Abstract
Objective
Intracerebral hemorrhage (ICH) is a devastating disorder with no current treatment. Whether peri-hematomal edema (PHE) is an independent predictor of neurologic outcome is controversial. We sought to determine whether PHE expansion rate predicts outcome after ICH.
Design
Retrospective cohort study
Setting
Tertiary medical center
Patients
139 consecutive supratentorial spontaneous ICH patients >18 years admitted between 2000–2013.
Interventions
None
Measurements and Main Results
ICH, intraventricular hemorrhage (IVH), and PHE volumes were measured from computed tomography (CT) scans obtained at presentation, 24 hours, and 72 hours post-ICH. PHE expansion rate was the difference between initial and follow-up PHE volumes divided by the time interval. Logistic regression was performed to evaluate the relationship between 1) PHE expansion rate at 24 hours and 90-day mortality and 2) PHE expansion rate at 24 hours and 90-day modified Rankin Scale score (mRS). PHE expansion rate between admission and 24-hours post-ICH was a significant predictor of 90-day mortality (OR 2.97, 95%CI 1.48–5.99, p=0.002). This association persisted after adjusting for all components of the ICH score (OR 2.21, 95% CI 1.05–4.64, p=0.04). Similarly, higher 24-hour PHE expansion rate was associated with poorer mRS in an ordinal shift analysis (OR 2.40, 95% CI 1.37–4.21, p=.002), even after adjustment for all ICH score components (OR 2.07, 95% CI 1.12–3.83, p=0.02).
Conclusions
Faster PHE expansion rate 24 hours post-ICH is associated with worse outcome. PHE may represent an attractive translational target for secondary injury after ICH.
Keywords: cerebral hemorrhage, brain edema
INTRODUCTION
Intracerebral hemorrhage (ICH) accounts for 10–15% of all strokes and is the deadliest stroke subtype. (1) A better understanding of ICH pathophysiology has led to interest in therapies that ameliorate secondary injury such as peri-hematomal edema (PHE). (2–4)
PHE results from peri-hemorrhagic inflammation, the toxicity of blood breakdown products, and other secondary processes. (4, 5) Therefore, PHE may cause neurological deterioration due to both biological injury (direct neurotoxicity, perturbations to water/solute homeostasis) and to additional mass effect beyond that of the hematoma. (4, 5) In animal models of ICH, PHE is consistently associated with worse neurological outcome. (6) Currently, however, the relationship between PHE and outcome in ICH patients is controversial, since studies examining this question have shown conflicting results. (7, 8) This critical gap in knowledge severely impedes translation of novel therapies for ICH.
It is widely appreciated that the rate at which a mass expands is critical in determining neurological injury. For example, tissue injury and displacement in a patient with a rapidly evolving acute subdural hematoma often leads to coma and death, whereas a patient with a brain tumor that has developed gradually may have few symptoms. Our aim was to understand the impact of edema formation over time on outcome after ICH. The main hypothesis of the study was that an increased rate of PHE expansion at 24 hours is independently associated with increased mortality and worse functional outcome. We also explored the potential association between PHE formation at 72 hours and functional outcome.
METHODS
Study Design
Subjects were retrospectively identified from an Institutional Review Board-approved prospective cohort study of ICH between 2000 and 2013 at Massachusetts General Hospital. Written informed consent was obtained from participants or their surrogates.
Eligible subjects for the prospective cohort (n = 497) were aged over 18 years and had a diagnosis of primary spontaneous ICH with a known time of ICH onset. (9) When an exact time of onset could not be determined, the time when the subject was last seen normal was substituted for the time of symptom onset. Subjects were admitted within the first 48 hours after symptom onset.
Clinical Data
Demographics and clinical characteristics were obtained by interviewing patients (or their families or surrogates) and reviewing hospital records. Clinical outcomes were 90-day mortality (assessed by telephone by trained study staff and supplemented by regular surveillance of the Social Security Death Index) and 90-day modified Rankin Scale (mRS) score (scale 0–6). (10) When the latter was not obtainable (n = 55), discharge mRS was used.
Primary Analysis
We created a primary analysis population (n = 139) by excluding infratentorial hemorrhages (n = 63), primary/isolated intraventricular hemorrhages (n = 35), and subjects who underwent subsequent surgery or ventriculostomy placement (n = 198) (Figure 1). Our goal was to exclude confounding variables that could mask the effects of PHE. We additionally excluded warfarin-related hemorrhages (n = 94) because the coagulation pathway may be involved in PHE formation. (11) Of 139 subjects, 110 had computed tomography (CT) scans on admission and ~24 hours post-ICH, 58 had scans on admission and ~72 hours post-ICH, and 71 had scans ~72 hours post-ICH (Table 1). We examined the following PHE variables: absolute PHE, relative PHE (absolute PHE/ICH volume) and PHE expansion rate (calculated as the difference between the initial and the follow-up PHE volumes, divided by the interval of time between the two CT scans). Specifically, we examined PHE expansion rate between admission and 24 hours post-ICH (primary analyses), PHE expansion rate between admission and 72 hours post-ICH, and absolute and relative PHE at 72 hours post-ICH.
