Abstract
Background & objectives:
Skin is an established tissue source for cell based therapy. The hair follicle has been introduced later as a tissue source for cell based therapy. The ease of tissue harvest and multipotent nature of the resident stem cells in skin and hair follicle has promoted basic and clinical research in this area. This study was conducted to evaluate skin stem cells (SSCs) and hair follicle stem cells (HFSCs) as candidate cells appropriate for neuronal and melanocyte lineage differentiation.
Methods:
In this study, SSCs and hair follicle stem cells (HFSCs) were expanded in vitro by explant culture method and were compared in terms of proliferative potential and stemness; differentiation potential into melanocytes and neuronal lineage.
Results:
SSCs were found to be more proliferative in comparison to HFSCs, however, telomerase activity was more in HFSCs in comparison to SSCs. Capacity to differentiate into two lineages of ectoderm origin (neuronal and melanocyte) was found to be different. HFSCs cells showed more propensities towards melanocyte lineage, whereas SSCs were more inclined towards neuronal lineage.
Interpretation & conclusions:
The study showed that SSCs had differential advantage over the HFSCs for neuronal cell differentiation, whereas, the HFSCs were better source for melanocytic differentiation.
Keywords: Differentiation, hair follicle stem cells (HFSCs), skin stem cells (SSCs)
The neural crest is a transient embryonic tissue that gives a wide array of adult cell types and tissues including melanocyte and neuronal cells1. Some neural crest stem cells bypass differentiation signal and persist within crest-derived tissues. The hair follicles have been shown to harbour pluripotent neural crest stem cells2,3,4 and these can be differentiated into melanocytes, neuronal cells, adipose cells and other lineages5.
In skin, apart from melanocyte and keratinocyte stem cells6,7, dermis harbours dermal stem cells (DSCs)8,9 and skin derived progenitors (SKPs)10,11,12. Both are of neural crest in origin and are capable of differentiating into melanocyte and neural lineages. Furthermore, SKPs are more close to the neuronal cell lineage, while, DSCs are more close to the melanocytes progenitors13.
The skin stem cells (SSCs) are in clinical set up for a long period of time and many cell based applications are there for the management of vitiligo, burn and other pigmentary disorders. Some investigators have used hair follicle stem cells (HFSC) for cell based clinical needs, especially in vitiligo14,15,16. We under-took this preliminary study to evaluate the HFSCs in comparison to SSCs to establish a candidate cell for the differentiation into melanocyte and neuronal lineage. These both being originated from neural crest cells could be exploited for research and therapeutic purpose.
Material & Methods
This study was conducted in Stem Cell Facility, All India Institute of Medical Sciences (AIIMS), New Delhi, India. The study protocol was approved by the Institute Ethics Committee and Institutional Committee for Stem Cell Research. Tissue samples were collected from patients after taking their written informed consent. Hair and skin tissues were collected from the patients undergoing surgical management for vitiligo at AIIMS, New Delhi. Five sample of each skin and hair follicle were collected.
Hair follicle tissue processing: Hair follicles isolated by punch biopsy method were transported in the transport media [consisted of 1x Dulbecco's modified Eagles medium (DMEM) supplemented with 150 U/ml of penicillin and 150 µg/ml streptomycin and amphoterecin B 2.5 µg/ml] within 30 min of tissue harvest to the laboratory in ice pack at 4°C. The fatty tissue and dermal part were removed from the hair follicles by enzymatic treatment. To remove the dermal and fattay tissue, hair follicles were incubated in 10 mg/ml dispase enzyme (Invitrogen, NY, USA) overnight at 4°C. Individual hair follicles rooted in the dermis were plucked with the help of an epilation forceps.
Skin tissue processing: Pigmented healthy skin tissues were transported to the laboratory in cool packs. The fatty tissue intact with the skin tissue was removed with the help of a scalpel blade. To further remove the epidermis from dermis, skin tissue sample was incubated in 10 mg/ml dispase overnight at 4°C. Chopped small pieces of epidermis of approximately 2 x 2 mm size were kept intact for the purpose of explant culture.
