Abstract
Background:
Of all Smooth muscle tumours originating from uterus are leiomyomas are the most common ones. Benign nature and smooth muscle origination of leiomyomas can be easily documented via histological examination. In present study it was tried to examine immunohistochemical profile of leiomyomas with different subtypes.
Material and Methods:
In this cross-sectional study 64 cases of smooth muscle tumors originating from uterus were included in study. As a control group 12 cases of conventional leiomyomas were selected. Then estrogen receptor, progesterone receptor, p53 and ki-67 were assessed. Statistical analysis was conducted using SPSS 16.0.
Results:
P 53 and ki-67 antibody status was diffusely positive in 12 out of 24 cases (50%) of leiomyosarcomas. Leiomyomas with bizzare nuclei were stained with Ki67 proliferative marker less than those in obviously malignant cases (P < 0.001). Estrogen and progesterone receptors had a reverse correlation with tumours malignancy potential.
Conclusion:
Since p53 is known as an important inhibitory trigger for proliferative cycle of cells, in current study it was concluded that p53 inhibitory role decreases as malignancy potential increases, also tumors dependence on steroids via steroid receptors decreases as malignancy potential increases.
Keywords: Immunohistochemistry, leiomyoma, uterus
INTRODUCTION
Of all Smooth muscle tumours originating from uterus are leiomyomas are the most common ones. Benign nature and smooth muscle origination of leiomyomas can be easily documented via histological examination.1 Moreover leiomyomas, which are the most common neoplasm of uterus, malignant tumours only constitutes 1% of uterine malignant smooth muscle tumours.2,3,4 In histological examinations malignant smooth muscle tumour can be easily recognized as sarcomas, although some smooth muscle tumours with borderline appearance create controversies in diagnosis. Although these cases are described using histological details, but this categorization and description lacks information regarding the clinical features and prognosis.5 Of these cases with difficulty in diagnosis leiomyoma with bizarre nuclei (LM-BN), atypical leiomyoma, and smooth muscle tumour with undetermined malignant potential (STUMP) can be mentioned. Although recently a distinguished categorization as tumour with little metastatic potential has been proposed.6,7,8 Clinical behaviour of uterine smooth muscle tumours can be predicted by application of routine gross and light microscopic features. Problems arise in classification and diagnosis when there is mismatching between clinical outcome and morphology.9
Histologically, multinucleated giant cells (diffusely or focal) with no abnormal mitotic activity and no coagulative necrosis is recognized as LM-BNs.10 leiomyosarcoma in situ is another term used to describe this category, because giant cell appearance concerns pathologists 8 whereas low mitotic activity and lack of tumour cell necrosis distinguishes leiomyosarcoma in situ from classic Leiomyosarcomas (LMS).11 Although most clinically aggressive smooth muscle tumors demonstrate cellular atypia.
Review of literature indicates a favourable prognosis and a negligible risk for distant metastasis for this category, which can be treated with myomectomy alone, although local recurrence of tumour must be considered.12,13,14,15 Likewise considerable controversy has surrounded the nature of atypical nuclei in LM-BNs.16 As far as histological examination with light microscope is difficult to distinguish between different theses subtypes because of difficulty in distinguishing between malignant and degenerative changes.17,18,19,20,21 In this study it was tried to examine immunohistochemical profile Leiomyomas with different subtypes and to examine how molecular differences distinguish histological classifications.
MATERIAL AND METHODS
In this cross-sectional study, 64 cases of smooth muscle tumours originating from uterus were included in study using whole tissue sections. Data was obtained from Tabriz University of Medical Sciences (Tabriz, Iran) data bases during 2010 to 2014. At last 20 cases of LM-BN, 8 cases of STUMP and 24 cases of LMS were included in study; all cases diagnoses were confirmed by oncological pathologists. Also 12 cases of conventional LMs were included as control group.
Diagnosis of LMS, STUMP, LM-BN and leiomyoma was based on criteria published earlier.22,23 Based on the published criteria, nuclear atypia, coagulative tumour cell necrosis and mitotic count more than 10/10 per high power field were used as necessary histologic criteria for classifying and diagnosis smooth muscle tumours originating from uterus. Two or more of these criteria is sufficient to warrant diagnosis for LMS. An unusual combination of criteria, which was not enough to fulfil LMS diagnosis, was defined as STUMP.24
LM-BN diagnosis was made when multi-focal or focal atypical cells consisted of bizzare, multi-lobulated and large nuclei associated with hyperchromasia, which could have been multi-nucleated with smudge chromatin and cytoplasmic pseudo-inclusion, in a background of routine atypical LM; LM-BN cells are found in diffusely or isolated nests in this background with less than 2-3/10 high power field. For each case a sample block was selected for examination. MIB1/ki-67 (BioGenex, Ca, USA), p53 monoclonal antibody (USBiological, MA, USA) and estrogen receptor monoclonal antibody (BioLegend, CA, USA) and progesterone receptor monoclonal antibody (PgR 636, Ready to use-Dako), was respectively used to detect ki-67, p53, ER and PR. were Avidin-biothine method was used for Immunohistochemistry. Antigens were retrieved by using citrate buffer 20 min for ER, PR and P53 and 30 min for Ki-67 was used for retrieving antigens by microwaving process.
