Abstract
Purpose
This study aims to assess outcomes and characteristics associated with resection of metastatic renal cell carcinoma (mRCC) to the pancreas.
Materials and Methods
From April 1989 to July 2012, a total of 42 patients underwent resection of pancreatic mRCC at our institution. We retrospectively reviewed records from a prospectively managed database and analyzed patient demographics, comorbidities, perioperative outcomes, and overall survival. Cox proportional hazards models were used to evaluate the association between patient-specific factors and overall survival.
Results
The mean time from resection of the primary tumor to reoperation for pancreatic mRCC was 11.2 years (range, 0–28.0 years). In total, 17 patients underwent pancreaticoduodenectomy, 16 underwent distal pancreatectomy, and 9 underwent total pancreatectomy. Perioperative complications occurred in 18 (42.9 %) patients; there were two (4.8 %) perioperative mortalities. After pancreatic resection, the median follow-up was 7.0 years (0.1–23.2 years), and median survival was 5.5 years (range, 0.4–21.9). The overall 5-year survival was 51.8 %. On univariate analysis, vascular invasion (hazard ratio, 5.15; p=0.005) was significantly associated with increased risk of death.
Conclusions
Pancreatic resection of mRCC can be safely achieved in the majority of cases and is associated with long-term survival. Specific pathological factors may predict which patients will benefit most from resection.
Keywords: Carcinoma, renal cell, Neoplasm metastasis, Pancreatectomy, Treatment outcome
Introduction
Renal cell carcinoma (RCC) is an important cause of mortality in the USA and will account for an estimated 13,680 deaths in 2013.1 For primary RCC, surgical resection by total or partial nephrectomy is the principal treatment modality and offers the possibility of cure. Unfortunately, 25 % of patients diagnosed with RCC have locally advanced or metastatic disease at the time of diagnosis and are therefore not candidates for resection.2 Additionally, of those who undergo surgical resection for localized disease, nearly half will develop metastases later in life.3 Prognosis is poor once metastatic disease has developed, with 5-year survival less than 15 %.3,4
The most common sites of RCC metastases are the lung, bone, liver, brain, and adrenal tissue.5 Another, less common, site of metastatic RCC (mRCC) is the pancreas. While pancreatic metastases of nonrenal neoplasms are generally encountered only in the setting of widespread systemic disease, RCC frequently spreads to the pancreas as an isolated lesion, making it amenable to surgical resection.6,7 In recent years, our institution and others have described this finding in multiple series.8–10 Improved recognition of this unique clinical entity, in combination with advances made in pancreatic surgery over the past several decades,11 has better defined the role of pancreatic resection in treating this patient population.
Previous reports on surgical resection of metastatic RCC, including our own, consist of relatively small series of patients, and our understanding of outcomes remains limited. We have previously reported preliminary findings in 21 patients who underwent pancreatic resection for metastatic RCC. In the present study, we update our experience with pancreatic metastatectomy for renal cell carcinoma in 42 patients—the largest single-institution series to our knowledge. Additionally, with a larger cohort, we attempted to further evaluate clinical and pathological factors associated with long-term survival, aiming to identify patient subgroups that may benefit most from surgical intervention.
Materials and Methods
The Johns Hopkins pancreatic resection database contains prospectively collected data for all pancreatic resections performed at our institution since 1970. Forty-two patients were identified who underwent pancreatectomy for metastatic renal cell cancer spanning from April 1989 to July 2012. This retrospective analysis was approved by the Institutional Review Board at the Johns Hopkins Medical Institutions and complied with Health Insurance Portability and Accountability Act regulations.
All patients had a diagnosis of primary RCC and a pancreatic specimen consistent with metastatic RCC on final pathology. Primary kidney lesions were not reviewed at our institution if resected at an outside institution. Patients with synchronous and metachronous metastases were included in this analysis. For this analysis, we excluded all patients with primary pancreatic malignancies or primary renal tumors that involved the pancreas by direct extension.