Figure 1.
Flow chart of primary analysis population selection. Abbreviation: ICH, intracerebral hemorrhage.
Table 1.
Subject Characteristics
| Primary Analysis Cohort (N = 139) |
Admission and 24 hours post- ICH scans (n = 110) |
Admission and 72 hours post-ICH scans (n = 58) |
72 hours post- ICH scan (n = 71) |
|
|---|---|---|---|---|
| Age, y, mean (SD) | 71.1 (12.8) | 71.8 (12.7) | 72.5 (12.6) | 72.6 (12.5) |
| Male, n (%) | 81 (58.3) | 63 (57.3) | 29 (50) | 39 (54.9) |
| Race, n (%) | ||||
| Caucasian | 117 (84.2) | 94 (85.5) | 46 (79.3) | 59 (83.1) |
| African American | 10 (7.2) | 7 (6.4) | 6 (10.3) | 6 (8.5) |
| Medical History, n (%) | ||||
| Hypertension | 107 (77) | 88 (80) | 47 (81) | 55 (77.5) |
| Diabetes | 32 (23) | 26 (23.6) | 13 (22.4) | 14 (19.7) |
| Medication History, n (%) | ||||
| Anti-hypertensive | 86 (61.9) | 72 (65.5) | 39 (67.2) | 41 (57.7) |
| Statin | 44 (31.7) | 37 (33.6) | 17 (29.3) | 20 (28.2) |
| Clinical Features | ||||
| Systolic BP, mm Hg, mean (SD) | 179 (34.3) | 182 (35.2) | 182 (33.9) | 180 (33.8) |
| GCS score, median (IQR) | 14 (11–15) | 14 (10–15) | 15 (10.8–15) | 15 (11.8–15) |
| Glucose, median (IQR) | 130 (112–154) | 132 (113–155) | 130 (114–161) | 131 (109–154) |
| DNR, n (%) | 30 (21.6) | 30 (27.3) | 15 (25.9) | 14 (19.7) |
| Location of Hematoma, n (%) | ||||
| Lobar | 69 (49.6) | 51 (46.4) | 26 (44.8) | 34 (47.9) |
| Deep | 70 (50.4) | 59 (53.6) | 32 (55.2) | 37 (52.1) |
| Intraventricular extension | 52 (37.4) | 50 (45.5) | 31 (53.4) | 37 (52.1) |
| Volume, mL, median (IQR) | ||||
| Admission ICH | 19 (9.0–40.7) | 16.2 (7.9–50.2) | NA | |
| Follow-up ICH | 19.9 (8.9–47.9) | 16.2 (7.6–46.8) | 15.5 (7.4–45.4) | |
| Admission PHE | 14.1 (7.3–34.3) | 12.8 (6.9–36.6) | NA | |
| Follow-up PHE | 18.4 (8.3–39.5) | 21.2 (11.8–54.4) | 21.2 (11.3–53.2) | |
| Follow-up relative PHE, mean (SD) | NA | NA | 1.6 (0.8) | |
| Time to CT scan, h, median (IQR) | ||||
| Onset to admission scan | 3.3 (1.8–6.1) | 3 (1.6–5.7) | NA | |
| Onset to follow-up scan | 22.5 (15.5–27.1) | 66.9 (60.3–75.8) | 74.3 (63.6–78.6) | |
| Clinical outcomes, n (%) | ||||
| 90-day mortality | 30 (21.6) | 29 (26.4) | 16 (27.6) | 15 (21.1) |
| 90-day mRS > 2 | 107 (77) | 91 (82.7) | 48 (82.8) | 53 (74.6) |
BP indicates blood pressure; DNR, do-not-resuscitate order; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; PHE, peri-hematomal edema
Imaging Analysis
ICH, PHE, and intraventricular hemorrhage (IVH) volumes were determined by a single rater (SU) from CT scans obtained closest to the relevant time-points, using Analyze 11.0 (AnalyzeDirect, Overland Park, KS, USA), as previously described. (12) Briefly, boundaries of the ICH, PHE, and IVH when present were outlined using the edge-detection tool and were adjusted after inspection of the volumes in each of the three orthogonal planes. PHE was defined as areas of hypodensity adjacent to the hemorrhage with the most hypodense region immediately adjacent to the hemorrhage. Additionally, the region of PHE was more hypodense than the same area in the contralateral hemisphere (Figure 2). We previously established excellent interrater reliability for CT-based PHE measurements in 20 subjects from this cohort and determined that measurements were comparable to those obtained on MRI. (12) A second rater (AL) performed PHE measurements 10 additional subjects. The intraclass correlation coefficient for the PHE measurements for these 10 subjects was 0.84 (95% CI 0.66–1.00, p=0.003) which indicates excellent interrater reliability.
Figure 2.
