Supplemental Digital Content is Available in the Text.
Key Words: adverse drug reactions, dry eye disease, randomized controlled trial, safety
Abstract
Purpose:
To evaluate the 1-year safety of lifitegrast ophthalmic solution 5.0% in patients with dry eye disease compared with placebo.
Methods:
SONATA (Safety Of a 5.0% coNcentrATion of lifitegrAst ophthalmic solution) was a multicenter, randomized, prospective, double-masked, placebo-controlled phase 3 study (NCT01636206). Adults (≥18 years) with dry eye disease (Schirmer test score ≥1 and ≤10 mm; corneal staining score ≥2.0) were randomized 2:1 to lifitegrast ophthalmic solution 5.0% or placebo twice daily for 360 days. The primary objective was percentage and severity of treatment-emergent adverse events (TEAEs). Secondary objectives were ocular safety measures: corneal fluorescein staining, drop comfort, best-corrected visual acuity, slit-lamp biomicroscopy, and intraocular pressure over 7 visits. Exploratory objectives included concentration of lifitegrast in plasma.
Results:
The safety population comprised 331 participants (220 lifitegrast; 111 placebo). There were no serious ocular TEAEs. Overall, 53.6% of participants receiving lifitegrast experienced ≥1 ocular TEAE versus 34.2% in the placebo group; most TEAEs were mild to moderate in severity. Rates of discontinuation because of TEAEs were 12.3% (lifitegrast) versus 9.0% (placebo). The most common (>5%) TEAEs occurring in either treatment group were instillation site irritation (burning), instillation site reaction, visual acuity reduced, dry eye, and dysgeusia (change in taste). Ocular safety parameters for lifitegrast were similar to placebo. The mean plasma lifitegrast concentration at 360 days (n = 43) was below the limit of detection. There was no indication of systemic toxicity or localized infectious complications secondary to chronic immunosuppression.
Conclusions:
Lifitegrast ophthalmic solution 5.0% seemed safe and well tolerated in this study, with no unexpected adverse events.
Lifitegrast is a small-molecule integrin antagonist that was developed as a treatment for dry eye disease (DED) by targeting an inflammatory pathway associated with DED. The efficacy and safety of lifitegrast ophthalmic solution 5.0%, when administered twice daily for 84 days in participants with DED, have been demonstrated in 3 randomized controlled studies. These are 1 phase 2 study1 and 2 phase 3 studies (OPUS-12 and OPUS-23). In OPUS-1, the coprimary sign endpoint of change from baseline to day 84 in inferior corneal staining score was significantly improved in patients with DED treated with lifitegrast compared with placebo. However, the coprimary symptom endpoint of change from baseline to day 84 on the visual-related function subscale was not met.2 The results of the OPUS-2 study were recently published; they showed that in lifitegrast-treated patients with DED with a recent history of artificial tear use and at least moderate baseline symptomology (eye dryness score ≥40), there was a significant improvement in the coprimary symptom endpoint of eye dryness score compared with placebo. The coprimary sign endpoint of inferior corneal staining score was not met in OPUS-2.3 In the phase 2 study, and in OPUS-1 and OPUS-2, lifitegrast was generally well tolerated, and no serious ocular adverse events (AEs) were reported.
Lifitegrast is designed to target the inflammation associated with DED by blocking the binding of the integrin, lymphocyte function-associated antigen 1 (LFA-1), to its cognate ligand, intercellular adhesion molecule 1 (ICAM-1). Inflammation at the cellular level of the lacrimal gland and ocular surface plays a major role in DED and is associated with symptoms of eye dryness and discomfort.4 T-cell activation is critical in the inflammatory process and is influenced by LFA-1/ICAM-1 binding.5 The interaction of LFA-1 and ICAM-1 is important in T-cell adhesion, migration, proliferation, and cytokine release at sites of inflammation.6–9 Infiltration of T-cells in the conjunctiva10 and increased expression of ICAM-1 in lacrimal and conjunctival epithelial cells11 have been demonstrated in patients with DED. Taken together, this evidence suggests that LFA-1/ICAM-1 binding is a logical pharmacological target in the treatment of DED. Lifitegrast has been shown in vitro to block the interaction between ICAM-1 and LFA-1, inhibiting T-cell activation and recruitment and reducing inflammation.12–14
The objective of the SONATA (Safety Of a 5.0% coNcentrATion of lifitegrAst ophthalmic solution) study was to examine the longer term safety profile of lifitegrast. To achieve this, we evaluated the 1-year safety of lifitegrast ophthalmic solution 5.0% in participants with DED compared with placebo.