Patient demographics, medical comorbidities, biochemical data, and operative and perioperative outcomes were assessed. Delayed gastric emptying, pancreatic fistula, and biliary anastomotic leak were defined as previously reported,12 and complications were classified by the Clavien grading system.13 Perioperative mortality was defined as death during the initial hospitalization or within 30 days of the operative date. Survival was determined from hospital records in conjunction with the US Social Security Death Index.14 Survival data were available for 41 of 42 subjects; one subject left the USA and was censored at the time of last follow-up. No other subjects were lost to follow-up. In patients who did not experience perioperative mortality, survival analysis was performed using the Kaplan–Meier method. Subjects who did not reach an outcome were censored at the time of analysis. Cox proportional hazard analysis was used to estimate hazard ratios based on specific covariates. Multivariate regression models were not created due to the low number of patients and events. A p value of <0.05 was considered statistically significant.
Results
Patient Characteristics
From April 1989 to July 2012, 42 patients underwent pancreatic resection at our institution for mRCC. Patient demographic information and presenting symptoms are listed in Table 1. Twenty-five patients (59.5 %) were male, and median patient age was 66.4 years (range, 32.0 to 86.8). The majority of subjects (54.8 %) were asymptomatic at the time of presentation. Among those with clinical findings, recent weight loss was the most common finding (23.8 %), and abdominal pain (16.7 %) was the most common symptom.
Table 1.
Patient demographics and presentation
| Characteristic | Number (%) |
|---|---|
| Total patients | 42 |
| Age, years | |
| Mean (SD) | 65.0 (10.8) |
| Median (range) | 66.4 (32.0–86.8) |
| Male sex | 25 (59.5 %) |
| White race | 39 (92.9 %) |
| Comorbidities | |
| Hypertension | 21 (50.0 %) |
| Ever tobacco use | 20 (47.6 %) |
| Diabetes | 8 (19.0 %) |
| Patient presentation | |
| Asymptomatic | 23 (54.8 %) |
| Symptomatic (≥1 symptom) | 19 (45.2 %) |
| Weight loss | 10 (23.8 %) |
| Abdominal pain | 7 (16.7 %) |
| Gastrointestinal bleeding | 6 (14.3 %) |
| Nausea/vomiting | 6 (14.3 %) |
| Jaundice | 4 (9.5 %) |
| Fever/chills | 2 (4.8 %) |
| Pancreatitis | 2 (4.8 %) |
Four patients (9.5 %) presented with synchronous primary RCC of the kidney and isolated metastases of the pancreas, while the remaining 38 (90.5 %) patients had metachronous disease. The mean time from nephrectomy to pancreatic resection was 11.2 years (median, 11.5; range, 0–28.0). Of the 38 patients with metachronous disease, eight (21.1 %) had undergone at least one metastatectomy prior to that of the pancreas. Table 2 lists the history of procedures and interval times in these patients.
Table 2.
Patients with previous metastatectomy
| Patient | Time (years): nephrectomy to M1 | Site | Time (years): M1 to M2 | Site | Time (years): M2 to M3 | Site |
|---|---|---|---|---|---|---|
| 1 | 5 | Adrenal | 1 | Pancreas | ||
| 2 | 5 | Lung | 3 | Pancreas | ||
| 3 | 7 | Brain | 10 | Pancreas | ||
| 4 | 11 | Liver | 4 | Pancreas | ||
| 5 | 11 | CL kidney | 2 | Pancreas | ||
| 6 | 17 | CL kidney | 1 | Pancreas | ||
| 7 | 2 | Lung | 8 | Adrenal | 2 | Pancreas |
| 8 | 20 | CL kidney | 6 | Lung, bone | 2 | Pancreas |
M1 first metastatectomy, M2 second metastatectomy, M3 third metastatectomy, CL contralateral to primary RCC site
Operative Details and Complications
Seventeen patients (40.5 %) underwent a pancreaticoduodenectomy, 11 (64.7 %) of which were pylorus preserving. Sixteen patients (38.1 %) underwent a distal pancreatectomy, and nine (21.4 %) had a total pancreatectomy. An en bloc splenectomy was performed with the distal pancreatectomy in 15 of 16 cases. No patients in this cohort underwent laparoscopic procedures. The median length of hospital stay was 8 days for all operations (range—5–57 for pancreaticoduodenectomy, 5–24 for distal pancreatectomy, and 7–28 for total pancreatectomy). The proportion of subjects who experienced one or more complications during their index admission was 46.7 % for pancreaticoduodenectomy, 43.8 % for distal pancreatectomy, and 33.3 % for total pancreatectomy.
