs with a similar structure and/or mechanism of action could represent potential therapeutic agents to enhance the antiproliferative effects of ionizing radiation. The observed, albeit modest, effectiveness of other aminoalkylindoles, JWH-015 and pravadoline, in enhancing the antiproliferative effects of radiation suggests that other aminoalkylindole derivatives might ultimately have utility as adjunctive cancer treatments without the limitations imposed by the cannabimimetic effects of WIN2.
Supplementary Material
Acknowledgments
The authors thank Dr. Mary Abood for sharing the CB1 and CB2 primer sequences used in the RT-PCR studies and to Dr. Laura Sim-Selley for guidance relating to the potential actions of WIN2 in the S1P system.
Abbreviations
- ANOVA
analysis of variance
- AM251
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-piperidinyl-1H-pyrazole-3-carboxamide
- AM630
[6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)-methanone
- BSA
bovine serum albumin
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CBD
cannabidiol
- CP55,940
(+)-rel-5-(1,1-dimethylheptyl)-2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-phenol
- DAPI
4,6-diamidino-2-phenylindole
- GW9662
2-chloro-5-nitrobenzanilide
- γH2AX
phosphorylated H2A histone family member X
- IR
ionizing radiation
- JWH-015
(2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenyl-methanone
- PBS
phosphate-buffered saline
- PPAR
peroxisome proliferator-activated receptor
- RT-PCR
reverse transcription-polymerase chain reaction
- S1P
sphingosine-1-phosphate
- SEW2871
5-[4-phenyl-5-(trifluoromethyl)-2-thienyl]-3-[3-(trifluoromethyl)phenyl]-1,2,4-oxadiazole
- THC
Δ9-tetrahydrocannabinol
- TRPV1
transient receptor potential cation channel subfamily V member 1
- WIN2 (WIN55,212-2)
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
- WIN3 (WIN55,212-3)
(−)-[2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate salt
Authorship Contributions
Participated in research design: Emery, Selley, Lichtman, Gewirtz.
Conducted experiments: Emery, Alotaibi, Tao.
Performed data analysis: Emery, Lichtman, Gewirtz.
Wrote or contributed to the writing of the manuscript: Emery, Selley, Lichtman, Gewirtz.
Footnotes
This work was supported by Department of Defense [Grants W81XWH-08-1-0096 (to D.A.G.) and W81XWH-08-1-0097 (to A.H.L.) and Predoctoral Fellowship W81XWH-11-1-0163 (to S.E.)].
Portions of this work were previously presented: International Cannabinoid Research Symposium (2011), Virginia Academy of Sciences (2011, 2012), American Association for Cancer Research (2012), and Carolina Cannabinoid Collaborative Conference (2012).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Belmokhtar CA, Hillion J, Ségal-Bendirdjian E. (2001) Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354–3362. [DOI] [PubMed] [Google Scholar]
- Biggers JW, Nguyen T, Di X, Gupton JT, Henderson SC, Emery SM, Alotaibi M, White KL, Jr, Brown R, Almenara J, et al. (2013) Autophagy, cell death and sustained senescence arrest in B16/F10 melanoma cells and HCT-116 colon carcinoma cells in response to the novel microtubule poison, JG-03-14. Cancer Chemother Pharmacol 71:441–455. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. (1998) Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem 273:16865–16873. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163. [PubMed] [Google Scholar]
- Bristol ML, Di X, Beckman MJ, Wilson EN, Henderson SC, Maiti A, Fan Z, Gewirtz DA. (2012) Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy 8:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol ML, Emery SM, Maycotte P, Thorburn A, Chakradeo S, Gewirtz DA. (2013) Autophagy inhibition for chemosensitization and radiosensitization in cancer: do the preclinical data support this therapeutic strategy? J Pharmacol Exp Ther 344:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Gironella M, Lorente M, Garcia S, Guzmán M, Velasco G, Iovanna JL. (2006) Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 66:6748–6755. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. (1992) Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther 263:1118–1126. [PubMed] [Google Scholar]
- Darmani NA, Janoyan JJ, Crim J, Ramirez J. (2007) Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew. Eur J Pharmacol 563:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. (2009) Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798–1806. [DOI] [PubMed] [Google Scholar]
- Doherty EM, Fotsch C, Bo Y, Chakrabarti PP, Chen N, Gavva N, Han N, Kelly MG, Kincaid J, Klionsky L, et al. (2005) Discovery of potent, orally available vanilloid receptor-1 antagonists. Structure-activity relationship of N-aryl cinnamides. J Med Chem 48:71–90. [DOI] [PubMed] [Google Scholar]
