Abstract
Purpose
Anthracyclines and concurrent whole-breast irradiation result in prohibitive cutaneous toxicity. We hypothesized that anthracycline-based chemotherapy and concurrent partial breast irradiation (PBI) is safe and conducted a single-arm feasibility trial testing this hypothesis with dose-dense doxorubicin and cyclophosphamide (ddAC).
Patients and Methods
Women with T1-2, N0-1 breast cancer with ≥ 3 mm lumpectomy margins received PBI (40.5 Gy, 15 daily 2.7-Gy fractions) concurrently with the first two of four cycles of ddAC (60 and 600 mg/m2 of doxorubicin and cyclophosphamide, respectively, every 14 days with colony-stimulating support). Primary end points were local and systemic toxicity. Additional systemic therapy was given at the physician's discretion.
Results
Twenty-seven patients enrolled between November 2004 and January 2007, but two patients did not receive protocol therapy (one found with additional local disease and one withdrew consent). Twenty-five women completed all planned PBI. Four (16%) of 25 did not complete all ddAC (febrile neutropenia [FN], n = 2; diverticulitis and neutropenia, n = 1; and social/economic reasons, n = 1). Four among the remaining 21 who completed all ddAC had a cycle delayed (FN, n = 1; acute respiratory illness, n = 1; foot blisters, n = 1; perianal dermatitis, n = 1). There was no grade 3 to 4 anemia or thrombocytopenia. Grade 3 nonhematologic toxicities (none grade 4) occurred in 28% (seven of 25) of patients (nausea/vomiting, n = 3; stomatitis, n = 2; contralateral breast abscess, n = 1; fatigue, n = 1; and cough/bronchospasms, n = 1). The observed rate of ≥ grade 2 skin toxicity was 0% (0 of 25; one-sided 95% CI, 0% to 11%).
Conclusion
PBI with concurrent ddAC is feasible, and local/systemic toxicity is acceptable. Larger studies are warranted to assess long-term locoregional control and late toxicities.
INTRODUCTION
Standard breast-conserving therapy (BCT) is defined as a lumpectomy followed by whole-breast irradiation (WBI).1–3 Unfortunately, fear of toxicity from WBI and the inconvenience of 5 to 7 weeks of daily radiation often cause women who would otherwise be candidates for BCT to choose mastectomy and therefore suffer an unnecessary anatomic loss. Alternative methods are needed to facilitate BCT. One promising alternative is partial breast irradiation (PBI). The goal of PBI is to deliver a therapeutic dose of radiation to the lumpectomy bed and surrounding tissue only. Conceptually, PBI is based on the observation that most local recurrences after traditional BCT arise in the same quadrant as the original tumor, suggesting that WBI may not be necessary.2,4,5 Treatment to the involved area only, PBI, may suffice.6–10 Large, randomized trials in both North America and Europe are assessing the efficacy of PBI. Small studies suggest that PBI may be equally effective as WBI.11,12
In BCT, chemotherapy, combined with radiation, improves local control when compared with radiation alone.2,13 However, controversy exists regarding the optimal sequencing of these modalities.13,14 Concurrent chemotherapy and radiation offer real and potential benefits. Concurrent therapy shortens the overall duration of therapy, allows both treatments to start temporally closer to surgery (theoretically maximizing the benefits of each modality), and potentially improves local control via the radiation-sensitizing effects of chemotherapy.
Concurrent therapy has successfully been achieved with cyclophosphamide, methotrexate and fluorouracil (CMF) and radiation.14–16 Concurrent use of taxanes and WBI seems feasible.17 Anthracycline regimens offer a survival benefit over CMF in the adjuvant setting, but available data suggest prohibitive toxicity if combined with WBI.18 Hoogenard et al19 reported a 30% rate of moist desquamation with the concomitant use of epirubicin and WBI. Fiets et al20 reported a 44% rate of grade 4 radiation dermatitis (RD) when combining an anthracycline with WBI. These authors also reported that the incidence of severe RD doubled when regional lymph nodes were included in the treatment portal. The severe toxicity seen in this and other studies helped to form a core belief in oncology: anthracyclines and radiation cannot be administered concurrently without excessive toxicity.21–23
However, with the advent of PBI and the resulting decrease in breast tissue irradiated, the prohibitive toxicity previously associated with concurrent chemotherapy and breast irradiation may no longer exist. To test this hypothesis we designed and executed a single-institution phase I trial to evaluate the feasibility of PBI with concurrent dose-dense doxorubicin and cyclophosphamide (ddAC). Our primary goal was to evaluate the local and systemic acute and late toxicity of this combination of therapies. This report presents the clinical results of our trial.