MATERIALS AND METHODS
The SONATA study was a phase 3, multicenter, randomized, prospective, double-masked, placebo-controlled, parallel-arm study conducted at 22 sites in the United States. The study was compliant with the Health Insurance Portability and Accountability Act, adhered to the tenets of the Declaration of Helsinki, and was registered at ClinicalTrials.gov (identifier, NCT01636206). Ethics committee approval was obtained before the study was started.
Participants
Eligible participants were adults aged ≥18 years who had a self-reported history of DED; best-corrected visual acuity (BCVA) of 0.7 logarithm of the minimum angle of resolution (logMAR) or better, corneal fluorescein staining score ≥2 (scale, 0–4) in ≥1 region (superior, inferior, or central), visual analog scale score ≥40 for either eye dryness or eye discomfort (scale, 0–100; 0 = no discomfort; 100 = maximal discomfort), use and/or desire to use artificial tears for DED in the past 6 months, and Schirmer test score (without anesthesia) ≥1 mm and ≤10 mm. All participants provided written informed consent. Individuals with secondary Sjögren syndrome were eligible to participate if they were not immunodeficient/immunosuppressed, were not taking systemic steroids, and met all other inclusion and exclusion criteria.
The following individuals were excluded from participation in the study: women who were pregnant or nursing an infant, those with contraindications or hypersensitivity to the investigational product, previous treatment with lifitegrast, use of any topical medication or antibiotics for the treatment of blepharitis or meibomian gland disease, ocular herpes or any other ocular infection within the last 30 days, blood donation or significant loss of blood within the last 56 days, ocular conditions or chronic illness that could affect study parameters, a disorder causing immunodeficiency, history of laser-assisted in situ keratomileusis or similar surgery within the last 12 months, history of neodymium:yttrium aluminum garnet laser posterior capsulotomy within the last 6 months, known history of alcohol/drug abuse that might interfere with study participation, and those with DED secondary to scarring or destruction of conjunctival goblet cells. Prohibited medications during the study were topical ophthalmic nonsteroidal antiinflammatory agents, topical ophthalmic cyclosporine, and systemic steroids.
After day 14 (visit 3), the use of the following was allowed: artificial tears (≤4 times daily, as needed), contact lenses (daily disposable lenses only), topical ophthalmic/nasal antihistamines, mast cell stabilizers, and steroids (only loteprednol etabonate for ≤4 weeks at a time); information on their use was collected beginning at day 90 (visit 4).
Study Design
The investigational product was supplied as a sterile liquid solution containing lifitegrast at a concentration of 5.0% with ∼0.2 mL in each dose vial. During the treatment period (days 0–360; Fig. 1), participants received twice-daily doses (in the morning and the evening just before bedtime) of either lifitegrast ophthalmic solution 5.0% or the vehicle as a placebo administered to the ocular surface as a single eye drop in both eyes. Compliance with treatment was assessed by reconciliation of used and unused investigational product vials collected from participants. Noncompliance was recorded as a protocol deviation if >20% of expected doses since last visit were missed or >120% of expected doses were taken.
FIGURE 1.
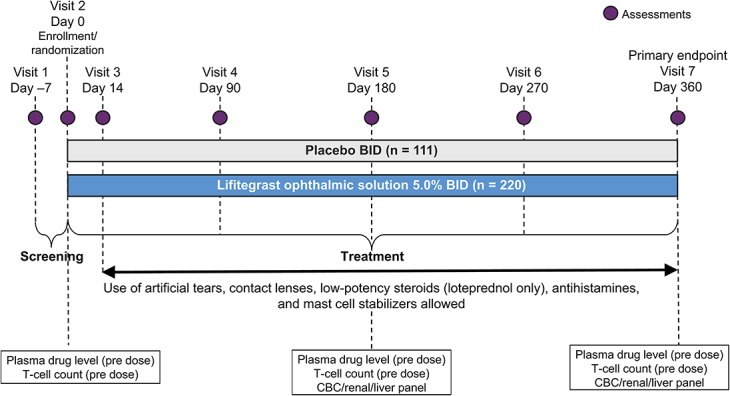
Study design schematic. BID, twice daily; CBC, complete blood count.
Outcome Measures
The primary safety assessment was based on ocular and nonocular treatment-emergent adverse events (TEAEs). AEs were considered treatment emergent if they occurred after the first dose of the investigational product; definition of AEs included intercurrent illnesses or injuries that represented an exacerbation (increase in frequency, severity, or specificity) of preexisting conditions, for example, the worsening of dry eye. TEAEs were assessed by the investigator for severity (mild, moderate, severe) and relatedness (not related, possibly related, probably related) to the investigational product. The secondary study objective was to evaluate ocular safety measures (including corneal fluorescein staining, drop comfort, BCVA, slit-lamp biomicroscopy, and intraocular pressure) over 7 visits in 360 days.
