Abstract
Purpose: 2-hydroxy estradiol (2-OHE2) is a catechol derivative of 17β -Estradiol (E2) and it is synthesized from E2 catalyzed by cytochrome P4501A1. Previous studies reported that 2-OHE2 is a physiologic antioxidant in lipoproteins, liver microsomes, and the brain. Catechol derivatives show an anti-inflammatory effect through the inhibition of prostaglandin endoperoxide synthase (PGS) activity. Corneal erosion caused by dry eye is related to an increase in oxidative stress and inflammation in ocular surface cells. We investigated the therapeutic effects of 2-OHE2 on corneal damage caused by dry eye.
Methods: Steroidal radical scavenging activity was confirmed through the electron spin resonance (ESR) method. PGS activity was measured using the COX Fluorescent Activity Assay Kit. To evaluate the effect of 2-OHE2 on the treatment for dry eye, 2-OHE2 was applied as an eye drop experiment using dry eye model rats.
Results: 2-OHE2 scavenged tyrosyl radical and possibly suppressed oxidative stress in corneal epithelial cells. In addition, 2-OHE2 inhibited PGS activity, and 2-OHE2 is probably a competitive inhibitor of PGS. Corneal PGS activity was upregulated in the dry eye group. Therefore, 2-OHE2 eye drops improved corneal erosion in dry eye model rats.
Conclusions: 2-OHE2 is a candidate for the treatment of dry eye through the suppression of inflammation and oxidative stress in the cornea.
Introduction
The ocular surface is strongly affected by physical and chemical factors, e.g., temperature [1], humidity [2], ultraviolet irradiation [3], airflow [4], and chemical compounds [5], and these factors contribute to the induction of oxidative stress and inflammation of the ocular surface [4-6]. To protect the corneal epithelium from oxidative stress, antioxidants, e.g., Glutathione, Tyrosine, vitamins [7], and lactoferrin [8,9], are contained in tear fluid, and antioxidative [5] and xenobiotic metabolizing enzymes [10] are expressed in corneal epithelial cells. Dry eye results not only from a desiccation of the ocular surface, but also from a lack of tear components essential for maintaining the ocular surface. As corneal erosion caused by dry eye is involved in an increase in oxidative stress [5,11] and inflammation [6,12], the development of a restrainer of these causes led us to establish a treatment for dry eye.
Our previous study showed vitamin A [13] and selenium compound [9,11] were beneficial to the treatment of dry eye, as they regulate oxidative stress in the cornea. Selenium is an essential trace element for most animals and it is indispensable for activities of selenium containing enzymes, e.g., glutathione peroxidase (GPx) and thioredoxin reductase (TrxR), which all have oxidoreductase functions [14]. GPx and TrxR participate in the reduction of hydrogen peroxide and lipoperoxide [14,15]; therefore, the physiologic role of GPx and TrxR is the regulation of oxidative stress.
The antioxidative ability of estrogens was shown in previous studies [16,17]. 2-hydroxy estradiol (2-OHE2), which is a catechol derivative of 17β-Estradiol (E2), is reported as a physiologic antioxidant in lipoproteins [18], liver microsomes [19], and the brain [20]. 2-OHE2 is physiological metabolite of E2 having an additional hydroxy group in position two of A-ring and synthesized from E2 catalyzed by cytochrome P4501A1 (CYP1A1) [21,22]. In the meantime, 2-OHE2 is a kind of catechol. Catechin and its derivatives, which are also a kind of catechol, showed anti-inflammatory effects and improved ocular inflammatory conditions caused by dry eye [23,24]. The anti-inflammatory effect of catechols contributed to the inhibition of prostaglandin endoperoxide synthase (PGS) [25]. Taken together with these studies [18-20, 23-25], 2-OHE2 is expected to have two therapeutic effects, i.e., antioxidative and anti-inflammatory effects. In this study, we investigate the therapeutic effects of 2-OHE2 on corneal damage caused by dry eye.
