Abstract
Background & objectives:
Under the outbreak-based measles surveillance in Maharashtra State the National Institute of Virology at Pune receives 3-5 serum samples from each outbreak and samples from the local hospitals in Pune for laboratory diagnosis. This report describes one year data on the measles and rubella serology, virus isolation and genotyping.
Methods:
Maharashtra State Health Agencies investigated 98 suspected outbreaks between January-December 2013 in the 20 districts. Altogether, 491 serum samples were received from 20 districts and 126 suspected cases from local hospitals. Samples were tested for the measles and rubella IgM antibodies by commercial enzyme immunoassay (EIA). To understand the diagnostic utility, a subset of serum samples (n=53) was tested by measles focus reduction neutralization test (FRNT). Further, 37 throat swabs and 32 urine specimens were tested by measles reverse transcription (RT)-PCR and positive products were sequenced. Virus isolation was performed in Vero hSLAM cells.
Results:
Of the 98 suspected measles outbreaks, 61 were confirmed as measles, 12 as rubella and 21 confirmed as the mixed outbreaks. Four outbreaks remained unconfirmed. Of the 126 cases from the local hospitals, 91 were confirmed for measles and three for rubella. Overall, 93.6 per cent (383/409) confirmed measles cases were in the age group of 0-15 yr. Measles virus was detected in 18 of 38 specimens obtained from the suspected cases. Sequencing of PCR products revealed circulation of D4 (n=9) and D8 (n=9) strains. Four measles viruses (three D4 & one D8) were isolated.
Interpretation & conclusions:
Altogether, 94 measles and rubella outbreaks were confirmed in 2013 in the State of Maharasthra indicating the necessity to increase measles vaccine coverage in the State.
Keywords: India, Maharashtra, measles, measles genotyping, outbreak-based surveillance, rubella
Measles is mainly a human disease caused due to measles virus (MeV) that belongs to the family Paramyxoviridae and genus Morbillivirus. MeV is enveloped, single stranded, negative-sense RNA virus consisting of 15894 nucleotides. MeV is highly contagious and causes a disease characterized by high-grade fever, cough, coryza, conjunctivitis and appearance of maculopapular rash. MeV is serologically a monotypic virus, but genetically distinguished into 24 genotypes1.
Measles is an endemic disease in India causing significant morbidity and mortality. The District Level Household Survey (DHLS)-32 conducted in 2007-2008 indicated an overall 68 per cent measles immunization coverage in the country; however, variations in the vaccine coverage within the States were also documented3. The Government of India had introduced one dose of measles vaccine in its Universal Immunization Programme (routine immunization) in 1985. A second dose of measles containing vaccine has been introduced in the routine immunization during 20104. As per the revised routine immunization schedule, every child should get two doses of measles vaccine; 1st at the age of 9-12 months and 2nd at the age of 16-24 months. If a child has missed the 1st or the 2nd dose, both doses can be offered up to 5 yr of age maintaining a gap of at least 4 wk between the doses5.
As per the Maharashtra State Government data, overall measles immunization coverage varied between 90-100 per cent in the last five years5. For laboratory confirmation, the National Institute of Virology (NIV), Pune, receives about 3-5 representative serum samples from each suspected outbreak. We report here one year data obtained in 2013 that focused on the measles and rubella serology, virus isolation, genotyping and phylogenetic analysis.
Material & Methods
Altogether, 98 suspected measles outbreaks were reported from the 20 districts of Maharashtra between January and December 2013. From the 20 districts, 491 serum samples were received for the laboratory diagnosis at the Measles Laboratory, NIV, Pune. Of these 491 suspected measles cases, 253 were male and 238 were female cases. Majority of the suspected cases were grouped in 0-15 yr (97.5%, n=479) and the remaining 12 were >15 yr old. Of these suspected cases, 126 had history of at least one dose of measles vaccine as per the record or by the parents recall.
Measles cases reported to the local hospitals: Between January and December 2013, altogether 126 suspected measles cases were reported to the local hospitals (n=10) and subsequently referred to the NIV for laboratory diagnosis. Generally, all the suspected measles cases were referred to NIV. Of the 126 suspected measles cases, 68 were males and 58 were females. Majority of the suspected cases belonged to 0-15 yr (94.4%, n=119) and the remaining seven were >15 yr old. Of these suspected cases, 53 had history of at least one dose of measles vaccine as per the record or by parents recall.
