Abstract
Ovarian hyperstimulation syndrome (OHSS) with the natural ovulatory cycle is extremely rare. We report a case of severe OHSS associated with a spontaneous normal singleton pregnancy in a 23-year-old woman presenting with severe abdominal pain, vomiting, and dyspnea. Ultrasonography revealed 10 weeks viable intra-uterine single fetus with bilateral multilocular cystic ovarian masses and ascites. She had supportive therapy inclusive of oral bromocriptine with complete resolution of OHSS and an eventual uncomplicated normal vaginal delivery at 39 weeks of pregnancy.
Keywords: Dopamine agonist, ovarian disease, ovarian hyperstimulation syndrome, pregnancy
INTRODUCTION
Ovarian hyperstimulation syndrome (OHSS) typically is an iatrogenic, serious, and potentially fatal complication of supraphysiological ovarian stimulation in assisted conception cycles. In its severe form, OHSS induces massive ovarian enlargement with multiple cysts, hemoconcentration, and third space accumulation of fluid in the forms of ascites, pleural and pericardial effusion and anasarca, other complications are renal failure/oliguria, hypovolemic shock, thromboembolic phenomena, adult respiratory distress syndrome, and even death.1,2
The syndrome occurs during either the luteal phase or early pregnancy, i.e. only after luteinization or following the administration of human chorionic gonadotropin (hCG).1,2 However, rarely, it may occur spontaneously at the beginning of a natural pregnancy and in the absence of any assisted reproductive treatment.2,3 Spontaneous OHSS has also been reported in pregnant women affected by polycystic ovary syndrome, hypothyroidism, hydatidiform mole, gonadotropin-producing pituitary adenoma, and multiple gestation.3,4
We present a case of spontaneous OHSS in a Nigerian woman with a singleton gestation conceived naturally and without any of the above-mentioned risk factors.
CASE REPORT
On October 11, 2011, a 23-year-old G3P2 (both alive) woman was referred to our department on a suspicion of a malignant ovarian disease. She had presented at the referring clinic 2 weeks earlier with abdominal distension, pain, dyspnea, nausea, and vomiting. She was 10 weeks pregnant; her last menstrual period was on August 1, 2011. The abdominal pain had increased 2 days prior to referral despite taking analgesics (paracetamol). She did not have any previous medical or surgical disease, and her gynecological history was unremarkable. Menarche was at the age of 12, and menstrual cycles were regular. She had two normal vaginal deliveries at term, in 2008 and 2010.
She was well-nourished and weighed 58 kg, in acute distress and mild dyspnea. Temperature, pulse rate, and blood pressure were within normal limits. Her physical examination revealed a moderately distended, tender, and tense abdomen with positive shifting dullness on percussion. The bowel sounds were normal. On pelvic examination, the uterine size and ovaries could not be made out because of the distention and tenderness, but bilateral irregular and mobile adnexal masses could be palpated with difficulty. Pelvic ultrasound revealed a single viable fetus at 11 weeks (crown-rump length 38 mm), with bilateral multilocular cystic ovarian masses, measuring 18.7 cm × 13.1 cm on the right and 14.6 cm × 12.6 cm on the left, associated with the moderate generalized peritoneal free fluid collection. The liver, gall bladder, spleen, and both kidneys were normal [Figure 1].
Figure 1.
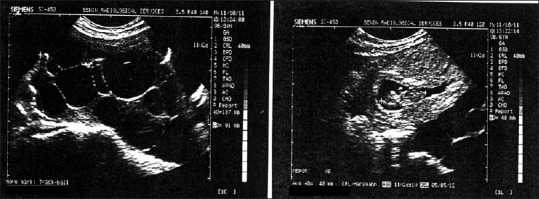
Intra-uterine fetus and enlarged ovarian follicles
Laboratory studies revealed: Hemoglobin; 12.1 g/dl, hematocrit (Hct); 35.5%, white blood cell; 8700, platelet; 406,000, Na; 133 mEq/L, K; 4.5 mEq/L, blood urea nitrogen; 23 mg/dl, creatinine; 0.5 mg/dl, fasting blood sugar; 90 mg/dl, aspartate aminotransferase; 44 U/l, alanine aminotransferase; 23 U/l, alkaline phosphatase; 105 U/l. Total protein; 5.6 g/dl, albumin 3.2 g/dl, liver function tests, prothrombin time, partial thromboplastin time, tests were also normal. Blood group was B rhesus positive and genotype AA.
A diagnosis of severe OHSS, with viable intrauterine pregnancy, was made. She was admitted for monitoring and supportive therapy. She had intravenous fluid (normal saline) at least 2000 ml per day, and liberal oral fluid intake encouraged. Medications included oral bromocriptine 2.5 mg daily for 5 days in addition to oral tramadol, piriton, metoclopramide, vasoprin, and ranitidine. During hospitalization, daily weighing, and abdominal girth measurements showed an increase from 58–63 kg to 89–92 cm, respectively, but there were no respiratory difficulties and her vital signs remained stable, the daily urine out-put was satisfactory. She responded well to treatment and at discharge, after 7 days; a full blood count, electrolytes, liver function, and coagulation profile gave results within normal limits. Hct was 34%; she weighed 63 kg, and abdominal girth was 88 cm.
