Abstract
Purpose
Patients with advanced, incurable thyroid cancer not amenable to surgery or radioactive iodine (131I) therapy have few satisfactory therapeutic options. This multi-institutional study assessed the activity and safety of axitinib, an oral, potent, and selective inhibitor of vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3 in patients with advanced thyroid cancer.
Patients and Methods
Patients with thyroid cancer of any histology that was resistant or not appropriate for 131I were enrolled onto a single-arm phase II trial to receive axitinib orally (starting dose, 5 mg twice daily). Objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors was the primary end point. Secondary end points included duration of response, progression-free survival (PFS), overall survival, safety, and modulation of soluble (s) VEGFR.
Results
Sixty patients were enrolled. Partial responses were observed in 18 patients, yielding an ORR of 30% (95% CI, 18.9 to 43.2). Stable disease lasting ≥ 16 weeks was reported in another 23 patients (38%). Objective responses were noted in all histologic subtypes. Median PFS was 18.1 months (95% CI, 12.1 to not estimable). Axitinib was generally well tolerated, with the most common grade ≥ 3 treatment-related adverse event being hypertension (n = 7; 12%). Eight patients (13%) discontinued treatment because of adverse events. Axitinib selectively decreased sVEGFR-2 and sVEGFR-3 plasma concentrations versus sKIT, demonstrating its targeting of VEGFR.
Conclusion
Axitinib is a selective inhibitor of VEGFR with compelling antitumor activity in all histologic subtypes of advanced thyroid cancer.
INTRODUCTION
Thyroid cancer is the 17th most common cancer worldwide; in 2002, the estimated incidence was more than 141,000 cases, more than three quarters of which occurred in women, with an estimated annual mortality of more than 35,000.1 The incidence and mortality rate of thyroid cancer in the United States are increasing for reasons that are unclear but may be a reflection of changing pathologic criteria or improved diagnosis.2 Although prognosis is generally good, with long-term survival rates typically better than 90%, patients who present with or develop advanced or refractory thyroid cancer represent an important health care challenge.3-5
Differentiated thyroid cancers (DTC), including papillary and follicular subtypes, account for 90% of thyroid malignancies, with some variants, particularly Hürthle and insular cell, being associated with more aggressive disease. Thyrocytes can also give rise to anaplastic thyroid cancer, which is rare (2% of thyroid cancers) but carries a poor prognosis.6 Medullary thyroid cancer (MTC) originates from parafollicular, calcitonin-producing cells and accounts for 3% to 5% of thyroid cancers but a disproportionate degree of mortality.
Patients with advanced thyroid cancer can sometimes undergo additional surgery with curative or significant palliative intent. Patients with iodine-avid disease from differentiated thyroid cancer may continue to receive periodic courses of radioactive iodine (131I). Many advanced thyroid cancers will eventually develop lack of iodine avidity, making chemotherapy the only viable option for systemic treatment. Doxorubicin is an approved therapy for incurable thyroid cancer based on response rates of 10% to 37%7,8 but has myelosuppressive and cardiac toxicities. Patients with advanced thyroid cancer that is iodine-refractory or iodine nonavid are either offered doxorubicin or referred for experimental therapies, usually phase I trials, underscoring the absence of useful therapies for this disease.
A common element to thyroid cancers is their associated vascularity, with elevated levels of vascular endothelial growth factor (VEGF) compared with normal thyroid tissue.9-11 Microvessel density is also higher in papillary thyroid cancer than in normal thyroid.12 In human thyroid tumor specimens, VEGF levels are correlated with stage, large tumor size, nodal involvement, extrathyroidal invasion, and distant metastasis.13 VEGF levels in papillary thyroid cancer are also correlated with risk of recurrence and inferior recurrence-free survival.14 It is well known that follicular thyroid cancer metastasizes hematogenously early in the disease process. Finally, increased expression of VEGF-C, which stimulates lymphangiogenesis via VEGF receptor (R) −3, is correlated with lymph node metastases in papillary thyroid cancer.15 Together, these observations support evaluation of axitinib in this disease.