In vitro expansion of skin stem cells (SSCs) and hair follicle stem cells (HFSCs) by explant culture method: One explant in case of skin was skin tissue measuring approximately 2 x 2 mm in size and in case of hair tissue individual hair follicles were used as explants. One explant/cm2 area was cultured according to the modified Rheinwald-Green system17 consisting of 3:1 DMEM and Ham's F12 nutrient mix (Sigma, USA), supplemented with 10 per cent foetal bovine serum (Hyclone, USA), 10 ng/ml epidermal growth factor (PeproTech, USA), 2.8 μg/ml hydrocortisone, 5 μg/ml insulin, 10 μg/ml transferrin (Sigma, USA), 10 ng/ml cholera toxin (Millipore, USA) and 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, USA) over fibronectin (Sigma, USA) coated culture dish (BD Falcon, USA).
Characterization of cells expanded from skin and hair follicle tissue for stemness: The SSCs and HFSCs were characterized for the expression of stem cell markers by immunofluorescence. The markers used for the immunofluoresence studies were cytokeratin (CK) 19, CK 15 and ß1 integrin.
Cell proliferation study by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay: Rate of cell proliferation was calculated by the MTT assay18. The SSCs and HFSCs were separately cultured in a 96-well culture plate (5,000 cells/well) with culture medium. MTT assay was done on day, 3, 5, 7, 10, 12, and 15; and 50 µl of MTT reagent (5mg/ml in PBS) was added to the wells in triplicate and incubated for 3.5 h at 37°C. After incubation, medium was removed and 300 µl of dimethylsulphoxide (Sigma, USA) was added to each well to dissolve the formazan crystals. The coloured solution (200 ul) was transferred to 96-well plates and read at 570 nm in a plate reader (EL 800, Biotek, USA).
Culture of immortalized human foreskin fibroblast (I-HFF) cell lines: I-HFF cells were maintained in growth medium containing Iscove's modified Dulbecco's medium (IMDM) (Sigma, USA) supplemented with 10 per cent foetal bovine serum (Hyclone, USA), 2 mM/ml L-glutamine (Gibco/Invitrogen, USA), 1 per cent non-essential amino acids (NEAA) and 100 IU/ml penicillin and 100 mg/ml streptomycin (Gibco/Invitrogen, USA).
Expression level of telomerase reverse transcriptase (TERT) gene transcript expression in SSCs and HFSCs by quantitative real time polymerase chain reaction (qRT-PCR)19: The relative expression of TERT gene transcript in SSCs and HFSCs was studied in comparison to the I-HFF cell line. SYBR green (KAPA Biosystems, South Africa) chemistry was used for relative quantification of the gene expression. Relative gene expression was evaluated by 2-∆∆Ct method20. ∆Ct was obtained by subtracting the CtReference from Cttarget (∆Ct = Cttarget - CtReference). ∆∆Ct was obtained by subtracting ∆CtControl cells from ∆CtTarget cells (∆∆Ct = ∆CtTarget - ∆CtControl cells). Realplex 4 epgradient Mastercycler (Eppendorf, Hamburg, Germany) was used for setting up the relative quantification experiments. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as the reference gene. The realplex software was used to analyze the data.
The primers used for the study were as follows: reference gene GAPDH forward- 5’ gagtcaacggatttggtcgt30 ‘ reverse-5’ gac aagcttcccgttctcag30 ‘; TERT forward-5’ ggcaagtcctacgtccagtg0 3’, reverse-5’ gggcatagctgaggaaggtt 30 ‘.