Investigator verbatim terms were coded using the Preferred Terms of the Medical Dictionary for Regulatory Activities (version 14.1). For example, the verbatim terms of decreased visual acuity, worsening visual acuity, decreased vision, logMAR change ≥22 from visit 2, decreased vision (lost glasses), and decreased visual acuity of 30 letters from baseline were coded to visual acuity reduced. A number of verbatim terms involving ocular burning upon instillation of study drug were coded to the Preferred Term of instillation site irritation. Blurred/blurry vision, ocular discharge, or ocular pressure sensation upon instillation were coded to instillation site reaction. Verbatim terms for dysgeusia (change in taste) included but were not limited to taste perversion or bitter or metallic taste in the mouth.
Exploratory objectives included the number and percentage of participants with TEAEs after using lifitegrast in conjunction with other topical eye drops, including artificial tears, steroids, mast cell stabilizers, and/or antihistamines, and after using lifitegrast in conjunction with contact lenses. Additional exploratory objectives included plasma lifitegrast concentration and whole-blood lymphocyte (CD3, CD4, and CD8) counts, which were collected from participants (n = 75) at selected study sites for each measure, and were obtained before administration of the investigational product at days 0 [visit 2 (baseline for lifitegrast levels and lymphocyte counts)], 180 (visit 5), and 360 (visit 7). Also in this category were clinical laboratory values (hematologic, renal, and liver panels) at days −7 [visit 1 (baseline for safety clinical laboratory tests)], 180 (visit 5), and 360 (visit 7). Participants were discontinued from the study if they requested to be withdrawn or at the discretion of the investigator and/or sponsor in accordance with their clinical judgment.
Randomization and Masking
An interactive web response system was used to randomly assign participants to receive lifitegrast or placebo based on a 2:1 ratio (lifitegrast:placebo). All study personnel were masked with regard to treatment assignments. No participants were unmasked during the study.
Statistical Methods
The safety population included all randomized participants who received ≥1 dose of the investigational product. Because this was a safety study, it was not powered for hypothesis testing to compare outcomes between the lifitegrast and placebo groups; statistical analyses were descriptive in nature. The study sample size was not based on statistical calculations or statistical assumptions, but on guidance provided by the US Food and Drug Administration and is consistent with the International Conference on Harmonisation guidance on exposure for drugs intended for long-term treatment of nonlife-threatening conditions (International Conference on Harmonisation 1995). The method for assaying plasma samples for lifitegrast was linear over the range of 0.500 to 100 ng/mL, with a lower limit of quantification of 0.500 ng/mL. Samples below the lower limit of quantification were treated as 0 in the calculation of summary statistics.
RESULTS
Participants
The study was conducted between October 2012 and March 2014. Of the 504 participants screened, 332 participants were randomized (lifitegrast, n = 221; placebo, n = 111; Fig. 2). One participant in the lifitegrast group was erroneously randomized but never received treatment. As such, 331 participants were included in the safety population (lifitegrast, n = 220; placebo, n = 111).
FIGURE 2.
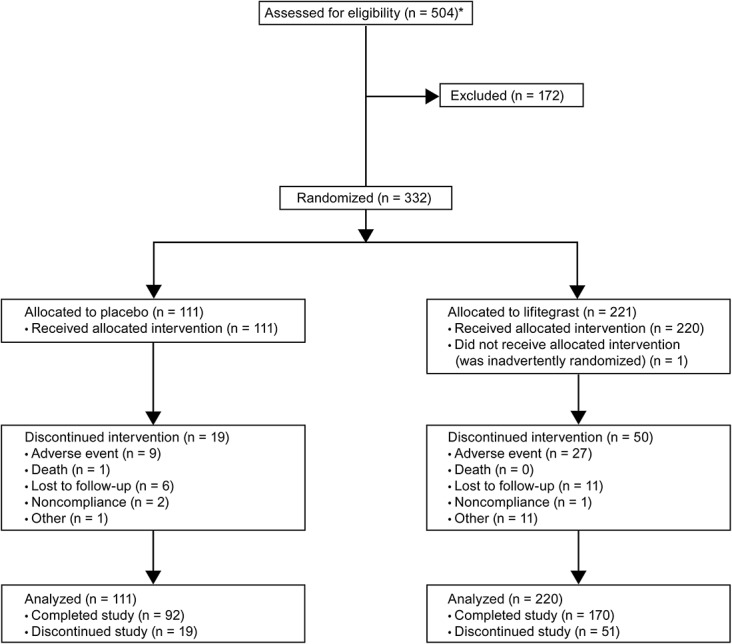
Participant flow. *Number may reflect multiple screenings for the same participant.