Information on postoperative complications is contained in Table 3. The majority of complications were either grade I (no intervention) or grade II (pharmacologic intervention or parenteral nutrition). The most common postoperative complication was delayed gastric emptying (n=6, 14.3 %); three such cases underwent endoscopy (grade IIIa). An additional six patients (14.3 %) had a pulmonary complication. Two reoperations (grade IIIb) were necessary, one due to an ISGPF grade C pancreatic fistula15 and one due to a biliary leak. An additional patient required transfer to the intensive care unit (grade IV) for hypoxemia as a result of aspiration. Two patients died in the immediate postoperative period (grade V). Retrospective review of these cases suggested aspiration leading to cardiac arrest and massive gastrointestinal hemorrhage, most likely resulting from a pseudoaneurysm, were the underlying causes of death, respectively.
Table 3.
Postoperative complications—Clavien grading
| Complication | Total | I | II | IIIa | IIIb | IV | V |
|---|---|---|---|---|---|---|---|
| Delayed gastric emptying | 6 | 1 | 2 | 3 | |||
| Pulmonary | 6 | 4 | 1 | 1 | |||
| Wound complication | 3 | 3 | |||||
| Abscess | 3 | 1 | 2 | ||||
| Bacteremia | 2 | 2 | |||||
| Cardiac | 2 | 2 | |||||
| Hepatic infarct | 1 | 1 | |||||
| Pancreatic fistula | 1 | 1 | |||||
| Biliary leak | 1 | 1 | |||||
| Bleeding | 1 | 1 | |||||
| Total | 26 | 6 | 12 | 3 | 2 | 1 | 2 |
Pathological Characteristics
Detailed pathological information is contained in Table 4. The median tumor size was 3.8 cm (range, 0.8–10.5), and 37 patients (88.1 %) had negative surgical margins (R0). Among distal pancreatectomy specimens, two were found to have a positive proximal pancreatic margin, and one had a positive splenic vein margin. In specimens obtained from pancreaticoduodenectomy, one revealed a positive margin at the uncinate process abutting the superior mesenteric vein, and another had microscopic evidence of tumor at the proximal duodenal margin. Lymph node status was available in 39 patients and positive in 2 (5.1 %). Pathology revealed vascular invasion in 11 patients (26.2 %).
Table 4.