Induction of SSCs and HFSCs into melanocyte differentiation: For melanocyte differentiation, 70-80 per cent confluent cultures of SSCs and HFSCs were incubated in the medium with the following composition: 50 per cent molecular cellular and developmental biology (MCDB) 201 medium, 40 per cent Ham's F12, nutrient mix (Sigma, USA), supplemented with 10 per cent foetal calf serum, 2mM/ml L-glutamine (Gibco, USA), 10-4 mol/l L-ascorbic acid, 10 nM/ml phorbol 12-myristate 13-acetate (PMA), 10 ng/ml cholera toxin, 20 ng/ml fibroblast growth factor (PeproTech, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin (Gibco, USA). Geneticin (Sigma, USA) at a concentration of 100 µg/ml was used to remove the contaminating fibroblast.
Functional assessment of melanocytes by L-DOPA staining: The functionality of the differentiated melanocytes was checked in vivo by the ability of the melanocytes to reduce the L-DOPA (L-3,4-dihydroxyphenylalanine) into DOPA-chrome with the help of tyrosinase enzyme. Cultured melanocytes were fixed with 10 per cent formalin in phosphate buffer saline (PBS) for 3 h at 4°C. Cells were rinsed with PBS and incubated with 0.05 mg/ml L-DOPA (Sigma-Aldrich, USA) in PBS for 3 h at 37°C. Following incubation, cells were rinsed with PBS and fixed with 10 per cent buffered formalin for 1 h. Functional melanocytes were stained brown in the presence of L-DOPA.
Induction of SSCs and HFSCs into neuronal differentiation: For neuronal differentiation 70-80 per cent confluent cultures of stem cells were incubated in the neurobasal medium (Invitrogen, USA) containing 100 IU/ml penicillin and 100 mg/ml streptomycin (Gibco, USA) supplemented with 20 ng/ml basic fibroblast growth factor (bFGF), 10 ng/ml epidermal growth factor (EGF) (PeroTech, USA), 10 ng/ml B-27 supplement (Invitrogen, USA) and 2 mM/ml L-glutamine (Gibco, USA). Geneticin (100 µg/ml) was added to remove the contaminating fibroblast. Medium was changed every third day, the cells started to change their morphology after 4 to 5 days of culture. This protocol was standardized in our laboratory (unpublished data).
Immunofluorescence staining for stem cells, melanocytes and neuronal cells: Cells grown over the cover slips or cytospin preparations were taken for immunofluorescence staining. Cells were fixed in 4 per cent paraformaldehyde for 10 min at room temperature, blocked with 2 per cent bovine serum albumin (BSA), and stained with primary antibodies. For HFSCs cells; CK15, CK19, and ß1-integrin, (Millipore, USA) antibodies were used. HMB45 (Human Melanoma Black 45) and S100 (S100 because of their solubility in a 100%-saturated solution with ammonium sulphate at neutral pH.) (Millipore, USA) antibodies were used to stain differentiated melanocytes. NF (neurofilament) and TH (tyrosine hydroxylase) (eBiosciences, USA) antibodies were used to stain differentiated neurons. Negative control was used by omitting the primary antibody. Fluorescein isothiocyanate (FITC) and Texas red (TR) (BD, USA) conjugated secondary antibodies were used to develop fluorescence signal. Propidium iodide (PI) and 4;6-diamidino-2-phenylindole (DAPI) (Sigma, USA) were used for nuclear staining.
qRT-PCR analysis for the gene expression pattern in melanocytes and neuronal cells derived from SSCs and HFSCs19: The relative expressions of MITF (microphthalmia-associated transcription factor)and TYR (tyrosinase) genes in melanocytes and NF and TH genes in neuronal cells were compared with their expression in native skin tissue using SYBR green chemistry as described earlier. The primers used for the study were as follows- MITF forward 5’ACCTCGGAACTGGGACTGAG 3’, reverse 5’GGGGACACTGAGGAAAGGAG 3’; TYR forward 5’ACGTCTTCCTGAACCACAGG 3’, reverse 5’CGTGGGGTCACTGTAACCTT 3’; NF forward 5’TGGGAAATGGCTCGTCATTT 3’ reverse 5’CTTCATGGAAGCGGCCACTT 3’ and TH forward 5’ggtcgcgctgcctgtact0 3’, reverse 5’tcatcacctggtcaccaagtt0 3’.