Measles and rubella IgM antibody detection: The serum samples collected from clinically suspected measles cases were tested for the measles specific IgM antibody detection by enzyme immunoassay (EIA) (Siemens, Margburg, Germany) according to the manufacturer's instructions. Due to similar clinical presentation of measles and rubella, measles negative samples were tested for the rubella specific IgM antibody detection by EIA (Siemens, Margburg Germany). For the qualitative evaluations, samples with a corrected ΔA of <0.100 were considered negative; those with a ∆A>0.200 were considered positive; and those between 0.100 and 0.200 was considered equivocal or weak positive.
Use of measles focus reduction neutralization test (FRNT) for laboratory diagnosis: To understand the diagnostic utility of neutralization test, a subset of samples (n=53) was tested in measles FRNT as described earlier6. Briefly, for the challenge experiments, wild type measles virus (D8 genotype) dilution giving 30-45 viral foci/10 µl inoculum per well was deduced for the virus stock and further used in neutralization test. Virus-serum neutralization reaction was performed at 37°C in 5 per cent CO2 incubator for 2 h. The un-neutralized measles virus was detected using established immunocolorimetric assay (ICA) by using mouse monoclonal antibodies to measles nucleoprotein (Abcam plc, Cambridge, UK). Rest of the protocol was followed as described previously6. FRNT results were compared with the clinical presentation and measles IgM antibody status.
Detection of measles virus by RT-PCR, genotyping and virus isolation: From the 38 suspected cases from the Pune and Thane, 37 urine specimens and 32 throat swabs were collected and transported on ice packs to the laboratory within 12 h. Measles reverse transcription (RT)-PCR was performed on urine and throat swab samples as per the WHO standard protocols described previously7,8,9,10. Eighteen clinical specimens (throat swab/urine) were used for the virus isolation. Five hundred microlitre of processed throat swab or one ml of centrifuged urine specimen was inoculated into the flask(s) containing 24 h old Vero hSLAM cell monolayer and cytopathic effect (CPE) was observed up to six days11. Specimens with negative CPE were passage three times to confirm virus isolation.
The study was conducted after obtaining approval from the institutional ethics committee.
Results
Of the 98 outbreaks, 71 (72.4%) suspected measles outbreaks were reported from the major cities like Mumbai, Thane, Nashik, Nagpur and Pune where majority of population movement occurs due to work/job. Rubella positivity was noted in seven districts; however, majority of rubella cases (73/76, 96%) were reported from Mumbai, Thane, Nashik, Nagpur and Pune. During February-May 2013, the State Government Health Authorities reported 51 outbreaks. Ten outbreaks were reported in the period of June-August and measles outbreaks peaked in September-December months resulting in 37 additional outbreaks. The seasonality of suspected measles outbreaks is presented in the Figure A. The month-wise data showed two major seasons of measles, i.e. first between February and May and second between September and December. However, it is interesting to note that measles cases were reported throughout the year.
Figure.