Methods
Assay for radical scavenging activity of steroids by ESR method
2-OHE2 was purchased from Sigma-Aldrich Co. LLC (St. Louis, MO). E2, estrone, and testosterone were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). The electron spin resonance (ESR) signals of tyrosine radical were measured according to a previous study [26]. The reaction mixture contained 400 μM myoglobin, 400 μM H2O2, and 100 mM 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) in a 50-mm PBS containing 1% ethanol, pH 7.4. Steroids were added to the reaction mixture before the start of the reaction as a radical scavenger. The reaction was started by adding H2O2, and the ESR spectra of the DMPO-tyrosyl radical adducts were recorded immediately at room temperature in a flat cell. The ESR settings were: microwave power, 10 mW; modulation frequency, 100 KHz; modulation field, 0.1 G; receiver gain, 1,000; and time constant, 0.3 s.
Inhibition of PGS activity by 2-OHE2
To determine the 2-OHE2 concentration to be used in the following experiments, the influence of 2-OHE2 on cellular viability was evaluated using human corneal epithelial cell line CEPI-17-CL4 (CEPI) cells [27]. An short tandem repeat (STR) analysis showed that CEPI cells most likely originated from a female individual (Appendix 1). CEPI cells were cultured to 80%–90% confluence in 96-well plates in EpiLife medium supplemented with HCGS (Cascade Biologics Inc., Portland, OR). EpiLife medium was serum-free medium of human epidermal keratinocytes and human corneal epithelial cell. 2-OHE2 was added to the medium, and CEPI cells were incubated for 24 h. The medium was changed to a fresh medium containing 10% Alamar Blue (Wako Pure Chemical Industries, Ltd., Osaka, Japan), which is a reagent for evaluating cellular viability. After 1 h of incubation, the fluorescence of Alamar Blue was measured using the ARVO SX multilabel reader (ARVO SX; PerkinElmer Japan Co., Ltd., Yokohama, Kanagawa, Japan).
As the anti-inflammatory effect of catechols was due to the inhibition of PGS activity, the effect of 2-OHE2 on PGS activity was evaluated using a cellular extract of CEPI cells. CEPI cells were homogenized using 50 mM Tris-HCl buffer (pH 7.4) containing 5 mM EDTA and 1 mM DTT and centrifuged at 10,500 × g for 5 min to prepare the cellular extract. PGS activity was measured using a COX Fluorescent Activity Assay Kit (Cayman Chemical Company, Ann Arbor, MI) with or without 10 μM steroids in the assay buffer. This assay kit provides a fluorescence-based method for detecting COX-1 or COX-2 activity in both crude (cell lysates/tissue homogenates) and purified enzyme preparations. The assay utilizes the peroxidase component of the COXs. In this assay, the reaction between prostaglandin G2, which is produced from arachidonic acid by COX, and 10-acetyl-3,7-dihydroxyphenoxazine produces the fluorescent compound resorufin, which can be analyzed using an excitation wavelength of 530–540 nm and an emission wavelength of 585–595 nm [28]. The fluorescence of resorufin was measured using the ARVO SX multilabel reader.
Effects of 2-OHE2 eye drops on treatment for dry eye
The effect of 2-OHE2 on the treatment for dry eye was evaluated by applying 2-OHE2 eye drops to the dry eye rat model. All animal experiments as follows were approved by the Laboratory Animal Care and Use Committee of Keio University School of Medicine. The dry eye rat model was prepared by removing the lacrimal glands [11,29]. Male 6-week-old Sprague-Dawley rats (SD; n = 10 in each experiment) were purchased from CLEA Japan, Inc. (Tokyo, Japan). Both lacrimal glands were surgically removed from the anesthetized rats. An eye drop experiment was performed on the day following lacrimal gland removal. As new medicines are usually used from the maximal concentration showing no toxicity to investigate the effectiveness, the concentration of 2-OHE2 was decided to be 0.1, 1, or 10 μM. 2-OHE2 was dissolved with ethanol to 10 mM, and the 2-OHE2 solution was diluted with PBS to 0.1, 1, or 10 μM. One eye in each dry eye rat was treated with 0, 0.1, 1 or 10 μM 2-OHE2 and the other side was treated with PBS (Vehicle, 0.1, 1, or 10 μM 2-OHE2 group, n = 10), and 5 μl of each eye drop was administrated 4 times per day for 2 weeks. Normal rats whose lacrimal glands were not removed were prepared as a normal group (n = 10) for a comparison with the vehicle and 2-OHE2 groups. After all the eye drop treatments, the corneal fluorescein scores and tear volumes were evaluated according to our previous study [11]. To estimate the degree of dry eye, the ocular surfaces of rats were photographed after being stained by fluorescein. The photographs of the corneas were divided into nine areas. Each area was scored from 0 to 3 points depending on the degree of staining, and the scores of all areas were totaled. This total was called the ‘fluorescein score.’ As the fluorescein score was varied based on the influences of humidity, temperature, air stream, etc., the fluorescein score of normal rats should be measured as a reference. The mean value of the fluorescein score of normal rats in each experiment was approximately 4 from 1. The mean value of fluorescein score of normal rats in each experiment was approximately 2.5 ± 1.5. Because the PBS eye drops did not reduce fluorescein staining, the fluorescein score of PBS eye drop rats is nearly equal to that of dry eye rats.
