Abstract
PD-L1 and PD-1 (PD) pathway blockade is a highly promising therapy and has elicited durable anti-tumor responses and long-term remissions in a subset of patients with a broad spectrum of cancers. How to improve, widen, and predict the clinical response to anti-PD therapy is a central theme in the field of cancer immunology and immunotherapy. Oncologic, immunologic, genetic and biological studies focused on the human cancer microenvironment have yielded significant insight into this issue. In this Review, we focus on tumor microenvironment; evaluate several potential therapeutic response markers including the PD-L1 and PD-1 expression pattern, genetic mutations within cancer cells and neoantigens, cancer epigenetics and effector T cell landscape, microbiota, and their mechanisms of action and roles in shaping, being shaped and/or predicting therapeutic responses. We also discuss a variety of combinations with PD pathway blockade and their scientific rationales for cancer treatment.
Keywords: immune checkpoint, cancer immunotherapy, CTLA-4, PD-1, B7-H1, PD-L1, biomarkers, combination, regulatory T cell, IDO, myeloid derived suppressor cell, macrophage, regulatory T cell, dendritic cell, tumor microenvironment, objective response, cancer stem cell and cancer
Introduction
The tumor microenvironment is the primary location in which tumor cells and the host immune system interact. Characterization of the nature of immune responses in the human cancer microenvironment holds the key to understanding protective tumor immunity and improving and empowering current cancer immunotherapy. Accumulating evidence has revealed that the interaction between tumor cells and the host immune system fosters tumor immune evasion and ultimately results in tumor dissemination, relapse, and metastasis (1-3). Study of different cancer infiltrating immune cell subsets including CD4+Foxp3+ regulatory T cells (Tregs) (4), antigen presenting cells (APCs) (5, 6), myeloid derived suppressor cells (MDSCs) (7) and effector T cell subsets (8-13), and immune signature networks (3) have defined the nature of immune responses in the human cancer microenvironment and have allowed for elucidation of the critical importance of reversing immune suppressive mechanisms, including programmed cell death 1 ligand (PD-L1, B7-H1, CD274) and programmed cell death receptor 1 (PD-1, CD279) pathway (herein PD pathway) blockade (1, 2, 14, 15) to engender potent anti-tumor immunity. The identification of PD-L1 (16, 17), the finding that PD-1 is a receptor for PD-L1 (18), and the demonstration of the expression, regulation, and function of the PD pathway in the human cancer microenvironment (5, 14, 16, 17, 19-23) have provided scientific rationales and direct support for the current clinical application of PD pathway blockade (Table 1).
Table 1.
Examples of clinical trials with PD-1 and PD-L1 blockade
| Target and drug information | Clinical response rate in different cancer types | Phase | Cases | References |
|---|---|---|---|---|
|
Target: PD-1 Name: Nivolumab,Opdivo, BMS-936558, MDX-1106, ONO-4538 Isotype: Humanized IgG4a Source: Bristol-Myers Squibb, Ono Pharmaceuticals |
12.8% in treatment-refractory metastatic melanoma, castrate-resistant prostate cancer, RCC, NSCLC, or CRC | I | 39 | (50) |
| 28% in advanced melanoma, 18% in NSCLC, 27% in RCC | I | 296 | (51) | |
| 40% in melanoma treated with nivolumab + ipilimumab, 20% in nivolumab followed by ipilimumab | I | 86 | (58) | |
| 87% in relapsed or refractory Hodgkin's lymphoma | I | 23 | (56) | |
| 14.5% in refractory NSCLC | II | 117 | (70) | |
| 31.7% in advanced melanoma progressed after anti-CTLA-4 | III | 405 | (65) | |
| 40% in previously untreated melanoma without BRAF mutation | III | 418 | (64) | |
| 17% in previously treated advanced NSCLC | II | 129 | (69) | |
| 29% in previously treated advanced RCC | I | 34 | (71) | |
| 20% in advanced squamous-cell NSCLC | III | 272 | (68) | |
| 57.6% (nivolumab + ipilimumab) vs 19% (ipilimumab) vs 43.7% (nivolumab) in untreated stage III or IV melanoma | III | 945 | (63) | |
|
Target: PD-1 Name: Pembrolizumab, Keytruda, MK-3475, lambrolizumab Isotype: Humanized IgG4 kappa Source: Merck |
38% in melanoma | I | 135 | (57) |
| 26% in Ipilimumab-refractory advanced melanoma | I | 173 | (61) | |
| 63% versus 0% in stage IV NSCLC patients with high and low nonsynonymous mutation burden | I | 29 | (67) | |
| 19.4% in advanced NSCLC | I | 495 | (66) | |
| 40% and 0% in mismatch repair-deficient/proficient CRC | II | 41 | (53) | |
| 33% (pembrolizumab) and 11.9% (ipilimumab) in advanced melanoma | III | 834 | (62) | |
|
Target: PD-1 Name: Pidilizumab or CT-011 Isotype: Humanized IgG1 Source: CureTech Ltd |
51% in diffuse large B-cell Lymphoma (after HSCT) | II | 66 | (54) |
| 66% in relapsed follicular lymphoma | II | 32 | (55) | |
|
Target: PD-L1 Name: MPDL3280A, RG7446 Isotype: Fc-modified human IgG1b Source: Genentech/Roche |
21% overall response rate in advanced incurable cancer NSCLC,SCLC, melanoma, RCC, CRC, gastric cancer, head and neck squamous cell carcinoma, breast cancer, ovarian, pancreatic cancer, uterine cancer, sarcoma, pancreatoduodenal cancer | I | 277 | (49) |
| 52% in metastatic bladder cancer | I | 68 | (47) | |
|
Target: PD-L1 Name: BMS-936559, MDX-1105 Isotype: Fully human IgG4a Source: Bristol-Myers Squibb |
17.3% in melanoma; 11.7% in RCC; 10.2% in NSCLC; 5.9% in ovarian cancer | I | 207 | (48) |
Note: Clinical trials with less than 20 cases or published after October 1, 2015 were not included in the table. Abbreviations: RCC, renal cell carcinoma; NSCLC, non-small-cell lung cancer; CRC, colorectal cancer, HSC, Hematopoietic Stem-Cell Transplantation
B7-H1 was cloned in 1999 (16). PD-1 has been subsequently identified as a counter-receptor for B7-H1 (18), and B7-H1 is therefore also known as PD-L1 to emphasize this receptor–ligand interaction. The expression profile of PD-L1 in human cancers has been previously reviewed (14). In addition to tumor cells (14, 17), high levels of PD-L1 protein expression have been observed in human tumor-associated APCs including tumor environmental dendritic cells (DCs), tumor draining lymph node DCs (5, 24), macrophages (20, 25), fibroblasts (26) and T cells (27, 28). PD-L1 expression can be induced or maintained by many cytokines (5, 17, 29, 30), of which interferon-γ (IFNγ) is the most potent. The association between tumor infiltrating T cells, IFNγ signaling genes, and PD-L1 expression suggests that effector T cell-derived IFNγ contributes to high levels of PD-L1 expression in the tumor microenvironment (31). Immune-induced tumor PD-L1 expression is considered to be an adaptive resistance mechanism for tumor cells in response to immune challenge (31-33). In addition, oncogenic phosphatase and tensin homolog (PTEN) loss results in enhanced PD-L1 expression in glioma (34) and triple negative breast cancer cells (35). In human T cell lymphoma, PD-L1 expression may depend on the expression and enzymatic activity of chimeric nucleophosmin (NPM) and anaplastic lymphoma kinase (ALK) (36). Thus, PD-L1 expression can also be regulated by intrinsic oncogenic pathways.