PATIENTS AND METHODS
Study Design and Eligibility
Women with stage I to II breast cancer who had a lumpectomy and received a recommendation for adjuvant chemotherapy were considered for trial enrollment. Eligibility criteria included age ≥ 40 years with histologically proven adenocarcinoma of the breast, a tumor ≤ 4.0 cm, ≥ 3.0-mm negative lumpectomy margins, and 0 to three involved axillary lymph nodes. Patients with a positive sentinel node biopsy were required to undergo an axillary node dissection.
Ineligibility criteria included other tumor histologies (eg, squamous cell or sarcoma), active neoplastic disease, current pregnancy, prior therapeutic radiation, or prior malignancy, except for basal cell or squamous cell skin cancer, in situ cervical cancer, or any other malignancy from which the patient has been disease-free for 5 years. Extensive intraductal component, extracapsular extension, and lymphovascular invasion were not exclusion criteria. The study protocol was approved by the Johns Hopkins institutional review board. The study was registered at the National Institutes of Health (www.clinicaltrials.gov No. NCT00278109).
Chemotherapy
Chemotherapy consisted of doxorubicin 60 mg/m2 administered intravenously over 15 minutes and cyclophosphamide 600 mg/m2 over 30 minutes repeated every 14 days for four cycles. On day 2, all patients received a single dose of pegfilgrastim, 6 mg administered subcutaneously, or began a 7- to 10-day course of filgrastim, approximately 5 μg/kg administered subcutaneously. Blood counts were assessed on day 1 of each cycle and not routinely monitored during a cycle in asymptomatic patients. An absolute neutrophil count ≥ 1,000/μL and platelet count more than 100,000/μL were required for continuation of chemotherapy. Decisions about additional systemic chemotherapy and endocrine therapy after completion of PBI and ddAC were made independently by the medical oncologist and the patient.
PBI
PBI began up to 2 days before but no later than day 1 of cycle 1 of ddAC. All patients underwent three-dimensional conformal or intensity-modulated radiation treatment planning, using five to seven noncoplanar photon beams only. The clinical target volume (CTV) was defined by uniformly expanding the lumpectomy cavity, as defined on computed tomography (CT), by 15 mm in all directions. The planning target volume (PTV) was defined by uniformly expanding the CTV by 5 mm. Both the CTV and PTV were limited to 5 mm from the skin surface and the chest wall lung interface. Patients received 15 weekday fractions of 2.7 Gy (40.5 Gy) to the PTV. The dosimetric limitations are listed in Table 1.
Table 1.
Dosimetric Limitations
| Structure | D(vol) | % of Prescribed Dose |
|---|---|---|
| CTV | D100 | 100 |
| PTV | D100 | 95 |
| Ipsilateral lung | D15 | < 30 |
| Heart | D10 | < 5 |
| Nontarget ipsilateral breast | D50 | < 50 |
NOTE. D(vol) is the dose to a volume of tissue as a percentage of the prescribed dose. For example, heart D10 < 5 means 10% of the heart is limited to less than 5% of the prescription dose.
Abbreviations: CTV, clinical target volume; PTV, planning target volume.
End Points and Statistical Analysis
The primary end points were acute and late systemic and local toxicities. Toxicities were defined as acute if they occurred during or less than 6 months after protocol therapy and late if they occurred ≥ 6 months after protocol therapy. The secondary end points were local control and cosmetic outcome. All toxicities were graded according to the Common Terminology Criteria for Adverse Events version 3.0. The sample size was calculated to estimate the incidence of acute or late radiation dermatitis. Based on historical estimates that concurrent anthracycline chemotherapy and breast irradiation resulted in frequent and severe tissue toxicity, we estimated that the use of PBI combined with ddAC would lead to acute grade 4 toxicities in fewer than 40% of patients. A sample size of 42 patients yields 89% power to detect a 50% reduction between the null hypothesis proportion of 0.40 and the alternative hypothesis proportion of 0.20 using a one-sided, exact binomial hypothesis test with a target significance level of .05. Results are demonstrated using a one-sided exact CI. An interim analysis was planned to assess toxicity. If eight or more of the first 20 patients were to have grade 4 RD, then the lower 85% confidence bound would be greater than 20% and thus the study would be terminated.