Corneas from the normal, vehicle, and 1-μM 2-OHE2 groups were collected to isolate the total RNA for real-time RT-PCR. Corneal total RNA was extracted using TRIzol (Life Technologies Corporation, Carlsbad, CA). Reverse transcription was performed using SuperScript III (Life Technologies Corporation). The gene expression of matrix metallopeptidase-9 (MMP-9), heme oxigenase-1 (Hmo×-1), PGS-2, Interleukin (IL)-6, or tumor necrosis factor (TNF)-α was estimated by TaqMan real-time RT-PCR (ABI PRISM 7500 Sequence Detection Systems, Life Technologies Corporation, Carlsbad, CA) according to the manufacturer’s protocol. Primer sets and TaqMan probes, as well as other reagents for TaqMan real-time PCR were purchased from Life Technologies Corporation. All data were analyzed with the ΔΔCt method, and the mRNA of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal standard. Corneas from the normal and vehicle groups were collected to assay for PGS activity of the corneal extract using the COX Fluorescent Activity Assay Kit.
Results
Radical scavenging activity of steroids
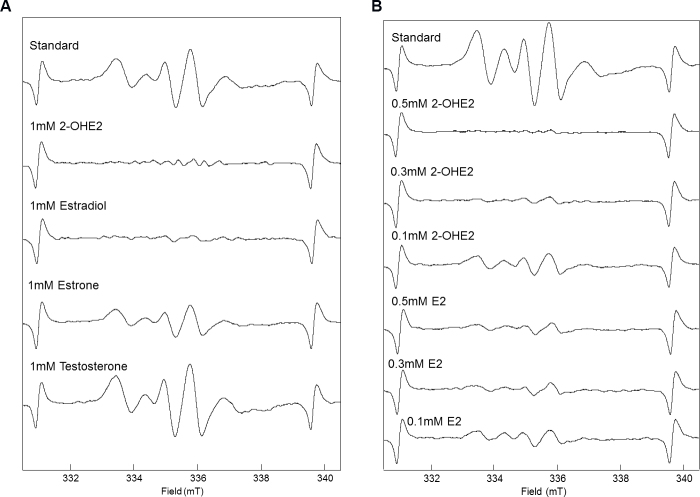
Figure 1 shows the results of the ESR study, where 1 mM 2-OHE2 and 1 mM estradiol reduced the ESR signal of tyrosyl radical and 1mM estrone weakly reduced and 1mM testosterone did not reduce the ESR signal (Figure 1A). In addition, 0.5 mM and 0.3 mM 2-OHE2 also reduced the ESR signal, whereas 0.5 mM and 0.3 mM estradiol weakly reduced the ESR signal (Figure 1B). These results mean estrogens, especially 2-OHE2, scavenge tyrosyl radical and possibly suppress oxidative stress in corneal epithelial cells.
Figure 1.
Diminution of the ESR signals of hydroxyl radicals by steroid. ESR signal of tyrosine radical was measured (Standard). A: Effect of 1mM steroid on ESR signals of hydroxyl radicals. B: Effect of 0.1, 0.3, 0.5 mM E2 and 2-OHE2 on ESR signals of hydroxyl radicals. The ESR setting was as follows: microwave power, 10 mW; modulation frequency, 100 kHz; modulation field, 0.1 G; receiver gain, 1000 and time constant, 0.3 s.