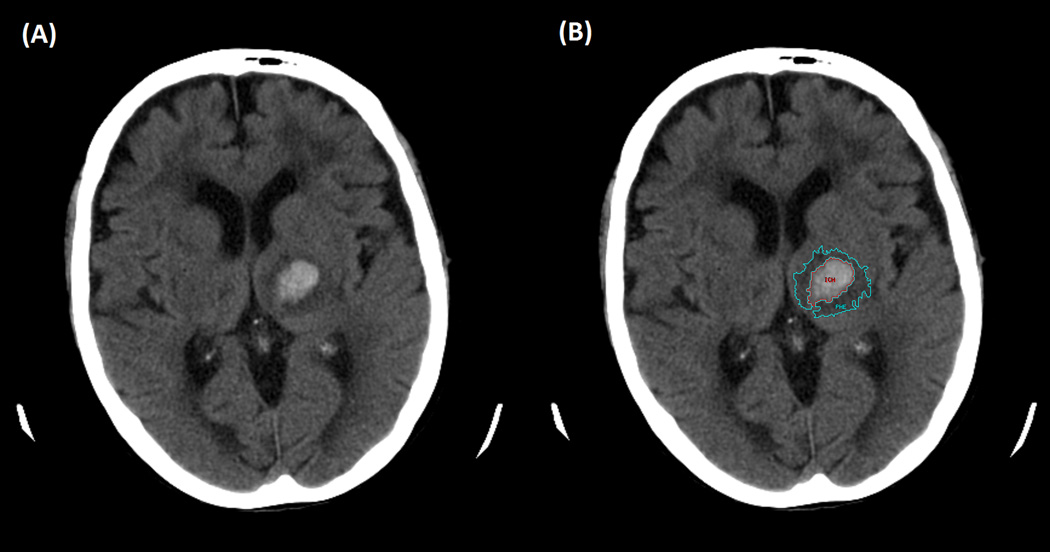
Representative Peri-hematomal Edema Measurement. A) Left thalamic hemorrhage. B) ICH (red) and PHE (cyan) outlined with an edge detection method. (12)
Statistical Analysis
Continuous variables were summarized using means [standard deviations (SDs)] or medians [interquartile ranges (IQRs)] when appropriate. Discrete variables were summarized using counts (percentages). Poor functional outcome was defined as an mRS score > 2. (13)
We examined associations between PHE variables and clinical outcomes in logistic regression models (binary for mortality and poor versus good outcome and ordinal for raw mRS score which assumed a natural ordinal level between each score from 0 to 6). We adjusted for known predictors of outcome following ICH in the following models: 1) All components of the ICH score (14) (age, ICH volume, IVH volume, GCS score, each as a continuous variable); 2) various definitions of hematoma expansion (HE) used in the literature (any HE, HE > 6 mL, > 12.5 mL, > 33% of initial hematoma volume, each as a dichotomous variable); 3) time from onset to first CT scan; and 4) do-not-resuscitate (DNR) status. DNR was defined as any subject who had a DNR order placed during the hospitalization; only one subject had a DNR order prior to the hospitalization for ICH.
All the statistical analyses were performed using SAS version 9.3 (SAS institute, Cary, NC). P-values ≤ 0.05 were considered statistically significant.
RESULTS
Characteristics of the 139 subjects in the primary analysis cohort, those with both admission and 24 hours post-ICH scans (n = 110), those with admission and 72 hours post-ICH scans (n = 58), and those with 72 hours post-ICH scans (n = 71) are shown in Table 1.
PHE expansion rate at 24 hours and absolute PHE expansion were weakly correlated to absolute hemorrhage expansion at 24 hours. (Spearman rank correlation coefficient = 0.22 and 0.34, respectively). Subjects with discharge mRS scores (n=55) were younger and had smaller baseline ICH and PHE volumes than subjects who had 90-day mRS scores (n=84) [67.1 years versus 75.6 years, p=0.01; 12.4 mL versus 23.4 mL, p=0.0004; 9.9 mL versus 20.2 mL, p=0.002; Wilcoxon rank-sum]. Among the 110 subjects in whom PHE expansion rate at 24 hours was calculable, median PHE expansion rate was not statistically different between those with discharge versus 90-day mRS scores.
PHE expansion rate between admission and 24 hours post-ICH
Subjects who died had an average PHE expansion rate of 0.74 mL/h (SE 0.2) compared to 0.17 mL/h (SE 0.1) in survivors (p = 0.0002) (Figure 3A). There was a strong independent association between PHE expansion rate and 90-day mortality (Table 2). Similarly, PHE expansion rate was an independent predictor of higher (worse) mRS score (Table 2). The association for both mortality and functional outcome persisted even after adjustment for baseline ICH severity, as captured in the ICH score (Table 2)
Figure 3.
Average peri-hematomal edema expansion rates. A) Comparison of the average rate of peri-hematomal edema expansion (mL/h) between admission and 24 hours post-intracerebral hemorrhage of subjects who died and subjects who survived. B) Comparison of the average rate of peri-hematomal edema expansion (mL/h) between admission and 72 hours post-intracerebral hemorrhage of subjects with a poor functional outcome and functionally independent subjects. Abbreviations: mRS, modified Rankin Scale; PHE, peri-hematomal edema
Table 2.