Cosmetic Evaluation
Because cosmetic outcome after BCT is influenced by both definitive (surgical) and adjuvant therapy (radiation and chemotherapy), we chose to use a modified Harvard cosmetic scale to delineate the cosmetic consequences most likely resulting from adjuvant therapy. In the Harvard cosmetic scale, the untouched contralateral breast serves as a reference. In our modified version, digital images of the ipsilateral, postsurgical breast, before adjuvant therapy, serve as a reference (Table 2). Physicians and nurses, who were not part of the treatment team, scored the cosmetic outcome for each patient by comparing digital images of the treated breast at baseline to images taken weekly during concurrent chemoradiation, biweekly during chemotherapy only, and every 3 to 6 months after protocol therapy.
Table 2.
Modified Harvard-Harris Cosmetic Scale
| Poor | Fair | Good | Excellent |
|---|---|---|---|
| When compared with baseline image, there is marked change in the appearance of the breast involving more than one quarter of the breast tissue. The skin changes are very obvious. There is severe scarring and thickening of the breast. In retrospect, mastectomy would have been a better option. | When compared with baseline image, there is moderate deformity with obvious difference in the size and shape of breast. This change involves one quarter or less of the breast. There is moderate thickening or scar tissue of the skin and the breast and obvious color changes. | When compared with the baseline image, there is mild asymmetry or slight difference in the size or shape of the breast. Mild reddening or darkening of the breast. The thickening or scar tissue within the breast causes only a mild change in the shape. | When compared with the baseline image, there is minimal or no difference in size or shape or consistency of the breast. There may be mild thickening or scar tissue within the breast or skin, but not enough to change the appearance. |
RESULTS
Patient and Treatment Characteristics
Twenty-seven patients signed an informed consent between November 2004 and February 2007. Two patients did not initiate study procedures: one was found to have residual gross disease during treatment planning, and another withdrew consent. Patient and tumor characteristics are detailed inTable 3. The median age was 49 years. The median tumor size was 1.8 cm (range, 0.4 to 3.0 cm). Inverse treatment planning was used in five of 25 patients. The CTV and PTV dosimetric limitations were satisfied in 24 of 25 and 25 of 25 patients, respectively. Nontarget tissue dosimetric limitations were met in all but one instance. In that patient, 15% of the heart (rather than the prescribed 10%) received 5% of the dose. The median follow-up is 27 months (range, 9 to 44 months).
Table 3.
Patient Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| ≤ 45 | 5 | 20 |
| > 45 | 20 | 80 |
| Menopausal status | ||
| Pre | 15 | 60 |
| Post | 10 | 40 |
| Tumor size, cm | ||
| ≤ 2.0 | 20 | 80 |
| > 2.0 | 5 | 20 |
| ER status | ||
| Positive | 12 | 48 |
| Negative | 13 | 52 |
| Positive axillary nodes | ||
| 0 | 17 | 68 |
| 1-3 | 8 | 32 |
| Chemotherapy | ||
| AC | 12 | 48 |
| AC + paclitaxel | 13 | 52 |
| GCSF | ||
| Pegfilgrastim | 21 | 84 |
| Filgrastim | 4 | 16 |
Abbreviations: ER, estrogen receptor; AC, doxorubicin and cyclophosphamide; GCSF, granulocyte colony-stimulating factor.
As planned, an interim analysis of the first 20 assessable patients was performed. It revealed that there was no grade 4 RD. In fact, after an additional five patients, there was no RD ≥ grade 2. Consequently, the research team concluded that our anticipated estimate of 20% toxicity was much higher than that observed in the trial and that strong evidence existed to reject the null hypothesis of 40% toxicity. Specifically, with 25 patients ultimately enrolled and no toxicities, the (two-sided) P value for testing the null hypothesis of a 40% RD toxicity rate is .000004. We then obtained permission from the Johns Hopkins IRB to terminate accrual to the study.
All patients completed PBI, and 84% (21 of 25) completed all planned cycles of ddAC; all 25 patients received at least three cycles of chemotherapy. Reasons for early discontinuation of treatment included grade 3 mucositis and febrile neutropenia in cycle 3 (n = 1), grade 3 nausea/vomiting and febrile neutropenia in cycles 2 and 3 (n = 1), diverticulitis requiring surgical intervention (complicated by repeated wound infections) and neutropenia after cycles 1 and 2 (n = 1), and social circumstances that precluded additional chemotherapy (n = 1).
Of the patients who completed chemotherapy, four patients had chemotherapy treatment delays. All delays were of cycle 4 and ranged from 1 to 2 weeks. One patient's treatment delay was due to a contralateral breast abscess with grade 4 neutropenia. The treatment delays in the remaining three patients were nonhematologic and included perianal dermatitis (grade 2), foot blisters (grade 2), and cough/bronchospasms (grade 3). Of the total number of cycles delivered, 5.3% were delayed.