Axitinib (AG-013736) is an oral, potent, and selective inhibitor of VEGFRs 1, 2, and 3. Axitinib was more than 10-fold less potent for inhibiting platelet-derived growth factor receptor beta (PDGFRβ) and c-KIT in cell-based assays. The relative selectivity for VEGFRs has been confirmed in in vivo pharmacokinetic and pharmacodynamic assays,16 with affinities (≥ nM) against other tyrosine kinases such as PDGFRβ and c-KIT reported in abstract form16 and submitted for publication (Dana Hu-Lowe, Pfizer Inc, data on file). Preclinical studies demonstrate that axitinib rapidly and selectively inhibits VEGF-dependent fenestrations and VEGFR-2 and -3 expression in endothelial cells and blocks angiogenesis and tumor blood flow in preclinical tumor models.17-20 Concomitant studies producing similar results by VEGF-Trap support the contention that axitinib exerts its antiangiogenic activity by inhibiting VEGF receptor signaling.17 A phase I trial of 36 patients with advanced solid tumors identified axitinib 5 mg twice daily as the starting clinical dose at which side effects were tolerable.21 Pharmacokinetic analyses showed that axitinib was rapidly absorbed, with peak plasma concentrations 2 to 6 hours after dosing.21 On the basis of the recognized importance of angiogenesis in thyroid cancer and preliminary evidence of antitumor activity, the activity of axitinib was investigated in this phase II trial.
PATIENTS AND METHODS
Patients
Patients with documented advanced thyroid cancer (papillary, follicular, anaplastic, or medullary) aged ≥ 18 years were eligible for this study if they met the following criteria: disease not controlled by 131I, or disease for which 131I is not an appropriate therapy; at least one Response Evaluation Criteria in Solid Tumors (RECIST) –defined target lesion that has not been externally irradiated; Eastern Cooperative Oncology Group performance status of 0 or 1; adequate bone marrow, hepatic, and renal function; and urinary protein less than 2+ by urine dipstick. Major exclusion criteria included the presence of central lung lesions involving major blood vessels; history of hemoptysis; preexisting uncontrolled hypertension defined as more than 140/90 mmHg despite adequate medical therapy; gastrointestinal abnormalities, including inability to take oral medication or malabsorption syndrome; previous treatment with antiangiogenesis agents; need for use of known potent CYP3A4 inhibitors or inducers; and history of malignancy other than thyroid cancer.
The study was approved by the institutional review board at each of the participating centers and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written, informed consent was obtained from all patients before study entry. This trial is registered on the clinical trials site of the United States National Cancer Institute Web site (NCT00094055).
Study Treatment
Axitinib was administered orally at a starting dose of 5 mg twice daily. Patients who tolerated axitinib with no grade 2 or worse treatment-related adverse events according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0) for consecutive 2-week periods could have their dose increased to 7 mg twice daily (and again to 10 mg twice daily), unless blood pressure was more than 150/90 mmHg or the patient was receiving antihypertensive medication. Those patients experiencing CTCAE v3.0 grade 2 toxicity or worse had their dose reduced to 3 mg twice daily (and again to 2 mg twice daily, if necessary). To assess compliance, patients were required to maintain diaries and include missed or changed doses. Axitinib treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent. Subsequent therapy was at the discretion of the investigator.
Assessment
Physical examinations and laboratory tests were performed at baseline and then repeated every 4 weeks. In addition, patients were provided with a blood pressure monitoring device and were instructed to measure their blood pressure twice daily before each dose and to notify their physician if blood pressure was more than 150/100 mmHg.
Tumor assessments were performed at baseline and every 8 weeks using RECIST. Response (complete response/partial response) had to be confirmed at least 4 weeks after first noted. Adverse events were reported and graded according to CTCAE v3.0.
Plasma Soluble Protein Biomarkers
Plasma samples were collected before dosing every 8 weeks (day 1, day 57, day 113, and so on) to assess effects of axitinib on soluble proteins as pharmacodynamic markers of the inhibition of VEGFR-mediated signaling.22 Each soluble protein was analyzed with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). Briefly, (VEGF)-A ELISA assay measures VEGF-A165 and VEGF-A121 isoforms. Soluble (s) VEGFR-2 was quantified with an ELISA that measures the extracellular (soluble) domain of VEGFR-2. Similarly, an ELISA kit that measures the extracellular (soluble) domain of VEGFR-3 and KIT (stem-cell factor receptor) was used. Both sVEGFR-2 and sVEGFR-3 assays were calibrated against recombinant proteins consisting of the full-length extracellular domain of the respective receptors; no cross-reactivity or interference was detected between the two receptors. ELISA assays were run by Alta Analytic (San Diego, CA) under Good Laboratory Practice conditions, and performance specifications of each ELISA were validated for their intended purpose per established guidelines.23
Soluble protein plasma concentrations were analyzed with Microsoft Excel. Differences with P < .05 from baseline results were considered statistically significant (Student's paired t test).