Effect of peri-hematomal edema expansion rate between admission and 24 hours post-intracerebral hemorrhage on clinical outcome determined using logistic regression analysis
| Binary outcome | Ordinal Outcome | |||||
|---|---|---|---|---|---|---|
| Mortality | Poor functional outcome (mRS >2) |
Higher (worse) mRS score | ||||
| Variable(s) | Odd Ratio (95% CI) |
P-value | Odd Ratio (95% CI) |
P-value | Odd Ratio (95% CI) |
P-value |
| PHE rate | 2.97 (1.48–5.99) | 0.002 | 2.63 (0.85–8.33) | 0.09 | 2.40 (1.37–4.21) | 0.002 |
| PHE rate, Age adjusted | 2.86 (1.44–5.69) | 0.003 | 2.56 (0.83–8.33) | 0.10 | 2.32 (1.33–4.04) | 0.003 |
| PHE rate, ICH vol adjusted | 2.23 (1.09–4.57) | 0.03 | 2.86 (0.64–12.5) | 0.17 | 1.94 (1.06–3.55) | 0.03 |
| PHE rate, IVH vol adjusted | 3.10 (1.48–6.49) | 0.003 | 2.78 (0.89–8.33) | 0.08 | 2.59 (1.43–4.68) | 0.002 |
| PHE rate, GCS adjusted | 3.05 (1.51–6.15) | 0.002 | 2.63 (0.84–8.33) | 0.10 | 2.36 (1.36–4.11) | 0.002 |
| PHE rate, All ICH score componentsa adjusted | 2.21 (1.05–4.64) | 0.04 | 3.13 (0.68–14.3) | 0.14 | 2.07 (1.12–3.83) | 0.02 |
| PHE rate, Any HE adjusted | 2.97 (1.46–6.02) | 0.003 | 2.50 (0.80–7.69) | 0.12 | 2.34 (1.34–4.10) | 0.003 |
| PHE rate, HE > 6 mL adjusted | 2.98 (1.40–6.33) | 0.005 | 2.70 (0.81–9.09) | 0.10 | 2.33 (1.30–4.20) | 0.005 |
| PHE rate, HE >12.5 mL adjusted | 2.78 (1.38–5.61) | 0.004 | 2.78 (0.82–9.09) | 0.10 | 2.29 (1.30–4.05) | 0.004 |
| PHE rate, HE > 33% adjusted | 2.59 (1.33–5.06) | 0.005 | 3.33 (0.91–12.5) | 0.07 | 2.20 (1.26–3.85) | 0.006 |
| PHE rate, Time to 1st scan adjusted | 2.91 (1.44–5.87) | 0.003 | 2.63 (0.82–8.33) | 0.10 | 2.33 (1.33–4.08) | 0.003 |
| PHE rate, DNR status adjusted | 3.06 (1.32–7.09) | 0.009 | 2.24 (0.69–7.28) | 0.18 | 2.03 (1.10–3.74) | 0.02 |
ICH score components: Age, ICH volume, IVH volume, GCS
DNR indicates do-not-resuscitate order; GCS, Glasgow Coma Scale; HE, hemorrhage expansion; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; mRS, modified Rankin Scale; OR, odds ratio; PHE, peri-hematomal edema
PHE expansion rate between admission and 72 hours post-ICH
At 72 hours, an exploratory analysis was performed that evaluated the relationship between PHE expansion rate and functional outcome. Using a binary outcome, there was an independent association between PHE expansion rate and poor functional outcome (Table 3). Subjects with a poor functional outcome had an average PHE expansion rate of 0.22 mL/h (SE 0.05) compared to 0.02 mL/h (SE 0.04) in functionally-independent subjects (p = 0.07) (Figure 3B). The relationship was not statistically significant after adjustment for components of the ICH score in the ordinal analysis of mRS.
Table 3.