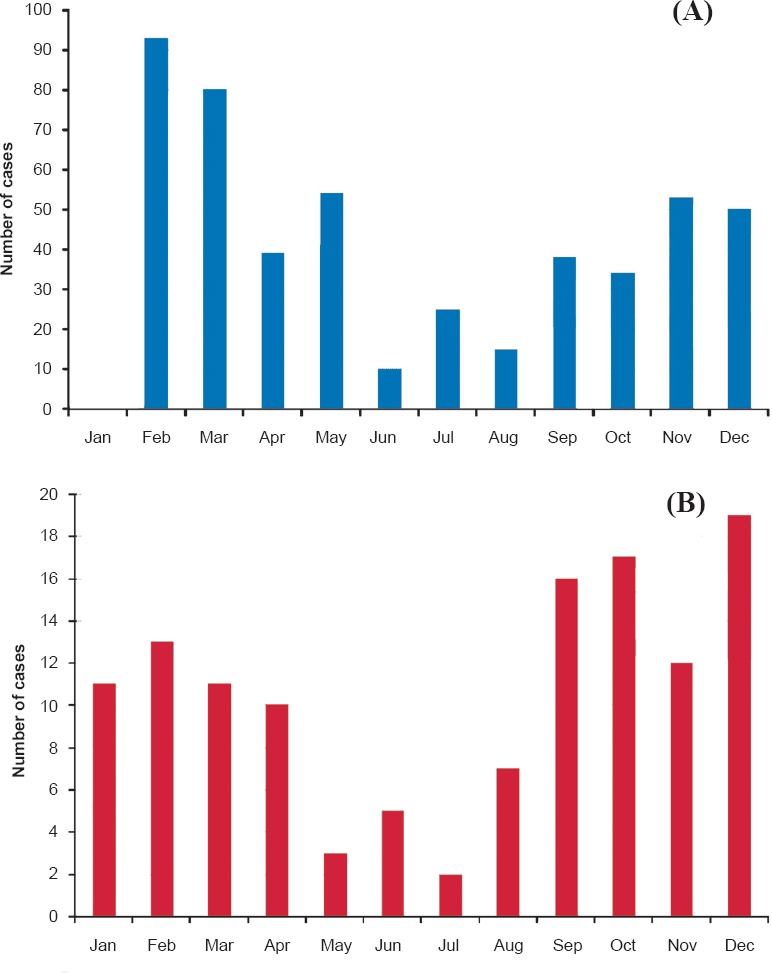
(A). Seasonality of suspected measles outbreaks reported in Maharashtra State (2013). (B). Seasonality of suspected measles cases reported in the local hospitals (2013).
Of the 98 suspected measles outbreaks, 61 were confirmed as a measles outbreaks and four outbreaks (from the districts; Bhandara, Chandrapur, Nagpur and Washim) could not be confirmed either for measles or rubella. Rubella IgM antibody positivity was noted in 33 suspected outbreaks where 12 were confirmed as rubella outbreaks and 21 were confirmed as mixed outbreaks of measles and rubella. Overall, 64.7 per cent (318/491) of suspected measles cases were confirmed by measles IgM EIA [92.4% (n=294) of laboratory confirmed cases in age group 0-15 yr] and 76 suspected measles cases were confirmed as rubella [92.1% (n=70) laboratory confirmed cases in 0-15 yr age group] (Table). Six suspected cases remained equivocal and the remaining 167 (34%) could not be confirmed serologically. Overall, 63.2 per cent (160/253) of suspected male cases and 66.4 per cent (158/238) suspected female cases were confirmed by measles specific IgM antibody EIA. Among the 491 suspected cases of measles, 126 had a history of measles immunization at childhood but only 68 (53.9%, males; 28, females; 40) showed laboratory confirmed measles.
Table.
Laboratory diagnosis for suspected measles outbreaks
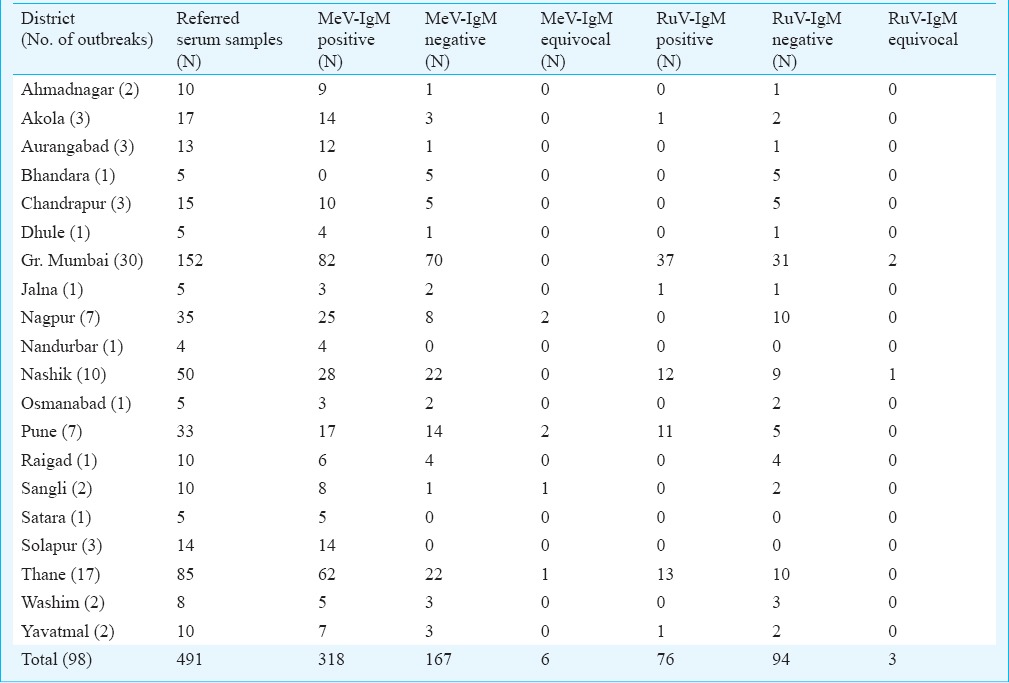
Of the 126 serum samples obtained from the local hospitals, 91 (79.2%) were found positive for measles IgM antibodies [97.8% (n=89) of laboratory confirmed cases in the age group 0-15 yr]. Measles positivity was 75.8 per cent (44/58) in females and 69.1 per cent (47/68) in males. From 35 measles negative cases, three were positive for rubella specific IgM antibodies. Interestingly, 53 cases had a history of measles immunization at childhood but 41 (77.3%, males; 34 & females; 19) individuals showed laboratory confirmed measles. Measles cases were reported to the local hospitals during entire year, however, the month-wise data showed two major seasons of measles, i.e. first between January and April and second between September and December (Figure B).
Altogether 53 of 617 serum samples were tested in measles FRNT. Presence of measles IgM antibody (EIA) detected in 25 samples correlated with measles neutralizing antibody results (FRNT). The neutralizing antibody titres to the wild type measles virus were higher (1/128 to 1/512, data not shown).
Measles RT-PCR showed presence of measles RNA in 18 samples, further sequencing of PCR products revealed circulation of D4 (n=9) and D8 (n=9) strains. All measles virus sequences (N gene) generated during study were submitted in GenBank. Four measles virus isolates were obtained on Vero hSLAM cells, further sequencing revealed three measles D4 and one D8 genotypes.