Pathological outcomes
| Factor | N (%) |
|---|---|
| Tumor size (cm) | |
| Mean (SD) | 4.1 (2.2) |
| Median (range) | 3.8 (0.8–10.5) |
| Multifocal pancreatic disease | 18 (42.9 %) |
| R0 margin | 37 (88.1 %) |
| Large vessel invasion | 11 (26.2 %) |
| Lymph node positive | 2 (5.1 %) |
Survival Analysis
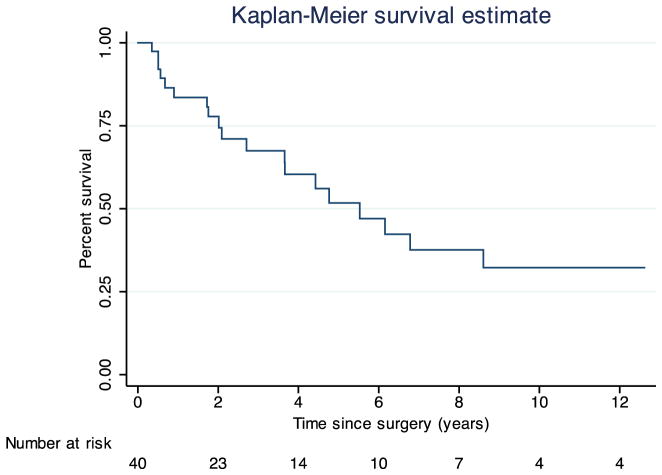
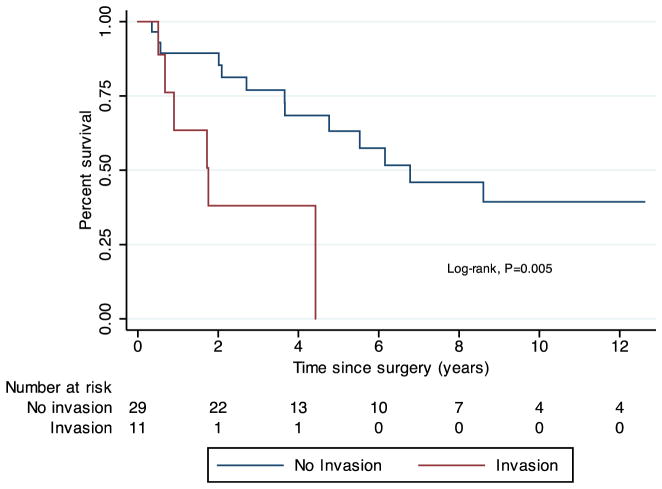
Using the Kaplan–Meier method, the median follow-up after pancreatic resection was 7.0 years (range, 0.1–23.2), and the median survival after resection was 5.5 years (range, 0.4–21.9) (Fig. 1). The 5-year survival rate was 51.8 %. When stratified by the presence of vascular invasion, median survival was 6.2 years in subjects without invasion and 1.8 years in those with invasion (log-rank test of survival, p=0.012) (Fig. 2). Table 5 demonstrates the Cox proportional hazards model for survival. On univariate regression, vascular invasion was a significant predictor of survival (hazard ratio (HR)=5.15, p=0.005). Positive surgical margin status and lymph node involvement were associated with increased hazard ratios for mortality, but these findings were not statistically significant. Presentation with synchronous primary RCC and pancreatic metastases did not significantly predict survival, nor did the time interval from nephrectomy to metastatectomy or history of a prior metastatectomy (Table 5).
Fig. 1.
Kaplan–Meier estimate of overall survival following pancreatectomy. Time zero was defined as the date of pancreatectomy
Fig. 2.
Kaplan–Meier estimates of overall survival following pancreatectomy in subjects with and without vascular invasion on final surgical pathology. Time zero was defined as the date of pancreatectomy
Table 5.
Cox proportional hazards model
| Univariate regression
|
||
|---|---|---|
| HR (95 % CI) | p value | |
| Demographic factors | ||
| Age (per year) | 1.02 (0.98–1.06) | 0.291 |
| Year of surgery | 1.00 (0.92–1.09) | 0.966 |
| Male | 1.82 (0.72–4.64) | 0.208 |
| Presentation | ||
| Synchronous presentation | 0.51 (0.07–3.82) | 0.509 |
| Interval from nephrectomy | 1.01 (0.95–1.07) | 0.738 |
| Previous metastatectomy | 1.80 (0.64–5.07) | 0.269 |
| Asymptomatic | 1.35 (0.54–3.36) | 0.523 |
| Pathological factors | ||
| Multifocal pancreatic disease | 0.85 (0.33–2.15) | 0.727 |
| Tumor size | 1.06 (0.85–1.32) | 0.602 |
| Positive surgical margin | 2.07 (0.67–6.41) | 0.205 |
| LN involvement | 6.62 (0.77–56.72) | 0.085 |
| Vascular invasion | 5.15 (1.64–16.13) | 0.005* |
Discussion
As previously described, RCC appears to have a predilection for metastasizing to the pancreas.5 Based on our data and those of others, pancreatic mRCC represents a unique clinical entity, largely characterized by three presenting features: an extended disease-free interval after initial nephrectomy, with the median interval ranging from 6 to 12 years; frequent discovery in the asymptomatic patient; and the presence of isolated metastasis in the absence of widespread disease.9 These findings emphasize the importance of long-term follow-up after initial nephrectomy, as well as maintaining a high index of suspicion during surveillance.