Hematologic and Nonhematologic Systemic Toxicity
Hematologic and nonhematologic toxicities are listed inTable 4. Grade 3 and 4 absolute neutrophil count toxicity occurred in 16% (four of 25) of the patients. There was no grade 3 or 4 thrombocytopenia or anemia. Grade 3 (none grade 4) nonhematologic toxicities occurred in 28% (seven of 25) of the patients. Nausea and vomiting were the most common toxicities (three of 25) followed by mucositis (two of 25), fatigue (one of 25), cough/bronchospasms (one of 25) and contralateral breast abscess in the setting of grade 4 neutropenia (one of 25).
Table 4.
Systemic Toxicity*
| Toxicity | Grade 3 (No.) | Grade 4 (No.) |
|---|---|---|
| Absolute neutrophil count | 1 | 3 |
| Platelets | 0 | 0 |
| Hemoglobin | 0 | 0 |
| Nausea/vomiting | 3 | 0 |
| Diarrhea | 0 | 0 |
| Stomatitis | 2 | 0 |
| Fatigue | 1 | 0 |
| Cough | 1 | 0 |
| Infection | 1 | 0 |
According to Common Terminology Criteria of Adverse Events version 3.0.
The etiology of the cough/bronchospasms is uncertain. This patient presented with complaints of cough, shortness of breath, and fatigue less than 2 weeks after completing PBI. A CT revealed nodular infiltrates limited to the left lung lingula adjacent to the area that received PBI. After a failed course of corticosteroids and fluconazole, the patient was treated with moxifloxacin hydrochloride and had marked symptomatic improvement with subsequent resolution of the nodular infiltrates previously seen on CT. It is possible that these symptoms were due to a unique form of radiation pneumonitis, given the novelty of the treatment. Because of the patient's improvement with antibiotics, the unusualpresentation, and the small amount of lung affected by radiation (< 8% of the ipsilateral lung received more than 10 Gy), we characterized her illness as an acute respiratory syndrome of unknown etiology.
RD and Cosmetic Outcome
Four patients had no skin changes during the course of therapy and the remaining 21 (84%) exhibited only grade 1 RD in the treated area. No patient had grade 2, 3, or 4 RD (one-sided 95% CI, 0% to 11%; Table 5).
Table 5.
Radiation Dermatitis
| Radiation Dermatitis | No. | % |
|---|---|---|
| No skin changes | 4 | 16 |
| Grade 1 | 21 | 84 |
| Grade 2, 3, or 4* | 0 | 0 |
One-sided 95% CI, 0% to 11%.
Baseline images were compared with images during the last week of radiation. Twenty-four of 25 patients were found to have a good or excellent cosmetic outcome. One patient was graded as having a poor cosmetic outcome as a result of hyperpigmentation in the treated area. (Fig 1A). At 6 months, 24 of 25 (including this one patient) were scored as having good or excellent cosmesis. A 6-month image was not available for another patient. However, an image of the treated breast at 10 months was available and scored as good/excellent when compared with baseline.
Fig 1.
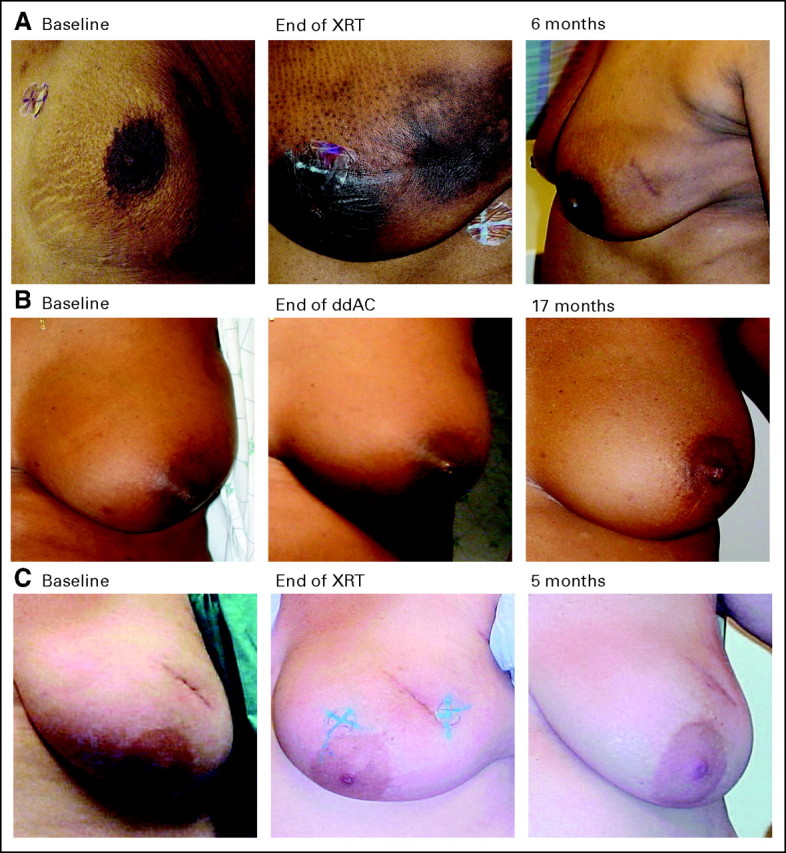
(A) Sole patient with poor cosmetic score at completion of radiation. Image demonstrates only hyperpigmentation. There is no moist desquamation. The cosmetic outcome was later scored as good/excellent at the 6-month follow-up. (B, C) Representative images of the majority of patients studied. XRT, external-beam radiation; ddAC, dose-dense doxorubicin and cyclophosphamide.
