Abstract
In this issue of Blood, Jin et al uncover how antibodies contribute to B- and T-cell pathology in sclerodermatous chronic graft-versus-host disease (cGVHD).1
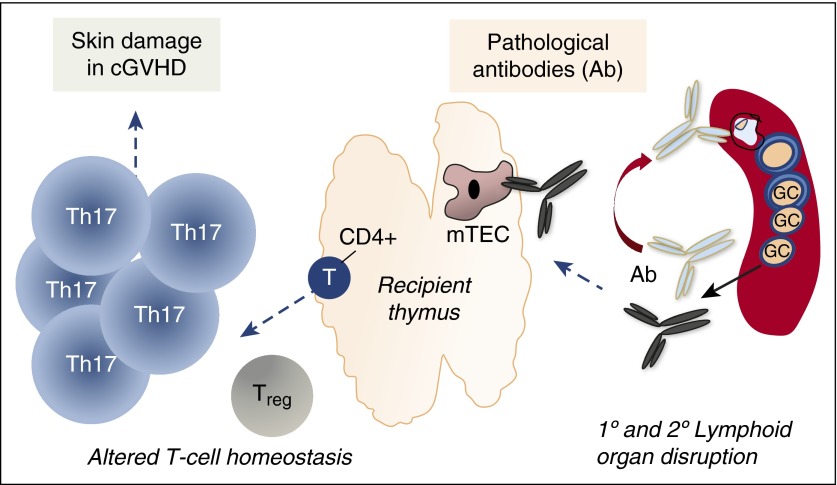
Working model of how donor-derived antibodies may help perpetuate cGVHD immune pathology. GCs are required for production of disease-inciting alloantibody early in the disease process. Due to altered T- and B-cell homeostasis, aberrant B cells develop over time that produce antibodies directed against primary (1°) and secondary (2°) lymphoid organs. Lymphoid-organ damage perpetuates loss of immune tolerance and the promotion of cGVHD of the skin. Treg, regulatory T cell.
Given that cGVHD patients are often cured of their cancer or other primary blood/marrow disease by allogeneic hematopoietic cell transplantation (HCT), the morbidity and sometimes lethality of cGVHD is especially tragic. For many long-term HCT survivors, cGVHD remains an untreatable, relentlessly morbid condition. Effective prophylaxis and treatment of cGVHD has been significantly hampered by the lack of understanding of the pathophysiology of cGVHD.
Studies in murine models continue to improve our understanding of the immunopathologic mechanisms of cGVHD, much as they did in acute GVHD. Animal studies, including some by the authors of this current paper and others, have confirmed that disease-mediating lymphocytes arise in recipients of allogeneic donor transplants and that these cells are capable of causing autoimmune disease in syngeneic animals. Insidious development of pleiotropic autoimmune disease manifestations in murine models and in patients after allogeneic HCT, but not autologous HCT, suggest that alloreactivity incites autoreactivity.2,3 Separating the distinct cGVHD events that result in ongoing broad reactivity to nonpolymorphic antigens and recipient tissues from specific immunologic reactions to malignant cells will be pivotal for developing more active and specific cGVHD treatments.
Elegant experiments in murine models have substantiated specific roles for B- and T-cell subsets in cGVHD development.4 Several studies suggested a role for B cells in cGVHD, and a seminal paper by Bruce Blazar’s group used transgenic mice either incapable of producing B cells or having B cells that cannot release immunoglobulin G (IgG) to demonstrate that B cells and secretion of anti-host antibody are required for cGVHD development.5 How B cells and antibodies might mediate cGVHD pathology remains unknown. Clinical trials assessing efficacy of rituximab in patients with steroid-refractory cGVHD initially showed promise, particularly in patients with sclerodermatous disease.6 Although these trials confirmed that B cells played a role in the disease process, more recent treatment studies were less encouraging.7 The abnormal antibody and aberrant B-cell signaling findings in patients have underscored the importance of targeting aberrant B-cell homeostasis while maintaining normal B-cell homeostasis in cGVHD.8 Although further evidence is needed to exclude an antitumor B-cell effect, the potential to preferentially eliminate cGVHD through suppression of B cells has emerged as a focus of therapeutic trials in cGVHD. Clearly, in conjunction with clinical trials, additional human and murine studies of immune mechanisms are urgently needed.