Outcomes following pancreatic resection of mRCC have been largely favorable. We have previously reported our experience in a series of 21 patients.10 Herein, we have updated this experience and describe 42 patients who have undergone pancreatic resection for mRCC at our institution. Notably, our series demonstrates a perioperative mortality rate (4.8 %) higher than other mRCC cohorts and higher than our overall experience with pancreatectomy. We believe this is secondary to the limited sample size on which we have reported and find no reason to believe that resection of mRCC presents greater mortality risk than resection of other pancreatic lesions. Aggregate data reporting on this phenomenon support this assertion. Our findings are otherwise consistent with other North American series16,17 but differ from some centers, which have reported 5-year survival rates exceeding 75 %.6,9 This discrepancy may be attributable to the duration of follow-up after pancreatic resection. While our median duration of follow-up was 7 years, studies citing higher 5-year survival report median follow-up of less than 3 years, indicating that survival rates were calculated using only a fraction of subjects in already underpowered studies. Another potential cause of observed differences is preoperative risk, as all patients who underwent resection in one series were in the favorable risk group based on the validated scoring scheme from Memorial Sloan-Kettering.9,18,19 Unfortunately, our database did not contain all necessary variables to calculate this risk score in our patient population. Nonetheless, additional study and follow-up will help to clarify long-term survival rates and better establish patient-level factors impacting prognosis.
While resection appears to be associated with positive outcomes, clinical predictors of survival are not well established in this population. Synchronous presentation of primary RCC and pancreatic metastasis is a rare phenomenon and occurred in only four (9.5 %) patients in this series. Our findings were consistent with accumulated data which have observed no relationship between synchronous disease and survival.20 In subjects with metachronous presentation, the mean interval from nephrectomy to pancreatic recurrence was 11.2 years. As demonstrated in composite data from previous reports, the disease-free interval from nephrectomy to pancreatic metastatectomy was not predictive of overall survival.5 While others have reported a trend toward increased survival in asymptomatic patients,21 we found no such association.
The biological factors that differentiate RCC of the pancreas from that of other sites remain unclear. Nonetheless, the behavior of renal cell tumors which reach the pancreas is encouraging. In fact, pancreatic metastatectomy compares favorably even to pulmonary metastatectomy, which is standard practice for mRCC of the lung.5,22 Even considering only those tumors confined to the pancreas, the biology of mRCC appears variable, as up to half of patients will have multiple foci of pancreatic disease.21,23 Accordingly, we observed multifocal disease in 43 % of patients. Also consistent with previous reports, we did not observe a significant relationship between multifocality and overall survival. This finding may support the notion that multifocal pancreatic RCC is not indicative of impending widespread metastases and should not preclude the possibility of resection.
The mechanism by which RCC reaches the pancreas is controversial, and both hematogenous and lymphatogenous spread have been considered. As we have previously described, there appears to be no relationship between the primary site of disease (i.e., right or left kidney) and the localization of pancreatic metastases (i.e., head, neck, tail). This may further support a hematogenous mechanism of metastasis, whereas a strong relationship between primary site and pancreatic localization would support local spread. Notably, we observed lymph node positivity in only 2 (5.1 %) of 39 patients in which lymph node status was assessed, and lymph node positivity has been exceedingly rare in other reports of this population.24 Conversely, vascular invasion was present in 26.2 % of patients. The high rate of vascular invasion and the rarity of lymphatic involvement in this analysis may further support the predilection of RCC to metastasize via the vasculature.
