Abstract
Although it is generally accepted that cognitive factors contribute to the pathogenesis of major depressive disorder (MDD), there are missing links between behavioral and biological models of depression. Nevertheless, research employing neuroimaging technologies has elucidated some of the neurobiological mechanisms related to cognitive-vulnerability factors, especially from a whole-brain, dynamic perspective. In this review, we integrate well-established cognitive-vulnerability factors for MDD and corresponding neural mechanisms in intrinsic networks using a dual-process framework. We propose that the dynamic alteration and imbalance among the intrinsic networks, both in the resting-state and the rest-task transition stages, contribute to the development of cognitive vulnerability and MDD. Specifically, we propose that abnormally increased resting-state default mode network (DMN) activity and connectivity (mainly in anterior DMN regions) contribute to the development of cognitive vulnerability. Furthermore, when subjects confront negative stimuli in the period of rest-to-task transition, the following three kinds of aberrant network interactions have been identified as facilitators of vulnerability and dysphoric mood, each through a different cognitive mechanism: DMN dominance over the central executive network (CEN), an impaired salience network–mediated switching between the DMN and CEN, and ineffective CEN modulation of the DMN. This focus on interrelated networks and brain-activity changes between rest and task states provides a neural-system perspective for future research on cognitive vulnerability and resilience, and may potentially guide the development of new intervention strategies for MDD.
Keywords: cognitive vulnerability, cross-network interaction, functional magnetic resonance imaging, intrinsic network, major depressive disorder
Major depressive disorder (MDD) is a common, recurrent, and severe psychiatric disorder and a leading source of disease burden,1 with a lifetime prevalence of 16.2% in adults and a prevalence of 11.7% among adolescents 13 to 18 years old.2,3 MDD is characterized not only by persistent negative mood and lack of motivation, but also by maladaptive thinking styles and specific impairments in integrating information. These cognitive disturbances present not only in people suffering from depressive disorders but also in people with elevated negative mood. Over the years, researchers have explored risk factors for, and potential treatment approaches to, MDD from both biological and psychological perspectives.
Cognitive factors, which have been highlighted in all psychological models, suggest that the interaction of stress and premorbid vulnerabilities contributes to depressive episodes throughout the life span.4 Recently, researchers suggested that cognitive vulnerability may represent an endophenotype for depression.5 Cognitive-vulnerability models themselves include a broad array of risk factors, as in Beck’s cognitive model (e.g., information-processing biases, negative self-schemas),6 hopelessness theory (e.g., depressogenic attribution styles to event causes, consequences, and the self),7 and response-styles theory (e.g., rumination).8 Given this array of vulnerability factors, researchers have sought to explore a new model that integrates the core factors of prominent theories in order to provide a more holistic understanding of cognitive vulnerability.
The dual-process theory of cognitive vulnerability, which has been supported by many behavior experiments,9–12 integrates the main vulnerability factors into one model from an information-processing perspective.13 According to this model, two information-processing modes correlate with the cognitive vulnerability to depression: one is called associative/implicit mode, which is characterized by quick, effortless processing based on well-learned associations, and the other is called reflective/explicit mode, which is characterized by slow, effortful processing that requires more intention and awareness.9,14 Dual-process theory emphasizes negatively biased, self-referential, associative processing as the foundation for cognitive vulnerability, which may be overcome by corrective reflective processing. Additionally, the theory raises three scenarios of interplay between associative and reflective processing that may lead to a downward spiral and then into more severe forms of dysphoria (see Figure 1). The dual-process theory thus provides a relatively parsimonious framework of cognitive vulnerability. It emphasizes the dynamic imbalance between two cognitive processes in the generation and persistence of negative mood. In addition, the model integrates the core factors of prominent cognitive-vulnerability theories, such as the negative information-processing biases and negative self-schemas of Beck’s cognitive model, the depressogenic attribution in hopelessness theory, and the rumination in response-styles theory.14 There remains a gap, however, between the symptom/behavior level of psychopathological theory and the associated biological underpinnings of depressive vulnerability.
Figure 1.
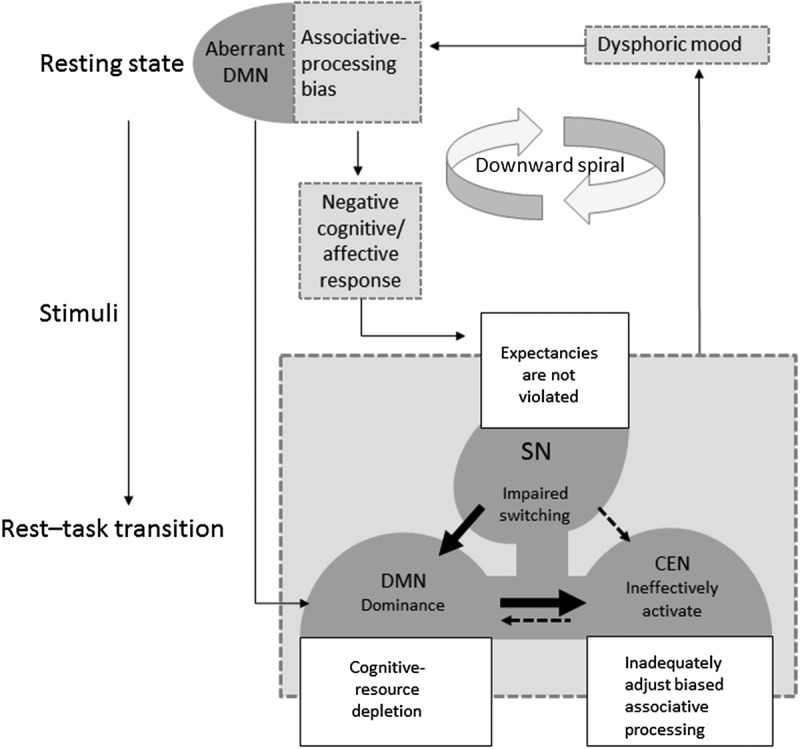
Aberrant intrinsic network interaction model that integrates the dual-process model of cognitive vulnerability, which has been adapted from Beevers (2005).14 The dual-process model includes two modes of information processing: (1) an associative mode involving quick, effortless processing, and (2) a reflective mode involving slow, effortful processing. We propose that abnormally increased resting-state DMN activity and connectivity, along with the corresponding biased associative processing (depressive rumination), contribute to the foundation of cognitive vulnerability. In the period of rest-to-task transition, aberrant network interactions contribute to three scenarios in which an associative bias cannot be corrected, thus promoting cognitive vulnerability. These three scenarios are (1) DMN dominance over the CEN (cognitive-resource depletion), (2) abnormal SN switching between the DMN and CEN (associative bias does not violate internal expectancies and trigger reflective processing), and (3) failure to activate the CEN effectively (reflective processing being triggered but failing to accurately adjust associative bias). As a result, a feedback loop between negative bias and dysphoria occurs, leading to a downward spiral. The solid lines in the triple-network model indicate enhanced interactions, while the dotted lines indicate attenuated interactions. CEN, central executive network; DMN, default mode network; SN, salience network.
Advances in brain imaging, especially in the field of intrinsic neural network research, may provide a useful tool to identify the missing neural-behavioral links (see Text Box 1). Over the past two decades, neuroimaging research using affective or cognitive tasks to study depressive vulnerability has shown that altered corticolimbic connectivity contributes to the generation of excessive and persistent negative affect.15,16 Additionally, given that substantial evidence has demonstrated the functional importance of spontaneous BOLD activity on task-related responses and has highlighted its predictive nature for subsequent behavioral and mental states,17–20 resting-state functional magnetic resonance imaging (fMRI) has been increasingly utilized to probe cognitive-vulnerability factors implicated in depression.21 Specifically, aberrant resting-state activity and functional connectivity in cortical midline structures have been reliably observed in at-risk,22,23 depressed,24,25 and formerly depressed populations.26,27 Moreover, abnormality in the default-mode network (DMN), a principal intrinsic brain network including cortical midline structures, is increasingly associated with cognitive vulnerability among at-risk and depressed individuals.28,29 Other large-scale neural networks, such as the central executive network (CEN) and salience network (SN), also seem to play a role in biased attention and processing of affective information in depression.30–32 Thus, understanding how there might be simultaneous dysfunction in multiple networks (see Text Box 2) may elucidate the neural nature of cognitive vulnerability.37,48,49
Text Box 1.