She was seen weekly at the clinic. Resolution of the ascites and decrease in follicular size occurred gradually, demonstrated by clinical and ultrasonographic studies. On November 01, 2011; she had no complaints, she weighed 60 kg, abdominal girth was 84 cm and Hct; 32%, ultrasonography revealed a single active fetus of 13 weeks gestation, with regressed adnexal masses with no ascites [Figure 2]. She remained stable, and at 26 weeks gestation ultrasound showed normal sized ovaries and there was no free fluid in the abdomen and pelvis. The fetus was compatible with 27 weeks of gestation, and no gross anomaly noted, the placenta was posteriorly located. Amniotic fluid was also within normal limits [Figure 3].
Figure 2.
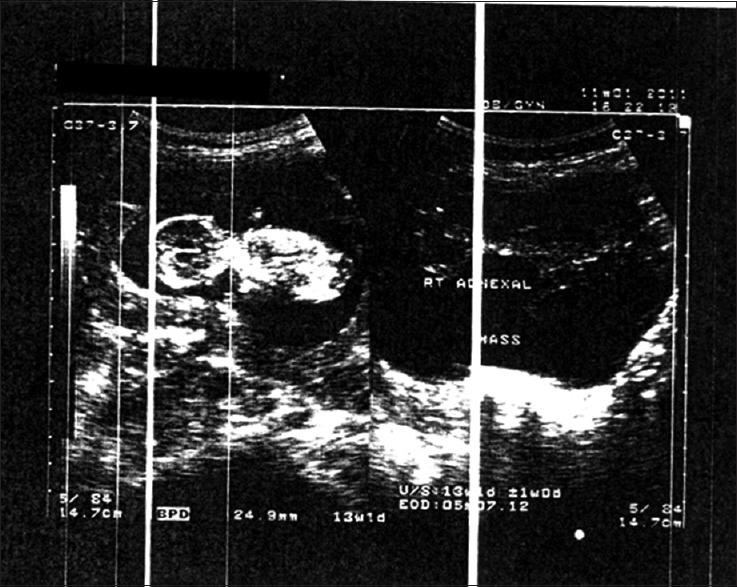
Reduction in enlarged ovarian follicles with viable fetus
Figure 3.
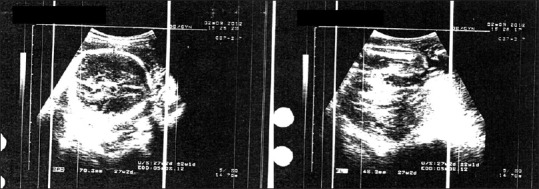
Viable fetus with no visible follicle or ascites
The pregnancy proceeded normally and at 39 weeks of gestation, the patient had spontaneous onset labor and underwent an uncomplicated vaginal delivery of male new born weighing 3.4 kg.
DISCUSSION
OHSS in spontaneous pregnancy is uncommon. In our case, OHSS occurred spontaneously in association with a singleton gestation. She did not receive any medication for ovulation induction and had no identified risk for OHSS. Published reports on spontaneous OHSS with a singleton pregnancy and no associated risk factors are few, majority being with an associated risk factor such as hypothyroidism, molar pregnancy, multiple gestation, or polycystic ovarian disease.3,4,5,6 Indeed, some authors have reported previous OHSS or familial history as identified risk factor.4,7
Although an extremely rare condition, OHSS is potentially fatal in its severest form if not managed properly. For the more familiar typical OHSS, which follows stimulated cycles in assisted reproduction, several preventive steps can be undertaken to avert the occurrence of OHSS when the patient is considered at high risk.1,2 However, this is not the situation in spontaneous OHSS, where cases are only recognized when the condition is already manifest. Thus, crucial to effective management is early recognition to prevent drastic, avoidable and sometimes invasive interventions.
Our patient was referred because of suspicion of an ovarian neoplasm owing to the ultrasound features in an unstimulated ovary. Previous reports have shown the similarity between ovarian tumors and OHSS8 also interesting to note that exploratory laparotomy was performed in some cases because ovarian cancer was suspected.3,8 Our experience in management of OHSS from a dedicated in vitro fertilization program9 helped in apt and prompt diagnosis/management of this case.