Inhibition of PGS activity by 2-OHE2
The toxic effect of 2-OHE2 on cellular viability was evaluated (Figure 2A). The cellular viability of CEPI cells was kept over 90% until 10 μΜ and then it sharply declined. The 2-OHE2 concentration for using cell culture and animal experiments was decided to be less than 10 μM.
Figure 2.
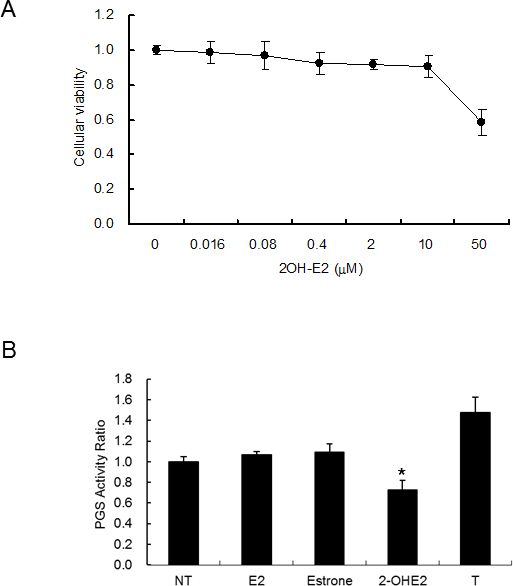
Suppression of PGS activity in CEPI cells by steroids. A: Effect of 2-OHE2 on cellular viability. The horizontal axis shows the concentration of 2-OHE2 added to the medium, and the vertical axis shows the cellular viability in CEPI cells. B: The PGS activity of the cellular extract of CEPI cells with or without 10 μM steroids. NT, E2, or T means without a steroid, estradiol, or testosterone addition. The vertical axis shows the PGS activity ratio. The results are expressed as mean ± SD. Dunnett’s test was used to determine the significance of differences. * indicates a significant difference from the result in NT, p<0.05.
The PGS activity of the cellular extract was measured at 3.0±0.2 nmol/minute/mg protein, and the PGS activity was significantly inhibited when 2-OHE2 was added to the assay buffer (Figure 2B). Estradiol and estrone were not affected by PGS activity, and testosterone enhanced PGS activity (Figure 2B). As only 2-OHE2 suppressed oxidative stress (Figure 1) and PGS activity (Figure 2B), we decided to apply 2-OHE2 for the treatment of dry eye.
Effects of 2-OHE2 eye drops on treatment for dry eye
Figure 3 shows the results after 2 weeks of 2-OHE2 eye drop use. The fluorescein-stained areas of the PBS treatment side (fluorescein score: 6, Figure 3A) were larger than those of the 2-OHE2 eye drop side (fluorescein score: 4, Figure 3B). The fluorescein score of the vehicle group largely increased compared to that of normal rats, and the fluorescein score of 2-OHE2 groups was shown at a low level compared with that of the vehicle group (Figure 3C). The 2-OHE2 eye drops were clearly effective in the suppression of corneal irritation. Tear secretion decreased by about 40% through lacrimal removal and it slightly recovered following the use of 2-OHE2 eye drops (Figure 3D).
Figure 3.