Postradiation Toxicity
All 25 patients are free of late skin or lung toxicities, with a median follow-up of 27 months (range, 9 to 44 months). One patient was hospitalized for nonradiating chest pain 42 days after completion of protocol therapy and 7 days after cycle 3 of paclitaxel. Her examination was significant for pleuritic chest pain and “subtle pericardial rub.” An ECG revealed sinus tachycardia, diffuse ST segment elevations, PR segment depressions in lead 2, and PR segment elevations in AVR lead. Cardiac enzymes analysis, echocardiogram, chest x-ray, and CT scan were all within normal limits. She was diagnosed with pericarditis and mild myocarditis, possibly related to the paclitaxel and pegfilgrastim, treated with ibuprofen successfully, then discharged the following day. The final cycle of paclitaxel was discontinued, and the patient has had no other episodes of chest pain. In review of her radiation treatment plan, and based on the free-breathing treatment planning CT scan, less than 0.05% of the cardiac tissue received more than 1 Gy.
DISCUSSION
Prospective randomized trials have established the role of concurrent chemoradiation in the treatment of head and neck, pancreas, rectum, lung, and brain malignancies.15,24–28 Unfortunately, our ability to test the concept of concurrent chemotherapy and radiation in patients undergoing BCT has been limited because of toxicity.15,19,20 Nonetheless, interest remains in this combined modality approach. Two recently published randomized prospective trials examined the role of concurrent chemoradiation in the management of breast cancer. Rouesse et al29 randomly assigned 416 women with node-positive breast cancer to breast and regional nodal irradiation with concurrent fluorouracil, mitoxantrone, and cyclophosphamide (FNC, 500, 12, and 500 mg/m2, respectively, every 21 days for four cycles) or sequential fluorouracil, epirubicin, and cyclophosphamide (500, 60, and 500 mg/m2, respectively, every 21 days for four cycles). In this study, the 5-year locoregional failure rates were 9% and 3% in the sequential and concurrent arms, respectively (P = .01). In separate trial, Toledano et al30 randomly assigned 716 women with stage I and II breast cancer to WBI with concurrent or sequential FNC (500, 12, and 500 mg/m2, respectively, every 21 days for six cycles). In the node-positive subgroup, there was a significant difference in locoregional recurrence-free survival between the sequential and concurrent arms: 91% and 97%, respectively (P = .02). The data suggest that a benefit to concurrent chemotherapy and radiation in breast cancer exists. A potential weakness of both trials is that the chemotherapy regimens, FNC and fluorouracil, epirubicin, and cyclophosphamide, may be considered by some to be outdated or nonstandard. The epirubicin dose of 60 mg/m2 in the Rouesse et al29 trial may be considered low. Regimens using doxorubicin and cyclophosphamide, frequently followed by a taxane, are currently now more common in the United States.
In our trial, we chose to combine one of the most commonly used and potentially radiation-sensitizing chemotherapy regimens, ddAC, with PBI. Also, we chose a PBI fractionation scheme that differs from that in the ongoing National Surgical Adjuvant Breast and Bowel Project B-39/Radiation Therapy Oncology Group 0413 PBI trial. In our trial, patients received 40.5 Gy (2.7 Gy × 15) in external-beam radiation to the lumpectomy bed plus margin. This regimen was initially based on the results of a trial by Whelan et al and later supported by the results of the United Kingdom national Standardization ofRadiotherapy (START) trial B.31,32 Both trials showed, in a randomized controlled fashion, that 2.67 Gy × 16 and 2.67 Gy × 15, respectively, were equivalent to the current WBI standard of 2.0 Gy × 25 with respect to local control. The fractionation scheme in National Surgical Adjuvant Breast and Bowel Project B-39/ Radiation Therapy Oncology Group 0413 (3.85 × 10 twice a day) has not been evaluated in a like manner.