P53 and Ki67 staining was considered positive when nuclear staining was positive. Positive p53 staining was defined as strong and diffuse (more than 75% of cell population) staining.
Percentage Ki67 positive cells to total number of cells was used after counting minimum of 1000 cells at high power field. Allred Score was used for PR and ER, which consisted of intensity score (3 = strong, 2 = intermediate, 1 = weak and 0 = none) and proportion score (0 = none, 1 <1/100, 2 = 1/100 to <1/10, 3 = 1/10 to < 1/3, 4 = 1/3to 2/3 and 5= >2/3). A total score was obtained by adding these scores which ranged from 0 to 8. Samples with score more than 4 were considered as positive.25
The study protocol was approved by the Ethics Committee of Tabriz University of Medical Sciences (TUMS), which was in compliance with the Declaration of Helsinki. Statistical Package for the Social Sciences TM Version 15 (SPSS ltd, Chicago, IL, USA) was used for statistical analyses. Fisher's exact test or Chi-square were used to compare the qualitative variables. P <0.05 was considered statistically significant.
RESULTS
Mentioned criteria and scoring methods was used for the samples based on immunohistochemical staining. P53 immunoreactivity in uterine smooth muscle tumours is shown in Table 1; 12 out of 24 LMSs were positive using p53 antibody (50%) while only 2 LM-BNs (11%) were positive for p53.
Table 1.
P53, ER and PR immunohistochemistry in uterine smooth muscle tumour
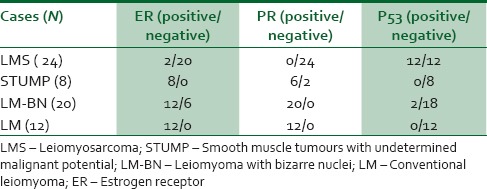
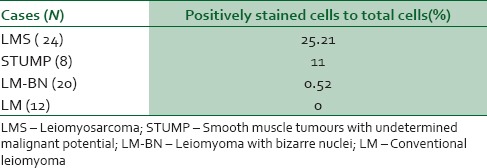
A similar result was obtained with regard to Ki67 staining. Ki67 immunohistochemistery staining is shown in Table 2; Ki67 proliferative marker increased as malignancy potential increased. Ki67 was positive in 25.21% of LMS cells and 0.52% of LM cells (P < 0.001). Considering steroid hormone receptors, PR and ER positivity decreased as malignancy potential increased through LM-BN and STUMP cases Table 1.
Table 2.
Ki67 immunohistochemistry in uterine smooth muscle tumours
DISCUSSION
LM-BN are one of rare subtypes of smooth muscle cell tumours originating from uterus which are seen among younger women than those LMS affects. This subtype is recognized with bizarre, hyperchromatic and multi-lobulated nuclei distributed in multi-focal or focal nests in a conventional LM background. Hyperchromatic and multi-lobulated nuclei in giant cells is also found in ~ 75% of LMS.26,27 Presence of this features in toumor cells provoke concern among pathologists about possible malignant potency although they are found in a background of routine LM.13 Considering biologic features of these toumoral cells, in many studies it has been reported that there is no difference between LM-BNs and LMs in recurrence rate.28
In a study by Croce et al. concluded that LM-BNs are associated with a favourable prognosis and highlights, so a conservative approach can be accepted, because many of them are of reproductive age.29 Considering this favourable outcome, the term LM-BN is preferable to alternative terminology “atypical leiomyoma.30”
Immunohistochemistry has led to a new spectrum of tools to distinguish between different subtypes. To overcome this dilemma molecular studies have tried to compare nature of these the immunohistochemical findings between malignant, atypical and benign smooth muscle cells and biologic features of these cells. In a study by Sung et al. it was reported that 5 of all 13 cases of LM-BNs showed immunoreactivity for p53, including 1 case with strong, diffuse positive p53. Also it was declared that none of patients had distant metastasis, only one case had local recurrence after myomectomy31; in present study 2/20 of LM-BN had positive immunoreactivity for p53, which is less than what this study have reported.