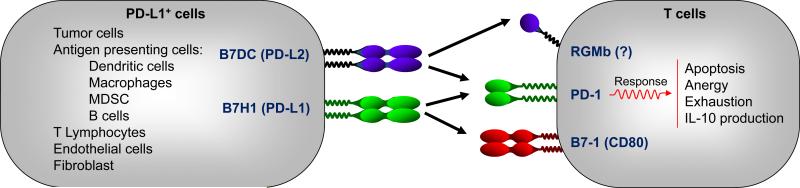
In addition to PD-L1, PD-L2 (B7-DC, CD273) also interacts with PD-1 with similar affinity to deliver a potentially suppressive signal. The immunologic function of this interaction in cancer immunity, however, may not be critical due to relatively rare expression of PD-L2 on cancer cells as well as its interaction with a potentially stimulatory receptor, repulsive guidance molecule b (RGMb) (15)(Figure 1).
Figure 1.
Mechanisms of action of the PD-L1 and PD-1 pathway. Tumor cells, APCs, and other cells express high levels of PD-L1. Engagement of PD-L1+ cells with T cells may induce T-cell apoptosis, anergy, functional exhaustion, or IL-10 production.
PD-1 is absent on resting T cells and was initially found in activated mouse T cells upon TCR engagement (37) and subsequently in exhausted T cells in chronic infection murine models (38, 39). In patients with different types of cancer, high levels of PD-1 expression are detected in tumor infiltrating T cells including tumor antigen-specific T cells, presumably due to chronic antigenic stimulation. These human tumor associated PD-1+ T cells are functionally impaired and their biological activity can be partially recovered with PD-1 or PD-L1 blockade (19-23). A recent study reports that a subset of human melanoma cells express PD-1 and melanoma cell-intrinsic PD-1 promotes melanoma cell growth (40). This finding, however, is inconsistent to previous result that PD-1 expression is predominant on tumor infiltrating lymphocytes but insignificant in melanoma based on immunohistochemistry analysis (41). The role of specific reagents and techniques in detecting PD-1 expression by tumor cells is important to further understand the biologic importance of the data. The magnitude of this surprising finding remains to be prospectively determined in additional studies across tumor types in patients. Thus, PD-L1 and PD-1 are expressed by various cellular components in the human tumor microenvironment, where they can inhibit anti-tumor T-cell immunity (Figure 1). This geographic expression profile is an important feature for PD pathway blockade.
In this review we focus on the human cancer microenvironment and PD pathway blockade. We propose that human anti-tumor immune responses are controlled and regulated by tumor somatic mutations, epigenetic alterations, and environmental cues. We discuss these three aspects and emphasize the relevant studies in the human cancer microenvironment and link scientific rationales to clinical combinational therapy with PD pathway blockade.
Mechanisms of action of the PD signaling pathway
How does PD signaling mediate dysfunctional tumor immunity? PD-L1+ cells, particularly PD-L1 expressing APCs and tumor cells, engage PD-1+ T cells, resulting in T cell dysfunction. Multiple modes of action are thought to explain T cell-immune evasion via the PD pathway. The engagement of PD-L1 and PD-1 may cause T cell apoptosis, anergy, exhaustion, and IL-10 expression (Figure 1). PD-L1 may function as a molecular ”shield” to protect PD-L1+ tumor cells from CD8+ T cell-mediated lysis (14, 15). In addition to PD-1, an interaction between PD-L1 and CD80 has been demonstrated in mouse models (42, 43) (Figure 1). Activated T cells and APCs may express CD80, and CD80 may function as a receptor and deliver inhibitory signals when engaged by PD-L1 (42, 43). It has been shown that PD-L1 can function as a receptor to ”back” transmit signals into T cells (44) and tumor cells (45) to affect their survival whereas the intracellular biochemistry of this “back” signaling is yet to be determined. Thus, PD-L1 could act as both ligand and receptor to execute immune-regulatory functions.
In addition to PD-L1, PD-1 is a receptor for PD-L2. RGMb is a binding partner for PD-L2 (46) (Figure 1). Thus, PD blockade may not be biologically identical and differing blockade may shift the balance in their interaction with their binding partners, leading to potential varied biological outcomes (Figure 1). Notably, the relationship between CD80, PD-1 and RGMb, PD-L1 and PD-L2 in their cellular expression profile, expression regulation, potential molecular interaction, and functional relevance in human tumor immunity and checkpoint immunotherapy have not been completely defined. Furthermore, as PD-L1 is expressed by different types of cells and mediates immune regulation via different mechanisms, it is not totally understood which major cellular and molecular mechanisms are correlated with clinical responses to PD pathway blockade in patients with cancer. Nonetheless, current clinical trials demonstrate similar clinical response patterns for anti-PD therapy (Table 1), suggesting that major cellular and molecular mechanisms associated with a clinical response may be shared in PD blockade. Future studies of the tumor microenvironment in patients with or without immunotherapy will hopefully demonstrate the dynamics of these various interactions and the underlying cellular and molecular mechanisms, which are relevant for the further understanding of how immune elements shape and predict therapeutic response and non-response (Figure 1).
Clinical trials and response biomarkers of PD pathway blockade
Clinically, PD pathway blockade has demonstrated important activity across a spectrum of different tumor types spanning both solid tumors and hematologic malignancies including bladder cancer(47), breast cancer(48, 49), colorectal cancer(48, 50-53), diffuse large B-cell lymphoma(54), follicular lymphoma(55), gastric cancer(48), head and neck squamous cell carcinoma(49), Hodgkin lymphoma(56), melanoma(48-52, 57-65), ovarian cancer(48, 49), non–small-cell lung cancer (NSCLC) (48-51, 66-70), pancreatic cancer(48, 49), renal cell carcinoma (RCC) (48-51, 71), prostate cancer(50, 51), sarcoma(49), small-cell lung cancer (SCLC) (49), and uterine cancer(49). The objective response rates are varied from different cancer types in different clinical trials (Table 1). Based on current clinical data, bladder cancer(47), melanoma(48-52, 57-65), mismatch repair–deficient colorectal cancer(53) and certain hematopoietic malignancies (54, 56) may be among the most responsive cancer types. Specific features of particular clinical trials are summarized (Table 1) and discussed in the following sections.
Although head-to-head comparisons of antibodies to PD-1 and PD-L1 have not been done, levels of clinical response and toxicities appear to be generally consistent with either approach. Immune-related toxicities occur with PD pathway blockade but are much less frequent than those observed with cytotoxic T lymphocyte antigen-4 (CTLA-4) blockade (62, 63). The most frequently observed toxicity encountered with PD pathway blockade is fatigue, which often does not require treatment and does not necessarily limit duration of therapy. However, inflammatory pneumonitis has been observed, which may be fatal if not addressed promptly with corticosteroids or other means of immune suppression. Rare, high grade events like pneumonitis or interstitial nephritis may necessitate cessation of therapy. Yet, clinical responses remain durable despite cessation of therapy and even after immune suppression, implying that the optimal duration of therapy with PD pathway blocking agents remains to be determined. Given that PD pathway blockade induces a clinical response in subsets of cancer patients (Table 1), a central question is whether and how therapeutic responsiveness is predicted and/or is shaped by host and tumor components. There are many ongoing efforts to identify predictive biomarkers of PD pathway blockade. Recent studies have provided hints to associate clinical responses with several potential biomarkers (Figure 2).