While vascular invasion was the lone significant predictor of outcome in this study, other factors may certainly play a role. However, due to small patient numbers and large confidence intervals, they may not be predictive in current modeling techniques. For instance, lymph node invasion was only present in two patients, one of which experienced a rapid recurrence of disease and demise. Consistent with this, there is evidence that lymphadenectomy may provide therapeutic benefit in some patients.25 In light of these findings, we should reconsider the suggestion that peripancreatic nodal resection is of limited importance in the setting of metastatic RCC. Regardless, nodal status can provide staging information helpful in selecting among new agents for treating metastatic disease.
Nonetheless, vascular invasion is emerging as a very important prognostic factor in the setting of RCC. Pichler et al. recently reported that supplanting the Leibovich score with vascular invasion status improved the ability to predict subsequent mRCC after primary resection.26 In this study, we have found vascular invasion after metastatectomy is a significant predictor of death. Combining insight gained in this report with previous information, such as the Memorial Sloan-Kettering scoring scheme, should allow for better identification of appropriate surgical candidates.
Although we believe this to be the largest single-institution series of pancreatic resection for metastatic RCC, the modest sample size limits our ability to draw more definitive conclusions. In addition, this was not a controlled study in which surgically resected patients were compared to a similar group of patients treated medically, and information regarding adjuvant therapies was not available and therefore was not included in our analysis. On the other hand, the consistency with which clinical and pathological data are measured and recorded at our institution is a notable strength of this study. Ultimately, based on our findings and others, we feel the most important considerations in selecting patients for operative treatment are control of primary cancer site, absence of extrapancreatic disease, resectability of metastases based on local vessel relationships, and the patient’s ability to tolerate pancreatectomy. Factors surrounding presentation such as disease-free interval, multifocality on imaging, and the presence or absence of symptoms seem to have little effect on survival and should not preclude surgery in otherwise good candidates. Pathological factors such as vascular invasion and nodal involvement may be best utilized in counseling patients regarding prognosis following metastatectomy.
Conclusions
Renal cell cancer metastatic to the pancreas is a unique clinical entity and necessitates long-term follow-up after treatment of primary RCC. Our findings indicate that pancreatic resection of metastatic RCC can be safely and feasibly achieved in the majority of cases and is associated with long-term survival. In light of advancements in systemic therapy for RCC, continued reporting of surgical outcomes will be necessary to better define the role of surgery in the management of metastatic RCC.
Contributor Information
J. J. Tosoian, The Department of Surgery, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, 604 Blalock Building, 600 N. Wolfe Street, Baltimore, MD 21287, USA. The James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Park Building 223, 600 N. Wolfe Street, Baltimore, MD 21287, USA
J. L. Cameron, The Department of Surgery, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, 604 Blalock Building, 600 N. Wolfe Street, Baltimore, MD 21287, USA
M. E. Allaf, The James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Park Building 223, 600 N. Wolfe Street, Baltimore, MD 21287, USA
R. H. Hruban, The Department of Pathology, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, Baltimore, MD, USA. Department of Oncology, Johns Hopkins Medical Institutions, Sol Goldman Pancreatic Research Center, Baltimore, MD, USA
C. B. Nahime, The Department of Surgery, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, 604 Blalock Building, 600 N. Wolfe Street, Baltimore, MD 21287, USA
T. M. Pawlik, The Department of Surgery, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, 604 Blalock Building, 600 N. Wolfe Street, Baltimore, MD 21287, USA. Department of Oncology, Johns Hopkins Medical Institutions, Sol Goldman Pancreatic Research Center, Baltimore, MD, USA
P. M. Pierorazio, The James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Park Building 223, 600 N. Wolfe Street, Baltimore, MD 21287, USA
S Reddy, The Department of Surgery, The University of Alabama at Birmingham, The Kirklin Clinic, 2000 6th Avenue South, Birmingham, AL 35233, USA.