Statistical analysis: Student t test was used for statistical calculations between two groups and One Way-ANOVA for multiple groups.
Results
Initiation of cell growth from the skin explant took a little more time i.e. 6-7 days in comparison to the hair follicle explant, which took 4-5 days. The cell sheet obtained from both the tissue explants showed a typical honey comb morphology, and growth pattern of keratinocyte stem cells. Hair follicle stem cells could be expanded for 10 passages as compared to skin stem cells which could be taken for up to eight passages. The doubling time was 3.7±0.8 and 4.6±0.4 days for skin stem cells and hair follicle stem cells, respectively.
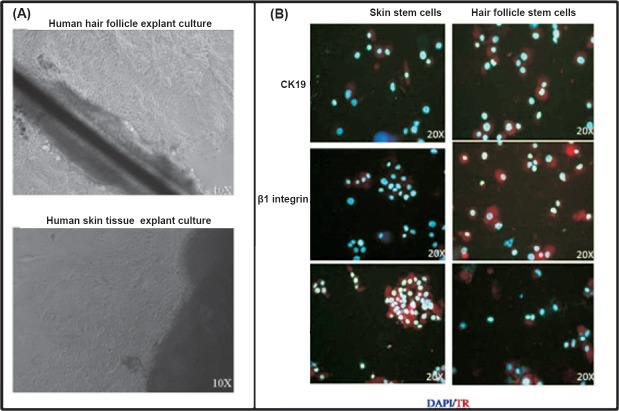
Characterization of in vitro expanded cells: The cells expanded from both the tissue were positive for keratinocyte stem cell markers K19, ß1-integrin and CK15 as revealed by immunofluorescence (Fig. 1). The cells were negative for the expression of the markers in the negative control slides.
Fig. 1.
Expansion and charcaterization of skin and hair follicle stem cells. (A). Photomicrograph showing the explant culture of hair and skin tissue over fibronectin coated culture dish. The cells started coming out from the explant periphery after a week in culture media. (B). The cells were positive for the expression of CK-19, β1-integrin and CK-15 as revealed by immunofluorescence. DAPI (blue colour) was used as a nuclear stain. The secondary antibody was conjugated with Texas red (red colour).
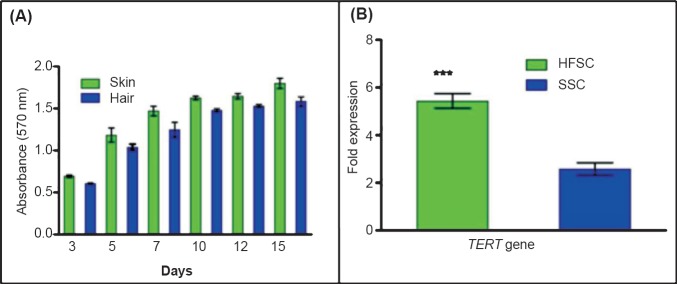
Cell proliferation assay by MTT: The MTT assay was performed for the determination of SSCs and HFSCs proliferation rate for upto 15 days in culture (Fig. 2A). The cell proliferation was evident from the increase in the absorbance of the purple formazan formed. There was a difference in the absorbance of the formazan formed from 3rd day onwards and the difference in the absorbance was maintained till 15th day. SSCs showed higher absorbance in comparison to the HFSCs, indicating a higher rate of cell proliferation for SSCs.
Fig. 2.
Analysis of skin and hair follicle stem cell proliferation. (A). The cell proliferation for SSCs and HFSCs at day 3, 5, 7, 10, 12 and 15. The data represent the mean ± SD of five independent experiments. (B). The expression of TERT gene was significantly (P<0.001) higher in hair follicle stem cells as compared to the skin stem cells. The data represent mean ± SD of five independent experiments.