Glossary
Functional magnetic resonance imaging (fMRI)
A form of noninvasive neuroimaging based on blood-oxygen-level-dependent (BOLD) signals in the brain in vivo.
Blood-oxygen-level-dependent (BOLD) signal
The measurement of metabolic activity in the brain based on the magnetic resonance imaging contrast of blood deoxyhemoglobin levels arising from changes in local blood flow.
Brain network
Network originally referred to a physical system that can be represented by a graph consisting of nodes and edges. In the field of neuroscience, brain networks can be defined by structural connectivity or functional interdependence. The former is based on the anatomical linkage of its neurons, whereas the latter refers to joint activity in different brain structures that is codependent under variation of a functional or behavioral parameter.
Independent component analysis
A computational technique that separates a multivariate signal into additive components based on the assumption that the components arise from statistically independent non-Gaussian sources.
Network node
Node refers the component of network linked by edges. In the field of neuroscience, the nodes in structural networks are typically considered to be brain areas defined by cytoarchitectonics, local circuit connectivity, output projection target commonality, and input projection source commonality. The functional nodes are commonly identified by inferences concerning the effects of brain lesions on cognitive function (historically) or by relating the joint activation or deactivation of brain areas to different cognitive functions.
Functional connectivity
The statistical interrelation of variables representing temporal changes in different network nodes. In other words, functional connectivity refers the temporal correlation of a neurophysiological index measured in different brain areas, with the consequence that coactivating brain regions are usually identified as functional brain networks.
Large-scale network
These networks are neural systems that are distributed across the entire extent of the brain. Based on the technological and methodological advances of structural and functional brain connectivity, large-scale network studies focus on revealing how cognitive functions arise from interactions within and between distributed brain systems.
Intrinsic network
Intrinsic networks are also called intrinsic connectivity networks. Originally, the intrinsic network referred to a large-scale network of interdependent brain areas observed at rest, which typically were identified by independent component analysis on BOLD spontaneous fluctuations. Recently, emerging evidence shows that the intrinsic network architecture also is present across a wide variety of task states, suggesting that this form of interconnectedness is an “intrinsic” standard architecture of functional brain organization.
Text Box 2.
Intrinsic Network Research and the Triple-Network Model
The intrinsic network research originally emerged from the resting-state brain activity or connectivity studies. After Raichle and Greicius33,34 identified “default mode” network, which exhibits high levels of activity at rest and becomes deactivated with tasks needing specific goal-directed behavior, Fox and colleagues35 formulated a model in 2005 that included two tightly locked, but anti-correlated, networks—namely, the task-negative network and task-positive network. In 2007, Seeley and colleagues36 demonstrated that the task-positive network actually comprises two dissociable networks: an executive network and a salience network (SN). Based on the previous studies, Menon and colleagues37 proposed a triple-network model comprising the default mode network (DMN), SN, and central executive network (CEN) as the core neurocognitive networks, especially for investigating psychopathology in psychiatric and neurological disorders. The triple-network model is now one of the most widely utilized models for understanding the neural bases of neuropsychiatric disorders. It is therefore the one that we have used in this review.
The DMN comprises mainly the medial prefrontal cortex and adjoining ventral anterior cingulate cortex, posterior cingulate cortex, bilateral inferior parietal cortex, and medial temporal lobe.33 The DMN is involved in self-referential/internally directed information processing and is typically deactivated during external stimulus–driven cognitive processing.38
The CEN comprises mainly the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex, and is responsible for high-level cognitive functions. The CEN is involved in control processes during goal-directed/externally oriented tasks and in regulating emotional responses, particularly those mediated via the DLPFC.36,39
The SN is anchored in the anterior insular cortex and dorsal anterior cingulate cortex, and also includes two key subcortical structures: the amygdala and substantia nigra/ventral tegmental area, which are important for detecting emotional and reward saliency.40 The SN is involved in detecting and orienting to both external and internal salient stimuli and events.36 The anterior insular cortex is critically involved in maintaining and updating representations of current and predictive salience, and contributes to appropriate behavioral responses to salient stimuli by switching between DMN-related self-referential, and CEN-related goal-directed, cognitive activity.41,42
Although originally identified from resting-state fMRI data, the CEN, DMN, and SN can also be readily identified across a wide range of cognitive tasks. The responses of those networks increase and decrease proportionately, and often antagonistically, with the demands of general external cognitive tasks. For instance, the CEN and SN typically show increased activation during external stimulus–driven cognitive and affective information processing, whereas the DMN shows decreased activation with tasks in which self-referential processing is not required.33,34 However, the DMN and CEN can also positively couple when organizing internal self-relevant thought (e.g., autobiographical planning).43–45 Dynamic engagement and disengagement of those core neurocognitive networks are prominent in many cognitive tasks.41,46,47
According to studies in healthy individuals, the CEN typically shows increased activation during external stimulus–driven cognitive and affective information processing, whereas the DMN shows decreased activation with tasks in which self-referential processing is not required.35,38 In addition, emerging evidence shows that the SN, especially the right fronto-insular cortex, is responsible for switching DMN-CEN interactions.40,41,50,51 Briefly, during the externally oriented processing there is typically anti-correlated relationship between the DMN and CEN, which is regulated by the SN (see Figure 2).43–45 Since vulnerability-stress models of depression emphasize an interaction of cognitive diatheses with negative life events leading to the development and onset of depressive episodes,4 investigating abnormal brain responses to emotional or cognitive stimuli in the rest-task transition period (from an internally oriented processing state to an externally oriented stimulus/task-induced processing state) are especially important to understanding the emergence of cognitive vulnerability and depression. In our section below on rest-task transition, we focus on the role of DMN suppression in support of externally oriented cognition to negative life events and on the dynamic interaction among three intrinsic networks. Previous studies demonstrated that patients with MDD, at-risk individuals, and remitted depression subjects consistently show the abnormal connectivity and interaction between the DMN and CEN, as well as dysregulation of the SN, when processing external cognitive or affective stimuli.27,55,56 Therefore, the resting state–related prominent abnormality of the DMN, along with an imbalance among intrinsic networks in the rest-task transition period, has been proposed as the specific neural susceptibility feature of depression.
Figure 2.