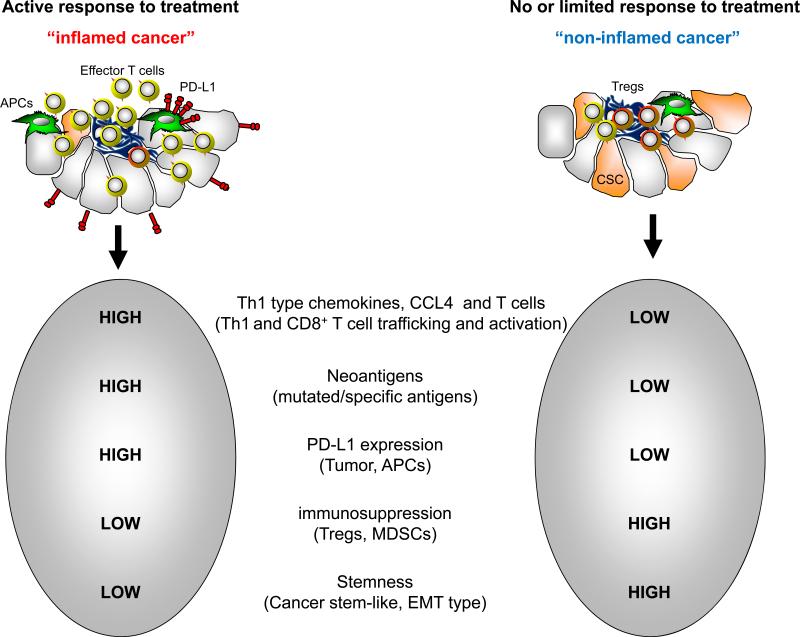
Figure 2.
Proposed potential response biomarkers of PD pathway blockade. Several biomarkers including high levels of PD-L1 expression, Th1-type chemokines, infiltrating T cells, mutations, low levels of immune suppressive elements, and EMT/stem-like features may be associated with an active response to PD pathway blockade.
Does expression level of PD-L1 predict clinical response (Figure 2)?
Tumor cells and APCs express high levels of PD-L1, and tumor associated PD-L1+ DCs mediate T cell suppression (5, 14). Tumor tissues of RCC (72), esophageal, gastric (73, 74), and ovarian (75) cancers show that PD-L1 expression is an indicator of poor prognosis for patient survival. It is reasonable to hypothesize that PD-L1 in tumor and/or APCs in the tumor microenvironment may predict or be associated with the clinical response of PD blockade. In support of this, a correlation has been observed between the expression of tumor tissue PD-L1 and the likelihood of the response to anti-PD therapy in patients with melanoma (48, 51), NSCLC (66), and RCC (41). An 87% objective response is observed in patients with relapsed or refractory Hodgkin's lymphoma treated with anti-PD-1 (Table 1). In line with this, an amplification of PD-L1 and PD-L2 is detected in lymphoma cells in these patients (56). In addition to PD-L1 expression in tumor, expression of PD-L1 in tumor infiltrating immune cells, particularly myeloid APCs (macrophages and myeloid DCs), is correlated with clinical responses to anti-PD-L1 treatment in several types of cancer (Table 1) (49). In contrast, most progressing patients show a lack of PD-L1 upregulation by either tumor cells or tumor-infiltrating immune cells (49). However, the results in trials with anti-PD-1 (64) and the combination of anti-PD-L1 and anti-CTLA-4 suggest that melanoma patients can have a clinical response regardless of the tumor cell PD-L1 status (58, 76). Notably, the host immune cell PD-L1 expression, particularly PD-L1 expression in DCs in the tumor microenvironment and draining lymph nodes (5), has not been specifically examined in majority of clinical trials. Furthermore, activated T cells or innate immune cells can release type I and II IFN and stimulate de novo PD-L1 expression. Presumably, blocking newly induced PD-L1 can affect therapeutic responses. In support of this possibility, across multiple cancer types, responses to anti-PD-L1 therapy are frequent in patients with high PD-L1 expression in tumor-infiltrating immune cells, particularly macrophages and DCs, in the course of tumor regression (41, 47, 49). Hence, the relevance of host PD-L1 expression in shaping the clinical response to PD pathway blockade is important to consider.
Furthermore, PD-L1 expression may be clustered rather than diffuse in tumor tissues (17, 31) and is likely localized to the area where IFNγ+ T cells infiltrate (31). Thus, current needle biopsy-based sampling of human tumor tissues may miss PD-L1 positive area and give false negative results. This problem may be overcome by an in vivo imaging method using radiolabeled high affinity PD-1 variants to assess PD-L1 expression in entire tumor as shown in tumor bearing mouse model (77). In addition, oncogene-driven expression of tumor PD-L1 may not correlate with tumor-infiltrating T cells. It remains to be determined whether the therapeutic efficacy of PD pathway blockade is similar between patients with oncogenic PD-L1+ tumors (31, 41, 78) and immune-driven PD-L1+ tumors.
With current limitations of clinical sampling methods, the expression of PD-L1 on the surface of tumor cells and immune cells prior to immunotherapy may be a useful, but not a definitive predictive biomarker of the response to the PD pathway blockade. Unlike the presence of oncogenic driver mutations, PD-L1 expression is a dynamic and inducible biomarker which is more of a relative indicator of the likelihood of response, rather than a binary predictor of response.
Do cancer neoantigens and/or somatic mutations predict a clinical response (Figure 2)?
The immune system can recognize developing cancers. Therapeutic manipulation of immunity can induce tumor regression. While tumor associated antigens (TAAs) are largely self-antigens, cancers contain somatic genetic mutations, which could be specific to the cancer. These mutation associated antigens can be tumor specific antigens and be presented to and recognized by T cells in patients with cancer. Aligned with this notion, the tumor mutational antigen (neoantigen) T cell response has been documented in a genetically engineered, autochthonous mouse model of sarcomagenesis (79), in a highly immunogenic methylcholanthrene (MCA) induced mouse sarcomas model (80), and in mouse vaccine models (81, 82). In patients with melanoma, tumor mutation-specific CD4+ (83, 84) and CD8+ (85) T cells are found, and these cells can mediate tumor regression. Vaccination with melanoma-derived mutant peptides augments T cell immunity directed at naturally occurring dominant neoantigens and subdominant neoantigens (86). Although the antigen specificity is unknown, the mismatch repair-defective subset of colorectal cancer displays high tumor infiltration of Th1-type T cells and CD8+ T cells along with high PD-L1 and PD-1 expression (87). Using large-scale genomic data sets of solid tumor tissues, the number of predicted MHC class I-associated neoantigens is correlated with the cytolytic activity of CD8+ T cells (88).