In contrast a study on nine cases of simplastic uterine leiomyomas, Sun et al. found no significant difference between atypical and non-atypical cells in expression of P53, ER and PR immunostaining except for Ki67, which was significantly higher in malignant cells.31 Also in a similar study Chen and Yang found 26% LM-BNs in mediate and 33% LM-BNs in diffuse p53 immunorreactivity staining patterns.at last they concluded that there is significant overlapping in distribution of staining and intensity of P53 and Ki67 between LM-BN and LMS.32
In contrast with this study, present study showed a distinguishable difference between tumoural cells in LMS and other subtypes regarding expression of p53 [Figure 1]; twelve cases of 24 LMS (50%) had positive immunoreactivity, while none of STUMPs and only one case of LM-BN was positive. These results are similar to what sun et al. reported; which there was no statistically significant difference was found between non-atypical and atypical cells regard to p53 staining.31
Figure 1.
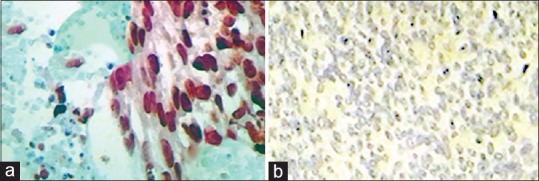
P53 immunostaining in uterine smooth muscle tumours originating from uterus. (a) Negative reaction in LM. (b) LMS; showing coagulative tumour cell necrosis and diffuse and strong P53 positivity. LMS – Leiomyosarcoma; LM – Conventional leiomyoma
Current findings are similar with what O'Nell et al.33 had concluded in which expression of p53 was found in only 3/11 symplastic leiomyoma. Although because of analytical limitations, statistical analysis was not performed there was a remarkable difference in staining pattern of P53 between different subtypes.
There was statistically significant in Ki-67 positivity between LMS cases as a group and STUMP, LM-BN and LMs as another group (P < 0.05); in a pattern which Ki67 positivity increased as malignancy potential increase [Figure 2]; from routine LM to LMS through LM-BN and STUMP. Also, increasing loss of ER and PR receptors in smooth muscle cell tumours was associated with increasing in malignancy potential [Figures 3 and 4].
Figure 2.
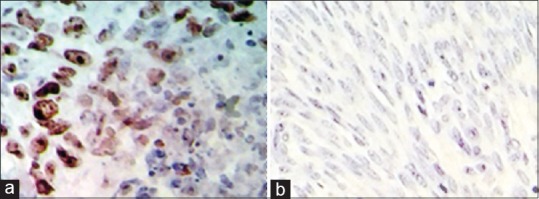
Ki67 immunostaining in uterine smooth muscle tumours originating from uterus. (a) High Ki67 positivity in LMS. (b) Negative reaction in LM. LMS – Leiomyosarcoma; LM – Conventional leiomyoma
Figure 3.
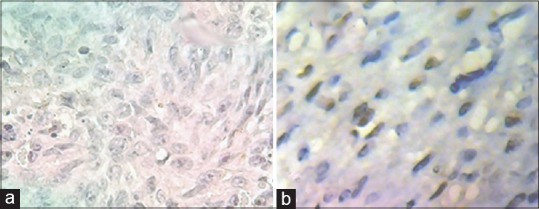
ER immunostaining in uterine smooth muscle tumours originating from uterus. (a) Complete negativity in LMS. (b) Good reaction for ER antibody LM. LMS – Leiomyosarcoma; LM – Conventional leiomyoma; ER – Estrogen receptor
Figure 4.
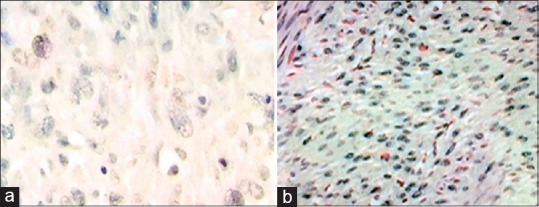
PR immunostaining in uterine smooth muscle tumours originating from uterus. (a) Negative reaction in LMS, (b) Positive reaction in LM. LMS – Leiomyosarcoma; LM – Conventional leiomyoma; PR – Progesterone receptor
World Health Organisation (WHO) has classified STUMP as smooth muscle tumours which cannot be distinguished as benign or malignant based on present criteria.34 Also, based on this classification, LM-BNs have been included in “atypical leiomyomas” category.