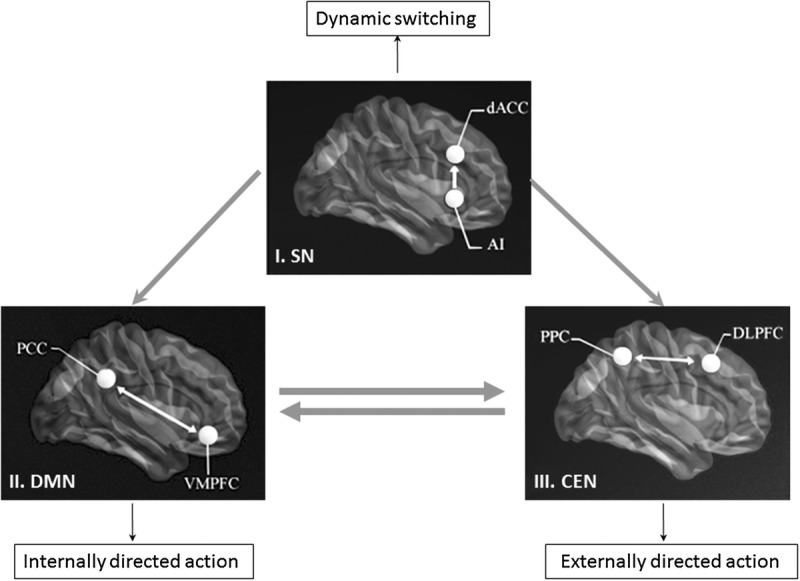
Schematic figure of triple-network model showing the SN-induced coordination between the DMN and CEN. According to this model, the SN (I) mediates the “switching” between the DMN (II) and CEN (III) to guide appropriate responses to salient stimuli.40 Salience signals are integrated in the anterior insular cortex of the SN, and then causally influence signals in the DMN and CEN, which support internally directed and externally directed cognition, respectively.41,47 In light of recent work that suggests the existence of distinct functional subdivisions within the insular cortex,52,53 the right anterior insular cortex is now thought to be the specific brain region that assists in switching between networks. The large dots show the key nodes of each network in the triple-network model. AI, anterior insular cortex; CEN, central executive network; dACC, dorsal anterior cingulated cortex; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; SN, salience network; PPC, posterior parietal cortex. Adapted from Uddin (2014)51 and Uddin & Menon (2009).54
The aim of this review is to synthesize current neuroimaging research to reinterpret the formation of cognitive vulnerability and subsequent depression from the perspective of intrinsic organization and cross-network interaction. Based on the dual-process model, we will describe how abnormal activities and interactions among the DMN, CEN, and SN can provide insights into the emergence of cognitive vulnerability and MDD. Specifically, as first proposed by Sheline and colleagues57,58 and subsequently discussed in multiple studies,24,28,59–61 the hyperactivity in the DMN leads to a negative associative-processing bias commonly associated with negative self-referential processes, which forms the neural foundation of cognitive vulnerability to depression. In addition, abnormal cross-network interactions during rest-task transitions, which include three scenarios, lead to the failure to correct negative associative-processing bias, thereby facilitating and stabilizing the cognitive vulnerability (see Figure 2). We will describe these mechanisms in detail in the next sections. Though research on functional connectivity and intrinsic networks does not provide a direct proxy for anatomic/structural connectivity, the integrative framework presented here extends beyond the phenomenological dimensions of depression and local functional impairments. It suggests new research directions addressing both behavioral and neuronal features in cognitive vulnerability, and potentially informs the development of targeted interventions for MDD.
ABERRANT RESTING-STATE DMN AND ASSOCIATIVE-PROCESSING BIAS
The dual-process model suggests that negatively biased self-referential associative processing is the foundation for cognitive vulnerability to depression.14 Though many vulnerability factors are associated with biased self-referent information, maladaptive rumination plays a primary role in mediating the internalization of negative self-representations and onset of depression symptoms.62 Research suggests that maladaptive rumination is characterized by spontaneous, narrowly focused, self-referential associative processing.63 In addition, there is a strong overlap between the DMN and the cortical network that mediates associative processing on the medial prefrontal cortex (medial PFC), medial temporal lobe, and medial-parietal cortex.64 Recently, increasing evidence suggests that maladaptive rumination-related resting-state DMN activity is associated with the generation of cognitive vulnerability.60 Specifically, Bar63 states that the neural basis of rumination is resting medial PFC hyperactivity coupled with increased functional connectivity between the medial PFC and anterior cingulate cortex (ACC). This increased functional connectivity, in turn, suppresses medial temporal lobe activity and constrains normally broad associative processing and the generation of a positive mood, leading to ruminative processes and negative mood states.
Neuroimaging studies have supported Bar’s hypothesis that persistent hyperactivity in the medial PFC and its neighboring ventral ACC regions, as well as their relationship with the medial temporal lobe, plays a core role in the cognitive vulnerability to depression. Berman and colleagues28 examined the relationship between DMN functional connectivity and rumination, both at rest and in a short-term memory task. During the rest period, MDD participants exhibited increased DMN connectivity in the subgenual ACC, which correlated with self-report rumination and hyperconnectivity between the medial PFC and medial temporal lobe. Similarly, a study on first-episode, unmedicated MDD subjects found significantly greater functional connectivity between the rostral ACC and parahippocampal gyrus at rest.65 Recently, Sambataro and colleagues66 investigated the function of multiple DMN subsystems at rest in MDD and showed increased connectivity, increased low-frequency band spectral power in the medial PFC, subgenual ACC, and rostral ACC, and aberrant interactions between the rostral ACC and hippocampus. Moreover, using an unsupervised machine-learning approach with a network derived from resting-state fMRI data, including the subgenual ACC, medial PFC, and superior temporal gyri, Zeng and colleagues67 demonstrated a highly discriminative power for accurate identification of MDD. Schilbach’s study using a meta-analytically informed network analysis68 also showed the subgenual ACC to be a hub of resting-state hyperconnectivity in the introspective socio-affective network in depressive individuals. Thus, converging evidence from a number of brain-imaging studies suggests that the generation of maladaptive rumination is associated with resting hyperactivity in the medial PFC and with hyperconnectivity between the medial PFC and both the ventral ACC and medial temporal lobe.
Bar’s hypothesis has been further supported by consistent evidence of enhanced functional connectivity among anterior DMN regions in MDD patients. Using independent component analysis, Greicius and colleagues69 first explored DMN abnormalities in major depression and suggested that the DMN functional connectivity in depression, which was associated with the ruminative nature of depressive subjects, was disproportionately driven by increased activity in the subgenual ACC. Importantly, numerous other studies have replicated the basic findings of that study.57,70,71 In addition, using multivariate Granger causality analysis of resting-state data, Hamilton and colleagues72 found that medial PFC and subgenual ACC activities were mutually reinforcing in MDD and that the medial PFC-to-subgenual ACC connectivity correlated with levels of depressive rumination. In 2012, we provided the first direct evidence of increased resting-state functional connectivity in the anterior medial cortex regions (especially the medial PFC and rostral ACC) that correlated with rumination scores from first-episode, treatment-naive MDD patients.24 That finding has been supported by studies in adolescents experiencing first-episode MDD.25 Using a unique four-year follow-up sample of children with a history of preschool-onset MDD, Gaffrey and colleagues73 also demonstrated abnormal subgenual ACC functional connectivity and found correlations between dysregulated emotional behavior and subgenual ACC/medial PFC connectivity. The subgenual ACC and rostral ACC formed the affective division of ACC, which is activated by tasks with affective or emotional content and also deactivated by cognitively demanding tasks.74 Hence, the joint hyperactivity in those DMN nodes, by leading to ruminative processes, may have a critical role in the formation of cognitive vulnerability.