Statistical Analysis of Response to Therapy
Sample size was based on a two-stage Simon minimax design24 to evaluate the null hypothesis that the true objective response rate (ORR) was 5% and the alternative hypothesis that the ORR was ≥ 20%, with a type I error (α) level of 0.10% and type II error (β) of 0.10. Thyroid cancer responds poorly to conventional therapy, and low response rates were set. With this design, there was target accrual of 18 patients in stage 1, with an additional 14 patients to be enrolled in stage 2 if one or more responses were observed. There was a maximum enrollment of an additional 28 patients for further assessment of safety and efficacy if four or more responses were observed in the first and second stages. Duration of response and progression-free survival were assessed, and 95% CIs for the medians were provided. Demographic data were analyzed by summary statistics.
RESULTS
In total, 60 patients were enrolled. The demographics and baseline characteristics are listed in Table 1. The median age of patients was 59 years (range, 26 to 84 years); 47 (78%) of 60 of the study participants were white and 35 (58%) of 60 were male. The most common type of thyroid cancer was papillary, accounting for 30 (50%) of 60 cancers. Almost all patients (56 of 60 patients, 93%) had received previous treatment; the most common previous treatments were surgery (52 of 60 patients, 87%), radiotherapy (49 of 60 patients, 82%), 131I (43 of 60 patients, 72%), and chemotherapy (nine of 60 patients, 15%).
Table 1.
Demographics and Baseline Characteristics (n = 60)
| Characteristic | No. | % | |
|---|---|---|---|
| Age, years | |||
| Median | 59 | ||
| Range | 26-84 | ||
| Race/ethnicity | |||
| White | 47 | 78 | |
| Black | 5 | 8 | |
| Hispanic | 5 | 8 | |
| Asian | 3 | 5 | |
| Male sex | 35 | 58 | |
| ECOG performance status | |||
| 0 | 24 | 40 | |
| 1 | 36 | 60 | |
| Histology | |||
| Papillary | 30 | 50 | |
| Follicular/Hürthle cell variant | 15/11 | 25/18 | |
| Medullary | 11 | 18 | |
| Anaplastic | 2 | 3 | |
| Other* | 2 | 3 | |
| Current disease stage | |||
| IV | 30 | 50 | |
| Recurrent | 30 | 50 | |
| Received previous treatment | 56 | 93 | |
| Surgery | 52 | 87 | |
| Radiotherapy | 49 | 82 | |
| 131I | 43 | 72 | |
| Chemotherapy | 9 | 15 | |
| Investigational therapy | 5 | 8 | |
| Chemotherapy/immunotherapy | 1 | 2 | |
| Immunotherapy, biologic | 1 | 2 | |
| Other | 8 | 13 | |
| Treatment-naive | 4 | 7 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; 131I, radioactive iodine.
One patient each had insular carcinoma and neuroendocrine carcinoma.
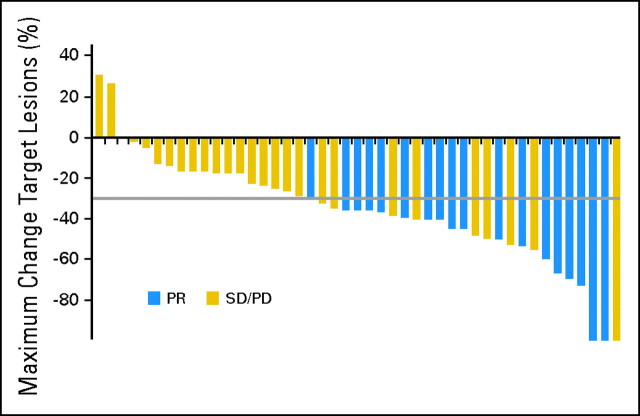
Fifteen patients (25%) were ineligible for response assessment (eight patients did not meet any response criteria, and postbaseline data were missing for seven patients). A partial response was observed in 18 patients, giving an overall ORR of 30% (95% CI, 18.9% to 43.2%) in an intent-to-treat analysis. Response to treatment by investigator assessment is listed in Table 2. Stable disease was reported in an additional 23 patients (38%), and four patients (7%) had progressive disease as best response. There was no apparent association between response rate and histology (Table 3). As shown in Figure 1, most patients experienced some tumor shrinkage during axitinib treatment. The median duration of response has not yet been reached. Of those patients who initially responded, 13 patients (72%) have not yet experienced disease progression and are alive. Of the nine patients treated with prior chemotherapy (of whom seven patients had received prior doxorubicin), a partial response was observed in five patients, with a maximum tumor regression range of 36% to 54%, stable disease in two patients, and progressive disease in two patients (Table 4).
Table 2.