Expression of TERT gene in SSC and HFSC: The fold expression of TERT gene in HFSCs and the SSCs was 5.433 ± 0.616 and 2.583 ± 0.518, respectively (Fig. 2B), indicating HFSCs to have a significantly (P<0.001) higher level transcription of TERT gene compared to SSCs.
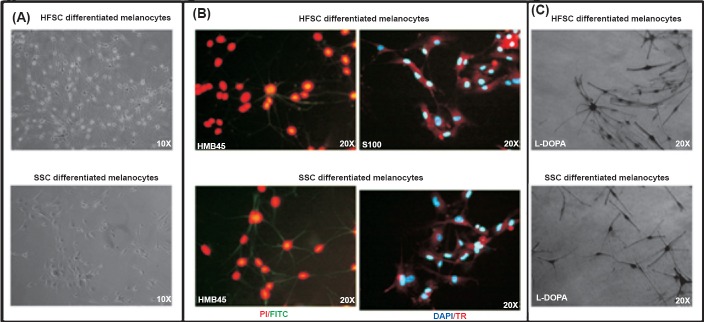
Differentiation into melanocytes: The protocol to differentiate the stem cells into melanocytes was of 21 days. The melanocytes differentiation initiated after 8-10 days in the differentiation medium for stem cells of both skin and hair origin. The culture dish became homogenously confluent with melanocytes in almost 25-30 days. The morphological appearance of the melanocytes obtained from the two sources appeared to be similar. There was no visible difference in the melanocytes stained with HMB-45 (recognizes melanosomal protein gp100) and S-100 (recognizes calcium binding protein within melanocytes) antibodies, for immunofluorescence, differentiated from HFSCs and SSCs (Fig. 3A,B)
Fig. 3.
Differentiation of SSCs and HFSCs into melanocytes and their characterization. (A). Photomicrographs depicting the differentiation of SSCs and HFSCs in melanocytes. (B). SSCs and HFSCs differentiated melanocytes were positive for the melanocyte markers HMB-45 and S-100 markers as revealed by immunofluorescence. PI (red colour) or DAPI (blue) has been used as nuclear stain. The secondary antibody was conjugated with Texas red (red colour) or FITC (green colour). (C). Functional assay of the differentiated melanocytes was done by L-DOPA staining.
Determination of in vitro functional aspect of melanocytes differentiated from SSCs and HFSCs by L-DOPA staining: The melanocytes derived from skin and hair follicle were functionally active as demonstrated by L-DOPA assay. The percentage of cells found to be functionally active were 55.06 ± 5.24 and 61.27 ± 3.64 for melanocytes derived from HFSCs and SSCs, respectively (Fig. 3C).
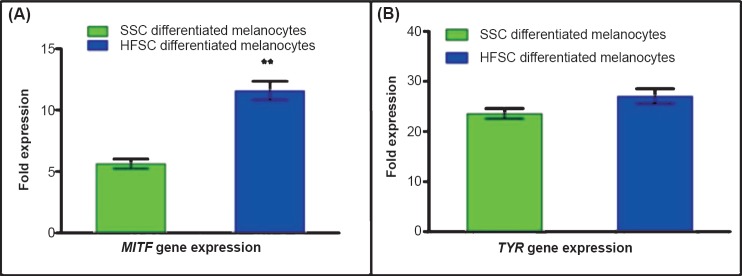
Quantitative RT-PCR for the relative expression of MITF and TYR gene in melanocytes derived from HFSCs and SSCs: The fold expression of MITF gene was 5.63 ± 0.66 folds for SSCs derived melanocytes and 11.58±1.32 folds for HFSCs derived melanocytes which was significantly (P<0.01) higher. The fold expression of TYR in HFSCs derived melanocytes and SSCs derived melanocytes was 27.09 ± 2.60 and 23.56 ± 1.75 folds, respectively (Fig. 4).