Neuroimaging evidence from subjects at risk for MDD and from nonclinical populations provides additional evidence that the DMN is involved in ruminative processes and contributes to cognitive vulnerability. Norbury and colleagues22 examined resting-state fMRI data from 15 healthy at-risk participants having a biological parent with MDD and from 15 healthy controls. Relative to the controls, the subjects with positive family history showed significantly greater DMN connectivity in the left dorsomedial PFC, left medial temporal lobe, orbitofrontal cortex, and left precuneus. The researchers suggest that this pattern may represent a ruminative vulnerability. Similarly, Kross75 reported that rumination induction in healthy subjects activated a network that included the subgenual ACC and medial PFC, and that this network activity was greater during induced rumination than during “analyze” or “accept” conditions. Moreover, activity in this network correlated positively with increases in negative affect during induced rumination. Recently, Liu76 and Fielder23 provided new evidence independently for the altered resting-state anterior DMN connectivity in healthy siblings of MDD patients76 and in women with subclinical depression but without a history of MDD.23
In summary, emerging evidence strongly indicates that elevated resting-state activity in anterior DMN regions and the greater functional connectivity among them are associated with rumination: a narrow associative-thinking pattern and cognitive vulnerability factor in depression. However, two points are worth mentioning. First, we propose that abnormal anterior DMN activity at rest may reflect only brooding, a subcomponent of rumination that involves negative self-focus and is hypothesized to be more strongly associated with depressive symptoms both in concurrent and longitudinal analyses.77 The other subcomponent of rumination—namely, reflection—more likely relates to reflective processing in the dual-process model, which is a purposeful turning inward to alleviate one’s depressive symptoms.14,78,79 Indeed, Hamilton and colleagues72 reported that greater DMN activity in MDD patients at rest was associated with higher levels of brooding and lower levels of reflection. Berman and colleagues28 reported a similar result. Second, there are some discrepant studies in the literature, which might be due to the differences in sample characteristics or analysis methods, such as using the a priori defined region-of-interest correlation approach versus whole-brain analyses,79,80 using independent component analysis at the group level versus on individual data sets,81 or applying a newly developed method.82 Deployment of more uniform methodologies may help resolve these discrepancies.
ABERRANT NETWORK INTERACTIONS DURING REST-TASK TRANSITIONS: THREE SCENARIOS
DMN Dominance: Cognitive-Resource Depletion
Although persistent resting-state DMN hyperactivity and heightened spontaneous rumination are core features of cognitive vulnerability, DMN hyperactivity may also occur in nondepressed individuals. In nondepressed individuals, however, that state is typically transient, and they are able to shift quickly toward task-based deactivation to support stimulus-driven, goal-directed cognitive processes. In other words, the resting-state “imbalance” within intrinsic networks may stop, and the development of cognitive vulnerability and dysphoric mood may be suspended.83 However, patients with MDD consistently show both a lack of DMN suppression and impaired performance during task-induced states, indicating that the DMN dominance continues and that its residual activity interferes with subsequent attentional and cognitive processing. Johnson’s study84 provided direct evidence that patients with MDD show less DMN deactivation during externally oriented cognitive processing—an effect that was positively correlated with rumination. Hence, the authors suggested that depression pathology may involve a difficulty with disengaging from self-reflection. Grimm and colleagues85 investigated negative BOLD responses in DMN regions during the presentation of emotional pictures and reported reduced rest-to-task attenuation of DMN activity in MDD patients. Importantly, that reduced attenuation was associated with strong feelings of hopelessness. Furthermore, Mitterschiffthaler and colleagues86 reported significant engagement of the rostral ACC and right precuneus in MDD subjects during an emotional Stroop task. The rostral ACC activation in MDD subjects was positively correlated with impaired performance on trials with negative words. A hyperactive DMN has also been shown to result in impaired cognitive performance in working-memory tasks, passive viewing, and implicit-recognition tasks for negative images.56,87,88
Based on the above neuroimaging studies, both the elevated DMN activity during resting state and the increased DMN activity (less suppression) during task-induced states play an important role in the pathophysiology of MDD. The latter is central to the first dual-processing scenario for developing cognitive vulnerability (Figure 1): reflective processing may not override the negatively biased associative processing when some cognitive load disrupts, which then leads to cognitive vulnerability. This finding was confirmed by Wenzlaff and colleagues89 using a series of behavior tasks in remitted and currently depressed individuals. Specifically, although remitted patients did not have a negative bias at baseline, biased performance under a cognitive-load condition was predictive of future depressive symptoms. In addition, behavioral,90 pupillary,91 and electrophysiological92 data support the notion that depressed individuals engage in increased intrinsic processing (e.g., focusing on negative automatic thoughts or rumination), which potentially taxes resources that would otherwise be allocated to cognitive tasks. Recently, Nixon and colleagues93 reported the first direct evidence of persistent excessive DMN activity during tasks in remitted MDD patients, which suggests that task-based DMN hyperconnectivity may result in cognitive and attentional bias and increase the risk of depression. Therefore, DMN dominance may be the neural basis for the role of cognitive-resource depletion in the development of vulnerability and then depression.
Recent work on DMN-CEN anti-correlation provides potential evidence for this mechanism. The optimal attunement between the DMN and CEN is thought to reflect efficient intrinsic brain organization.35,94 During the processing of externally directed stimuli, the deactivation of the DMN and the increased activation in the CEN occur concurrently and timely for ensuring appropriate and successful responses.36,46 In the rest-task transition period, however, both depressive and at-risk individuals show abnormal DMN dominance, which induces decreased DMN-CEN anti-correlation and subsequently depletes cognitive resources.95 This phenomenon may arise in two ways. In the first, exaggerated spontaneous DMN activity may persist following the rest-task transition, reduce recruitment of cognitive-control resources, and prevent distraction from rumination.25,96 This DMN persistence is evidenced by the rumination-related lack of DMN suppression in a non-self-referential task.84 Similarly, using a new quantitative method to compute the extent to which DMN activity levels exceed task-related network activity over the course of the resting-state scan, Hamilton and colleagues72 showed that increasing levels of DMN dominance were associated with higher levels of brooding and lower levels of reflection in MDD. In addition, Davey and colleagues97 reported that the subgenual ACC–ventromedial PFC connectivity in MDD increased in resting state and that this change in functional connectivity could predict the relative activity of CEN regions, thus suggesting that dysfunctional connectivity with the subgenual ACC may influence executive/attentional processes. Thus, the attenuated DMN-CEN anti-correlation stemming from abnormal DMN dominance in the rest-task transition may induce cognitive-resource depletion leading to cognitive vulnerability to depression.
The second way in which abnormal DMN dominance in MDD may arise is from automatic, effortless, negatively biased information processing at a preconscious level, which activates the DMN and attenuates the influence of otherwise distracting cognitive processing. Many studies related to negative stimulus processing mentioned above support this mode of developing DMN dominance.56,86,98 Such studies consistently reported heightened responses in the amygdala, along with higher activity in the DMN and lower activity in the CEN. Moreover, spontaneous activity in the amygdala has been shown to positively predict the activity of the medial PFC and to negatively predict the activity of the middle frontal gyrus.99 A longitudinal study further showed that amygdala-ventromedial PFC connectivity was predicted by early-life stress and associated positively with depressive symptoms in female adolescents.100 A recent study further reported increased, sustained amygdala reactivity involving the involuntary processing of salient negative information associated with all the dimensions of trait rumination.101 Thus, aberrant DMN dominance during prior processing of negative emotional stimuli in MDD may be caused by excessive input from the SN nodes, and be associated with aberrant attention allocation and switching. We will interpret the related mechanisms in the next section.
In summary, the DMN-dominant state during rest-task transition may arise from the persistence of resting DMN activity or from quick activation of the DMN during negative stimulus processing. This persistence depletes cognitive resources and leads to a sustained negative cognitive bias, which then facilitates the development of cognitive vulnerability to depression.