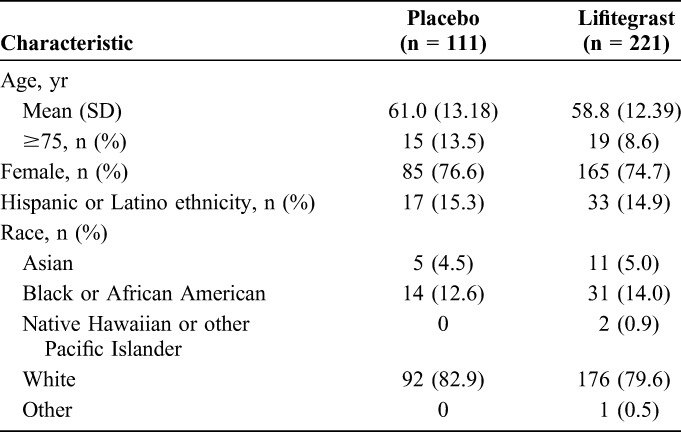
Baseline characteristics were similar between treatment groups (Table 1). Participants' ages ranged from 21 to 89 years, with a mean (SD) age of 59.5 (12.68) years. The majority of participants were female and white. All participants had an ocular medical history of DED (the primary diagnosis). Other than the primary diagnosis, the most common (>10%) occurrences in ocular medical history were cataract (41.1%), punctate keratitis (27.2%), nuclear cataract (19.0%), pinguecula (14.8%), and cataract surgery (13.9%). Within nonocular medical history, the most common (>10%) occurrences were postmenopause (45.9%), hypertension (44.7%), hysterectomy (20.8%), gastroesophageal reflux disease (20.5%), hypercholesterolemia (18.1%), depression (15.4%), seasonal allergy (14.8%), increased blood glucose (14.5%), hypothyroidism (13.0%), drug hypersensitivity (13.9%), insomnia (13.6%), osteoarthritis (11.8%), and type 2 diabetes mellitus (11.5%).
TABLE 1.
Participant Demographics
Overall, 38.1% of participants took an ocular concomitant medication with a start date on or after the first dose of investigational product, most commonly polyvinyl alcohol in artificial tear preparations (12.4%). Similar proportions of participants in each treatment group used contact lenses [lifitegrast, 2.6% (5/195) vs. placebo, 4.1% (4/98)], topical ophthalmic/nasal antihistamine [lifitegrast, 5.1% (10/195) vs. placebo, 5.1% (5/98)], topical ophthalmic/nasal steroids [lifitegrast, 6.7% (13/195) vs. placebo, 5.1% (5/98)], and topical ophthalmic/nasal mast cell stabilizers [lifitegrast, 1.5% (3/195) vs. placebo, 1.0% (1/98)]. Data on concomitant use of medications or contact lenses were based on numbers too small to show trends in TEAE severity, relatedness, or seriousness. Based on investigational product vials returned, 81.1% of placebo-treated and 84.1% of lifitegrast-treated participants were compliant with study treatment.
Treatment-Emergent Adverse Events
A higher proportion of participants in the lifitegrast group experienced TEAEs compared with the placebo group (Table 2). TEAEs were categorized by the investigator as mild, moderate, or severe; in most participants with TEAEs, TEAEs were mild to moderate in severity (Table 2).
TABLE 2.
Summary of Ocular and Nonocular TEAEs
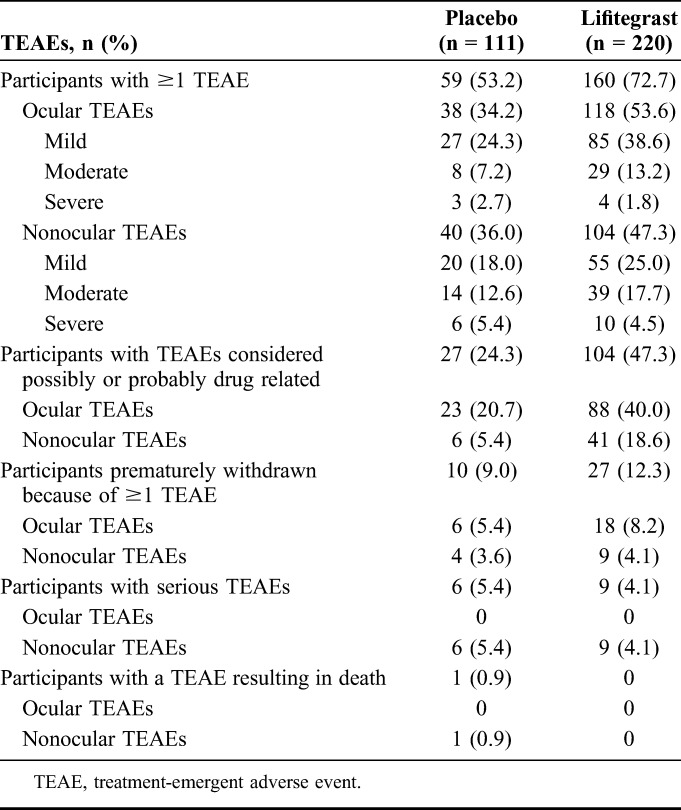
The most common (>5%) ocular TEAEs occurring in either treatment group were instillation site irritation (burning), instillation site reaction, visual acuity reduced, and dry eye; the most common (>5%) nonocular TEAE was dysgeusia (change in taste). Most TEAEs were mild to moderate in severity (Fig. 3; also see Table, Supplemental Digital Content 1, http://links.lww.com/ICO/A396). All cases of instillation site irritation, instillation site reaction, and dysgeusia (change in taste) were considered possibly/probably related to the investigational product; a small proportion of participants experienced visual acuity reduced (lifitegrast, 6.4%; placebo, 1.8%) and dry eye (lifitegrast, 1.8%; placebo, 3.6%) that were not considered related to the investigational product.