Response to Treatment: Investigator-Assessed Response
| Response | No. | % | |
|---|---|---|---|
| CR | 0 | 0 | |
| PR | 18 | 30 | |
| SD | 23 | 38 | |
| PD | 4 | 7 | |
| IND | 8 | 13 | |
| Missing | 7 | 12 | |
| Objective response rate | 18 | 30 | |
| 95% CI | 18.9 to 43.2 | ||
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; IND, indeterminate (includes eight patients who did not meet any response criteria and seven patients without postbaseline scans).
Table 3.
Response to Treatment by Histology
| Histology | No. of Patients |
||||||
|---|---|---|---|---|---|---|---|
| PR | SD | PD | IND | ||||
| Papillary, n = 30 | 8 | 12 | 2 | 8 | |||
| Follicular, n = 15 | 6 | 7 | 1 | 1 | |||
| Medullary, n = 11 | 2 | 3 | 0 | 6 | |||
| Anaplastic, n = 2 | 1 | 0 | 1 | 0 | |||
| Other, n = 2 | 1 | 1 | 0 | 0 | |||
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; IND, indeterminate (includes eight patients who did not meet any response criteria and seven patients without postbaseline scans).
Fig 1.
Maximum percentage of tumor reduction for target lesions by Response Evaluation Criteria in Solid Tumors. The gray line represents zero change in tumor size. Each bar represents an individual patient. PR, partial response; SD, stable disease; PD, progressive disease.
Table 4.
Response to Treatment in Patients Receiving Prior Chemotherapy
| Patient | Prior Chemotherapy | Treatment Duration (months) | Best Response | Tumor Regression on Axitinib (%) |
|---|---|---|---|---|
| 1 | Doxorubicin | 2 | PR | −54 |
| 2 | Liposomal doxorubicin + docetaxel | 2 | PR | −40 |
| 3 | Irinotecan + thalidomide | 3 | PR | −36 |
| 4 | Doxorubicin followed by single-agent cisplatin | 6/3 | PR | −50 |
| 5 | Ecteinascidin + liposomal doxorubicin | 10 | PR | −54 |
| 6 | Paclitaxel | 4 | SD | −14 |
| 7 | Liposomal doxorubicin | 4 | SD | −42 |
| 8 | Doxorubicin | 2 | PD | 30 |
| 9 | Doxorubicin + cisplatin | 1/unknown | PD | 26 |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease.
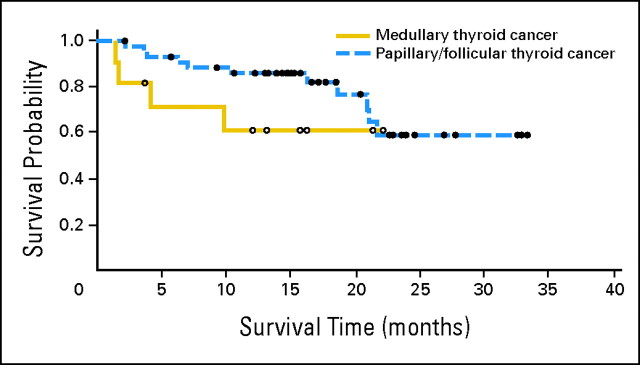
With a median follow-up of 16.6 months (95% CI, 15.0 to 21.2 months), 25 patients (42%) have experienced disease progression or died. The median progression-free survival is 18.1 months (95% CI, 12.1 to not estimable). Forty-two patients (70%) are still alive, and median overall survival has not yet been reached (95% CI, 20.8 to not estimable; Fig 2).
Fig 2.
Kaplan-Meier curve for overall survival in patients with medullary thyroid cancer (solid line) and differentiated thyroid cancer (dashed line). Patients (n = 2) with anaplastic carcinoma or “other” histology (n = 2) were excluded from this analysis.
The median duration of axitinib therapy was 4.8 months (range, 0.07 to 24.5 months). The median daily dose of axitinib was 9.87 mg (range, 2.1 to 13.6 mg), with eight patients being dose-escalated to receive ≥ 7 mg twice per day. In total, 32 patients discontinued axitinib treatment. Of these, 10 patients (17%) discontinued treatment because of lack of efficacy, eight patients (13%) discontinued treatment because of adverse events (including hemoptysis, proteinuria, headache, pericardial effusion, dysphagia, and dyspnea), four patients died of causes related to adverse events (not considered related to study treatment), and the remainder discontinued treatment for other causes. In addition, the axitinib dose was reduced in 23 patients (38%) because of adverse events, most commonly fatigue, hematuria, and diarrhea.