Several lines of evidence support a link between PD pathway blockade and tumor mutation-derived antigen specific T cell responses. In the mouse MCA sarcoma model, treatment with anti-PD-1 and/or anti-CTLA-4 activates tumor mutational antigen specific T cells and results in tumor rejection (89). In patients with colorectal cancers, mismatch-repair status predicts clinical benefit of PD-1 blockade (53). In patients with NSCLC treated with anti-PD-1, high nonsynonymous mutation burden is associated with improved objective response, durable clinical benefit, and progression-free survival, and mutated antigen-specific CD8+ T cell responses parallel tumor regression (67). Lung cancer and melanoma are found to be clinically responsive in two early clinical trials with anti-PD therapy (51). These two types of cancer have high numbers of somatic mutations as a result of exposure to cigarette smoke and ultraviolet radiation. Biopsied specimens of regressing melanoma lesions are infiltrated by CD8+ T cells in patients treated with anti-PD therapy (57). A high objective response rate to anti-PD-1 therapy is observed in patients with microsatellite instable colorectal cancer, but not in patients with mismatch repair-proficient colorectal cancer (53). Similarly, in 29 stage IV NSCLC patients, there were 63% and 0% response rates respectively with high and low nonsynonymous mutation burden (67) (Table 1). The data suggest that the increased number of mutation-associated neoantigens may be associated with the enhanced responsiveness to PD pathway blockade in some cancer patients.
Notably, we are at the beginning of our understanding of the relationship between mutant tumor neoantigens, their T cell responses, and cancer immunotherapy responses. Several observations are worth noting in this regard: (a) Melanoma and lung cancer naturally possess high levels of mutations. (b) High levels of mutations may not necessarily form high levels of immunogenic neoantigens; this may simply be a probabilistic phenomenon. (c) There is no direct evidence demonstrating that neoantigen specific T cells mediate tumor elimination in patients treated with PD pathway blockade. (d) Multiple factors including PD-L1 and PD-1 expression levels, effector T cell tumor trafficking, and environmental cues (microbiota) may contribute to immunotherapeutic responses. Nonetheless, recognizing the intrinsic ability of the immune system to specifically target the unique mutations potentially shared by many cancer types (90) is an important part of understanding the mechanism of cancer immunotherapy (91).
Do Th-1 type chemokines, tumor infiltrating T cells, and T cell clonality predict clinical responses (Figure 3)?
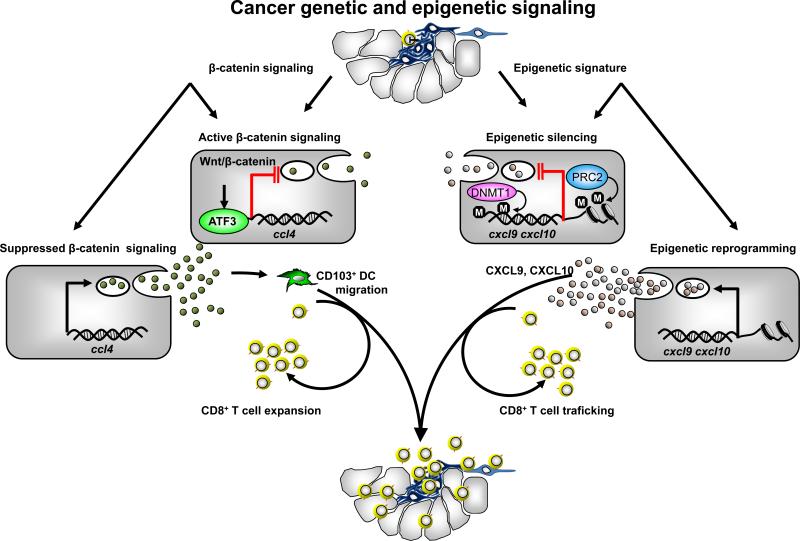
Figure 3.
Mechanisms of poor tumor T cell infiltration. Active tumor β-catenin inhibits CCL4 expression and limits CD103+ DC recruitment and CD8+ T cell activation. Th1-type chemokines CXCL9 and CXCL10 are repressed by EZH2 and DNMT-mediated epigenetic silencing. Consequently CD8+ T cells poorly infiltrate tumor.
Th1-type chemokines are correlated with effector T cell density in some human tumors and are also positively associated with cancer patient survival (8, 10, 92). However, a crucial question is why some tumors are “inflamed” with effector T cell infiltration while others are not. Two research teams have proposed plausible mechanisms to address this question. In a murine melanoma model, tumor intrinsic β-catenin signaling negatively controls chemokine CCL4 expression. CCL4 mediates DC tumor trafficking. Thus, tumor β-catenin activation results in poor CCL4 expression and subsequently limits DC recruitment and DC-mediated T cell activation (Figure 3) (93). In human ovarian cancer (94) and colon cancer (95), poor T cell tumor infiltration is attributed to potent epigenetic silencing of tumor Th1-type chemokines CXCL9 and CXCL10, which mediate effector T cell and natural killer (NK) cell tumor migration (Figure 3) (94, 95). Polycomb repressive complex 2 (PRC2), the demethylase JMJD3-mediated histone H3 lysine 27 trimethylation (H3K27me3), and DNA methylation repress the expression of Th1-type chemokines and subsequently restrain effector T cell trafficking into the cancer microenvironment (94, 95). These studies suggest that intrinsic tumor oncogenic (93) and epigenetic (94, 95) pathways control T cell activation and/or migration. Both epigenetic silencing (96) and β-catenin signaling are intrinsic tumorigenic mechanisms and are associated with cancer stem cell properties. Thus, oncogenic genetic and epigenetic pathways may play dual biologic and immunologic roles in supporting tumor progression and limiting spontaneous and therapeutic-induced tumor specific T cell immunity (Figure 3).
PD-L1 expression is clearly important in the tumor microenvironment (5, 14, 15, 33). A major feature of PD pathway blockade is immune regulation specifically at the tumor site (15). Hence, it is reasonable to assume that pre-existing tumor infiltrating immune cells and Th1 type chemokines may correlate with clinical response to PD pathway blockade. In support of this possibility, recent clinical trials suggest that intra-tumoral T cell infiltration and Th-1 type gene expression and a clonal TCR repertoire are associated with improved clinical responses to anti-PD therapy (47, 97). The frequency of mutational antigen specific T cells and their functional status in the tumor infiltrating T cell populations remain to be investigated and compared prior to and post cancer immunotherapy. Given that oncogenic genetic (93) and epigenetic (94, 95) pathways control effector T cell tumor trafficking, epigenetic marks, enhancer of zeste homolog 2 (EZH2) and DNA methyltransferase 1 (DNMT1), are negatively associated with CD8+ T cells and patient survival (94, 95), and epigenetic reprograming synergistically increases the effect of PD-L1 blockade(94), it will be interesting to explore whether tumor specific genetic and epigenetic marks are associated with the clinical response to anti-PD therapy (Figure 2).
Do microbiota contribute to clinical responses to PD pathway blockade?