Impaired SN Switching Between DMN and CEN: Expectancies Are Not Violated
In Menon’s triple-network model,37 the SN—which is anchored in the dorsal anterior cingulate cortex, fronto-insular cortex, amygdala, and ventral striatum—is involved in bottom-up detection of salient events and in switching between other large-scale networks to facilitate appropriate allocation of attentional and cognitive/executive resources when a salient event is detected. Using the Granger causality analysis method in both of resting-state and task-state data, Sridharan and colleagues41 discovered that the right fronto-insular cortex–ACC network, especially the right fronto-insular cortex, plays a causal role in DMN-CEN switching. Recently, and using a novel technique for analyzing resting-state data, Goulden and colleagues50 independently validated the role of SN in driving the switching between the DMN and CEN. Another study involving a combination of fMRI and diffusion tensor imaging data also supported the critical role of the right fronto-insular cortex in switching between brain networks for complex, flexible cognitive processing.47 Furthermore, by combining transcranial magnetic stimulation (TMS) and fMRI, Chen and colleagues102 successfully demonstrated that single-pulse TMS of an SN site enhanced both within-SN and within-CEN connectivity, whereas TMS stimulation of a CEN site enhanced only within-CEN psychophysiological connectivity. In summary, the SN switching role between the DMN and CEN (see Figure 2) has been strongly supported by converging evidence in healthy subjects and highlights the possibility that the atypical engagement of the SN is a feature of neuropsychiatric disorders.51
A growing body of evidence demonstrates that the SN’s switching function is impaired in MDD and at-risk individuals.103,104 For instance, medication-naive adolescents with MDD showed reduced activation in the fronto-insular cortex and dorsolateral PFC during an attention-switching task.105 Strigo and colleagues106 further demonstrated that in MDD, altered right fronto-insular cortex function, along with increased activity in the ventromedial PFC and rostral ACC and decreased activity in the dorsolateral PFC, is associated with an impaired ability to effectively prepare for environmental changes. Similarly, Hamilton and colleagues72 examined the right fronto-insular cortex response during initiation of ascents in DMN and CEN activity and showed that right fronto-insular cortex plays a differential role in DMN/CEN dominance switching in MDD subjects versus controls. This model is further supported by Manoliu and colleagues’ recent study.107 In addition, through comparing task-based brain function of individuals with high or low risk of developing MDD, Peterson and colleagues104 described a neural system, including insular and other nodes in cortical attention circuits, as the risk endophenotype for MDD. Recently, Kaiser and colleagues56 compared the neural activation during emotion-word and color-word Stroop tasks in participants with subclinical depression. They suggested that affective interference stems from the increased salience of negative emotional information, coupled with the difficulty in shifting resources toward the external environment. That study further supported the SN’s abnormal function in switching between the DMN and CEN in vulnerable individuals.
Besides the direct measurement of activity or functional connectivity of the SN nodes in MDD and vulnerable individuals, many studies have showed that the abnormal switching function of the SN is associated with mood-congruent, negative-response biases, a well-known vulnerability factor emphasized in Beck’s cognitive model of depression.108 Specifically, the amygdala contributes to the biased processing of both explicitly and implicitly negative emotional stimuli in at-risk depressive individuals,31,109,110 MDD,111–114 and remitted MDD patients.115 The anterior insula and dorsal ACC also participate in the formation of these biases.116,117 A recent fMRI study showed that MDD patients develop a pessimistic attitude toward the emotional meaning of external events—an attitude associated with abnormal activation in the medial PFC, dorsolateral PFC, and insular cortex.118 Therefore, the misinterpretation of external cues by the insula due to unpleasant previous experiences may be a central underlying mechanism of depression-related dysregulation. Similarly, in a voxel-wise, whole-brain meta-analysis, Hamilton and colleagues119 showed that MDD patients had elevated activity in the amygdala, insula, and dorsal ACC, but decreased activity in the dorsolateral PFC, during the process of negative emotional stimuli. The authors suggested that in depression, negative information could induce a heightened neural response in the SN but fail to be propagated to the CEN for contextual processing and reappraisal. Indeed, in 2008, a pathway-mapping analysis provided direct fMRI evidence for that hypothesis: regulation of emotion through the amygdala to the ventrolateral PFC could predict reappraisal failure and more sustained negative emotion.120 To sum up, several core regions in the SN are critically involved in the formation of mood-congruent negative-response biases and subsequently in the change of CEN activity associated with cognitive control.
The aforementioned SN-related, mood-congruent, negative-bias processing could play a role in the second scenario of failing to correct biased associative processing in the dual-process model (see Figure 1): when the results of associative processing are congruent with one’s negative internal expectancy (e.g., negative self-referential schemas or representations related to one’s self-worth), expectancies will not be violated. By contrast, when healthy individuals with optimism biases confront negative external stimuli, cognitive conflict would be recognized automatically, and attention would then be disengaged from the negative thoughts. Previous studies showed that conflict signaling was reduced in individuals with negative thoughts/mood due to their mood-congruent attention and memory biases.121 Accordingly, in individuals at risk for depression, attentional resources could not reallocate appropriately to disengage from the situation; instead, the resources continue to focus on self-referring negative information.122 Along the same lines, observations in healthy individuals with high brooding scores show that more attentional control is needed to successfully disengage from negative information.123 Therefore, the “impaired disengagement” of attention based on the mood-congruent negative bias in MDD would facilitate not only sustained maladaptive rumination but also impaired cognitive performance. In summary, we propose that in the context of mood-congruent, negative-bias processing, impaired SN switching between the DMN and CEN underlies, at least in part, the cognitive vulnerability to depression.
Failure to Effectively Activate CEN: Reflective Processing Does Not Adequately Adjust Biased Associative Processing
In the third scenario of the dual-process model, which may involve a failure of CEN activation (see Figure 1), it is posited that cognitive vulnerability to depression is facilitated when reflective processing does not adequately adjust output from associative processing. In other words, when an individual tries to regulate emotion voluntarily (i.e., to reappraise) through reflective processing but fails, an associative bias is maintained or even amplified. Two cognitive-vulnerability factors are likely involved in reflective processes related to emotion regulation: reflective rumination74 and depressogenic attributional styles7 (i.e., regarding the self and the causes and consequences of negative events) based on hopelessness theory. When vulnerable individuals confront negative events, both of those factors will guide them to spontaneously use emotion-regulation strategies that could be directed against the increased negative emotion stemming from elaboration on the meaning of unpleasant events. However, failure to regulate will lead the individual to fall into more negative cognition, depressive rumination, and dysphoria.
Emotion-regulation deficits are a central characteristic of depressed individuals. Though previous studies showed that different types of emotion-regulation strategies (e.g., automatic vs. voluntary, distraction vs. reappraisal) are associated with different neural mechanisms, both strategies are subserved by common top-down control areas in the CEN (especially the dorsolateral PFC node) to effectively allocate attention to the external stimuli.124,125 Furthermore, numerous studies suggest that aberrant voluntary emotion-regulation in MDD is related to hypoactivity in CEN nodes.126,127 However, recent evidence has also pointed to hyperactivity in DMN components. Smoski and colleagues128 found that remitted MDD showed significantly greater rostral ACC activation and simultaneously decreased midfrontal gyrus activation during the reappraisal of sad images (in contrast to attending to sad images). Similarly, Sheline and colleagues58 reported that when subjects with MDD reappraised negative pictures actively, widely distributed elements of their DMNs failed to deactivate. Erk129 and Johnstone130 also found that MDD patients show significantly diminished responses in the dorsolateral PFC, but increased activation in DMN nodes, when performing an effortful reappraisal task for negative emotion. In a small study including only 12 MDD patients, Dillon and colleagues131 reported recently that dorsolateral PFC activity correlated inversely with depressive severity, though no group differences in reappraising negative pictures were found. Another follow-up study of remitted MDD participants employing sad mood provocation has added new evidence showing that expansive medial PFC reactivity can predict subsequent depressive relapse over an 18-month follow-up period.16 The authors linked medial PFC reactivity in remitted MDD participants to inefficient recruitment of the PFC in attempts to cognitively regulate negative emotion. Recently, two studies in remitted MDD reported similarly reduced PFC reactivity during negative feedback, which was associated with the rumination and possibly impaired the adaptive reappraisal of negative experience.132,133 Thus, emerging evidence suggests that during the cognitive regulation of negative events, the failure to regulate and the generation of cognitive vulnerability are not only associated with the decreased activation in CEN nodes but also with increased activation in DMN nodes.