The most common treatment-related adverse events occurring in ≥ 20% of patients are listed in Table 5 and were fatigue (30 of 60 patients, 50%), diarrhea, (29 of 60 patients, 48%), nausea (20 of 60 patients, 33%), anorexia (18 of 60 patients, 30%), hypertension (17 of 60 patients, 28%), stomatitis (15 of 60 patients, 25%), weight decrease (15 of 60 patients, 25%), and headache (13 of 60 patients, 22%). A total of 19 patients (32%) reported at least one grade ≥ 3 treatment-related adverse event. Three patients experienced grade 4 toxicity that resolved in all cases (one case each of stroke, reversible posterior leukoencephalopathy syndrome related to hypertension, and proteinuria).
Table 5.
Summary of Treatment-Related Adverse Events
| Adverse Event | Total |
Grade ≥ 3 |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Fatigue | 30 | 50 | 3 | 5 | ||
| Diarrhea | 29 | 48 | 2 | 3 | ||
| Nausea | 20 | 33 | 0 | 0 | ||
| Anorexia | 18 | 30 | 0 | 0 | ||
| Hypertension | 17 | 28 | 7 | 12 | ||
| Stomatitis | 15 | 25 | 0 | 0 | ||
| Weight decrease | 15 | 25 | 2 | 3 | ||
| Headache | 13 | 22 | 2 | 3 | ||
| Proteinuria | 11 | 18 | 3 | 5 | ||
| Hand-foot syndrome | 9 | 15 | 0 | 0 | ||
| Rash | 9 | 15 | 0 | 0 | ||
| Vomiting | 8 | 13 | 0 | 0 | ||
The effect of axitinib on soluble proteins as exploratory pharmacodynamic markers was also studied. Baseline concentrations of VEGF, sVEGFR-2, sVEGFR-3, and sKIT in blood (mean ± standard deviation) were 69 ± 47 (n = 36), 9,029 ± 1,909 (n = 36), 38,574 ± 13,627 (n = 27), and 51,994 ± 11,227 pg/mL (n = 36), respectively.
Treatment with axitinib led to a 2.8-fold increase in mean VEGF concentrations that generally plateaued by week 12 (P < .0001; data not shown). In contrast, axitinib produced a 32% decrease in mean sVEGFR-2 concentrations (P < .001), 35% decrease in mean sVEGFR-3 concentrations (P < .0001), and 13% decrease in mean sKIT concentrations (P < .01) in blood by week 12 compared with baseline (Fig 3A). The decrease in soluble VEGFR plateaued within the first 12 weeks of treatment and was sustained for the duration of therapy in most patients. Given the paucity of patients with progressive disease, it was not possible to make a definitive assessment of the potential correlation of changes in sVEGFR-2 or sVEGFR-3 with objective response. However, these data demonstrate the selectivity of axitinib for modulating soluble VEGFRs versus sKIT in patients with thyroid cancer.
Fig 3.
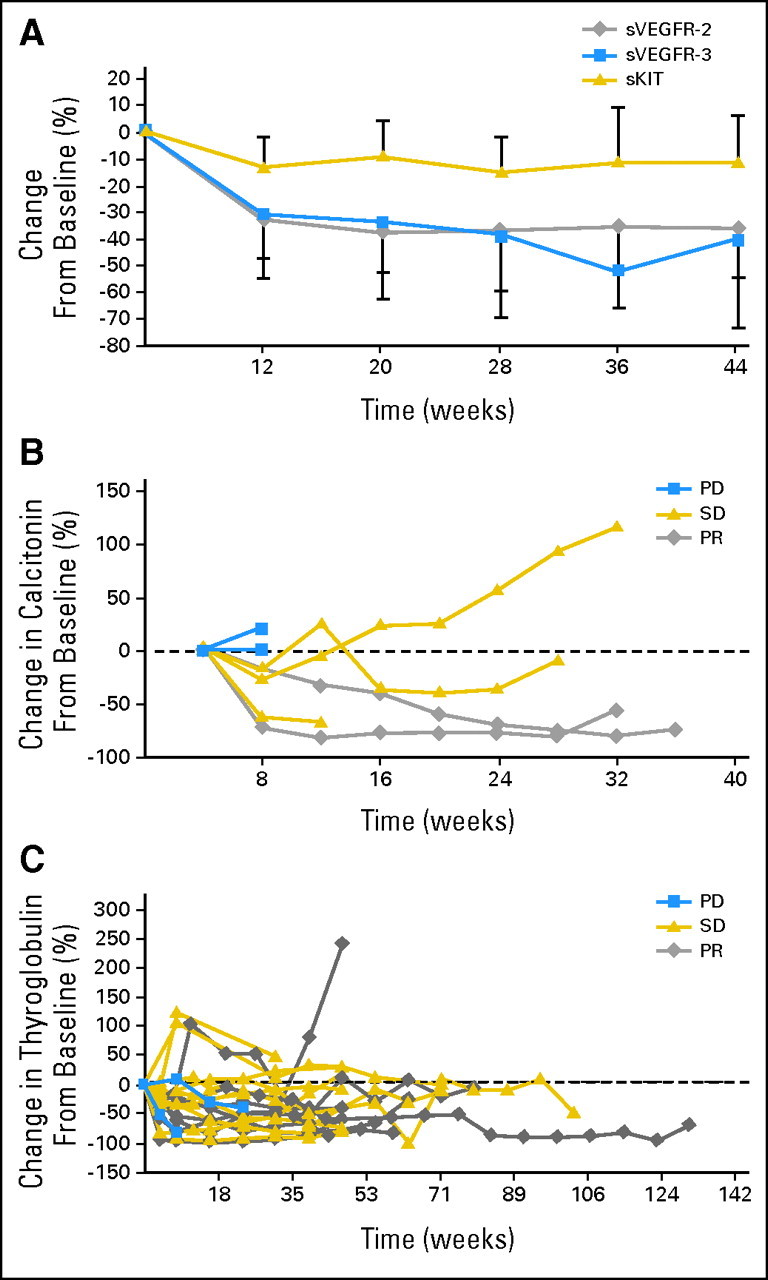
(A) Preferential suppression of soluble vascular endothelial growth factor receptor (sVEGFR) −2 and sVEGFR-3 by axitinib in patients with thyroid cancer. (B) Percentage change in calcitonin from baseline. (C) Percentage change in thyroglobulin from baseline. PD, progressive disease; SD, stable disease; PR, partial response.