Recent studies have shown that the gut microbiome can affect the outcome of cancer chemotherapy in murine models (98, 99). Chemotherapy induced Th1 and Th17 responses are enhanced by translocating commensals and contributes to tumor eradication (98, 99). This is in line with the anti-tumor role of polyfunctional Th17 cells in human ovarian cancer (11, 92). Similarly, commensal bacteria can alter the therapeutic effect of anti-PD-L1 therapy in mice bearing subcutaneous tumors, and the response to PD-L1 blockade is enhanced by supplementation with “good” bacteria, Bifidobacterium, during treatment (100). Interestingly, the antitumor effects of anti-CTLA-4 also depend on distinct Bacteroides species. In mice and patients, T cell responses specific for B. thetaiotaomicron or B. fragilis are associated with efficacy of CTLA-4 blockade (101). It appears that immune responses modulated by the gut microbiome can have systemic effects on tumor immunity and cancer therapy. It remains to be defined if the gut microbiome of cancer patients will have an important impact on PD pathway blockade including cancer neoantigen specific T cell responses and effector T cell tumor infiltration. Nonetheless, these studies raise the possibility that beneficial microorganisms may be an adjuvant for cancer immunotherapy. Thus, it will be scientifically and clinically interesting to profile patient gut microbiota and dissect the relationship with immune responses and clinical outcomes in the course of cancer immunotherapy.
We have discussed several biomarkers in shaping and predicting the clinical response to PD pathway blockade (Figure 2). Are there definite translational biomarkers for PD pathway blockade? Based on the immune profile, cancers may be classified into “inflamed” and “non-inflamed” types. The former is enriched with a Th1-type immune signature including Th1-type chemokines and effector T cells (presumably containing mutated antigen specific T cells) (94) and likely expresses a high amount of PD-L1. The latter is poorly immune infiltrated and likely expresses a limited amount of PD-L1. Recent clinical studies, largely from patients with melanoma, suggest that the “inflamed”, but not the “non-inflamed” tumor type, is highly responsive to PD pathway blockade (Figure 2). However, lymphocyte-rich regions may not be always associated with PD-L1 expression (41, 78, 102). Biologically, the “non-inflamed” tumor type may be closely associated with an epithelial-mesenchymal-transition (EMT) and stem-like type subgroup. In line with this possibility, the Th1-type immune profile is controlled by stem-like associated oncogenic and epigenetic pathways including β-catenin and PRC2 complex (93-95). Thus, immune “inflamed” cancers may be a “non-EMT/stem like type” and are more likely responders to PD blockade therapy. Analogously, the non-responders (or minimal responders) may be lacking T cell infiltration and Th1-type chemokines, less specific mutations and neoantigens, and enriched with multiple layers of immune suppressive mechanisms and potential EMT/stem-like types (Figure 2). An urgent next step is to define and develop combinatorial therapy to improve and enhance the clinical response in patients with different types of cancer.
Combinatorial regimens with PD pathway blockade
Because of the complexity of immune regulatory mechanisms and the heterogeneity of tumor and host, it is envisioned that combination immunotherapies will be required to efficiently treat a larger proportion of cancer patients (1). Continuing advances in our understanding of immune regulation and tumor immunity will allow for the development of new combination(s) for the treatment of different types of cancer. Based on particular limitations of single agent therapy and combinatorial scientific rationales, we have discussed a few examples of therapeutic combinations (Figure 4).
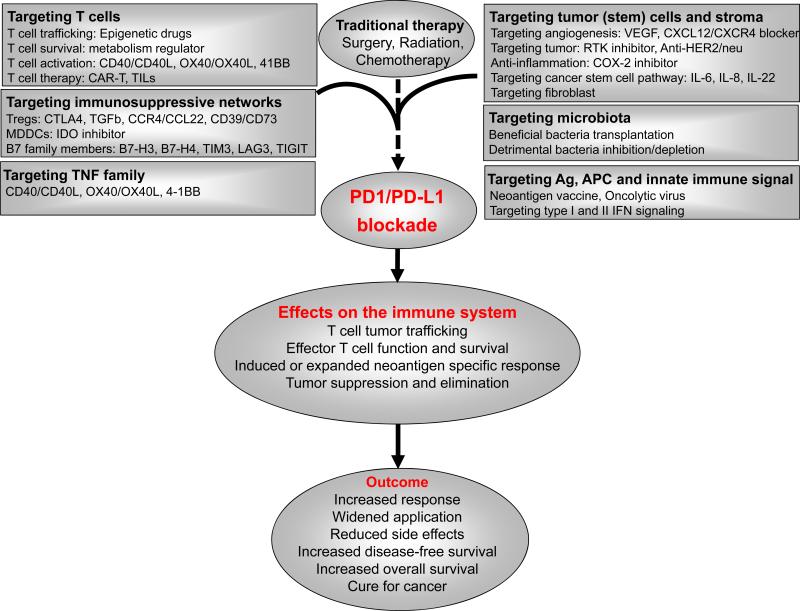
Figure 4.
Scientific rationales of potential therapeutic combinations with PD pathway blockade. Multiple layers of immunosuppressive mechanisms, weak T cell activation, tumor intrinsic biological pathways contribute to cancer progression and therapy resistance. The different combinations with PD pathway blockade may yield a synergistic or additive clinical response.
Enforcing effector T cell trafficking with epigenetic reprogramming drugs
Th1-type chemokines and effector T cell tumor infiltration are associated with therapeutic responses to PD pathway blockade (Figure 2). Histone modification and DNA methylation epigenetically repress tumor Th1-type chemokines and subsequently determine effector T cell trafficking into the tumor microenvironment (94, 95). It may be reasonable to surmise that cancer epigenetic reprograming may remove Th1-type chemokine repressive marks and promote effector T cell trafficking into the tumor microenvironment and improve the therapeutic efficacy of PD pathway blockade. In support of this, treatment with cancer epigenetic reprograming drugs including EZH2 inhibitors, DZNep (103), a selective inhibitor of EZH2 methyltransferase activity, GSK126 (104), and a DNMT inhibitor, 5-aza-2′deoxycytidine (5-AZA dC), enhance tumor Th1-chemokine production and T cell trafficking into tumor (94, 95) and augment therapeutic effects of PD-L1 blockade and T cell therapy in a preclinical model (94). Furthermore, treatment with azacitidine up-regulates IFN signature genes in several human cancer cell lines (105, 106). 5-AZA dC treatment enhances the cancer and germline antigen NY-ESO-1 expression in human ovarian cancer cells (107) and promotes chemokine expression and T cell tumor trafficking in a mouse ovarian cancer model (108). Thus, epigenetic reprogramming can unlock the repressed Th1-type chemokines, IFN-signature genes, and tumor antigen expression, and may therefore condition tumor from poor T cell infiltration to rich T cell infiltration and ultimately potentiate PD blockade therapy (94, 95, 108).
Supplementation of effector T cells with adoptive T cell therapy (ACT)
There may be insufficient functional effector T cells in the tumor microenvironment. ACT, including in vitro expanded peripheral blood or tumor infiltrating T cells (TILs) and genetically engineered chimeric antigen receptor (CAR) T cells, is an important therapeutic approach. However, activated human TILs and TAA-specific T cells express PD-1(19-23, 109). These data suggest a potential benefit for the combination of ACT and PD pathway blockade. In further support of the role of PD-1 blockade in ACT, melanoma TILs with zinc finger nuclease (ZFN)-mediated gene editing of PD-1 display increased effector function in vitro (110). New clinical trials will be warranted to further evaluate this strategy. Mechanistically, PD pathway blockade prior to ACT may prepare the specific “soil”, the less suppressive tumor microenvironment (1), for the transferred T cells to home and function. Given that transferred T cells are often activated and express PD-1 and immune activation can stimulate PD-L1 expression in tumor cells and APCs in the tumor microenvironment, it is assumed that concurrent or post PD pathway blockade to ACT may improve the functionality of the transferred T cells.