Since CEN hypoactivity and DMN hyperactivity could be occurring simultaneously during impaired cognitive regulation in MDD, it raised a question whether attenuated CEN modulation is a primary effect of CEN hypoactivity or a secondary effect of interference from DMN activity. In a recent study combining TMS with fMRI, Chen and colleagues102 reported direct evidence of a causal regulatory relationship primarily from the CEN to the DMN. Specifically, single-pulse excitatory stimulation of a CEN node (posterior middle frontal gyrus) induced negative DMN connectivity with the CEN, whereas inhibitory repetitive TMS to the same stimulation site induced the disinhibition of DMN activity on the medial PFC. Two other TMS studies presented further evidence that repetitive TMS on the dorsolateral PFC could modulate interactions between the DMN and CEN by normalizing depression-related DMN hyperconnectivity.134,135 As more anti-correlation between the two networks was induced, the clinical efficacy of the TMS increased. Interestingly, in a recent double-blind, six-month trial examining changes in the neural circuitry involved in emotion regulation after antidepressant treatment, only changes in CEN nodes (dorsolateral PFC and Brodmann area 10) that were are engaged while subjects performed a negative affect–regulating task correlated with changes in depression severity over the following six months.136
In summary, previous studies suggest that failure to effectively activate the CEN and the corresponding reduced effect on the DMN mainly contribute to the unsuccessful voluntary regulation of negative emotion, thus allowing negative cognitive/affective responses to persist and facilitating the development of depressive vulnerability.
CONCLUSIONS AND FUTURE DIRECTIONS
In this review, we used a dual-process framework and built upon the literature of intrinsic networks to integrate well-established MDD cognitive-vulnerability factors and corresponding neural mechanisms. We propose that abnormally increased resting-state DMN activity and connectivity (mainly in anterior DMN regions) and corresponding depressive rumination contribute to the development of cognitive vulnerability. Furthermore, in the period of rest-to-task transition, three kinds of aberrant network interactions may facilitate the occurrence of cognitive vulnerability. Specifically, when confronting negative life events or stimuli, DMN dominance (persisting due to increased resting activity and facilitated by automatic, biased information processing), abnormal SN-mediated switching between the DMN and CEN (related to a negative schema and mood-congruent negative bias), and failure to effectively activate the CEN (related to reflective rumination, negative attribution style, and corresponding emotion-regulation impairment). A focus on interrelated networks and brain activity changes between rest-task transitions provides an approach for future research into inter-individual differences in vulnerability and resilience. Several outstanding questions remain, however, and need to be explored in depth.
First, although an increasing number of neuroimaging studies have investigated cognitive vulnerability to depression, more systematic research is required to test and confirm the validity of our framework. For instance, longitudinal fMRI studies with comprehensive assessment of cognitive-vulnerability factors would allow us to investigate the intrinsic network changes in predicting future occurrences of depression. To date, only a few studies have compared brain system activity among never-depressed individuals with vulnerability factors, currently depressed individuals, and remitted depressed individuals. A more systematic research approach, including both cross-sectional and longitudinal studies, is needed to develop the models for mapping the neural patterns.
Second, the neurobiological underpinnings of network dysfunction and impaired interactions remain poorly understood. Recently, however, some theoretical hypotheses have been elaborated for the complex dynamics between large-scale neural systems. For example, Anticevic and colleagues95 proposed a model for the synaptic mechanisms of altered DMN suppression and DMN-CEN interactions: the anti-correlation between CEN and DMN activities during task performance is derived from the reciprocal network interaction through net inhibitory long-range projections, which are related to disrupted NMDA conductance onto GABAergic interneurons. According to this model, local disinhibition induces hyperactivity of DMN-type microcircuitry and hyposensitivity to long-range suppressive inputs from task-activated, cognitive-related microcircuits—which precludes silencing the already high-firing DMN at task onset. The specific mechanisms of GABAergic/glutamatergic neural interaction, as well as their regulation on the brain network activity, need to be explored in future research.
Third, despite the strong evidence for the clinical effectiveness of psychotherapy in treating MDD, the neural underpinning of depression-specific psychotherapies remains unclear. Our framework may help to explore this issue. For instance, in cognitive-behavioral therapy, patients are given explicit instructions on how to regulate their negative thoughts and emotions, which may increase CEN activity and decrease DMN activity, and thereby enhance patients’ ability to complete reflective processing successfully.137–139 Moreover, mindfulness therapy, which highlights present-moment awareness and acceptance, may alter activity in the SN, especially anterior insular activity, by decreasing the incongruence between the outcome of associative processing and the individual’s expectations.140,141 Based on a meta-analysis, Ma124 further proposed that antidepressant medication and cognitive-behavioral therapy have different neuropsychological mechanisms: the latter targets prefrontal function by increasing inhibitory executive control, whereas the former may act more directly on the network associated with abnormal emotion generation/experience. Furthermore, a recent review proposed that psychotherapy may facilitate recovery and plasticity at the brain network level after antidepressant drugs reactivate a window of juvenile-like plasticity in the adult cortex.142 Ongoing composite research on cognitive vulnerability, treatment effects, and brain network–level plasticity is very promising.
Fourth, evidence shows some overlap of dysfunctional neural processing among different disorders. For instance, similar DMN abnormalities reflecting internally oriented attention and thought are found in both depression and schizophrenia.143 Our diagnostic categories are heterogeneous and likely encompass multiple biologically distinct entities. Future work using the research domain criteria on brain system dysfunction may provide valuable insights into which patients demonstrate these network-level abnormalities and how those relate across diagnoses.
Finally, much work remains for relating network models explicitly to cognition and neural computation.144–146 The intrinsic functional connectivity and network model undoubtedly provides a unique and powerful tool to provide insight into the organization of distributed association brain networks, especially as an organizing framework for characterizing biological substrates in mental disorders. Some methodological problems remain, however, in studying such networks—such as the sensitivity of functional connectivity MRI to head motion and physiological artifacts,147,148 and the effect of global signal regression on detecting anti-correlation between networks.46,149 Difficulties also exist with the definition of node/edge and the interpretation of network measures; for examples, as an indirect, relative measure of neural-activity fluctuations, functional connectivity MRI measures have no proven biological interpretation. Therefore, more studies are needed to promote the translation from the network properties to the realities of behavior and neurobiology.
In summary, a growing body of neuroimaging research reveals that cognitive vulnerability, the most generally accepted psychological risk factor for depression, is associated with neural functional abnormalities. Under a dual-processing framework, we integrated the MDD-related cognitive-vulnerability factors in the context of an intrinsic network and cross-network interaction perspective. Although the hypothesis presented in this review remains somewhat speculative, it provides a unique understanding of the link between in vivo brain measurements and cognitive vulnerability, and it enhances our insight into the biological underpinnings in the development, maintenance, and treatment of depression. The integrative framework suggests a paradigmatic shift in cognitive-vulnerability research and potentially informs the development of targeted interventions for MDD.
Declaration of interest
Dr. Öngür was on the Scientific Advisory Board for Lilly Inc. in 2013.
Footnotes
Supported by Chinese Ministry of Education’s Humanities and Social Science Research Project grant no. 13YJA190015 and Program for New Century Excellent Talents in University grant no. NCET-12-0557 (Dr. Wang); National Institutes of Health grant nos. R01MH094594 (Dr. Öngür) and K23MH097786 (Dr. Auerbach); Harvard Medical School Kaplen Fellowship on Depression (Dr. Auerbach); and National Natural Science Foundation of China grant no. 81071104 (Dr. Yao).
Original manuscript received 22 August 2014; revised manuscript received 27 November 2014, accepted for publication 12 January 2015.