Discussion
Despite high measles vaccine coverage in the Maharashtra State, 98 suspected measles outbreaks were reported in the 20 districts in 2013. It was interesting to note that of the 33 suspected measles outbreaks, 12 were confirmed as rubella and 21 mixed outbreaks of measles and rubella. This indicated towards a need to establish rubella surveillance to understand rubella virus transmission in the population.
Previous studies conducted during 1996-1998 revealed circulation of measles genotypes A, D4 and D8 in the Pune city8. Further continuation of the measles surveillance revealed circulation of measles D7 virus in three cities (Bengaluru, Chennai and Pune) from India9. Molecular epidemiology of measles has been extensively studied during 2005-2010 and during this period, circulation of measles D4, D7 and D8 strains was detected in different parts of the country10. However, circulation of measles D4 and D8 genotypes was detected in the Maharashtra State during 2013.
In this study, measles in the immunized children has been detected indicating primary vaccine failure and supports introduction of second dose of measles to boost immunity in the population. This needs careful interpretation since individual's vaccination status was procured from the health records or by parent's recall. Therefore, a possibility of bias could not be ruled out. A few adult measles cases were observed suggesting that these individuals remained either unexposed to wild type virus or were not immunized for the measles during their childhood. Another possible reason may be the accumulation of unvaccinated individuals in the population that may remain susceptible for measles. As a result, many measles outbreaks may be detected in the State.
The WHO SEARO has setup a target for the elimination of measles and control of rubella before 202012. For this purpose, a case based measles and rubella surveillance has been initiated in the phased manner in South-East Asian countries. In case-based measles surveillance all suspected measles cases presented with fever and maculopapular rash (non-vesicular) with cough, coryza or conjunctivitis are notified, investigated and subsequently confirmed either in laboratory or epidemiologically or clinically. In India the measles case-based surveillance has not been introduced yet, but those States which can utilize their existing surveillance system (like Integrated Disease Surveillance Programme or Polio network) may enter into a case-based surveillance.