Calcitonin and thyroglobin were both measured in subsets of patients in this study. Carcinoembryonic antigen was not measured. Calcitonin was measured in seven patients with MTC: two patients with progressive disease, three patients with stable disease, and two patients with partial response (Fig 3B). The number of patients is small and from this data set show that patients with progressive disease did not have a change in calcitonin concentrations; those with partial response had a marked decrease in calcitonin concentrations, and patients with stable disease had a slight decrease in calcitonin.
Thyroglobulin was measured in two patients with progressive disease, 11 patients with stable disease, and eight patients with partial response (Fig 3C). The preliminary assessment demonstrates that most patients, regardless of their clinical response to therapy, had initial decreases in thyroglobulin. However, because of the small number of patients with progressive disease in this trial, no definitive conclusions could be made for either calcitonin or thyroglobulin with regard to their utility as potential biomarkers of response to axitinib.
DISCUSSION
These results demonstrate that axitinib is active in all histologic subtypes of metastatic thyroid cancer, with an observed ORR of 30%. An additional 38% of patients experienced stable disease for 16 weeks or more by RECIST, and the median progression-free survival time in excess of 18 months also suggests that axitinib is efficacious. These results are notable given that the majority of patients were men and had metastatic disease, which are risk factors for poor prognosis. There clearly exists a subset of patients with refractory, advanced DTC or MTC whose disease will progress slowly, making uncontrolled reports of progression-free survival difficult to interpret. Studies evaluating chemotherapy regimens in either DTC or MTC have observed median progression-free survival durations of 5 to 6 months, suggesting that axitinib therapy would compare favorably in this regard.25,26
In addition to the clinical activity observed, dramatic effects on VEGF, sVEGFR-2, and sVEGFR-3, along with minimal effects on sKIT, indicate that axitinib behaves as a selective VEGFR inhibitor. This finding of minimal effect on sKIT contrasts with sunitinib, where sKIT was found to be markedly decreased.27 The decrease in sVEGFR occurred within the first 12 weeks of treatment and was sustained for the duration of therapy in most patients. Additional studies examining the effects of axitinib on sPDGFR concentrations would further confirm the VEGFR selectivity of the compound in patients with cancer.
In general, treatment with axitinib was well tolerated. Adverse events included fatigue, diarrhea, nausea, anorexia, hypertension, stomatitis, and proteinuria, a side effect profile consistent with data from the phase I study of axitinib and its mechanism of action.21 Hypertension has been observed with other VEGF-targeting agents and underscores the inhibitory effect of axitinib against VEGFRs. Importantly, the patients who experienced hypertension were generally managed with standard antihypertensive medication, and no episodes of hypertension led to permanent study discontinuation. Bleeding, another adverse event associated with anti-VEGF agents, was uncommon (10%), and all events were grade 1.