One potential criticism is that our dose of 40.5 Gy (2.7 Gy × 15), which is based on the WBI dose of 50 Gy (2.0 Gy × 25), is insufficient for PBI. In the Boost–No Boost trial, 5,318 patients were randomly assigned to additional radiation (boost) or not after completing 50 Gy of WBI. The authors originally reported a local control benefit in patients who received a boost.33 However, a recent subset analysis on more than 1,700 patients, who underwent central pathologic review revealed that there was no local control benefit associated with a boost in patients with surgical margins ≥ 2 mm.34 Given the results of this subset analysis and the fact that participation in our trial required ≥ 3-mm surgical margins, we remain confident that our fractionation regimen is sufficient.
Our trial results show that PBI with concurrent ddAC has an acceptable hematologic and nonhematologic toxicity profile. The likelihood of RD ≥ grade 2 is ≤ 11% (one-sided 95% CI, 0% to 11%). Our results not only stand in stark contrast to those reported by Fiets et al,20 but also call into question one of the most strongly held oncologic beliefs, that radiation and anthracyclines cannot be administered concurrently without excessive toxicity. These findings have significant implications. They suggest that we can, perhaps for the first time, safely evaluate concurrent versus sequential therapy in early-stage breast cancer using both modern chemotherapy and modern radiation techniques.
Although promising, there are several reasons why this regimen should not be offered outside of a clinical trial. First, PBI has not yet been shown to be equivalent to WBI. Those studies are ongoing. Second, although the toxicity profile in our trial seems to be acceptable, the results are preliminary and based on a small sample size. Finally, 8% to 12% (two to three of 25) of the patients did not complete chemotherapy because of treatment-related toxicities. This percentage may seem higher than expected at first, but review of the literature suggests otherwise. In Cancer and Leukemia Group B 9741, a trial that established the benefit of ddAC, the authors do not explicitly state the percentage of patients who did not complete all four cycles of chemotherapy.35 However, Citron et al35 report that 31% of the patients who received ddAC had at least one cycle delayed and 6% of all cycles were delayed. This compares favorably with 32% and 5.6% in our patients, respectively.
Our trial shows that anthracyclines and PBI can be administered concurrently without acute toxicity. However, verification in a larger trial and longer follow-up are necessary to determine the ultimate cosmetic outcome and late toxicity profile. Presently, we are conducting a feasibility trial evaluating the toxicity of concurrent PBI, with taxane as well as longer anthracycline-based chemotherapy regimens. Our preliminary findings support the development of a larger-scale randomized trial of PBI with concurrent or sequential chemotherapy. This proposed trial will compare not only the safety and cosmetic outcomes of these two schedules but will also test the hypothesis that concurrent chemotherapy and PBI will result in improved breast cancer outcomes.
Acknowledgment
We thank Michele G. Donehower, Stacie Jeter, Pendleton Powers, and Laurie K. Wright for the help in enrolling and monitoring study participants, Gary Chamness for editorial assistance, and Abe Recht for mentorship.
Footnotes
Supported by the Breast Cancer Research Foundation.