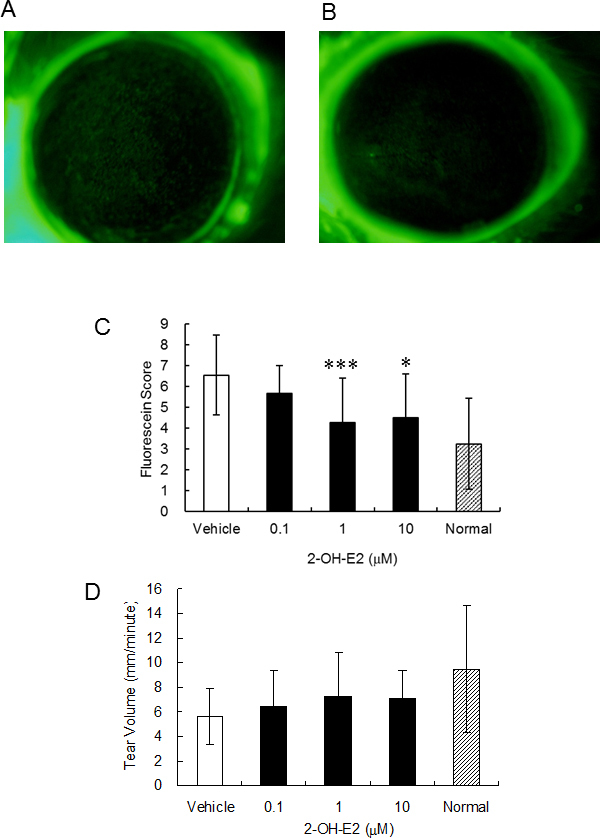
Effects of 2-OHE2 eye drops on the cornea of a dry eye rat model. Photo of cornea stained by fluorescein. A: PBS treatment, B: 2-OHE2 eye drops. C, D: Fluorescein score of cornea (C) and tear volume (D) in normal, vehicle, and 2-OHE2 groups (n = 10). Results are expressed as mean ± S.D. Dunnett’s test was used to determine the significance of differences. * and *** indicate a significant difference from the result in vehicle group, p< 0.05 and p< 0.005, respectively.
Figure 4 shows the fold change of mRNA prepared from the corneas in the normal, vehicle, and 1 μM 2-OHE2 eye drop groups. The fold change was calculated from ΔCt value, shown in Table 1. The expressions of MMP-9 and IL-6 increased markedly in the vehicle group, and this increase was suppressed by 2-OHE2 eye drops. The expressions of HO-1 and PGS-2 increased weakly, but they were not suppressed by 2-OHE2 eye drops, unlike MMP-9 and IL-6. The expression of TNF-α in the vehicle and 2-OHE2 eye drop groups did not change compared with that in the normal group.
Figure 4.
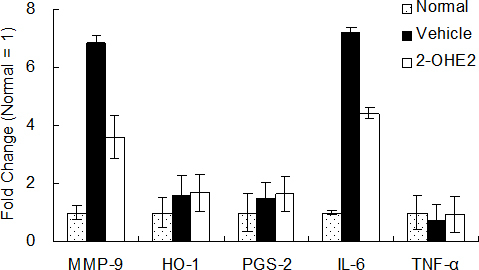
Fold change of mRNA expression in cornea. The vertical axis shows fold change calculated from ΔCt value (Table 1). Fold change of normal group defined as 1. Results are expressed as the mean ± SD (n = 6).
Table 1. mRNA expression in cornea.
| MMP-9 | HO-1 | PGS-2 | IL-6 | TNF-α | |
|---|---|---|---|---|---|
| NT |
11.3±1.9 |
10.5±1 |
9.6±0.6 |
18.6±3.8 |
9.1±0.7 |
| Vehicle |
8.±1.7 |
9.8±0.7 |
9.1±0.8 |
15.7±2.6 |
9.6±0.7 |
| 2-OHE2 | 9.4±0.4 | 9.8±0.7 | 8.9±0.7 | 16.4±2.2 | 9.2±0.7 |
The relative expression level of mRNAs was determined by real-time RT–PCR and was estimated using the ΔCt value. ΔCt value was calculated using Ct (cycle threshold) values in each gene and GAPDH (ΔCt value=Ct value of each gene - Ct value of GAPDH). If the ΔCt value is lower, the relative RNA level is higher. Results are expressed as the mean ± SD (n=6).
PGS activity in the corneas of dry eye model rat
The unilateral removal of lacrimal glands led to a decrease in tear secretion and resulted in corneal erosion on the dry eye side. As an increase in the fluorescein score for the dry eye side compared with the normal side was confirmed (Figure 5A), the PGS activity of the corneas on both sides was measured. The PGS activity of the corneal extract on the dry eye side was higher than that on the normal side (Figure 5B). Dry eye was considered to be related with PGS activation in the cornea. The PGS activity of the corneal extract was significantly inhibited by the addition of 2-OHE2 to the assay buffer (Figure 5C).