Other multitargeted tyrosine kinase inhibitors are being studied in advanced thyroid cancer, including motesanib diphosphate28 (an inhibitor of VEGF, PDGF-β, KIT receptors, and RET), sorafenib29 (an inhibitor of Raf, VEGFR, PDGF-β, c-KIT, and RET), and vandetanib30 (ZD6474, an inhibitor of VEGFR and EGFR). The latter is currently being studied in MTC. Although the single-arm design of the present study does not allow our results to be compared directly with historical controls or with results obtained with other agents, the high response rate and substantial duration of effect with axitinib are noteworthy. For example, in a phase II study of motesanib diphosphate in patients with progressive thyroid cancer, an ORR of 12% (by independent review) was reported.28 A small study (19 assessable patients) of sorafenib in thyroid cancer reported a response rate of 26% (all patients except one had papillary thyroid cancer).29 A common mechanistic theme among these agents is inhibition of VEGFR pathways, providing preliminary validation of antiangiogenesis as an approach for the treatment of this disease.
In conclusion, axitinib has significant antitumor activity in all histologic subtypes of thyroid cancer, as evidenced by the high response rate, prolonged duration of response, and overall survival. Modulation of sVEGFR-2 and sVEGFR-3 by axitinib demonstrates the selectivity of this oral inhibitor against VEGFRs. Together with a generally favorable safety profile, the data suggest that axitinib may represent a useful option for a refractory thyroid cancer patient population with few therapeutic options. These results also validate the therapeutic efficacy of VEGFR inhibition in patients with advanced thyroid cancer. Confirmation of these data in a larger trial is needed to further evaluate axitinib in patients with thyroid cancer.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Sinil Kim, Pfizer (C); Paul Bycott, Pfizer (C); Michael Tortorici, Pfizer (C); David R. Shalinsky, Pfizer (C); Katherine F. Liau, Pfizer (C) Consultant or Advisory Role: Everett E. Vokes, Pfizer (C) Stock Ownership: Ezra E.W. Cohen, Pfizer; Sinil Kim, Pfizer; David R. Shalinsky, Pfizer Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Sinil Kim, Paul Bycott, Michael Tortorici
Administrative support: Katherine F. Liau
Provision of study materials or patients: Ezra E.W. Cohen, Lee S. Rosen, Everett E. Vokes, Merrill S. Kies, Arlene A. Forastiere, Francis P. Worden, Madeleine A. Kane, Eric Sherman, Roger B. Cohen
Collection and assembly of data: Ezra E.W. Cohen, Lee S. Rosen, Sinil Kim, Paul Bycott, Michael Tortorici, David R. Shalinsky, Katherine F. Liau
Data analysis and interpretation: Ezra E.W. Cohen, Sinil Kim, Paul Bycott, Michael Tortorici, David R. Shalinsky, Katherine F. Liau, Roger B. Cohen
Manuscript writing: Ezra E.W. Cohen, Everett E. Vokes, Merrill S. Kies, Arlene A. Forastiere, Francis P. Worden, Madeleine A. Kane, Sinil Kim, Paul Bycott, Michael Tortorici, David R. Shalinsky, Katherine F. Liau, Roger B. Cohen
Final approval of manuscript: Ezra E.W. Cohen, Everett E. Vokes, Sinil Kim, Katherine F. Liau, Roger B. Cohen
Acknowledgments
We thank ACUMED (funded by Pfizer) for expert writing assistance, Brian Hee for expert technical support, and Jerry Wu for analytic support. We also thank Charlotte Cione and Christine Jerome.
Footnotes
published online ahead of print at www.jco.org on June 9, 2008.
Supported by Pfizer Inc, La Jolla Laboratories, Clinical Development Department, San Diego, CA (institutional grant support to E.E.W.C., L.S.R., M.S.K., E.S., R.B.C.).