FIGURE 3.
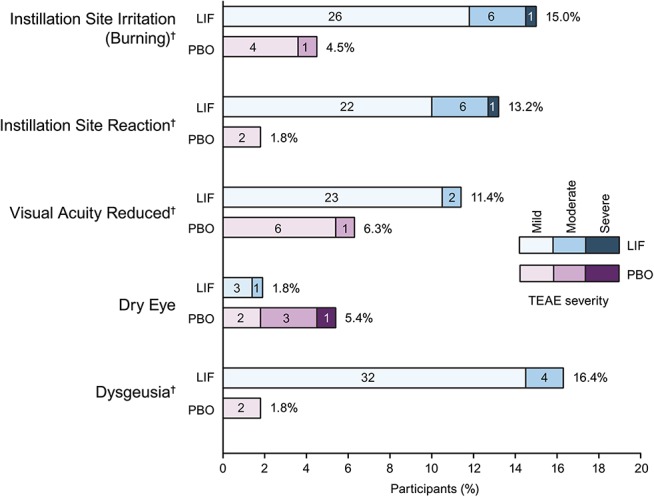
Incidence and severity of most frequent (>5%) TEAEs*. Percentage value indicates the proportion of participants who experienced each type of TEAE. Values inside bars = numbers of participants. *TEAEs occurring in >5% of participants in either treatment group. †Verbatim terms coding to dysgeusia, instillation site irritation, instillation site reaction, and visual acuity reduced are given in the Materials and Methods section. LIF, lifitegrast; PBO, placebo; TEAE, treatment-emergent adverse event.
No serious ocular TEAEs occurred during the study, while 15 participants [lifitegrast, 4.1% (9/220) vs. placebo, 5.4% (6/111)] had serious nonocular TEAEs. One of these participants (in the placebo group) had a severe TEAE of sudden cardiac arrhythmia that resulted in death. All serious TEAEs were considered by the investigator to be not related to the investigational product, moderate to severe in severity, and resolved, except for arrhythmia (fatal outcome), spinal fracture (unknown outcome), and chronic obstructive pulmonary disease (resolved with sequelae). Chronic obstructive pulmonary disease was the only serious TEAE that occurred in >1 participant (lifitegrast, n = 0; placebo, n = 2).
Discontinuations Resulting From TEAEs
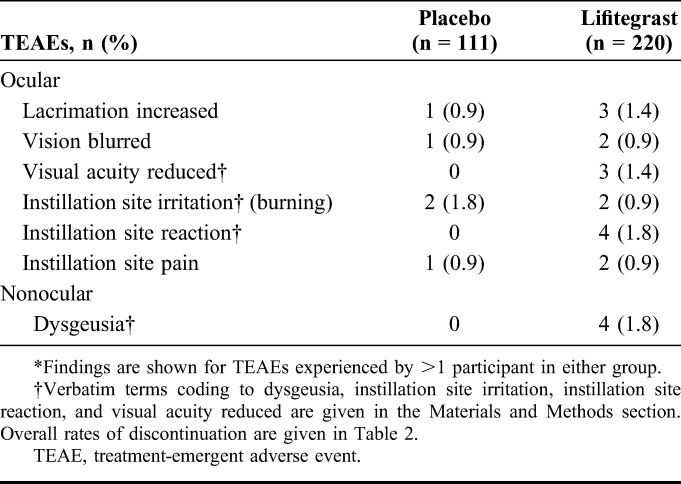
A total of 24 participants [lifitegrast, 8.2% (18/220) vs. placebo, 5.4% (6/111)] had ≥1 ocular TEAE and 13 participants had ≥1 nonocular TEAE [lifitegrast, 4.1% (9/220) vs. placebo, 3.6% (4/111)] that resulted in discontinuation. The most common TEAEs (experienced by >1 participant in either group) that resulted in discontinuation are presented in Table 3. In both treatment groups, most ocular TEAEs that led to discontinuation were considered mild to moderate in severity.