Promotion of T cell function via targeting TNF family and T cell metabolism
The tumor microenvironment is highly immunosuppressive in patients with advanced cancer. Targeting immunosuppressive mechanisms is considered an effective strategy to treat patients with cancer (1). On the other hand, it remains logical to directly stimulate and activate the immune cells in combination with PD pathway blockade (111). To this end, we can manipulate certain TNF family signaling ways in patients with cancer.
(a) CD40 and CD40L
The interaction of CD40 and CD40L delivers a potent costimulatory signal to T cells. The humanized CD40 agonist antibody CP-870893 in combination with chemotherapy is currently in clinical trials to treat patients with pancreatic cancer (112). Two other anti-CD40 antibodies, dacetuzumab and HCD122 are currently being tested in hematologic malignancies (113).
(b) OX40 and OX40L
The interaction of OX40 and OX40L may potentiate T cell activity (114) and agonistic anti-OX40 antibodies and OX40 ligands are currently being studied in clinical trials (115) (NCT02219724, NCT02410512, and NCT02221960).
(c) 4-1BB (CD137)
CD137 engagement may preferentially stimulate CD8+ T cells and NK cells. Administration of agonistic anti-4-1BB antibody improves T cell immunity in various murine tumor models (116). Recent clinical trials have shown promising results in single agent or in combination with anti-PD therapy for the treatment of advanced solid tumors (NCT02179918, NCT02554812).
(d) Targeting T cell metabolism
Recent studies reveal that abnormal metabolism impairs effector T cell function in the tumor microenvironment (13, 117, 118). Reprogramming tumor metabolism would be an interesting option in combination with PD pathway blockade (Fig. 4).
Thus, there are scientific rationales to support the combination with all of these agonistic antibodies and approaches with PD pathway blockade. Clinical studies will be needed to conclusively demonstrate the precise indications, effectiveness and side effects of given combination in treating specific type of cancer.
Subversion of immunosuppressive networks in the tumor microenvironment
Immunosuppressive networks are major obstacles for spontaneous and therapy-induced anti-tumor immunity (1). The clinical responses observed by blocking inhibitory B7 family members provides solid evidence to target additional immunosuppressive components in the tumor microenvironment.
(a) Targeting Tregs
Tregs actively inhibit T cell-mediated anti-tumor immunity in the human cancer microenvironment (4). Targeting Tregs is proposed as a therapeutic strategy to treat patients with cancer (119-121). Tregs express CTLA-4. Anti-CTLA-4 antibody may deplete Tregs in the tumor (122). Ipilimumab, a fully human anti-CTLA-4 mAb was approved by the U. S. Food and Drug Administration (FDA) in 2011 for the treatment of patients with advanced melanoma (123). Ipilimumab monotherapy produces clinical responses in 10-20% of patients and extends overall survival in metastatic melanoma, with 20% of patients surviving 3 years or longer (123, 124). In a phase I trial of anti-PD-1 combined with anti-CTLA-4 in patients with advanced melanoma, rapid and deep tumor regressions were observed in 53% of patients treated at the optimal dose level (58). Phase II and III clinical trials have confirmed these observations and further demonstrate the beneficial effects on the objective-response rate and the progression-free survival among patients with advanced melanoma who had not previously received treatment (63, 76) (Table 1). Interestingly, in patients with tumors demonstrating < 5% PD-L1 expression, this combination appears to be more effective than either agent alone (63). However, the response rate for monotherapy with CTLA-4 blockade is generally lower than PD blockade in patients with melanoma (Table 1) (62, 63). Durability of responses with each approach is a topic of current study. Nonetheless, anti-CTLA-4 treatment may deplete Tregs and potentially increase the ratio between effector T cells and Tregs in the tumor microenvironment. Thus, this combination therapy has been approved by the FDA for treatment of BRAF V600 wild-type unresectable or metastatic melanoma. This treatment, however, may carry a relatively high frequency of immune-related toxicity. Therefore, a sequenced model with PD pathway blockade therapy first followed by anti-CTLA-4 later may be considered. In addition to anti-CTLA-4, other strategies targeting Tregs (121) including transforming growth factor (TGF) β signaling blockade may be used in combination with PD blockade (125). Tregs migrate into the human cancer microenvironment through CCL22 and CCR4 signaling pathway (4, 119). Blocking this pathway may enhance the effects of PD blockade. An anti-CCR4 antibody (mogamulizumab) can deplete circulating Tregs in patients with T cell leukemia and lymphoma (126). Ongoing studies are evaluating the efficacy of anti-CCR4 in combination with PD pathway blockade (NCT02301130). Human tumor associated Tregs express the ectonucleotidases CD39 and CD73, convert ATP to adenosine, and inhibit T cell activation by the adenosinergic pathway (92). A CD73-specific antibody has demonstrated an additive activity when combined with PD-1 antibodies in murine tumor models (127). Thus, CD39, CD73, adenosine and adenosine A2a receptor (ADORA2A) signaling blockade may be combined with PD pathway therapy to treat patients with cancer.
(b) Targeting myeloid cells
Human tumor associated MDSCs and inhibitory APCs including macrophages actively inhibit T cell-mediated anti-tumor immunity (5-7) and promote cancer stemness (7, 128) in the human cancer microenvironment. Given the roles of indoleamine-2,3-dioxygenase (IDO) in MDSC-mediated T cell suppression (129), the use of IDO inhibitor(s) (INCB024360) (32) with PD blockade is a potential option for combinatorial therapy.
(c) Targeting additional potentially immune inhibitory immunoglobulin superfamily molecules
The expression of B7-H3 (B7RP-2, CD276) and B7-H4 (B7x, B7S1) is found in different types of human tumor tissues (6, 14, 130, 131). In human hepatocellular carcinoma (HCC) B7-H3 expression is linked to limited T cell proliferation and IFN-γ production (132). B7-H3 blockade resulted in increased CD8+ T cell influx in murine pancreatic cancer (133) while some studies showed that B7-H3 could promote tumor immunity (132). Tumor-associated macrophages express B7-H4 and are able to suppress tumor-specific T cells (6). Therefore, immune modulatory role of B7-H3 and B7-H4 in different types of tumor or host cells is under debate (134, 135). Several clinical studies, however, have been initiated to test the effect of anti-B7-H3 antibodies in single agent (NCT02628535) or in combination with anti-CTLA-4 (NCT02381314) or with anti-PD-1 (NCT02475213) for treating solid tumors.
PD-1H (Dies1, VISTA, DD1α) is a more recently identified B7-CD28 family molecule and was shown to be an immune inhibitory ligand in a mouse tumor model and in a mouse experimental autoimmune encephalomyelitis (EAE) model (136). However, using PD-1H deficient mice and agonistic antibodies, this molecule was shown also to be an immune inhibitory receptor on T cells (137, 138). Thus, VISTA blockade in single agent or in the combination of anti-PD blockade may be potential regimens to be examined in future clinical trials once the necessary reagents are available.