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00094055.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al: Global cancer statistics, 2002. CA Cancer J Clin 55::74,2005-108, [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG: Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 295::2164,2006-2167, [DOI] [PubMed] [Google Scholar]
- 3.Gilliland FD, Hunt WC, Morris DM, et al: Prognostic factors for thyroid carcinoma: A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer 79::564,1997-573, [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2008. CA Cancer J Clin 58::71,2008-96, [DOI] [PubMed] [Google Scholar]
- 5.Sherman SI: Thyroid carcinoma. Lancet 361::501,2003-511, [DOI] [PubMed] [Google Scholar]
- 6.Green LD, Mack L, Pasieka JL: Anaplastic thyroid cancer and primary thyroid lymphoma: A review of these rare thyroid malignancies. J Surg Oncol 94::725,2006-736, [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb JA, Hill CS: Chemotherapy of thyroid cancer with Adriamycin: Experience with 30 patients. N Engl J Med 290::193,1974-197, [DOI] [PubMed] [Google Scholar]
- 8.Shimaoka K, Schoenfeld D, DeWys WD, et al: A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56::2155,1985-2160, [DOI] [PubMed] [Google Scholar]
- 9.Schoenberger J, Grimm D, Kossmehl P, et al: Effects of PTK787/ZK222584, a tyrosine kinase inhibitor, on the growth of a poorly differentiated thyroid carcinoma: An animal study. Endocrinology 145::1031,2004-1038, [DOI] [PubMed] [Google Scholar]
- 10.Bauer AJ, Patel A, Terrell R, et al: Systemic administration of vascular endothelial growth factor monoclonal antibody reduces the growth of papillary thyroid carcinoma in a nude mouse model. Ann Clin Lab Sci 33::192,2003-199, [PubMed] [Google Scholar]
- 11.Viglietto G, Maglione D, Rambaldi M, et al: Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene 11::1569,1995-1579, [PubMed] [Google Scholar]
- 12.Kilicarslan AB, Ogus M, Arici C, et al: Clinical importance of vascular endothelial growth factor (VEGF) for papillary thyroid carcinomas. APMIS 111::439,2003-443, [DOI] [PubMed] [Google Scholar]
- 13.Klein M, Picard E, Vignaud JM, et al: Vascular endothelial growth factor gene and protein: Strong expression in thyroiditis and thyroid carcinoma. J Endocrinol 161::41,1999-49, [DOI] [PubMed] [Google Scholar]
- 14.Lennard CM, Patel A, Wilson J, et al: Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery 129::552,2001-558, [DOI] [PubMed] [Google Scholar]
- 15.Yu XM, Lo CY, Chan WF, et al: Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res 11::8063,2005-8069, [DOI] [PubMed] [Google Scholar]
- 16.Hu-Lowe D, Hallin M, Feeley R, et al: Characterization of potency and activity of the VEGF/PDGF receptor tyrosine kinase inhibitor AG013736. Proc Am Assoc Cancer Res 43::A5357,2002, (abstr) [Google Scholar]
- 17.Inai T, Mancuso M, Hashizume H, et al: Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 165::35,2004-52, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso MR, Davis R, Norberg SM, et al: Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116::2610,2006-2621, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamba T, Tam BY, Hashizume H, et al: VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290::H560,2006-H576, [DOI] [PubMed] [Google Scholar]
- 20.Baffert F, Le T, Sennino B, et al: Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 290::H547,2006-H559, [DOI] [PubMed] [Google Scholar]
- 21.Rugo HS, Herbst RS, Liu G, et al: Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: Pharmacokinetic and clinical results. J Clin Oncol 23::5474,2005-5483, [DOI] [PubMed] [Google Scholar]
- 22.Deprimo SE, Bello CL, Smeraglia J, et al: Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: Modulation of VEGF and VEGF-related proteins. J Transl Med 5::32,2007, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeSilva B, Smith W, Weiner R, et al: Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res 20::1885,2003-1900, [DOI] [PubMed] [Google Scholar]
- 24.Simon R: Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10::1,1989-10, [DOI] [PubMed] [Google Scholar]
- 25.Sturgeon C, Angelos P: Identification and treatment of aggressive thyroid cancers: Part 2. Risk assessment and treatment. Oncology 20::397,2006-404, [PubMed] [Google Scholar]
- 26.Sarlis NJ: Metastatic thyroid cancer unresponsive to conventional therapies: Novel management approaches through translational clinical research. Curr Drug Targets Immune Endocr Metabol Disord 1::103,2001-115, [PubMed] [Google Scholar]
- 27.Blackstein M, Huang X, Demetri GD, et al: Evaluation of soluble KIT (sKIT) as a potential surrogate marker for TTP in sunitinib malate (SU)-treated patients (pts) with advanced GIST. J Clin Oncol 25::546s,2007, (suppl; abstr 10007) [Google Scholar]
- 28.Sherman SI, Schlumberger MJ, Droz J, et al: Initial results from a phase II trial of motesanib diphosphate (AMG 706) in patients with differentiated thyroid cancer. J Clin Oncol 25::303s,2007, (suppl; abstr 6017) [Google Scholar]
- 29.Gupta V, Puttaswamy W, Lassoued W, et al: Sorafenib targets BRAF and VEGFR in metastatic thyroid cancer. J Clin Oncol 25::303s,2007, (suppl; abstr 6019) [Google Scholar]
- 30.Wells S, You YN, Lakhani V, et al: A phase II trial of ZD6474 in patients with hereditary metastatic medullary thyroid cancer. J Clin Oncol 25::303s,2007, (suppl; abstr 6018) [Google Scholar]