Effect of peri-hematomal edema expansion rate between admission and 72 hours post-intracerebral hemorrhage on clinical outcome determined using logistic regression analysis
| Binary outcome | Ordinal Outcome | |||||
|---|---|---|---|---|---|---|
| Mortality | Poor functional outcome (mRS >2) |
Higher (worse) mRS score | ||||
| Variable(s) | Odd Ratio (95% CI) |
P-value | Odd Ratio (95% CI) |
P-value | Odd Ratio (95% CI) |
P-value |
| PHE rate | 1.08 (1.00–1.18) | 0.07 | 1.28 (1.01–1.64) | 0.04 | 1.11 (1.01–1.20) | 0.02 |
| PHE rate, Age adjusted | 1.07 (0.99–1.17) | 0.10 | 1.28 (1.01–1.64) | 0.04 | 1.09 (1.01–1.19) | 0.04 |
| PHE rate, ICH vol adjusted | 1.04 (0.95–1.14) | 0.38 | 1.43 (1.02–2.04) | 0.04 | 1.07 (0.98–1.17) | 0.16 |
| PHE rate, IVH vol adjusted | 1.09 (1.00–1.20) | 0.05 | 1.28 (1.01–1.61) | 0.04 | 1.12 (1.03–1.23) | 0.01 |
| PHE rate, GCS adjusted | 1.07 (1.00–1.16) | 0.14 | 1.30 (1.01–1.67) | 0.04 | 1.09 (1.00–1.19) | 0.04 |
| PHE rate, All ICH score componentsa adjusted | 1.01 (0.92–1.11) | 0.85 | 1.54 (1.04–2.22) | 0.03 | 1.06 (0.97–1.15) | 0.21 |
| PHE rate, Any HE adjusted | 1.09 (0.99–1.19) | 0.07 | 1.30 (1.02–1.64) | 0.04 | 1.10 (1.01–1.20) | 0.02 |
| PHE rate, HE > 6 mL adjusted | 1.08 (0.99–1.18) | 0.10 | 1.28 (1.01–1.64) | 0.04 | 1.10 (1.01–1.20) | 0.03 |
| PHE rate, HE >12.5 mL adjusted | 1.05 (0.95–1.16) | 0.35 | 1.28 (1.01–1.64) | 0.05 | 1.08 (0.99–1.18) | 0.10 |
| PHE rate, HE > 33% adjusted | 1.08 (0.99–1.17) | 0.07 | 1.28 (1.00–1.61) | 0.05 | 1.10 (1.01–1.19) | 0.03 |
| PHE rate, Time to 1st scan adjusted | 1.08 (0.99–1.17) | 0.09 | 1.28 (1.02–1.64) | 0.03 | 1.10 (1.01–1.19) | 0.04 |
| PHE rate, DNR status adjusted | 1.09 (0.98–1.21) | 0.13 | 1.60 (1.01–2.53) | 0.04 | 1.09 (1.00–1.19) | 0.05 |
ORs are per 0.04 mL/h increase
ICH score components: Age, ICH volume, IVH volume, GCS
DNR indicates do-not-resuscitate order; GCS, Glasgow Coma Scale; HE, hemorrhage expansion; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; mRS, modified Rankin Scale; OR, odds ratio; PHE, peri-hematomal edema
PHE volume at 72 hours post-ICH
In univariable analysis, absolute PHE volume was significantly associated with 90-day mortality (Table 4). Similarly, absolute PHE was an independent predictor of higher (worse) mRSscore in unadjusted analysis and when adjusting for time to first scan or DNR status (Table 4).
Table 4.
Effect of absolute peri-hematomal edema volume at 72 hours post-intracerebral hemorrhage on clinical outcome determined using logistic regression analysis
| Binary outcome | Ordinal Outcome | |||||
|---|---|---|---|---|---|---|
| Mortality | Poor functional outcome mRS >2) |
Higher (worse) mRS score | ||||
| Variable(s) | Odd Ratio (95% CI) | P-value | Odd Ratio (95% CI) | P-value | Odd Ratio (95% CI) | P-value |
| Absolute PHE volume | 1.03 (1.01–1.05) | 0.008 | 1.01 (0.99–1.03) | 0.19 | 1.02 (1.01–1.04) | 0.005 |
| Absolute PHE volume, Age adjusted | 1.03 (1.01–1.05) | 0.01 | 1.01 (0.99–1.03) | 0.19 | 1.02 (1.01–1.04) | 0.007 |
| Absolute PHE volume, ICH vol adjusted | 1.01 (0.98–1.04) | 0.51 | 0.98 (0.94–1.02) | 0.39 | 1.00 (0.98–1.03) | 0.81 |
| Absolute PHE volume, IVH vol adjusted | 1.03 (1.01–1.05) | 0.01 | 1.01 (0.99–1.03) | 0.23 | 1.02 (1.01–1.04) | 0.004 |
| Absolute PHE volume, GCS adjusted | 1.02 (1.00–1.05) | 0.02 | 1.02 (1.00–1.05) | 0.08 | 1.02 (1.01–1.04) | 0.005 |
| Absolute PHE volume, All ICH score componentsa adjusted | 1.01 (0.97–1.05) | 0.61 | 1.00 (0.95–1.05) | 0.91 | 1.01 (0.98–1.04) | 0.52 |
| Absolute PHE volume, Time to 72 h scan | 1.03 (1.01–1.05) | 0.008 | 1.01 (0.99–1.04) | 0.16 | 1.02 (1.01–1.04) | 0.004 |
| Absolute PHE volume, DNR status | 1.03 (1.01–1.06) | 0.02 | 1.01 (0.99–1.03) | 0.33 | 1.02 (1.00–1.04) | 0.01 |
ICH score components: Age, ICH volume, IVH volume, GCS
DNR indicates do-not-resuscitate order; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; mRS, modified Rankin Scale; OR, odds ratio; PHE, peri-hematomal edema
There was no significant association between relative PHE and clinical outcomes (data not shown).