TABLE 3.
Most Frequent TEAEs Leading to Discontinuations*
Secondary Safety Results
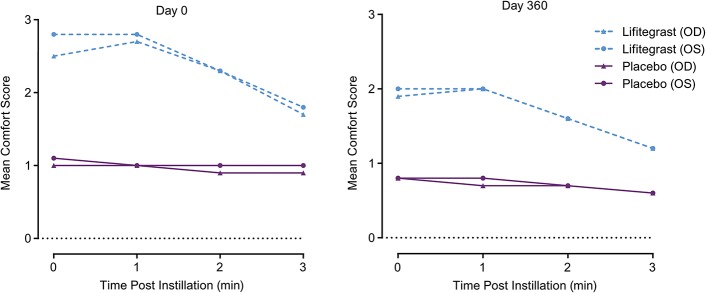
At each time point and visit, the mean drop comfort score (scale, 0–10; 0 = very comfortable, 10 = very uncomfortable) of placebo-treated participants was numerically lower (more comfortable) than the drop comfort of lifitegrast-treated participants. However, in general, numerical improvement in comfort was observed within each visit (at each time point postinstillation) in the lifitegrast group. By 3 minutes postinstillation at each study visit, the lifitegrast group had mean drop comfort scores below 2 (Fig. 4).
FIGURE 4.
Drop comfort in a study of lifitegrast compared with placebo for dry eye disease. OD, right eye; OS, left eye.
The lifitegrast group had almost twice the frequency of participants with visual acuity reduced as the placebo group (11.4% vs. 6.3%, respectively). However, mean changes in logMAR BCVA from baseline to day 360 were minimal in both treatment groups [lifitegrast: no change (right eye, OD), +0.003 (left eye, OS); placebo: −0.026 (OD), −0.018 (OS); see Table, Supplemental Digital Content 2, http://links.lww.com/ICO/A395]. In addition, there were similar improvements in corneal fluorescein staining at each visit for all ocular regions examined (change from baseline to day 360), and no worsening in staining was observed in any region for either treatment group (see Table, Supplemental Digital Content 2, http://links.lww.com/ICO/A395).
Similar mean intraocular pressures of the right and left eyes were measured between treatment groups at days −7, 180, and 360 (visits 1, 5, and 7; see Table, Supplemental Digital Content 2, http://links.lww.com/ICO/A395). In addition, generally, assessment of each eye on these days using slit-lamp biomicroscopy did not reveal any clinically significant abnormalities and there was no increased incidence of cataract formation in participants receiving lifitegrast [0.9% (2/220)] compared with those in the placebo group [0.9% (1/111)].
Exploratory Endpoint Results
Concomitant Artificial Tear Use
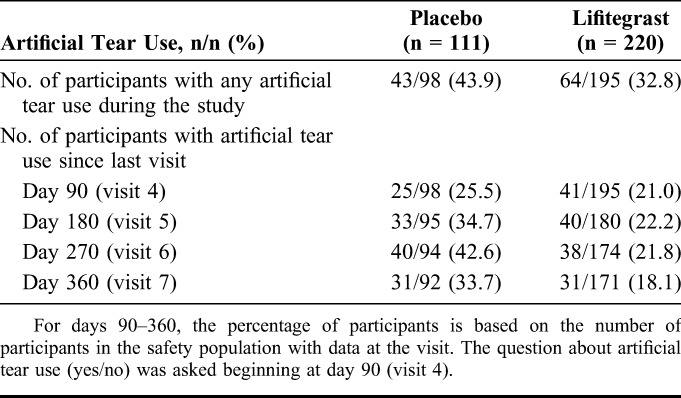
Overall, a lower proportion of participants in the lifitegrast group used artificial tears at any time after day 14 compared with the placebo group (Table 4). In addition, at each visit (days 90–360), the proportion of participants in the lifitegrast group who reported use of artificial tears since the last visit was numerically lower than that in the placebo group (Table 4).
TABLE 4.
Artificial Tear Use in the Treatment Groups
Participants in both treatment groups who used artificial tears had higher rates of TEAEs compared with those not using artificial tears, respectively (ocular: lifitegrast, 67.2% vs. 45.0%; placebo, 44.2% vs. 25.5%; nonocular: lifitegrast, 60.9% vs. 42.7%; placebo, 44.2% vs. 32.7%). In general, a lower proportion of participants who used artificial tears had TEAEs that led to discontinuation compared with those who did not use artificial tears (ocular: lifitegrast, 3.1% vs. 3.1%; placebo, 0% vs. 1.8%; nonocular: lifitegrast, 0% vs. 2.3%; placebo, 0% vs. 3.6%).