Figure 5.
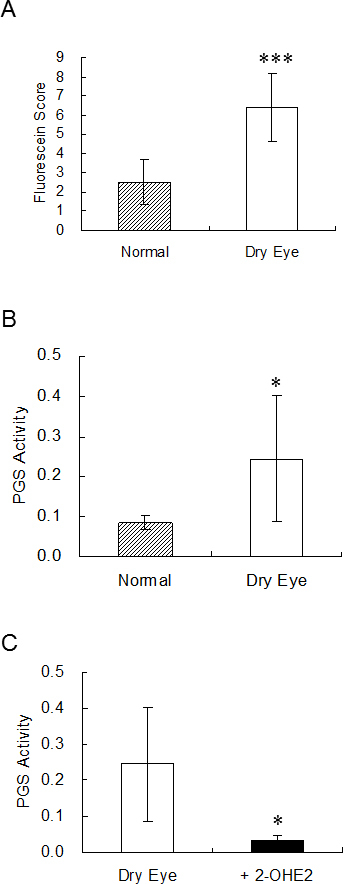
PGS activity in corneas of dry eye model rat. Fluorescein score (A) and PGS activity (B) of cornea in normal and dry eye rats (n = 10). C: PGS activity in corneal extract with or without 10 μM 2-OHE2 (n = 6). 2-OHE2 was added to the assay buffer. Results are expressed as mean ± S.D. t-test was used to determine the significance of differences. * and *** indicate a significant difference from the result in normal (A, B) or dry eye group (C), p< 0.05 and p< 0.005, respectively.
Discussion
The number of dry eye patients increases year by year [30]. Several eye drops for the treatment of dry eye are commercially available and many kinds of drug candidates are under development. This study showed that 2-OHE2 was effective in treating corneal damage in dry eye model rats. A rat with the lacrimal gland removed is suitable as a dry eye model to estimate candidate drugs for the treatment of dry eye. This model was widely used as a typical dry eye model because this model was easily prepared and drug was not used to cause dry eye. We already showed that selenium compounds were useful for the treatment of dry eye using this model [9,11]. Both saline and PBS solutions hardly showed an effect in this dry eye model. Saline and PBS solutions could be thought of as negative controls and used as vehicles.
Previous studies show that the expressions of PGS-2, MMP-9, and IL-6 are induced in the corneas of dry eyes [6,9,31,32], and the expressions of IL-6 and IL-8 increase through desiccation in corneal epithelial cells [6]. In this study, increases in MMP-9 and IL-6 in the cornea of the dry eye were suppressed by 2-OHE2 eye drops (Figure 4), which means that 2-OHE2 eye drops improved dry eye. The PGS-2 expression was not suppressed by 2-OHE2 eye drops (Figure 4). This result suggested that 2-OHE2 was not affected on PGS-2 expression. When 2-OHE2 was added to the culture medium in cultivating CEPI cells, the PGS activity in the cellular extract was not suppressed (the PGS activity of no addition was 3.0 ± 0.2 and that of 2-OHE2 addition was 3.3 ± 0.3 nmol/minute/mg protein). This means that 2-OHE2 was dissociated from PGS during cellular extract preparation. It was thought that 2-OHE2 inhibited the PGS activity as a competitive inhibitor.
It is unlikely that 2-OHE2 does not uptake to CEPI cells, because 2-OHE2 in the medium affects the viability of CEPI cells (Figure 2A). Furthermore, as 2-OHE2 is an amphipathic compound having three hydroxyl groups, 2-OHE2 is soluble in water and apt to pass through the cell membrane. 2-OHE2 captured radicals, such as reactive oxygen species, lipid radical, tyrosine radical, and, consequently, the affected cellular redox system, as catechol acts as a radical capture agent. The inhibition mechanism of PGS activity by 2-OHE2 is related to the tyrosine radical scavenging effect of 2-OHE2 (Figure 1). Tyrosine radical was essential for the activity of PGS and so the tyrosine radical scavenger acted as a PGS inhibitor [33-35]. In Figures 5B,C, the dry eye with 2-OHE2 eye drops group showed less PGS activity than the normal group. It was probably that 2-OHE2 inhibited not only induced but also endogenous PGS. The expression of TNF-α in the vehicle and 2-OHE2 eye drop groups did not change compared with the normal group. Similar results were obtained from our previous study using a dry eye model [6,9].