C. L. Wolfgang, Email: cwolfga2@jhmi.edu, The Department of Surgery, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, 604 Blalock Building, 600 N. Wolfe Street, Baltimore, MD 21287, USA. The Department of Pathology, Johns Hopkins Medical Institutions Sol Goldman Pancreatic Research Center, Baltimore, MD, USA. Department of Oncology, Johns Hopkins Medical Institutions, Sol Goldman Pancreatic Research Center, Baltimore, MD, USA
References
- 1.American Cancer Society. Cancer Facts and Figures 2013. Atlanta: GACS; 2013. [Last accessed March 10, 2013]. Available online. [Google Scholar]
- 2.Campbell SC, Flanigan RC, Clark JI. Nephrectomy in metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:363–372. doi: 10.1007/s11864-003-0037-4. [DOI] [PubMed] [Google Scholar]
- 3.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287–293. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]
- 6.Law CH, Wei AC, Hanna SS, Al-Zahrani M, Taylor BR, Greig PD, Langer B, Gallinger S. Pancreatic resection for metastatic renal cell carcinoma: presentation, treatment, and outcome. Ann Surg Oncol. 2003;10:922–926. doi: 10.1245/aso.2003.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Lavu H, Yeo CJ. Metastatic renal cell carcinoma to the pancreas. Gastroenterol Hepatol (N Y) 2011;7:699–700. [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn TA, Yeo CJ, Cameron JL, Nakeeb A, Lillemoe KD. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001;5:346–351. doi: 10.1016/s1091-255x(01)80060-3. [DOI] [PubMed] [Google Scholar]
- 9.Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. 2008;15:1161–1168. doi: 10.1245/s10434-007-9782-0. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N, Wolfgang CL. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008;15:3199–3206. doi: 10.1245/s10434-008-0140-7. [DOI] [PubMed] [Google Scholar]
- 11.Lillemoe KD, Yeo CJ, Cameron JL. Pancreatic cancer: state-of-the-art care. CA Cancer J Clin. 2000;50:241–268. doi: 10.3322/canjclin.50.4.241. [DOI] [PubMed] [Google Scholar]
- 12.Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. doi: 10.1097/00000658-199510000-00014. discussion 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Social Security death index (SSDI) Search performed. 2012 Jul 30; Available at: http://ssdi.rootsweb.ancestry.com.
- 15.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Kassabian A, Stein J, Jabbour N, Parsa K, Skinner D, Parekh D, Cosenza C, Selby R. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000;56:211–215. doi: 10.1016/s0090-4295(00)00639-7. [DOI] [PubMed] [Google Scholar]
- 17.Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002;9:675–679. doi: 10.1007/BF02574484. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, Mazumdar M. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 19.Mekhail TM, Abou-Jawde RM, Boumerhi G, Malhi S, Wood L, Elson P, Bukowski R. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 20.Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75–85. doi: 10.1245/ASO.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 21.Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96:579–592. doi: 10.1002/bjs.6606. [DOI] [PubMed] [Google Scholar]
- 22.Ferretti S, Fornia S, Ampollini L, Rusca M, Salsi P, Vaglio A, Cortellini P. Lung metastasectomy in patients with renal cell cancer (RCC). A 17-year experience in Parma Hospital. Acta Biomed. 2007;78:41–45. [PubMed] [Google Scholar]
- 23.Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C, Warshaw AL, Ferrone CR. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010;211:749–753. doi: 10.1016/j.jamcollsurg.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerbi A, Pecorelli N. Pancreatic metastases: An increasing clinical entity. World J Gastrointest Surg. 2010;2:255–259. doi: 10.4240/wjgs.v2.i8.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamal JE, Jarrett TW. The current role of lymph node dissection in the management of renal cell carcinoma. Int J Surg Oncol. 2011;2011:816926. doi: 10.1155/2011/816926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Groselj-Strele A, Kampel-Kettner K, Pummer K, Zigeuner R. Prognostic value of the Leibovich prognosis score supplemented by vascular invasion for clear cell renal cell carcinoma. J Urol. 2012;187:834–839. doi: 10.1016/j.juro.2011.10.155. [DOI] [PubMed] [Google Scholar]