The expression of T cell immunoglobulin mucin 3 (Tim-3) (139, 140), lymphocyte activation gene 3 protein (LAG3) (23), and T cell immunoglobulin and ITIM domain (TIGIT) (141, 142) are reported in human cancer infiltrating T cells. Blockade of Tim-3 (139, 140) and LAG3 (23, 143) and TIGIT (141, 142) with anti-PD antibody increases effector T cell function. Thus, the combinations of PD pathway with Tim-3, LAG3, and TIGIT blockade have been proposed and tested in clinical trials (NCT01968109, NCT02460224).
Targeting tumor specific antigen and antigen presentation
(a) Neoantigen vaccine
Traditional vaccines can activate T cells and induce immune responses to targets on tumor cells, but there is not substantial evidence of reproducible clinical responses in patients with established tumors (144). Interestingly, PD pathway blockade can increase the anti-tumor efficacy of conventional vaccines in animal models (145-147). As somatic genetic mutations could generate unique tumor specific neoantigens and neoantigen specific T cell responses (148), vaccination with immunogenic neoantigens has been examined in animal models (81, 82, 89). Mutated antigen specific T cell responses can be found in patients with cancer (67, 83-86). Thus, a novel vaccination platform will be likely incorporated with specific neoantigens and potentially in combination with PD pathway blockade. Ongoing research efforts are aimed at increasing the efficiency of producing personalized neo-epitope vaccines and also possibly identifying shared neoantigens for use along with immune modulating antibodies. As the tumor microenvironment is immunologically suppressive (1) and metabolically dysregulated (13, 117, 118), in addition to inducing and/or expanding neoantigen specific T cells via specific vaccination, it is also crucial to ensure that these T cells can efficiently traffic into and survive in the cancer microenvironment (Figure 4).
(b) Oncolytic viral therapy
Talimogene laherparepvec (T-VEC) is a herpes simplex virus type 1–derived oncolytic immunotherapy designed to selectively replicate within tumors and produce granulocyte macrophage colony-stimulating factor (GM-CSF) (149). The US FDA has approved T-VEC for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. T-VEC may likely trigger DC differentiation and enhance antigen presentation, promote T cell activation and IFN-γ production, and induce PD-L1 expression. Thus, T-VEC may be a high priority agent for combination trials with PD blockade. A phase III study is currently exploring T-VEC with pembrolizumab (anti-PD-1) for unresected melanoma (NCT02263508).
Targeting inflammatory mediators with COX-2 inhibitor
Prostaglandin E2 (PGE2) and its key synthesizing enzyme cyclooxygenase 2 (COX2) can directly mediate pro-tumor activities and recruit and induce MDSCs in the tumor microenvironment (150). However, PD pathway blockade may increase the expression of PGE2 and pro-tumor inflammatory cytokines, which potentially offsets the therapeutic effects of this blockade (151). Several pre-clinical models demonstrate that inhibition of COX-2 synergizes with PD pathway blockade in eradicating tumors (151, 152), suggesting that COX inhibitors could be useful adjuvants for immune-based therapies including PD blockade in cancer patients.
Targeting innate immune signaling pathway
The innate immune system and its major signaling type I and II IFN contribute to the antitumor immune response (3, 153). Human tumor associated plasmacytoid DCs induce IL-10+ regulatory T cells (154, 155). However, after activation, these human tumor associated plasmacytoid DCs are capable of producing large amount of type-I IFN (154, 155). In tumor bearing mouse models, type I and II IFN signaling pathway is essential for therapeutic responses to chemotherapy (156, 157), radiation therapy (158, 159), and anti-HER2/neu therapy (160). In line with mouse studies, in women with metastatic breast cancer, response to anti-HER2/Neu therapy correlates with natural killer (NK) cell associated antibody-dependent cell-mediated cytotoxicity (ADCC) (161). Furthermore, PD-L1 expression can be potently stimulated through IFN signaling pathway. Thus, targeting innate immune signaling pathway in combination with PD pathway blockade is scientifically rationalized.
Targeting cancer cells
(a) Localized radiation
Radiation therapy is a well-recognized means to achieve local tumor destruction. It has been reported to trigger innate immune signaling pathways, impair regulatory T cells, activate CD8+ T cells, stimulate chemokine expression, and promote immune infiltration into tumors (158, 159, 162-164). However, radiation-induced inflammation (including IFN signaling) can enhance tumor PD-L1 expression (159, 163) which may reduce radiation-induced protective tumor immunity. Interestingly, increased PD-L1 expression may provide a window of opportunity for PD pathway blockade. In line with this notion, radiation therapy combined with anti-PD therapy can synergistically promote anti-tumor immunity in several tumor bearing mouse models (159, 163, 165, 166). Although there is a solid scientific rationale to support the combination of radiation and PD pathway blockade, radiation parameters including dose, site, and time may be critical to the success of such a combination and need to be further explored. Given the challenges in recapitulating human radiation fractionation regimens in animal models, clinical trials will be essential to carefully sort out the feasibility of this approach. Such clinical trials will also provide an opportunity to examine if radiation induces immunogenic mutation-associated neoantigens and whether the induced mutations are associated with treatment response.
(b) Chemotherapy
Mouse studies suggest that therapeutic responses to some chemotherapy agents including anthracyclines and oxaliplatin, may partially depend on immune responses, particularly type-I IFN signaling mediated immunity (156, 167). Interestingly, PD-L1 can be induced by the IFN signaling pathway. Oxaliplatin treatment promotes PD-L1+ plasmocyte tumor infiltration in a mouse prostate cancer model (168). These preclinical studies suggest that PD pathway blockade may enhance the efficacy of chemotherapy. As chemotherapy induces genetic mutations, it is also reasonable to hypothesize that such combinations may induce mutation specific neoantigen-specific T cell responses and affect clinical outcomes. Nonetheless, chemotherapy also modulates the immune system and PD pathway blockade depends on ongoing immune responses, especially those in the tumor microenvironment (15). Future clinical studies will determine which therapeutic modality including agents, doses, and timing will increase clinical responses in combination with PD pathway blockade.
(c) Targeting oncogenic signals
Anti-HER2/neu antibody therapy and multiple receptor tyrosine kinases (RTK) inhibitors (sunitinib, imatinib) interrupt oncogenic signals and mediate tumor regression. Recent studies indicate that anti-HER2/neu therapy (160) and RTK inhibitors (169) can promote T cell activation and trafficking. PD pathway blockade in combination with anti-HER2/neu antibody or RTK inhibitors may be considered in the treatment of certain cancers. Careful attention will need to be paid to proper dose and scheduling of targeted therapies with immunotherapies to avoid potential suppression of T cell or APC activity given the physiologic role for some of the targeted pathways.