Presented at the 49th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 28-November 1, 2007, Los Angeles, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Vered Stearns, Abraxis; Antonio C. Wolff, RocheExpert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Richard C. Zellars, Vered Stearns, Lee Myers, Nancy E. Davidson, Antonio C. Wolff
Administrative support: Richard C. Zellars
Provision of study materials or patients: Richard C. Zellars, Vered Stearns, Deborah Frassica, Fariba Asrari, Theodore Tsangaris, Shirley DiPasquale, Julie R. Lange, Lisa K. Jacobs, Leisha A. Emens, Deborah K. Armstrong, John H. Fetting, Nancy E. Davidson, Antonio C. Wolff
Collection and assembly of data: Richard C. Zellars, Vered Stearns, Deborah Frassica, Fariba Asrari, Theodore Tsangaris, Lee Myers, Shirley DiPasquale, Lisa K. Jacobs, Leisha A. Emens, Deborah K. Armstrong, John H. Fetting, Elizabeth Garrett-Mayer, Nancy E. Davidson, Antonio C. Wolff
Data analysis and interpretation: Richard C. Zellars, Vered Stearns, Lee Myers, Elizabeth Garrett-Mayer, Nancy E. Davidson, Antonio C. Wolff
Manuscript writing: Richard C. Zellars, Vered Stearns, Elizabeth Garrett-Mayer, Nancy E. Davidson, Antonio C. Wolff
Final approval of manuscript: Richard C. Zellars, Vered Stearns, Deborah Frassica, Fariba Asrari, Lee Myers, Julie R. Lange, Lisa K. Jacobs, Deborah K. Armstrong, John H. Fetting, Elizabeth Garrett-Mayer, Nancy E. Davidson, Antonio C. Wolff
REFERENCES
- 1.Clark RM Whelan T Levine M, etal: Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: An update—Ontario Clinical Oncology Group J Natl Cancer Inst 88:1659–1664,1996 [DOI] [PubMed] [Google Scholar]
- 2.Fisher B Anderson S Bryant J, etal: Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer N Engl J Med 347:1233–1241,2002 [DOI] [PubMed] [Google Scholar]
- 3.Forrest AP Stewart HJ Everington D, etal: Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial—Scottish cancer trials breast group Lancet 348:708–713,1996 [DOI] [PubMed] [Google Scholar]
- 4.Liljegren G Holmberg L Adami HO, etal: Sector resection with or without postoperative radiotherapy for stage I breast cancer: Five-year results of a randomized trial—Uppsala-Orebro Breast Cancer Study Group J Natl Cancer Inst 86:717–722,1994 [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U Luini A Del Vecchio M, etal: Radiotherapy after breast-preserving surgery in women with localized cancer of the breast N Engl J Med 328:1587–1591,1993 [DOI] [PubMed] [Google Scholar]
- 6.Vicini FA Remouchamps V Wallace M, etal: Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy Int J Radiat Oncol Biol Phys 57:1247–1253,2003 [DOI] [PubMed] [Google Scholar]
- 7.Vaidya JS Baum M Tobias JS, etal: The novel technique of delivering targeted intraoperative radiotherapy (targit) for early breast cancer Eur J Surg Oncol 28:447–454,2002 [DOI] [PubMed] [Google Scholar]
- 8.Orecchia R Ciocca M Lazzari R, etal: Intraoperative radiation therapy with electrons (ELIOT) in early-stage breast cancer Breast 12:483–490,2003 [DOI] [PubMed] [Google Scholar]
- 9.Orecchia R Luini A Veronesi P, etal: Electron intraoperative treatment in patients with early-stage breast cancer: Data update Expert Rev Anticancer Ther 6:605–611,2006 [DOI] [PubMed] [Google Scholar]
- 10.Benitez PR Chen PY Vicini FA, etal: Partial breast irradiation in breast conserving therapy by way of interstitial brachytherapy Am J Surg 188:355–364,2004 [DOI] [PubMed] [Google Scholar]
- 11.Benitez PR Streeter O Vicini F, etal: Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ: A phase II clinical study Am J Surg 192:427–433,2006 [DOI] [PubMed] [Google Scholar]
- 12.Formenti SC Truong MT Goldberg JD, etal: Prone accelerated partial breast irradiation after breast-conserving surgery: Preliminary clinical results and dose-volume histogram analysis Int J Radiat Oncol Biol Phys 60:493–504,2004 [DOI] [PubMed] [Google Scholar]
- 13.Bellon JR Lindsley KL Ellis GK, etal: Concurrent radiation therapy and paclitaxel or docetaxel chemotherapy in high-risk breast cancer Int J Radiat Oncol Biol Phys 48:393–397,2000 [DOI] [PubMed] [Google Scholar]
- 14.Dubey AK Recht A Come S, etal: Why and how to combine chemotherapy and radiation therapy in breast cancer patients Recent Results Cancer Res 152:247–254,1998 [DOI] [PubMed] [Google Scholar]
- 15.Dubey A Recht A Come SE, etal: Concurrent CMF and radiation therapy for early stage breast cancer: Results of a pilot study Int J Radiat Oncol Biol Phys 45:877–884,1999 [DOI] [PubMed] [Google Scholar]