Fig. 4.
Characterization of differentiated melanocytes for specific transcripts by qRT-PCR. (A). Expression of MITF gene was significantly (P<0.01) higher in melanocytes derived from HFSCs as compared to the SSCs derived melanocytes. (B). There was no significant difference in the expression of tyrosinase (TYR) in melanocytes differentiated from either SSCs or HFSCs. The data represent mean ± SD fold expression of five independent experiments.
Differentiation into neuronal cells: Protocol for neuronal differentiation was of 14 days. The stem cells started to change their morphology in the differentiation medium by 4-5 days. The differentiated cells were positive for neuronal markers NF and TH by immunofluorescence (Fig. 5).
Fig. 5.
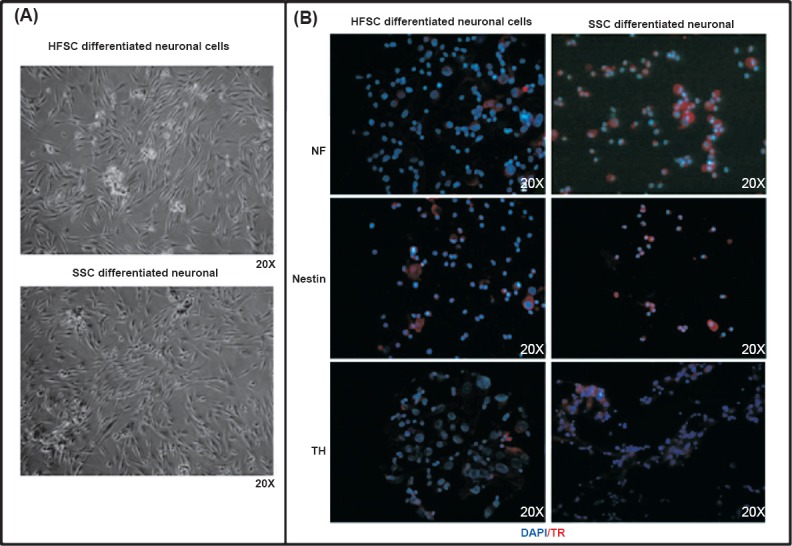
Differentiation of SSCs and HFSCs cells into neuronal cells and their characterization. (A). Photomicrographs depicting differentiation of SSCs and HFSCs into neuronal cells. (B). Immunofluorescence image of SSCs and HFSCs derived neuronal cells stained neuronal cell specific markers NF, nestin and TH antibody. DAPI (blue colour) was used as a nuclear stain. The secondary antibody was conjugated with Texas red (red colour).
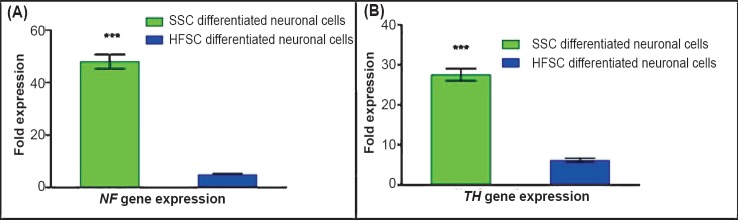
Quantitative RT-PCR for the relative expression of TH and NF genes in neuronal cells differentiated from HFSCs and SSCs: The fold expression of TH gene was 27.56 ±3.44 folds for SSCs derived neuronal cells and 6.2±1.158 folds for HFSCs derived neuronal cells. The fold expression of NF gene in SSCs derived neuronal cells and HFSCs derived neuronal cells was 48.03 ± 6.07 folds and 4.89 ± 1.03 folds, respectively (Fig. 6). The fold expression of both the genes was significantly (P<0.001) higher in SSCs derived neuronal cells.
Fig. 6.