There is a vast body of literature on the management of OHSS and pregnancy.1,2,10 The management is tailored to the degree of severity. Severe OHSS requires hospital admission and replacement of lost intravascular volume to prevent grave complications such as renal failure and thromboembolic events.2,10 The patients should be closely monitored to ensure they do not progress into the critical category. For patients with significant ascites, paracentesis is beneficial by decreasing intra-abdominal pressure and improving renal blood flow with a subsequent increased production of urine.1,10 Other helpful interventions that may be necessary include thoracocentesis for pleural effusion, heparin for thromboembolism, surgical laparotomy for torsion or hemorrhage of the cyst. However, our patient required none of these. In this case, we used the dopamine agonist, bromocriptine in an attempt to reduce the severity of OHSS; Prophylactic administration of dopamine agonist has been associated with a significant reduction in the incidence of symptoms and signs of moderate/severe OHSS. It is thought that dopamine agonist inhibits vascular endothelial growth factor receptor 2 phosphorylation and signaling and thus vascular permeability.11,12 Its use is safe and not associated with any detrimental effect on pregnancy or fetal development.11,12,13
Our case emphasizes the importance of scrupulous evaluation of all women with ovarian masses and/or ascites complicating pregnancy and the need for clinicians to bear the differential diagnosis of OHSS. By taking all possible differential diagnoses into account, an unnecessary laparotomy may be avoided. Furthermore, this case highlights the importance of astute ultrasound evaluation in making diagnosis and treatment follow-up. Furthermore, the safety and beneficial effect of bromocriptine in OHSS is re-emphasized.
CONCLUSION
OHSS is a potentially life-threatening complication that can occur sequel to the influence of hCG (whether exogenous or endogenous). Early recognition and appropriate supportive therapy is advocated to ensure a good outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): A review. Hum Reprod Update. 2002;8:559–77. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 2.Vloeberghs V, Peeraer K, Pexsters A, D'Hooghe T. Ovarian hyperstimulation syndrome and complications of ART. Best Pract Res Clin Obstet Gynaecol. 2009;23:691–709. doi: 10.1016/j.bpobgyn.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Ayhan A, Tuncer ZS, Aksu AT. Ovarian hyperstimulation syndrome associated with spontaneous pregnancy. Hum Reprod. 1996;11:1600–1. doi: 10.1093/oxfordjournals.humrep.a019452. [DOI] [PubMed] [Google Scholar]
- 4.Zalel Y, Orvieto R, Ben-Rafael Z, Homburg R, Fisher O, Insler V. Recurrent spontaneous ovarian hyperstimulation syndrome associated with polycystic ovary syndrome. Gynecol Endocrinol. 1995;9:313–5. doi: 10.3109/09513599509160465. [DOI] [PubMed] [Google Scholar]
- 5.Oztekin O, Soylu F, Tatli O. Spontaneous ovarian hyperstimulation syndrome in a normal singleton pregnancy. Taiwan J Obstet Gynecol. 2006;45:272–5. doi: 10.1016/S1028-4559(09)60241-2. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso CG, Graça LM, Dias T, Clode N, Soares L. Spontaneous ovarian hyperstimulation and primary hypothyroidism with a naturally conceived pregnancy. Obstet Gynecol. 1999;93 (5 Pt 2):809–11. doi: 10.1016/s0029-7844(98)00435-9. [DOI] [PubMed] [Google Scholar]
- 7.Di Carlo C, Bruno P, Cirillo D, Morgera R, Pellicano M, Nappi C. Increased concentrations of renin, aldosterone and Ca125 in a case of spontaneous, recurrent, familial, severe ovarian hyperstimulation syndrome. Hum Reprod. 1997;12:2115–7. doi: 10.1093/humrep/12.10.2115. [DOI] [PubMed] [Google Scholar]
- 8.Lipitz S, Grisaru D, Achiron R, Ben-Baruch G, Schiff E, Mashiach S. Spontaneous ovarian hyperstimulation mimicking an ovarian tumour. Hum Reprod. 1996;11:720–1. doi: 10.1093/oxfordjournals.humrep.a019240. [DOI] [PubMed] [Google Scholar]
- 9.Orhue AA, Aziken ME, Osemwenkha AP, Ibadin KO, Odoma G. In vitro fertilization at a public hospital in Nigeria. Int J Gynaecol Obstet. 2012;118:56–60. doi: 10.1016/j.ijgo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins JM, Drakeley AJ, Mathur RS, editors. The Management of Ovarian Hyperstimulation Syndrome. RCOG Green Top Guideline no.5. 2006 [Google Scholar]
- 11.Soares SR, Gómez R, Simón C, García-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008;14:321–33. doi: 10.1093/humupd/dmn008. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez C, Martí-Bonmatí L, Novella-Maestre E, Sanz R, Gómez R, Fernández-Sánchez M, et al. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab. 2007;92:2931–7. doi: 10.1210/jc.2007-0409. [DOI] [PubMed] [Google Scholar]
- 13.Ricci E, Parazzini F, Motta T, Ferrari CI, Colao A, Clavenna A, et al. Pregnancy outcome after cabergoline treatment in early weeks of gestation. Reprod Toxicol. 2002;16:791–3. doi: 10.1016/s0890-6238(02)00055-2. [DOI] [PubMed] [Google Scholar]