REFERENCES
- 1. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289: 3095– 105. [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593– 602. [DOI] [PubMed] [Google Scholar]
- 3. Merikangas KR, He JP, Burstein M. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010; 49: 980– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. New York: Guilford, 1998; 88– 115. [Google Scholar]
- 5. Haeffel GJ, Eastman M, Grigorenko EL. Using a cognitive endophenotype to identify risk genes for depression. Neurosci Lett 2012; 510: 10– 3. [DOI] [PubMed] [Google Scholar]
- 6. Beck AT. Cognitive therapy of depression: new perspectives. In: Clayton PJ, Barrett JE, eds. Treatment of depression: old controversies and new approaches. New York: Raven, 1983; 265– 90. [Google Scholar]
- 7. Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: a theory-based subtype of depression. Psychol Rev 1989; 96: 358– 72. [Google Scholar]
- 8. Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol 1991; 100: 569– 82. [DOI] [PubMed] [Google Scholar]
- 9. Haeffel GJ, Abramson LY, Brazy PC, Shah JY, Teachman BA, Nosek BA. Explicit and implicit cognition: a preliminary test of a dual-process theory of cognitive vulnerability to depression. Behav Res Ther 2007; 45: 1155– 67. [DOI] [PubMed] [Google Scholar]
- 10. Phillips WJ, Hine DW. Exploring the factor structure of implicit and explicit cognitions associated with depression. Assessment 2013; 20: 474– 83. [DOI] [PubMed] [Google Scholar]
- 11. Elgersma HJ, Glashouwer KA, Bockting CL, Penninx BW, de Jong PJ. Hidden scars in depression? Implicit and explicit self-associations following recurrent depressive episodes. J Abnorm Psychol 2013; 122: 951– 60. [DOI] [PubMed] [Google Scholar]
- 12. Carver CS, Johnson SL, Joormann J. Major depressive disorder and impulsive reactivity to emotion: toward a dual-process view of depression. Br J Clin Psychol 2013; 52: 285– 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaiken S, Trope Y. Dual-process theories in social psychology. New York: Guilford, 1999. [Google Scholar]
- 14. Beevers CG. Cognitive vulnerability to depression: a dual process model. Clin Psychol Rev 2005; 25: 975– 1002. [DOI] [PubMed] [Google Scholar]
- 15. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35: 192– 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farb NAS, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol Psychiatry 2011; 70: 366– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 2007; 56: 171– 84. [DOI] [PubMed] [Google Scholar]
- 18. Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014; 83: 238– 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 2013; 80: 360– 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends Neurosci 2010; 33: 277– 84. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Hermans DF, Hickie IB, Lagopulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord 2012; 142: 6– 12. [DOI] [PubMed] [Google Scholar]
- 22. Norbury R, Mannie Z, Cowen PJ. Imaging vulnerability for depression. Mol Psychiatry 2011; 16: 1067– 8. [DOI] [PubMed] [Google Scholar]
- 23. Felder JN, Smoski MJ, Kozink RV, et al. Neural mechanisms of subclinical depressive symptoms in women: a pilot functional brain imaging study. BMC Psychiatry 2012; 12: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry 2012; 71: 611– 7. [DOI] [PubMed] [Google Scholar]
- 25. Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry 2013; 74: 898– 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li B, Liu L, Friston KJ, et al. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry 2013; 74: 48– 54. [DOI] [PubMed] [Google Scholar]
- 27. Jacobs RH, Jenkins LM, Gabriel LB, et al. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One 2014; 9: e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci 2011; 6: 548– 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev 2012; 22: 229– 51. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis 2013; 52: 4– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pilhatsch M, Vetter NC, Hubner T, et al. Amygdala-function perturbations in healthy mid-adolescents with familial liability for depression. J Am Acad Child Adolesc Psychiatry 2014; 53: 559– 68. [DOI] [PubMed] [Google Scholar]
- 32. Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci 2014; 7: 13– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A 2001; 98: 676– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 2003; 100: 253– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005; 102: 9673– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349– 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011; 15: 483– 506. [DOI] [PubMed] [Google Scholar]
- 38. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124: 1– 38. [DOI] [PubMed] [Google Scholar]
- 39. Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 2005; 360: 781– 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 214: 655– 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008; 105: 12569– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uddin LQ, Supekar K, Lynch CJ, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 2013; 70: 869– 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 2010; 53: 303– 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spreng RN. The fallacy of a “task-negative” network. Front Psychol 2012; 3: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat Neurosci 2011; 14: 830– 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004; 101: 4637– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci 2011; 31: 18578– 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 2012; 379: 1045– 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin 2013; 4: 209– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 2014; 99: 180– 90. [DOI] [PubMed] [Google Scholar]
- 51. Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015; 16: 55– 61. [DOI] [PubMed] [Google Scholar]
- 52. Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 2011; 21: 1498– 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 2013; 23: 739– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev 2009; 33: 1198– 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Auerbach RP, Admon R, Pizzagalli DA. Adolescent depression: stress and reward dysfunction. Harv Rev Psychiatry 2014; 22: 139– 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kaiser RH, Andrews-Hanna JR, Spielberg JM, et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc Cogn Affect Neurosci 2014; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 2010; 107: 11020– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A 2009; 106: 1942– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci 2013; 7: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hamilton JP, Chen G, Thomason ME, Schwartz Gotlib ME. Investigating neural primacy in major depressive disorder: multivariate granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry 2011; 16: 763– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Northoff G, Wiebking C, Feinberg T, Panksepp J. The ‘resting-state hypothesis’ of major depressive disorder—a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev 2011; 35: 1929– 45. [DOI] [PubMed] [Google Scholar]
- 62. Hankin BL. Stability of cognitive vulnerabilities to depression: a short-term prospective multiwave study. J Abnorm Psychol 2008; 117: 324– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bar M. A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci 2009; 13: 456– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus 2007; 17: 420– 8. [DOI] [PubMed] [Google Scholar]
- 65. Peng DH, Shen T, Zhang J, et al. Abnormal functional connectivity with mood regulating circuit in unmedicated individual with major depression: a resting-state functional magnetic resonance study. Chin Med J (Engl) 2012; 125: 3701– 6. [PubMed] [Google Scholar]
- 66. Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med 2013; 31: 1– 11. [DOI] [PubMed] [Google Scholar]
- 67. Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. Hum Brain Mapp 2014; 35: 1630– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schilbach L, Müller VI, Hoffstaedter F, et al. Meta-analytically informed network analysis of resting state FMRI reveals hyperconnectivity in an introspective socio-affective network in depression. PLoS One 2014; 9: e94973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62: 429– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou Y, Yu C, Zheng H, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord 2010; 121: 220– 30. [DOI] [PubMed] [Google Scholar]
- 71. Davey CG, Harrison BJ, Yücel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med 2012; 42: 2071– 81. [DOI] [PubMed] [Google Scholar]
- 72. Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 2011; 70: 327– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gaffrey MS, Luby JL, Botteron K, Repovš G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry 2012; 53: 964– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4: 215– 22. [DOI] [PubMed] [Google Scholar]
- 75. Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry 2009; 65: 361– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu CH, Ma X, Wu X, et al. Resting-state brain activity in major depressive disorder patients and their siblings. J Affect Disord 2013; 149: 299– 306. [DOI] [PubMed] [Google Scholar]
- 77. Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cogn Ther Res 2003; 27: 247– 59. [Google Scholar]
- 78. Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behav Ther 2006; 37: 269– 80. [DOI] [PubMed] [Google Scholar]