Based on present study application of current criteria for diagnosis of smooth muscle cell tumours originating from uterus is confirmed, but the main this advantage of present study along with most of the literature mentioned in present study is lack of sufficient case to make a strong and precise.
CONCLUSION
Based on present findings loss of p53 gene as an important inhibitory factor in smooth muscle cell tumours originating from uterus is a crucial event that leads to malignant transformation of LM-NBs and STUMPs. In addition, presence of mutual features of malignant tumours and LM-BNs may indicate pathogenic mechanism recurrent cases in this subtype.
Financial support and sponsorship
Tabriz University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cotran RS, Kumar V, Collins T, Robbins SL. Robbins pathologic basis of disease. 1999 [Google Scholar]
- 2.Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: An international internet-based survey of 21,746 women. BMC women's health. 2012;12(1):6. doi: 10.1186/1472-6874-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo DL, Bulun SE. Uterine fibroids. New England Journal of Medicine. 2013;369(14):1344–55. doi: 10.1056/NEJMra1209993. [DOI] [PubMed] [Google Scholar]
- 4.Asadi H, Orangi M, Shanehbandi D, Babaloo Z, Delazar A, Mohammadnejad L, et al. Methanolic Fractions of Ornithogalum cuspidatum Induce Apoptosis in PC-3 Prostate Cancer Cell Line and WEHI-164 Fibrosarcoma Cancer Cell Line. Advanced pharmaceutical bulletin. 2014;4(Suppl 1):455. doi: 10.5681/apb.2014.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caraffi S, Corradi D, Campanini N, Govoni P, Rocchi L, Perris R, et al. Microcirculation density and maturity in uterine and soft tissue leiomyosarcomas: An immunohistochemical study. Histology and histopathology. 2015;30(1):69–76. doi: 10.14670/HH-30.69. [DOI] [PubMed] [Google Scholar]
- 6.Matoso A, Chen S, Plaza JA, Osunkoya AO, Epstein JI. Symplastic leiomyomas of the scrotum: A comparative study to usual leiomyomas and leiomyosarcomas. The American journal of surgical pathology. 2014;38(10):1410–7. doi: 10.1097/PAS.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 7.Prat J, Mbatani N. Uterine sarcomas. International Journal of Gynecology and Obstetrics. 2015;131:S105–S10. doi: 10.1016/j.ijgo.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Ly A, Mills AM, McKenney JK, Balzer BL, Kempson RL, Hendrickson MR, et al. Atypical leiomyomas of the uterus: A clinicopathologic study of 51 cases. The American journal of surgical pathology. 2013;37(5):643–9. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 9.Kempson RL, Hendrickson MR. Smooth Muscle, Endometrial Stromal, and Mixed Mullerian Tumors of the Uterus. Mod Pathol. 0000;13(3):328–42. doi: 10.1038/modpathol.3880055. [DOI] [PubMed] [Google Scholar]
- 10.Hubele F, Averous G, Rust E, Imperiale A, Namer IJ. FDG-PET/CT findings of Atypical (bizarre/symplastic) Uterine Leiomyoma in a patient with abdominal leiomyosarcoma. Radiology Case Reports. 2012;7(3) doi: 10.2484/rcr.v7i3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przybora LA. Leiomyosarcoma in situ of the uterus. Cancer. 1961;14(3):483–92. doi: 10.1002/1097-0142(199005/06)14:3<483::aid-cncr2820140305>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Yanai H, Wani Y, Notohara K, Takada Si, Yoshino T. Uterine leiomyosarcoma arising in leiomyoma: Clinicopathological study of four cases and literature review. Pathology international. 2010;60(7):506–9. doi: 10.1111/j.1440-1827.2010.02549.x. [DOI] [PubMed] [Google Scholar]
- 13.Ip PP, Cheung AN, Clement PB. Uterine Smooth Muscle Tumors of Uncertain Malignant Potential (STUMP): A Clinicopathologic Analysis of 16 Cases. The American journal of surgical pathology. 2009;33(7):992–1005. doi: 10.1097/PAS.0b013e3181a02d1c. [DOI] [PubMed] [Google Scholar]
- 14.Fisher C, Goldblum JR, Epstein JI, Montgomery E. Leiomyosarcoma of the paratesticular region: A clinicopathologic study. The American journal of surgical pathology. 2001;25(9):1143–9. doi: 10.1097/00000478-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Masood J, Voulgaris S, Atkinson P, Carr TW. A rare symplastic or bizarre leiomyoma of the scrotum: A case report and review of the literature. Cases journal. 2008;1(1):381. doi: 10.1186/1757-1626-1-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Angelo E, Prat J. Uterine sarcomas: A review. Gynecologic oncology. 2010;116(1):131–9. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Veras E, Zivanovic O, Jacks L, Chiappetta D, Hensley M, Soslow R. “Low-grade leiomyosarcoma” and late-recurring smooth muscle tumors of the uterus: A heterogenous collection of frequently misdiagnosed tumors associated with an overall favorable prognosis relative to conventional uterine leiomyosarcomas. The American journal of surgical pathology. 2011;35(11):1626–37. doi: 10.1097/PAS.0b013e31822b44d2. [DOI] [PubMed] [Google Scholar]