- Goehe RW, Di X, Sharma K, Bristol ML, Henderson SC, Valerie K, Rodier F, Davalos AR, Gewirtz DA. (2012) The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep? J Pharmacol Exp Ther 343:763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaerts SJ, Hermans E, Lambert DM. (2004) Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur J Pharm Sci 23:233–243. [DOI] [PubMed] [Google Scholar]
- Guerrero AV, Quang P, Dekker N, Jordan RC, Schmidt BL. (2008) Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci Lett 433:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202. [DOI] [PubMed] [Google Scholar]
- Jonathan EC, Bernhard EJ, McKenna WG. (1999) How does radiation kill cells? Curr Opin Chem Biol 3:77–83. [DOI] [PubMed] [Google Scholar]
- Jones KR, Elmore LW, Jackson-Cook C, Demasters G, Povirk LF, Holt SE, Gewirtz DA. (2005) p53-Dependent accelerated senescence induced by ionizing radiation in breast tumour cells. Int J Radiat Biol 81:445–458. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. (1999) Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem 42:769–776. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, Almanza C, Pakdel A, Lee J, Limbad C, et al. (2011) Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat 129:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS. (2005) Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol 174:3281–3289. [DOI] [PubMed] [Google Scholar]
- Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, et al. (2012) Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res 72:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Selley DE, Sim-Selley LJ. (2010) Statistical parametric mapping reveals ligand and region-specific activation of G-proteins by CB1 receptors and non-CB1 sites in the 3D reconstructed mouse brain. Neuroimage 52:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreja-Guevara C. (2012) Clinical efficacy and effectiveness of Sativex, a combined cannabinoid medicine, in multiple sclerosis-related spasticity. Expert Rev Neurother 12(4, Suppl)3–8. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE. (2007) Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol 152:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB₁ and CB₂. Pharmacol Rev 62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkmajer S, Chibalin AV. (2011) Serum starvation: caveat emptor. Am J Physiol Cell Physiol 301:C272–C279. [DOI] [PubMed] [Google Scholar]
- Preet A, Qamri Z, Nasser MW, Prasad A, Shilo K, Zou X, Groopman JE, Ganju RK. (2011) Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev Res (Phila) 4:65–75 Phila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamri Z, Preet A, Nasser MW, Bass CE, Leone G, Barsky SH, Ganju RK. (2009) Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther 8:3117–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146:905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG. (1999) Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol 126:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB. (2008) Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag 4:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C, Torres S, García S, et al. (2009) Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 119:1359–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. (2005) Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett 579:5313–5317. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Lawrence WD, Christensen C, Wappler NL, Ruan H, Deppe G. (1997) Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells. Int J Cancer 70:214–220. [DOI] [PubMed] [Google Scholar]
- Scuderi MR, Cantarella G, Scollo M, Lempereur L, Palumbo M, Saccani-Jotti G, Bernardini R. (2011) The antimitogenic effect of the cannabinoid receptor agonist WIN55212-2 on human melanoma cells is mediated by the membrane lipid raft. Cancer Lett 310:240–249. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. (1996) Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:8057–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait L, Soule HD, Russo J. (1990) Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res 50:6087–6094. [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51. [DOI] [PubMed] [Google Scholar]
- Wang F, Nohara K, Olivera A, Thompson EW, Spiegel S. (1999) Involvement of focal adhesion kinase in inhibition of motility of human breast cancer cells by sphingosine 1-phosphate. Exp Cell Res 247:17–28. [DOI] [PubMed] [Google Scholar]
- Wasik AM, Almestrand S, Wang X, Hultenby K, Dackland AL, Andersson P, Kimby E, Christensson B, Sander B. (2011) WIN55,212-2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell Death Dis 2:e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR. (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem 43:527–550. [DOI] [PubMed] [Google Scholar]
- Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, Gewirtz DA. (2011) A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer 2:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.