Other Exploratory Endpoints
The mean changes in CD3, CD4, and CD8 counts from baseline to days 180 and 360 (visits 5 and 7) were minimal and similar between the lifitegrast and placebo groups, with no trends to suggest chronic suppression of lymphocyte subset counts. No opportunistic infections or AEs to suggest chronic T-cell suppression were reported. In the hematologic, renal, and liver panels, the changes from baseline (day −7, visit 1) to days 180 and 360 (visits 5 and 7) were minimal and similar between treatment groups for all parameters.
The mean concentration of lifitegrast in plasma was below the lower limit of quantification (0.500 ng/mL) at days 0, 180, and 360. The mean plasma lifitegrast concentration at day 360 (n = 43) was 0.047 ng/mL.
DISCUSSION
SONATA is the first study to investigate the long-term safety of lifitegrast in the treatment of DED, and is one of a few multicenter, double-masked, placebo-controlled studies to investigate the safety of a drug treatment for DED over 12 months. In SONATA, no serious ocular TEAEs were reported, and the rate of discontinuations because of ≥1 TEAE was low in participants receiving lifitegrast (12.3%), albeit slightly higher than among those receiving placebo (9.0%). Approximately one-half of the participants who received lifitegrast reported ≥1 ocular TEAE across the 1-year study period; however, most TEAEs were mild to moderate in severity. Findings for the ocular safety measures of corneal fluorescein staining, drop comfort, BCVA, slit-lamp biomicroscopy, and intraocular pressure were comparable between the lifitegrast and placebo groups.
The safety profile observed in SONATA was consistent with that reported previously in shorter term studies of lifitegrast (84 days).1–3 The most common ocular TEAEs attributed to lifitegrast were instillation site irritation, instillation site reaction, and visual acuity reduced, occurring in 15.0%, 13.2%, and 11.4% of participants, respectively. Importantly, discontinuation because of burning (coded under instillation site irritation) occurred in only 2 participants receiving lifitegrast, or 0.9% of the lifitegrast treatment group. Ocular TEAEs for lifitegrast seemed to be transient given that the most common ocular TEAEs were related to administration of the drug and that drop comfort improved within 3 minutes of instillation.
As with SONATA, the most commonly reported ocular TEAEs in earlier studies were administration site symptoms (instillation site irritation, pain, and reaction) and visual acuity reduced, and the most common nonocular TEAE was dysgeusia (change in taste).1–3 No new safety signals were identified in this 1-year study.
Visual acuity reduction was reported by participants in both lifitegrast and placebo groups. Visual acuity changes have been reported previously in studies of topical ophthalmic agents, including over-the-counter artificial tears.15 The tear film constitutes a critical component of the refractive properties along the visual axis,16 and instillation of eye drops may cause transient disturbances of the tear film, which may account for some of the visual acuity reduction in this study, although it should be noted that a slightly higher proportion of participants experienced visual acuity reduction in the lifitegrast group compared with the placebo group (11.4% vs. 6.3%, respectively).
The most commonly reported nonocular TEAE was dysgeusia (change in taste), which occurred in 16.4% of participants in the lifitegrast group and 1.8% of the placebo group. Clinically, this is a relatively common AE associated with instillation of some topical ophthalmic medications, because of normal tear drainage through the nasolacrimal duct into the nose and then into the oropharynx. The event is usually self-limited and short in duration. In the present study, discontinuations because of dysgeusia (change in taste) occurred in 4 participants receiving lifitegrast, or 1.8% of the lifitegrast treatment group. Fifteen participants (6 placebo, 9 lifitegrast) experienced serious nonocular TEAEs, which was consistent with medical complications in an older population.
There was no evidence of accumulation of lifitegrast in plasma over the course of the study, with most concentrations below the limit of detection on days 180 and 360. The very low plasma levels of lifitegrast in this study suggest that systemic side effects would not be expected after topical ophthalmic administration of lifitegrast. Indeed, there was no evidence of systemic toxicity or localized infectious complications secondary to chronic immunosuppression.
Artificial tear substitutes augment the tear film and are commonly used as a first-line therapy for DED. In the United States alone, artificial tears are used by ∼7 to 10 million people.17 In this study, although TEAEs were more frequent in participants using artificial tears (in both treatment groups) than those who did not, concomitant use of artificial tears with lifitegrast did not result in a higher frequency of discontinuations because of TEAEs. Interestingly, a lower proportion of participants receiving lifitegrast used artificial tears compared with those receiving placebo, suggesting that the perceived need for artificial tears was lower in the lifitegrast group.
A limitation of this study was that patients with at least moderate baseline symptomology were enrolled, as inclusion criteria included corneal fluorescein staining score of ≥2, eye dryness score ≥40, and use and/or desire to use artificial tears in the past 6 months. Therefore, milder cases of DED would not have been studied. In addition, patients with a history of corneal surgery, such as laser-assisted in situ keratomileusis, within the past year also were excluded from this study, so the safety of lifitegrast in this group was not evaluated.