DISCUSSION
There is robust evidence that secondary brain injury, in the form of PHE, leads to worse outcome in experimental models of ICH. (15, 16) However, whether PHE is an independent predictor of neurologic outcome in human ICH remains controversial, since studies have produced contradictory results. (7, 8, 17) Here we have shown that the rate of PHE expansion predicts outcome after ICH. To our knowledge, this index of edema formation has not been investigated previously. (4) Prior studies have traditionally focused on absolute PHE, relative PHE (absolute PHE/ICH volume), and absolute and relative PHE growth. (7, 8) The main findings of our study are that 1) the rate of PHE expansion between admission and 24 hours post-ICH predicts 90-day mortality, and 2) the rate of PHE expansion between admission and 24 hours post-ICH predicts poor functional outcome, after controlling for known predictors of outcome after ICH.
We investigated the rate of PHE expansion because there are other examples in clinical neurology in which the rate at which a mass expands influences patient outcome. Romero et al. showed that the rate of ICH expansion is an independent risk factor for mortality. (18) Mechanistically, PHE may cause neurological deterioration by exerting both biological injury (e.g. perturbations to water/solute homeostasis) and brain-tissue shifts that compress vital structures. (4, 19) Our results suggest that some studies may not have found an association between PHE and outcome because this parameter was not explored. (4) In support of this, Arima et al. conducted an analysis of 270 patients and showed that absolute growth of edema between admission and 72 hours post-ICH did not independently predict poor outcome (defined as death or dependency at 90 days). (8) However, the investigators subsequently showed that absolute edema growth does independently predict poor outcome in an analysis of 1138 patients. (17) In view of our results, the rate of PHE expansion may be a more robust parameter in ICH studies, capable of discerning the relationship between PHE and clinical outcome in smaller datasets.
The contribution of PHE to patient outcome may depend on the rate of expansion, with PHE that expands more rapidly producing more stress on the system than PHE that accumulates gradually. (20) We found that acutely (i.e. up to 24 hours after the ictus), rapid PHE expansion predicts death. In this analysis, we observed a consistent relationship between PHE rate and outcome at 24 hours but not at 72 hours; however, the number of follow-up scans available at the 72 hour time were limited, and we may have been underpowered to detect an association. The optimal time point for PHE assessment must be further investigated in future studies since this may have implications for potential therapeutic time windows and clinical trial endpoint ascertainment.
Importantly, different biological mechanisms underlie PHE formation during these time frames. (4) Acutely, the blood-brain barrier (BBB) is still intact and ionic edema accumulates due to the transcapillary flux of electrolytes and water from the intravascular to the interstitial compartment. (4) The inflammatory response to ICH then leads to the progressive breakdown of the BBB, resulting in vasogenic edema. (4) Peri-hematomal inflammation peaks by two days after ICH. (21) As has been previously described, edema formation after ICH is likely a multistage process in terms of the pathophysiology. (4) The principle stages of edema formation include ionic edema and progressive vasogenic edema, which includes clot retraction. (4) The current analysis captures the potential effect of edema across all stages but does not identify the relative contributions of rate at each individual stage. Prospective studies should use advanced imaging techniques that provide insights regarding the source of edema formation (ionic versus vasogenic versus cytotoxic).
The findings that a faster PHE expansion rate between admission and 24 hours post-ICH is detrimental is significant from a translational perspective, because it provides a relatively wide window for therapeutic intervention. Most ICH clinical trials conducted to date have focused on reducing hemorrhage expansion, since it is strongly associated with poor outcome. (22) However, most hemorrhage expansion occurs within the first 24 hours after the ictus, limiting the therapeutic window. (23) Targeting PHE post-ICH could be a valuable adjunct therapeutic approach to improve clinical outcome in a larger pool of patients.
We confirmed the findings of a previous investigation that showed that absolute PHE volume at 72 hours post-ICH was independently associated with worse clinical outcome in an ordinal shift analysis of the mRS. (24) Additionally, we showed an independent association between absolute PHE volume at 72 hours post-ICH and mortality. The small sample size in our study likely limits our power to detect an association with poor functional outcome (mRS 3–6).