Although lacrimal glands were removed from rats, tear secretion was partially recovered with 2-OHE2 eye drops. This was possibly caused by the induction of tear secretion from intraorbital lacrimal glands or the recovery of water retention in the ocular surface.
P4501A1, which synthesizes 2-OHE2 from E2, is localized in the corneal epithelium [5]. As E2 is contained in tear fluid, 2-OHE2 is supposed to be physiologically produced from E2 in the corneal epithelium for the maintenance of the ocular surface. Therefore, 2-OHE2 is a candidate for the treatment of dry eye through the suppression of inflammation and oxidative stress in the ocular surface.
Acknowledgments
This work was supported in part by a grant from the Smoking Research Foundation.
Appendix 1. STR analysis.
To access the data, click or select the words “Appendix 1.”
References
- 1.Craig JP, Singh I, Tomlinson A, Morgan PB, Efron N. The role of tear physiology in ocular surface temperature. Eye (Lond) 2000;14:635–41. doi: 10.1038/eye.2000.156. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11040913&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 2.Cekiç O, Ohji M, Hayashi A, Fang XY, Kusaka S, Tano Y. Effects of humidified and dry air on corneal endothelial cells during vitreal fluid-air exchange. Am J Ophthalmol. 2002;134:75–80. doi: 10.1016/s0002-9394(02)01472-1. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12095811&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Zuclich JA, Connolly JS. Ocular damage induced by near-ultraviolet laser radiation. Invest Ophthalmol Vis Sci. 1976;15:760–4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=822714&dopt=Abstract [PubMed] [Google Scholar]
- 4.Nakamura S, Shibuya M, Saito Y, Nakashima H, Saito F, Higuchi A, Tsubota K. Effect of D-beta-hydroxybutyrate on ocular surface epithelial disorder in dry eye conditions through suppression of apoptosis. Invest Ophthalmol Vis Sci. 2003;44:4682–8. doi: 10.1167/iovs.03-0198. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14578386&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Higuchi A, Ito K, Dogru M, Kitamura M, Mitani F, Kawakita T, Ogawa Y, Tsubota K. Corneal damage and lacrimal glands dysfunction in a smoking rat model. Free Radic Biol Med. 2011;51:2210–6. doi: 10.1016/j.freeradbiomed.2011.09.025. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22001743&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Higuchi A, Kawakita T, Tsubota K. IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol Vis. 2011;17:2400–6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21976951&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 7.Tsubota K, Higuchi A. Serum application for the treatment of ocular surface disorders. Int Ophthalmol Clin. 2000;40:113–22. doi: 10.1097/00004397-200010000-00009. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11064861&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Fujihara T, Nagano T, Endo K, Nakamura M, Nakata K. Lactoferrin protects against UV-B irradiation-induced corneal epithelial damage in rats. Cornea. 2000;19:207–11. doi: 10.1097/00003226-200003000-00015. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10746454&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Higuchi A, Inoue H, Kawakita T, Ogishima T, Tsubota K. Selenium compound protects corneal epithelium against oxidative stress. PLoS ONE. 2012;7:e45612. doi: 10.1371/journal.pone.0045612. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23049824&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offord EA, Sharif NA, Macé K, Tromvoukis Y, Spillare EA, Avanti O, Howe WE, Pfeifer AM. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci. 1999;40:1091–101. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10235542&dopt=Abstract [PubMed] [Google Scholar]
- 11.Higuchi A, Takahashi K, Hirashima M, Kawakita T, Tsubota K. Selenoprotein P controls oxidative stress in cornea. PLoS ONE. 2010;5:e9911. doi: 10.1371/journal.pone.0009911. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20360971&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10487957&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Higuchi A, Shimmura S, Takeuchi T, Suematsu M, Tsubota K. Elucidation of apoptosis induced by serum deprivation in cultured conjunctival epithelial cells. Br J Ophthalmol. 2006;90:760–4. doi: 10.1136/bjo.2005.088203. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16531423&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17508906&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–35. doi: 10.1007/PL00000664. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11215509&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano M, Sugioka K, Naito I, Takekoshi S, Niki E. Novel and potent biological antioxidants on membrane phospholipid peroxidation: 2-hydroxy estrone and 2-hydroxy estradiol. Biochem Biophys Res Commun. 