In conclusion, the use of twice-daily lifitegrast ophthalmic solution 5.0% for 360 days seemed safe and well tolerated, with no unexpected TEAEs, and a safety profile that was similar to previous 12-week studies. The incidence of drug discontinuation was low, with 0.9% of participants withdrawing because of instillation site irritation (burning). In addition, there was no evidence of systemic toxicity or localized infectious complications secondary to chronic immunosuppression.
Supplementary Material
ACKNOWLEDGMENTS
E. D. Donnenfeld has been a consultant for Abbott Medical Optics, AcuFocus, Alcon, Allergan, Aquesys, Bausch & Lomb, CRST, Elenza, Glaukos, Icon Bioscience, Kala, Katena, LacriPen, Mati Therapeutics, Merck, Mimetogen, NovaBay, Novaliq, OcuHub, Odyssey, Omeros, Physician Recommended Nutriceuticals, Rapid Pathogen Screening, Shire, Strathspey Crown, TearLab, TLC Laser Eye Centers, TrueVision, Versant Ventures, WaveTec, and Zeiss and is an investor in AcuFocus, Aquesys, Elenza, Glaukos, LacriPen, Mati Therapeutics, Mimetogen, NovaBay, OcuHub, Rapid Pathogen Screening, SARcode Bioscience, Strathspey Crown, TearLab, TrueVision, Versant Ventures, and WaveTec. P. M. Karpecki has received consulting funding from Abbott Medical Optics, Akorn, Alcon, Allergan, Bausch & Lomb, Beaver-Visitech, Bio-Tissue, Bruder Healthcare, Focus Laboratories, Nicox, OCuSOFT, Reichert, Rysurg, SARcode Bioscience, ScienceBased Health, Shire, TearScience, and Topcon and has received research funding from Allergan, Bausch & Lomb, Eleven Biotherapeutics, Fera, Rigel, and Shire for US Food and Drug Administration clinical trials. P. A. Majmudar has received research funding from Shire and has been a consultant for Allergan, Rapid Pathogen Screening, TearScience, and Valeant. K. K. Nichols has received research funding from Alcon, Allergan, Eleven Biotherapeutics, SARcode Bioscience, TearLab, and TearScience and has been a consultant for Alcon, Allergan, Bausch & Lomb, Eleven Biotherapeutics, InSite, Kala, Nicox, SARcode Bioscience, ScienceBased Health, Shire, and TearLab. A. Raychaudhuri and M. Roy are full-time employees of Shire. C. P. Semba was an employee of Shire at the time this research was conducted.
The authors thank Nasser Malik, PhD, of Excel Scientific Solutions, who provided medical writing support funded by Shire.
Footnotes
This study was funded by SARcode Bioscience (now a wholly owned subsidiary of Shire) and Shire.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
REFERENCES
- 1.Semba CP, Torkildsen GL, Lonsdale JD, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol. 2012;153:1050.e1–1060.e1. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard JD, Torkildsen GL, Lonsdale JD, et al. ; OPUS-1 Study Group. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121:475–483. [DOI] [PubMed] [Google Scholar]
- 3.Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015;122:2423–2431. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern® Guidelines. Dry Eye Syndrome. 2013. Available at: http://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp–2013. Accessed August 26, 2015. [Google Scholar]
- 5.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali H, Haribabu B, Richardson RM, et al. Mechanisms of inflammation and leukocyte activation. Med Clin North Am. 1997;81:1–28. [DOI] [PubMed] [Google Scholar]
- 7.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu Y. LFA-1: more than just T cell Velcro. Nat Immunol. 2003;4:1052–1054. [DOI] [PubMed] [Google Scholar]
- 9.Smith A, Stanley P, Jones K, et al. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol Rev. 2007;218:135–146. [DOI] [PubMed] [Google Scholar]
- 10.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren′s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 11.Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögrens syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78:823–835. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci. 2011;52:3174–3180. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Zhang R, Gadek TR, et al. Corneal inflammation is inhibited by the LFA-1 antagonist, lifitegrast (SAR 1118). J Ocul Pharmacol Ther. 2013;29:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong M, Gadek TR, Bui M, et al. Discovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eye. ACS Med Chem Lett. 2012;3:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung CI, Kottaiyan R, Koh S, et al. Noninvasive, objective, multimodal tear dynamics evaluation of 5 over-the-counter tear drops in a randomized controlled trial. Cornea. 2012;31:108–114. [DOI] [PubMed] [Google Scholar]
- 16.Montés-Micó R. Role of the tear film in the optical quality of the human eye. J Cataract Refract Surg. 2007;33:1631–1635. [DOI] [PubMed] [Google Scholar]
- 17.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.