Our results are particularly relevant now that a better understanding of the pathophysiology of PHE has led to the emergence of potential molecular targets and therapies to ameliorate edema. (4) For example, an early-phase clinical trial showed that an anti-inflammatory agent, fingolimod, given for 72 hours after ICH onset, reduced the progression of PHE and was associated with an increased likelihood of functional independence at 90 days compared with controls. (2) In addition, there is preliminary evidence that the SUR1-TRPM4 (sulfonylurea receptor 1 – transient receptor potential M4 cation) channel is up-regulated after human ICH. (4) This channel contributes to edema formation in various animal models of acute brain injury (e.g. cerebral ischemia) and it is inhibited glyburide, a second-generation sulfonylurea. (25)
A distinctive feature of our study is the selection of an ICH population to evaluate the clinical impact of PHE. We believe that our primary analysis population represents the best cohort in which to reconcile conflicting reports in the literature, because it excludes patients in which the independent contribution of PHE on outcome may be masked due to uniformly high mortality. (4) For example, we excluded patients who underwent ventriculostomy placement because it could represent development of significant IVH leading to acute obstructive hydrocephalus, which could be a confounder. A crucial next step is to determine whether the observed associations are generalizable to other ICH populations (e.g. warfarin-associated hemorrhages), and if there are variations by topography (e.g. basal ganglia versus cortical ICHs) or underlying etiology (e.g. hypertensive, amyloid angiopathy). (4)
Our study has a number of limitations. Given the retrospective design, our imaging analysis was limited to scans available at admission, and closest to 24 and 72 hours after ICH. While this time frame includes the contribution of key pathways implicated in PHE formation (e.g. inflammatory pathways), there is evidence that edema continues to accumulate for 2–3 weeks after ICH. (26, 27) In particular, hemoglobin-degradation products promote delayed vasogenic edema starting three days after ICH. (4, 28) Also, CT scans were obtained at different times in all patients, although we did include models adjusting for the time to scan. Lack of a pre-specified imaging protocol also means there is a possibility of selection bias (e.g. subjects that had CTs at 72 hours post-ICH might have been deteriorating). Additionally, while many retrospective and prospective stroke studies use discharge outcome as the 90 day outcome when 90 day outcome is not available (29), this method may misclassify subjects because a particular subject’s functional status could either improve or get worse. This limitation is further highlighted by the fact that subjects in whom only discharge mRS was available were younger and had smaller hemorrhages with less PHE. Prospective studies, with sufficient power to examine multivariable models and that have careful follow-up will be needed to replicate our results, particularly with regard to functional outcome.
Although our analysis adjusted for key factors implicated in ICH outcome (i.e. the ICH score, various definitions of hemorrhage expansion, DNR orders), important variables were not available due to the retrospective nature of the study. For example, we lacked data on use of osmotherapy, which may modify PHE volume and affect patient outcome. (30, 31,) However, a previous study found that a small number of ICH patients develop high intracranial pressure, suggesting osmotic agents may not be used as widely as in the management of other neurocritical care conditions. (2) DNR status is a known mediator of a potential “self-fulfilling prophecy” in ICH outcomes. In this analysis, even when adjusting for DNR, rate of edema was still associated with poor outcome. Prospective studies will need to perform a survivors’ only analysis of functional outcome and record administration of osmotic therapies in order to understand any potential effects further.
CONCLUSIONS
In conclusion, this study provides evidence that the rate of PHE expansion independently predicts clinical outcomes after ICH. PHE may represent an attractive translational target for this disorder. Future prospective studies with neuroimaging acquired systematically at various time-points post-ICH are warranted to validate these findings.
ACKNOWLEDGEMENTS
S.U. is supported by the Leon Rosenberg, MD Medical Student Research Fund in Genetics (Yale University School of Medicine) and a 2014 Student Scholarship in Cerebrovascular Disease and Stroke (American Heart Association Stroke Council). L.A.B. is supported by the National Institute of Neurological Disorders and Stroke (NINDS; K12-NS049453). W.T.K is supported by NINDS K23NS076597. J.M.S. is supported by grants from the Department of Veterans Affairs (Baltimore; BX001629), the NINDS (NS060801, NS061808), and the National Heart, Lung and Blood Institute (HL082517).
Dr. Urday received support for article research from the National Institutes of Health (NIH). He received funding from Leon Rosenberg, MD Medical Student Research Fund in Genetics (Yale University School of Medicine) and a 2014 Student Scholarship in Cerebrovascular Disease and Stroke (American Heart Association Stroke Council). He disclosed that W.T.K., L.A.B. and K.N.S. are investigators in GAMES-RP, a phase II study of an investigational compound aimed at preventing swelling after large stroke. J.M.S. holds a US patent (7,285,574, Methods for treating neural cell swelling). J.R. has a consulting relationship with Boehringer Ingelheim. Dr. Dai received support for article research from the NIH. His institution received funding from the NIH. Dr. Zhang received support for article research and received funding from the NIH. Dr. Vashkevich received support for article research from the NIH. Dr. Ayres received support for article research from the NIH. Dr. Selim received support for article research from the NIH. Dr. Rosand received support for article research from the NIH. His institution received funding from the NIH. Dr. Kimberly received support for article research from the NIH. Her institution received funding from the American Heart Association; NIH; and Remedy Pharmaceuticals, Inc.