- 18.Khaki A. Effect of Allium Cepa Effects on Uterus Apoptosis and Serum Antioxidants in Rats Exposures in Electromagnetic Fields. Medical Journal of Tabriz University of Medical Sciences and Health Services. 2014;36(4):38–43. [Google Scholar]
- 19.Somchit MN, Sanat F, Hui GE, Wahab SI, Ahmad Z. Mefenamic Acid Induced Nephrotoxicity: An Animal Model. Advanced pharmaceutical bulletin. 2014;4(4):401. doi: 10.5681/apb.2014.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barar J, Omidi Y. Translational approaches towards cancer gene therapy: Hurdles and hopes. BioImpacts: BI. 2012;2(3):127. doi: 10.5681/bi.2012.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S. Nanomaterials as non-viral siRNA delivery agents for cancer therapy. BioImpacts: BI. 2013;3(2):53. doi: 10.5681/bi.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downes KA, Hart WR. Bizarre leiomyomas of the uterus: A comprehensive pathologic study of 24 cases with long-term follow-up. The American journal of surgical pathology. 1997;21(11):1261–70. doi: 10.1097/00000478-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Evans HL, Chawla SP, Simpson C, Finn KP. Smooth muscle neoplasms of the uterus other than ordinary leiomyoma: A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer. 1988;62(10):2239–47. doi: 10.1002/1097-0142(19881115)62:10<2239::aid-cncr2820621028>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Ip PP, Tse KY, Tam KF. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: A review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Advances in Anatomic Pathology. 2010;17(2):91–112. doi: 10.1097/PAP.0b013e3181cfb901. [DOI] [PubMed] [Google Scholar]
- 25.Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanh LD, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: A validation study. Modern pathology. 2004;17(12):1545–54. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 26.van Meurs HS, Dieles JJ, Stel HV. A uterine leiomyoma in which a leiomyosarcoma with osteoclast-like giant cells and a metastasis of a ductal breast carcinoma are present. Annals of diagnostic pathology. 2012;16(1):67–70. doi: 10.1016/j.anndiagpath.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Othman M, Rashid A, Wang H, Li Z, Katz MH, et al. Solid pseudopapillary neoplasm of the pancreas with prominent atypical multinucleated giant tumour cells. Histopathology. 2013;62(3):465–71. doi: 10.1111/his.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miettinen M, Fetsch J. Evaluation of biological potential of smooth muscle tumours. Histopathology. 2006;48(1):97–105. doi: 10.1111/j.1365-2559.2005.02292.x. [DOI] [PubMed] [Google Scholar]
- 29.Croce S, Young RH, Oliva E. Uterine Leiomyomas With Bizarre Nuclei: A Clinicopathologic Study of 59 Cases. The American Journal of Surgical Pathology. 2014;38(10):1330–9. doi: 10.1097/PAS.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 30.Skinner GN, Louden KA. Non-puerperal uterine inversion associated with an atypical leiomyoma. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2001;41(1):100–1. doi: 10.1111/j.1479-828x.2001.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Mittal K. MIB-1 (Ki-67), estrogen receptor, progesterone receptor, and p53 expression in atypical cells in uterine symplastic leiomyomas. International Journal of Gynecologic Pathology. 2010;29(1):51–4. doi: 10.1097/PGP.0b013e3181b0259b. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. International Journal of Gynecologic Pathology. 2008;27(3):326–32. doi: 10.1097/PGP.0b013e31815ea7f5. [DOI] [PubMed] [Google Scholar]
- 33.O'neill C, McBride H, Connolly L, McCluggage W. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007;50(7):851–8. doi: 10.1111/j.1365-2559.2007.02699.x. [DOI] [PubMed] [Google Scholar]
- 34.D'Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Human pathology. 2009;40(11):1571–85. doi: 10.1016/j.humpath.2009.03.018. [DOI] [PubMed] [Google Scholar]