Characterization of differentiated neuronal cells for specific transcripts by qRT-PCR. (A). The expression of NF gene was significantly (P<0.001) higher in SSCs derived neuronal cells in comparison to HFSCs derived neuronal cells. (B). The expression of TH gene was significantly (P<0.001) higher in SSCs derived neuronal cells in comparison to HFSCs derived neuronal cells. Values are mean ± SD of five independent experiments.
Discussion
Epidermal tissues of hair and skin are rich source of multipotent stem cells. Hair follicle and skin tissue, apart from the bone marrow, are perhaps the only tissues, which have got the niche for diverse kind of stem cells- melanocyte stem cells, keratinocyte stem cells, and mesenchymal stem cells21,22. The skin largely contains the keratinocyte stem cells (KSCs), in the basal layer. Lately, two distict population of stem cells have been identified in skin dermis known as skin derived progenitors (SKPs) and dermal sheath cells (DSCs)8,9,10,11,13,23,24.
The cell expanded in culture contained a mixed population of cell types. The stem cells can be maintained in culture for longer period of time compared to other cell types. The culture expanded cells from skin and hair were positive for the keratinocyte stem markers. There was no significant difference in the positivity for the expression of stem cell markers. There was no significant difference in the proliferation rate of SSCs versus HFSCs, however, the telomerase activity was significantly higher in HFSCs. This indicates that with almost same proliferation rate in both groups of the cells, HFSCs had the advantage of being passaged for longer period of time as indicated by higher telomerase gene expression.
The melanocytes showed multidendritic morphology, similar to that expected in situ. We used PMA, as inducer of melanocyte, in the induction medium which promoted cell proliferation and helped multiple dendrite formation25,26. Contaminating fibroblasts in cultures were eliminated by the addition of geneticin, which resulted in homogenous population of differentiated cells25,26. The mode of action of geneticin is by blocking the protein synthesis which ultimately kills the cells. The differentiated cells from both the origins were positive for the melanocytes markers, MITF and tyrosinase (TYR) by qRT-PCR and HMB-45, and S-100 by immunofluorescence. The melanocytes were also positive for L-DOPA stain indicating their functionality, however, the efficiency of generation of functional melanocytes was higher in SSCs derived melanocytes. There was a population of cells which acquired the dentritic phenotype but lacked L-DOPA staining. These are the amelanotic melanocytes (AMMC)27,28. These AMMC are considered to be melanocyte stem cells population29,30. The importance of SSC and HFSC is not just limited to the cells of the epidermal lineage. Nestin positive cells have been isolated and differentiated from skin and hair follicle tissue into neuronal cells30,31. Neuronal cells differentiated from SSCs showed significantly higher expression of the neuronal cell specific genes (TH and NF)32,33 in comparison to the HFSCs derived neuronal cells. The observation may be explained in view of skin tissue harbouring a special niche of stem cells, which are known as SKPs20,25. The SKPs are known to have close relationship with neuronal cells. The SKPs tend to have spontaneous differentiation tendency towards neuronal lineage. However, there is no report on comparative study of the neuronal cells differentiated from SSCs and HFSCs.
This was a preliminary study which investigated the candidate cells appropriate for neuronal and melanocyte lineage differentiation. The differentiation studies indicated hair to be a better source for melanocyte differentiation and skin to be more inclined for neuronal differentiation. Future studies involving more number of samples and exploring the functional aspects of differentiated melanocytes and neuronal cells need to be initiated.
Acknowledgment
This work was supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India, through grant number BT/01/COE/07/03. The first author (AK) was a recipient of Research Fellowship from University Grants Commission, Government of India. Authors acknowledge Dr Anis Feki, University of Geneva, Switzerland, for providing I-HFF cell line.
Footnotes
Conflicts of Interest: None.