Footnotes
Institution where work was performed: Yale University School of Medicine
Copyright form disclosures:
The other authors declare no competing interests.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 3.Sheth KN, Rosand J. Targeting the immune system in intracerebral hemorrhage. JAMA Neurol. 2014;71:1083–1084. doi: 10.1001/jamaneurol.2014.1653. [DOI] [PubMed] [Google Scholar]
- 4.Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, Simard JM, Sheth KN. Targeting secondary injury in intracerebral haemorrhage-perihaematomal oedema. Nat Rev Neurol. 2015;11:111–122. doi: 10.1038/nrneurol.2014.264. [DOI] [PubMed] [Google Scholar]
- 5.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 7.Appelboom G, Bruce SS, Hickman ZL, Zacharia BE, Carpenter AM, Vaughan KA, Duren A, Hwang RY, Piazza M, Lee K, Claassen J, Mayer S, Badjatia N, Connolly ES., Jr Volume-dependent effect of perihaematomal oedema on outcome for spontaneous intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2013;84:488–493. doi: 10.1136/jnnp-2012-303160. [DOI] [PubMed] [Google Scholar]
- 8.Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Morgenstern LB, Chalmers J, Anderson CS Investigators I. Significance of perihematomal edema in acute intracerebral hemorrhage: The interact trial. Neurology. 2009;73:1963–1968. doi: 10.1212/WNL.0b013e3181c55ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcone GJ, Biffi A, Brouwers HB, Anderson CD, Battey TW, Ayres AM, Vashkevich A, Schwab K, Rost NS, Goldstein JN, Viswanathan A, Greenberg SM, Rosand J. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013;70:988–994. doi: 10.1001/jamaneurol.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojcik NC, Huebner WW, Jorgensen G. Strategies for using the national death index and the social security administration for death ascertainment in large occupational cohort mortality studies. Am J Epidemiol. 2010;172:469–477. doi: 10.1093/aje/kwq130. [DOI] [PubMed] [Google Scholar]
- 11.Levine JM, Snider R, Finkelstein D, Gurol ME, Chanderraj R, Smith EE, Greenberg SM, Rosand J. Early edema in warfarin-related intracerebral hemorrhage. Neurocrit Care. 2007;7:58–63. doi: 10.1007/s12028-007-0039-3. [DOI] [PubMed] [Google Scholar]
- 12.Urday S, Beslow LA, Goldstein DW, Vashkevich A, Ayres AM, Battey TW, Selim MH, Kimberly WT, Rosand J, Sheth KN. Measurement of perihematomal edema in intracerebral hemorrhage. Stroke. 2015;46:1116–1119. doi: 10.1161/STROKEAHA.114.007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B, Hata J, Arima H, Parsons M, Li Y, Wang J, Heritier S, Li Q, Woodward M, Simes RJ, Davis SM, Chalmers J Investigators I. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Fan RM, Chen JL, Wang CM, Zeng YC, Han C, Jiao S, Xia XP, Chen W, Yao ST. Neuroprotective effects of argatroban and c5a receptor antagonist (pmx53) following intracerebral haemorrhage. Clin Exp Immunol. 2014;175:285–295. doi: 10.1111/cei.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Arima H, Wu G, Heeley E, Delcourt C, Zhou J, Chen G, Wang X, Zhang S, Yu S, Chalmers J, Anderson CS Investigators I. Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: Pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke. 2015;46:1009–1013. doi: 10.1161/STROKEAHA.114.007154. [DOI] [PubMed] [Google Scholar]
- 18.Romero JM, Heit JJ, Delgado Almandoz JE, Goldstein JN, Lu J, Halpern E, Greenberg SM, Rosand J, Gonzalez RG. Spot sign score predicts rapid bleeding in spontaneous intracerebral hemorrhage. Emerg Radiol. 2012;19:195–202. doi: 10.1007/s10140-012-1020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 20.Caplan LR. Caplan's stroke : A clinical approach. Philadelphia: Saunders/Elsevier; 2009. [Google Scholar]
- 21.Wu H, Zhang Z, Li Y, Zhao R, Li H, Song Y, Qi J, Wang J. Time course of upregulation of inflammatory mediators in the hemorrhagic brain in rats: Correlation with brain edema. Neurochem Int. 2010;57:248–253. doi: 10.1016/j.neuint.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35:195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi GH, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 24.Sansing LH, Messe SR, Cucchiara BL, Lyden PD, Kasner SE. Anti-adrenergic medications and edema development after intracerebral hemorrhage. Neurocrit Care. 2011;14:395–400. doi: 10.1007/s12028-010-9498-z. [DOI] [PubMed] [Google Scholar]
- 25.Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: A focused review. J Cereb Blood Flow Metab. 2012;32:1699–1717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, Wijman CA. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke. 2011;42:73–80. doi: 10.1161/STROKEAHA.110.590646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- 28.Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2008;39:1165–1170. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriquez GJ, Suri MF. Discharge destination as a surrogate for modified Rankin Scale defined outcomes at 3- and 12-months poststroke among survivors. Arch Phys Med Rehabil. 2012;93:1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner I, Hauer EM, Staykov D, Volbers B, Dorfler A, Schwab S, Bardutzky J. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke. 2011;42:1540–1545. doi: 10.1161/STROKEAHA.110.609479. [DOI] [PubMed] [Google Scholar]
- 31.Ryu JH, Walcott BP, Kahle KT, Sheth SA, Peterson RT, Nahed BV, Coumans JV, Simard JM. Induced and sustained hypernatremia for the prevention and treatment of cerebral edema following brain injury. Neurocrit Care. 2013;19:222–231. doi: 10.1007/s12028-013-9824-3. [DOI] [PubMed] [Google Scholar]
- 32.Kamel H, Hemphill JC., 3rd Characteristics and sequelae of intracranial hypertension after intracerebral hemorrhage. Neurocrit Care. 2012;17:172–176. doi: 10.1007/s12028-012-9744-7. [DOI] [PubMed] [Google Scholar]