1987;142:919–24. doi: 10.1016/0006-291x(87)91501-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3827906&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Rifici VA, Khachadurian AK. The inhibition of low-density lipoprotein oxidation by 17-beta estradiol. Metabolism. 1992;41:1110–4. doi: 10.1016/0026-0495(92)90295-l. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1328822&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi S, Yanase T, Kobayashi K, Takayanagi R, Haji M, Umeda F, Nawata H. Catechol estrogens are more potent antioxidants than estrogens for the Cu(2+)-catalyzed oxidation of low or high density lipoprotein: antioxidative effects of steroids on lipoproteins. Endocr J. 1994;41:605–11. doi: 10.1507/endocrj.41.605. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7704084&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–8. doi: 10.1016/0039-128x(94)90006-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7940617&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Teepker M, Anthes N, Krieg JC, Vedder H. 2-OH-estradiol, an endogenous hormone with neuroprotective functions. J Psychiatr Res. 2003;37:517–23. doi: 10.1016/s0022-3956(03)00068-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14563383&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57:237–57. doi: 10.1016/0163-7258(93)90057-k. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8361994&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Mense SM, Chhabra J, Bhat HK. Preferential induction of cytochrome P450 1A1 over cytochrome P450 1B1 in human breast epithelial cells following exposure to quercetin. J Steroid Biochem Mol Biol. 2008;110:157–62. doi: 10.1016/j.jsbmb.2008.03.029. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18456490&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie K, Kodani I, Dickinson DP, Ogbureke KU, Camba AM, Wu M, Looney S, Chu TC, Qin H, Bisch F, Sharawy M, Schuster GS, Hsu SD. Effects of oral consumption of the green tea polyphenol EGCG in a murine model for human Sjogren's syndrome, an autoimmune disease. Life Sci. 2008;83:581–8. doi: 10.1016/j.lfs.2008.08.011. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18809413&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011;17:533–42. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21364905&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 25.Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8:229–35. doi: 10.2174/187152809788681029. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19601883&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 26.Miura T. A mechanistic study of the formation of hydroxyl radicals induced by horseradish peroxidase with NADH. J Biochem. 2012;152:199–206. doi: 10.1093/jb/mvs068. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22718789&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 27.Sharif NA, Wiernas TK, Howe WE, Griffin BW, Offord EA, Pfeifer AM. Human corneal epithelial cell functional responses to inflammatory agents and their antagonists. Invest Ophthalmol Vis Sci. 1998;39:2562–71. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9856766&dopt=Abstract [PubMed] [Google Scholar]
- 28.https://www.caymanchem.com/app/template/Product.vm/catalog/700200.
- 29.Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001;42:96–100. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11133853&dopt=Abstract [PubMed] [Google Scholar]
- 30.O'Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004;4:314–9. doi: 10.1007/s11882-004-0077-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15175147&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 31.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26:431–9. doi: 10.1089/jop.2010.0019. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20874497&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. doi: 10.1167/iovs.03-1145. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15557435&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 33.Miura T. Reactivity of nonsteroidal anti-inflammatory drugs with peroxidase: a classification of nonsteroidal anti-inflammatory drugs. J Pharm Pharmacol. 2012;64:1461–71. doi: 10.1111/j.2042-7158.2012.01524.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22943177&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 34.Karthein R, Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur J Biochem. 1988;171:313–20. doi: 10.1111/j.1432-1033.1988.tb13792.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2828053&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Karthein R, Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. Rapid electronic spectroscopy detected two spectral intermediates during the peroxidase reaction with prostaglandin G2. Eur J Biochem. 1988;171:313–20. doi: 10.1111/j.1432-1033.1988.tb13793.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2828053&dopt=Abstract [DOI] [PubMed] [Google Scholar]