- 79. Cullen KR, Gee DG, Klimes-Dougan B, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett 2009; 460: 227– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bluhm R, Williamson P, Lanius R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci 2009; 63: 754– 61. [DOI] [PubMed] [Google Scholar]
- 81. Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci 2010; 4: 1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo W, Liu F, Dai Y, et al. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2013; 41: 24– 9. [DOI] [PubMed] [Google Scholar]
- 83. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 2011; 18: 251– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Soc Cogn Affect Neurosci 2009; 4: 313– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grimm S, Boesiger P, Beck J, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology 2009; 34: 932– 43. [DOI] [PubMed] [Google Scholar]
- 86. Mitterschiffthaler MT, Williams SC, Walsh ND, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med 2008; 38: 247– 56. [DOI] [PubMed] [Google Scholar]
- 87. Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med 2009; 39: 977– 87. [DOI] [PubMed] [Google Scholar]
- 88. Ho TC, Yang G, Wu J, et al. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord 2014; 155: 65– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rude SS, Wenzlaff RM, Gibbs B, Vane J, Whitney T. Negative processing biases predict subsequent depressive symptoms. Cogn Emot 2002; 16: 423– 40. [Google Scholar]
- 90. Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol 2008; 117: 182– 92. [DOI] [PubMed] [Google Scholar]
- 91. Jones NP, Siegle GJ, Muelly ER, Haggerty A, Ghinassi F. Poor performance on cognitive tasks in depression: doing too much or not enough? Cogn Affect Behav Neurosci 2010; 10: 129– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry 2008; 65: 179– 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nixon NL, Liddle PF, Nixon E, Worwood G, Liotti M, Palaniyappan L. Biological vulnerability to depression: linked structural and functional brain network findings. Br J Psychiatry 2014; 204: 283– 9. [DOI] [PubMed] [Google Scholar]
- 94. Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci 2014; 26: 501– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012; 16: 584– 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 2011; 12: 467– 77. [DOI] [PubMed] [Google Scholar]
- 97. Davey CG, Yücel M, Allen NB, Harrison BJ. Task-related deactivation and functional connectivity of the subgenual cingulate cortex in major depressive disorder. Front Psychiatry 2012; 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn 2009; 69: 218– 25. [DOI] [PubMed] [Google Scholar]
- 99. Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 2009; 45: 614– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Burghy CA, Stodola DE, Ruttle PL, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci 2012; 15: 1736– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mandell D, Siegle GJ, Shutt L, Feldmiller J, Thase ME. Neural substrates of trait ruminations in depression. J Abnorm Psychol 2014; 123: 35– 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen AC, Oathes DJ, Chang C, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A 2013; 110: 19944– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci 2012; 6: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Peterson BS, Wang Z, Horga G, et al. Discriminating risk and resilience endophenotypes from lifetime illness effects in familial major depressive disorder. JAMA Psychiatry 2014; 71: 136– 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Halari R, Simic M, Pariante CM, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. J Child Psychol Psychiatry 2009; 50: 307– 16. [DOI] [PubMed] [Google Scholar]
- 106. Strigo IA, Matthews SC, Simmons AN. Right anterior insula hypoactivity during anticipation of homeostatic shifts in major depressive disorder. Psychosom Med 2010; 72: 316– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Manoliu A, Meng C, Brandl F, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci 2013; 7: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 2002; 59: 597– 604. [DOI] [PubMed] [Google Scholar]
- 109. Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry 2007; 61: 231– 9. [DOI] [PubMed] [Google Scholar]
- 110. Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 2008; 165: 90– 8. [DOI] [PubMed] [Google Scholar]
- 111. Zhong M, Wang X, Xiao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol 2011; 88: 233– 42. [DOI] [PubMed] [Google Scholar]
- 112. Suslow T, Konrad C, Kugel H, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 2010; 67: 155– 60. [DOI] [PubMed] [Google Scholar]
- 113. Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 2010; 67: 1128– 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stuhrmann A, Dohm K, Kugel H, et al. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci 2013; 38: 249– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pulcu E, Lythe K, Elliott R, et al. Increased amygdala response to shame in remitted major depressive disorder. PLoS One 2014; 9: e86900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34: 418– 32. [PMC free article] [PubMed] [Google Scholar]
- 117. Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 2004; 8: 539– 46. [DOI] [PubMed] [Google Scholar]
- 118. Herwig U, Brühl AB, Kaffenberger T, Baumgartner T, Boeker H, Jäncke L. Neural correlates of “pessimistic” attitude in depression. Psychol Med 2010; 40: 789– 800. [DOI] [PubMed] [Google Scholar]
- 119. Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169: 693– 703. [DOI] [PubMed] [Google Scholar]
- 120. Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Neural mechanisms of emotion regulation: evidence for two independent prefrontal-subcortical pathways. Neuron 2008; 59: 1037– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Koster EH, De Raedt R, Leyman L, De Lissnyder E. Mood-congruent attention and memory bias in dysphoria: exploring the coherence among information-processing biases. Behav Res Ther 2010; 48: 219– 25. [DOI] [PubMed] [Google Scholar]
- 122. Rochat L, Billieux J, Van der Linden M. Difficulties in disengaging attentional resources from self-generated thoughts moderate the link between dysphoria and maladaptive self-referential thinking. Cogn Emot 2012; 26: 748– 57. [DOI] [PubMed] [Google Scholar]
- 123. Vanderhasselt MA, Kühn S, De Raedt R. Healthy brooders employ more attentional resources when disengaging from the negative: an event-related fMRI study. Cogn Affect Behav Neurosci 2011; 11: 207– 16. [DOI] [PubMed] [Google Scholar]
- 124. Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry 2014; 20: 24. [DOI] [PubMed] [Google Scholar]
- 125. Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 2011; 21: 1379– 88. [DOI] [PubMed] [Google Scholar]
- 126. Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev 2013; 37: 2529– 53. [DOI] [PubMed] [Google Scholar]
- 127. Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251: E1– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Smoski MJ, Keng SL, Schiller CE, Minkel J, Dichter GS. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. J Affect Disord 2013; 151: 171– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci 2010; 30: 15726– 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007; 27: 8877– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dillon DG, Pizzagalli DA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Res 2013; 212: 99– 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schiller CE, Minkel J, Smoski MJ, Dichter GS. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord 2013; 151: 756– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nixon NL, Liddle PF, Worwood G, Liotti M, Nixon E. Prefrontal cortex function in remitted major depressive disorder. Psychol Med 2013; 43: 1219– 30. [DOI] [PubMed] [Google Scholar]
- 134. Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012; 7: 595– 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76: 517– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry 2013; 70: 1181– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yoshimura S, Okamoto Y, Onoda K, et al. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Soc Cogn Affect Neurosci 2014; 9: 487– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res 2011; 45: 577– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Anthes E. Depression: a change of mind. Nature 2014; 515: 185– 7. [DOI] [PubMed] [Google Scholar]
- 140. Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Soc Cogn Affect Neurosci 2013; 8: 56– 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kirk U, Gu X, Harvey AH, Fonagy P, Montague PR. Mindfulness training modulates value signals in ventromedial prefrontal cortex through input from insular cortex. Neuroimage 2014; 100: 254– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Castrén E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry 2013; 70: 983– 9. [DOI] [PubMed] [Google Scholar]
- 143. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 2012; 8: 49– 76. [DOI] [PubMed] [Google Scholar]
- 144. Jonathan D, Power Bradley L, et al. Studying brain organization via spontaneous fMRI signal. Neuron 2014; 84: 681– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 2013; 16: 832– 7. [DOI] [PubMed] [Google Scholar]
- 146. Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat Neurosci 2014; 17: 652– 60. [DOI] [PubMed] [Google Scholar]
- 147. Bright MG, Murphy K. Removing motion and physiological artifacts from intrinsic BOLD fluctuations using short echo data. Neuroimage 2013; 64: 526– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012; 59: 431– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 2009; 44: 893– 905. [DOI] [PMC free article] [PubMed] [Google Scholar]