References
- 1.Le Douarin NM, Kalcheim C. 2nd ed. Cambridge, UK: Cambridge University Press; 1999. The neural crest. [Google Scholar]
- 2.Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258–69. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 3.Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–54. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- 4.Lavker RM, Sun TT, Oshima H, Barrandon Y, Akiyama M, Ferraris C, et al. Hair follicle stem cells. J Investig Dermatol Symp Proc. 2003;8:28–38. doi: 10.1046/j.1523-1747.2003.12169.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–88. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbar T. Epithelial skin stem cells. Methods Enzymol. 2006;419:73–99. doi: 10.1016/S0076-6879(06)19004-7. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Fukunaga-Kalabis M, Herlyn M. Isolation and cultivation of dermal stem cells that differentiate into functional epidermal melanocytes. Methods Mol Biol. 2012;806:15–29. doi: 10.1007/978-1-61779-367-7_2. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, et al. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2010;123:853–60. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–93. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 12.Kajahn J, Gorjup E, Tiede S, von Briesen H, Paus R, Kruse C, et al. Skin-derived human adult stem cells surprisingly share many features with human pancreatic stem cells. Eur J Cell Biol. 2008;87:39–46. doi: 10.1016/j.ejcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Zabierowski S, Fukunaga-Kalabis M, Li L, Herlyn M. Dermis-derived stem cells: a source of epidermal melanocytes and melanoma? Pigment Cell Melanoma Res. 2011;24:422–9. doi: 10.1111/j.1755-148X.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 14.Vanscheidt W, Hunziker T. Repigmentation by outer-root-sheath-derived melanocytes: proof of concept in vitiligo and leucoderma. Dermatology. 2009;218:342–3. doi: 10.1159/000197467. [DOI] [PubMed] [Google Scholar]
- 15.Mohanty S, Kumar A, Dhawan J, Sreenivas V, Gupta S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br J Dermatol. 2011;164:1241–6. doi: 10.1111/j.1365-2133.2011.10234.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Mohanti S, Sahni K, Kumar R, Gupta S. Extracted hair follicle outer root sheath cell suspension for pigment cell restoration in vitiligo. J Cutan Aesthet Surg. 2013;6:121–5. doi: 10.4103/0974-2077.112679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–43. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–93. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 19.Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–62. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 23.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 24.Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–23. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eves PC, Beck AJ, Shard AG, Mac Neil S. A chemically defined surface for the co-culture of melanocytes and keratinocytes. Biomaterials. 2005;26:7068–81. doi: 10.1016/j.biomaterials.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Czajkowski R, Placek W, Drewa T, Olszewska-Slonina D, Sir J, Kowaliszyn B, et al. Establishing melanocyte cultures in a serum-free system for transplantation in vitiligo patients. Med Sci Monit. 2006;12:CR63–9. [PubMed] [Google Scholar]
- 27.Zhu WY, Zhang RZ, Ma HJ, Wang DG. Isolation and culture of amelanotic melanocytes from human hair follicles. Pigment Cell Res. 2004;17:668–73. doi: 10.1111/j.1600-0749.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–10. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishikawa-Torikai S, Osawa M, Nishikawa S. Functional characterization of melanocyte stem cells in hair follicles. J Invest Dermatol. 2011;131:2358–67. doi: 10.1038/jid.2011.195. [DOI] [PubMed] [Google Scholar]
- 30.Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530–4. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA. 2005;102:17734–8. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higashida T, Jitsuki S, Kubo A, Mitsushima D, Kamiya Y, Kanno H. Skin-derived precursors differentiating into dopaminergic neuronal cells in the brains of Parkinson disease model rats. J Neurosurg. 2010;113:648–55. doi: 10.3171/2010.2.JNS091432. [DOI] [PubMed] [Google Scholar]
- 33.Gingras M, Champigny MF, Berthod F. Differentiation of human adult skin-derived neuronal precursors into mature neurons. J Cell Physiol. 2007;210:498–506. doi: 10.1002/jcp.20889. [DOI] [PubMed] [Google Scholar]