- 16.Faul C Brufsky A Gerszten K, etal: Concurrent sequencing of full-dose CMF chemotherapy and radiation therapy in early breast cancer has no effect on treatment delivery Eur J Cancer 39:763–768,2003 [DOI] [PubMed] [Google Scholar]
- 17.Formenti SC Volm M Skinner KA, etal: Preoperative twice-weekly paclitaxel with concurrent radiation therapy followed by surgery and postoperative doxorubicin-based chemotherapy in locally advanced breast cancer: A phase I/II trial J Clin Oncol 21:864–870,2003 [DOI] [PubMed] [Google Scholar]
- 18.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials Lancet 365:1687–1717,2005. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) [DOI] [PubMed] [Google Scholar]
- 19.Hoogenraad W, Franssen J, van Turnhout J: Enhanced toxicity of radiotherapy due to epirubicin containing adjuvant chemotherapy in breast carcinoma patients Radiother Oncol 24:S42,1992abstr 158 [Google Scholar]
- 20.Fiets WE van Helvoirt RP Nortier JW, etal: Acute toxicity of concurrent adjuvant radiotherapy and chemotherapy (CMF or AC) in breast cancer patients: A prospective, comparative, non-randomised study Eur J Cancer 39:1081–1088,2003 [DOI] [PubMed] [Google Scholar]
- 21.Boal DK, Newburger PE, Teele RL: Esophagitis induced by combined radiation and adriamycin AJR Am J Roentgenol 132:567–570,1979 [DOI] [PubMed] [Google Scholar]
- 22.Clements IP, Davis BJ, Wiseman GA: Systolic and diastolic cardiac dysfunction early after the initiation of doxorubicin therapy: Significance of gender and concurrent mediastinal radiation Nucl Med Commun 23:521–527,2002 [DOI] [PubMed] [Google Scholar]
- 23.Umsawasdi T Valdivieso M Barkley HT Jr, etal: Esophageal complications from combined chemoradiotherapy (cyclophosphamide + adriamycin + cisplatin + XRT) in the treatment of non-small cell lung cancer Int J Radiat Oncol Biol Phys 11:511–519,1985 [DOI] [PubMed] [Google Scholar]
- 24.Glynne-Jones R, Harrison M: Locally advanced rectal cancer: What is the evidence for induction chemoradiation? Oncologist 12:1309–1318,2007 [DOI] [PubMed] [Google Scholar]
- 25.Kumar S Dimri K Khurana R, etal: A randomised trial of radiotherapy compared with cisplatin chemo-radiotherapy in patients with unresectable squamous cell cancer of the esophagus Radiother Oncol 83:139–147,2007 [DOI] [PubMed] [Google Scholar]
- 26.Schild SE Bonner JA Hillman S, etal: Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95-20-53) J Clin Oncol 25:3124–3129,2007 [DOI] [PubMed] [Google Scholar]
- 27.Sher DJ Henson JW Avutu B, etal: The added value of concurrently administered temozolomide versus adjuvant temozolomide alone in newly diagnosed glioblastoma J Neurooncol 88:43–50,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suntharalingam M: Definitive chemoradiation in the management of locally advanced esophageal cancer Semin Radiat Oncol 17:22–28,2007 [DOI] [PubMed] [Google Scholar]
- 29.Rouëssé J de la Lande B Bertheault-Cvitkovic F, etal: A phase III randomized trial comparing adjuvant concomitant chemoradiotherapy versus standard adjuvant chemotherapy followed by radiotherapy in operable node-positive breast cancer: Final results Int J Radiat Oncol Biol Phys 64:1072–1080,2006 [DOI] [PubMed] [Google Scholar]
- 30.Toledano A Azria D Garaud P, etal: Phase III trial of concurrent or sequential adjuvant chemoradiotherapy after conservative surgery for early-stage breast cancer: Final results of the ARCOSEIN trial J Clin Oncol 25:405–410,2007 [DOI] [PubMed] [Google Scholar]
- 31.Whelan T MacKenzie R Julian J, etal: Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer J Natl Cancer Inst 94:1143–1150,2002 [DOI] [PubMed] [Google Scholar]
- 32.START Trialists' Group Bentzen SM Agrawal RK, etal: The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial Lancet 371:1098–1107,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartelink H Horiot JC Poortmans P, etal: Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation N Engl J Med 345:1378–1387,2001 [DOI] [PubMed] [Google Scholar]
- 34.Jones H Antoni N Colette L, etal: The impact of boost dose and margins on the local recurrence rate in breast conserving therapy: Results from the EORTC boost-no boost trial Presented at the Plenary Session of the American Society of Therapeutic Radiation and Oncology annual meeting October 28-November 1, 2007 Los Angeles, CA abstr [Google Scholar]
- 35.Citron ML Berry DA Cirrincione C, etal: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of intergroup trial C9741/Cancer and leukemia group B trial 9741 J Clin Oncol 21:1431–1439,2003 [DOI] [PubMed] [Google Scholar]