Use of measles FRNT for the laboratory diagnosis has been documented on clinically and laboratory confirmed measles cases. Additionally, the neutralization test is preferred for the sero-epidemiological studies or vaccine studies to characterize the quantitative or qualitative immune response. FRNT may serve as an alternative for the gold standard plaque reduction neutralization test (PRNT).
Overall, 94 measles outbreaks were reported during a year indicating scope to increase measles vaccine coverage (by using a two-dose strategy) in the State of Maharashtra. Additionally, there is a need to increase measles and rubella surveillance to cover all the districts of Maharashtra. More efforts are needed to understand the circulating measles and rubella viruses (or genotypes) in Maharashtra and other parts of India, ultimately useful to track measles transmission.
Acknowledgment
Authors acknowledge Drs Sirima Pattamadilok and Lucky Sangal from the WHO SEARO for their support during this surveillance programme. Authors also thank Maharashtra State Health Authorities and the local hospitals for referring clinical specimens at NIV, Pune.
References
- 1.WHO. Measles virus nomenclature update: 2012. Wkly Epidemiol Rec. 2012;87:73–81. [PubMed] [Google Scholar]
- 2.District level household and facility survey (DLHS-3), 2007-08. India, Mumbai: IIPS; 2010. [accessed on February 24, 2016]. International Institute for Population Sciences (IIPS) Available from: www.rchiips.org/pdf/india_report_dlhs-3.pd . [Google Scholar]
- 3.Morris SK, Awasthi S, Kumar R, Shet A, Khera A, Nakhaee F, et al. Measles mortality in high and low burden districts of India: estimates from a nationally representative study of over 12,000 child deaths. Vaccine. 2013;31:4655–61. doi: 10.1016/j.vaccine.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SK, Sosler S, Haldar P, Hombergh HV, Bose AS. Introduction strategy of a second dose measles containing vaccine in India. Indian Pediatr. 2011;48:379–82. doi: 10.1007/s13312-011-0066-1. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health and Family Welfare Government of India 2010. Measles vaccine 2nd dose in routine immunization. [accessed on January 2, 2014]. Available from: http://www.unicef.org/india/Measles_2nd_dose_in_routine_immunization-_guideor_health_workers_(_English_).pdf .
- 6.Vaidya SR, Brown DW, Jin L, Samuel D, Andrews N, Brown KE. Development of a focus reduction neutralization test (FRNT) for detection of mumps virus neutralizing antibodies. J Virol Methods. 2010;163:153–6. doi: 10.1016/j.jviromet.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Rota PA, Brown K, Mankertz A, Santibanez S, Shulga S, Muller CP, et al. Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis. 2011;204(Suppl 1):S514–23. doi: 10.1093/infdis/jir118. [DOI] [PubMed] [Google Scholar]
- 8.Wairagkar N, Rota PA, Liffick S, Shaikh N, Padbidri VS, Bellini WJ. Characterization of measles sequences from Pune, India. J Med Virol. 2002;68:611–4. doi: 10.1002/jmv.10228. [DOI] [PubMed] [Google Scholar]
- 9.Vaidya SR, Wairagkar NS, Raja D, Khedekar DD, Gunasekaran P, Shankar S, et al. First detection of measles genotype D7 from India. Virus Genes. 2008;36:31–4. doi: 10.1007/s11262-007-0172-2. [DOI] [PubMed] [Google Scholar]
- 10.Wairagkar N, Chowdhury D, Vaidya S, Sikchi S, Shaikh N, Hungund L, et al. Molecular epidemiology of measles in India, 2005-2010. J Infect Dis. 2011;204(Suppl 1):S403–13. doi: 10.1093/infdis/jir150. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya SR, Kumbhar NS, Bhide VS. Detection of measles, mumps and rubella viruses by immuno-colorimetric assay and its application in focus reduction neutralization tests. Microbiol Immunol. 2014;58:666–74. doi: 10.1111/1348-0421.12201. [DOI] [PubMed] [Google Scholar]
- 12.Thapa A, Khanal S, Sharapov U, Swezy V, Sedai T, Dabbagh A, et al. Progress toward measles elimination - South-East Asia Region 2003-2013. MMWR Morb Mortal Wkly Rep. 2015;64:613–7. [PMC free article] [PubMed] [Google Scholar]