In their current study, Jin et al further our understanding of antibody-mediated cGVHD. They use their previously established T-cell dose-dependent model of cGVHD (DBA/2 CD25+ cell–depleted spleen cells across a minor histocompatibility antigen barrier) to induce cGVHD. For their experiments, they employ transgenic IgHμγ1 mice that do not secrete either IgM or IgG. After first confirming that IgHμγ1 donor cells develop into mature B cells in similar proportion to the wild-type (WT) controls after HCT, with similar subset frequencies and cell surface phenotypes, the authors show that the B-cell defect is confined to immunoglobulin secretion capacity. By comparing IgHμγ1 donor bone marrow cells to WT controls, the authors demonstrate mechanistically how secreted immunoglobulin may contribute to disease promotion. IgG deposits in diseased skin and the lack of disease manifestations in the absence of immunoglobulin suggest a pathological role for these antibodies. Interestingly, IgG was found in the diseased thymus, and its specificity was confirmed via passive transfer experiments in which IgG-containing sera led to IgG deposition in both the skin and thymus.
Although T cells have been elegantly shown to promote thymic destruction in acute GVHD,9 a mechanistic role for antibodies in development of cGVHD had not been described until the current Jin et al paper. A decrease in UEA-1+ medullary thymic epithelial cell (TEC) number suggests that these cells may be targeted by cGVHD antibodies, akin to findings in myasthenia gravis patients. A limitation of the current study is whether cGVHD antibodies are directly or indirectly cytotoxic and to what antigens they are directed. A concomitant infiltration by T helper 17 (Th17) cells in diseased skin suggests that altered T-cell homeostasis may result from antibody-mediated thymic destruction. If this bears out in future studies, we might find that antigens localized in TECs may be crucial for promotion of the altered T-cell homeostasis found in patients.10
Collectively, this manuscript and other cGVHD murine studies point out that the kinetics of immunologic events appear to be highly relevant in the pathobiology of cGVHD. Although robust germinal center (GC) formation appears to be critical for disease initiation,5 the current study reveals that GC disruption is also important for disease maintenance. The data suggest that antibody targeting of both primary and secondary lymphoid organs results in a vicious self-destructive cycle of these tissues in cGVHD. Perhaps ongoing primary lymphoid destruction leads to critical levels of lymphopenia such that out-competition by autoreactive B- and/or T-cell clones for factors and niches can occur. Additional experiments addressing this possibility are warranted.
Taken together, it appears that the immune system over time after allogeneic HCT becomes its own worst enemy (see figure). Organs that produce cGVHD-inciting cells are subsequently destroyed by those very cells or their cellular products, IgG. Further studies addressing this paradigm will lead us to pathways that afford targeting of pathological immune cells while preserving normal integrity of hematolymphoid organ systems.
Allogeneic HCT is the only known curative option for many patients afflicted with life-threatening blood, bone marrow, or lymph node disorders. Immune pathology in the form of cGVHD develops in 30% to 80% of HCT patients, carrying an impact on morbidity and survival that has not improved significantly over the last 30-plus years. It appears the old adage is correct: persistence is a virtue. With National Institutes of Health consensus conference initiatives and strong collaborative efforts by the cGVHD research community, things are now moving rapidly in the right direction! Including this current paper, a surge of human and murine studies over the last several years has led to significant improvement in the understanding of the cGVHD process. As we gain more knowledge about the importance of immune recovery and immune homeostasis, along with the distinct functional capacities of key lymphoid subsets, increasing numbers of viable treatments will become available for our patients.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Jin H, Ni X, Deng R, et al. Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood. 2016 doi: 10.1182/blood-2015-09-668145. 127(18):2249-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107(7):2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 3.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105(12):4885–4891. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai S, Pidala J, Pusic I, et al. A Randomized Phase II Crossover Study of Imatinib or Rituximab for Cutaneous Sclerosis after Hematopoietic Cell Transplantation. Clin Cancer Res. 2016;22(2):319–327. doi: 10.1158/1078-0432.CCR-15-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood. 2015;125(11):1703–1707. doi: 10.1182/blood-2014-12-567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na IK, Lu SX, Yim NL, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. J Clin Invest. 2010;120(1):343–356. doi: 10.1172/JCI39395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]