Other potential combinations
In addition to T cell and APC subsets and tumor cells, vascular endothelial cells, stromal fibroblasts, cancer stem cells, and microbiota may be targeted in combination with PD pathway blockade. Vascular endothelial growth factor A (VEGF-A) and CXCL12 (154, 170, 171) are highly expressed in the tumor microenvironment and mediate tumor angiogenesis. Targeting tumor stromal fibroblasts (172), CXCL12 and CXCR4 blockade (172), and VEGF targeted therapy (173) have been tested as combinatorial partners for immunotherapy in the literature. As human cancer microenvironmental immune cells including macrophages (128), MDSCs (7), Th22 cells (12) and inflammatory Tregs (174) and their associated cytokines IL-6 (128, 175, 176), IL-8 (177) and IL-22 (12) can promote and maintain the cancer stem cell pool, targeting cancer stem cell pathway with PD blockade may be an important option. The gut microbiota influence the host responsiveness to immunotherapy (100, 101). Beneficial bacteria inoculation or detrimental bacteria inhibition may be combined with PD pathway blockade in specific type of cancer. Additional potential combinatory strategies have been discussed in the literature (111).
Concluding remarks
PD pathway blockade has elicited durable clinical responses in patients with a broad spectrum of cancers with a reasonable toxicity profile (Table 1). This therapy largely relies on efficient T cell infiltration into tumor and effector T cell function in the tumor microenvironment. The human cancer immune microenvironment thus holds the key to understanding the nature of immunity in response to tumor progression and tumor immunotherapy (1, 15, 33). PD blockade may potentially induce and/or expand T cells specific to mutated neoantigen in patients with cancer. Accumulating evidence points towards mechanism-based combination of various treatment regimens with PD pathway blockade to establish new standard of care for patients with cancer. Dynamic immunologic studies along with genetics and epigenetics in the human cancer microenvironment will guide the development of different combination therapies and generate novel insight into how the human immune system responds to and is shaped by a variety of tumor types.
Acknowledgements
We would like to thank our former and current collaborators and trainees for their intellectual input and hard work. The work described in this Review was supported by grants from the United States National Institutes of Health CA193136, CA190176, CA171306, CA152470, CA099985, CA156685, CA123088, CA133620, CA092562, CA100227 for W.Z; P30 CA008748, R01 CA056821, the Ludwig Trust, the Lloyd Charitable Trust and the CRI/SU2C Immunotherapy Dream Team for J.D.W.; CA121974, CA177444, CA016359 for L.C. and Ovarian Cancer Research Fund and the United States Department of Defense for W.Z., Stand Up to Cancer fund for L.C. The review largely focuses on the human cancer immune microenvironment and cancer patient-oriented studies. Due to the plethora of literature related to the topic described in this review, it makes a complete and extensive review extremely challenging. We apologize in advance for any inadvertent omission.
Footnotes
Competing financial interest
W.Z. is a consultant to NGM and Lycera, and has sponsored research grants from Medimmune and Lycera.
J.D.W. is a consultant to Bristol-Myers Squibb, Merck, Genentech and Medimmune.
L.C. is scientific founder of NextCure Inc. and receives loyalty payment from Bristol-Myers Squibb, Ventana and ImmunNext. He is a consultant to Pfizer, MedImmune and NextCure and has sponsored research grants from Boehringer Ingelheim, Pfizer and NextCure.
References
- 1.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature reviews. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 6.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. The Journal of experimental medicine. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, Kotarski J, Tarkowski R, Wicha M, Cho K, Giordano T, Liu R, Zou W. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England journal of medicine. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 11.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 Cells Are Long-Lived Effector Memory Cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S, Zgodzinski W, Majewski M, Wallner G, Fang J, Huang E, Zou W. IL-22CD4 T Cells Promote Colorectal Cancer Stemness via STAT3 Transcription Factor Activation and Induction of the Methyltransferase DOT1L. Immunity. 2014 doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, Wei S, Crespo J, Wan S, Vatan L, Szeliga W, Shao I, Wang Y, Liu Y, Varambally S, Chinnaiyan AM, Welling TH, Marquez V, Kotarski J, Wang H, Wang Z, Zhang Y, Liu R, Wang G, Zou W. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nature immunology. 2016;17:95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. The Journal of clinical investigation. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 18.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 20.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. Journal of immunology. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 25.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. The Journal of experimental medicine. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr., Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. Journal of immunology. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. Journal of immunology. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 30.Kryczek I, Wei S, Gong W, Shu X, Szeliga W, Vatan L, Chen L, Wang G, Zou W. Cutting edge: IFN-gamma enables APC to promote memory Th17 and abate Th1 cell development. Journal of immunology. 2008;181:5842–5846. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- 31.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Science translational medicine. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 35.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 38.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 40.Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q, Lian CG, Thomi R, Hoetzenecker W, Cozzio A, Dummer R, Mihm MC, Jr., Flaherty KT, Frank MH, Murphy GF, Sharpe AH, Kupper TS, Schatton T. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell. 2015;162:1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the b7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J-J, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. The Journal of clinical investigation. 2003;111:363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH, DeKruyff RH, Freeman GJ. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. The Journal of experimental medicine. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng S.-l., Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 48.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA., Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen Y-B, Kaminski MS, Holland HK, Winter JN, Mason JR, Fay JW, Rizzieri DA, Hosing CM, Ball ED, Uberti JP, Lazarus HM, Mapara MY, Gregory SA, Timmerman JM, Andorsky D, Or R, Waller EK, Rotem-Yehudar R, Gordon LI. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, Feng L, Baladandayuthapani V, Wang Z, Ma W, Gao Y, Wallace M, Vence LM, Radvanyi L, Muzzafar T, Rotem-Yehudar R, Davis RE, Neelapu SS. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. The Lancet Oncology. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elassaiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet (London, England) 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 62.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, K.-. investigators. Carlino MS, Cebon J, Hersey P, Long G, McNeil C, Millward M, Walpole E, Holler C, Kehrer H, Richtig E, Schmuth M, Baurain J-F, Neyns B, Wolter P, Butler M, McWhirter E, Miller W, Petrella T, Acevedo A, Caglevic C, Morales L, Sanchez J, Yepes A, Zambrano AR, Avril M-F, Dutriaux C, Grob JJ, Guilot B, Lacour J-P, Leccia M-T, Legoupil D, Lebbe C, Machet L, Mortier L, Robert C, Berking C, Loquai C, Mohr P, Schadendorf D, Utikal J, Bar-Sela G, Lotem M, Schachter J, Blank C, Barrow C, Fitzharris B, Kersten C, Nyakas M, Straume O, Arance A, Berrocal A, Cortes J, Espinosa E, Lopez-Martin J, Martin-Algarra S, Masucci G, Papworth K, Ullenhag G, Brown E, Chao D, Evans T, Larkin J, Lorigan P, Middleton M, Skaria S, Steven N, Agarwala S, Bhatia S, Daud A, Gangadhar T, Gonzalez R, Hamid O, Kumar P, Kuzel T, Lutzky J, O'Day S, Perry D, Pecora A, Puzanov I, Ribas A, Slezak K, Sznol M, Tarhini A, Weiss G. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 63.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 65.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr., Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob J-J, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The Lancet Oncology. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 66.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn M-J, Felip E, Lee J-S, Hellmann MD, Hamid O, Goldman JW, Soria J-C, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, K.-. Investigators. Hui R, Chu Q, Leighl N, Chomy F, Dansin E, Lena H, Quere G, Soria J-C, Cappuzzo F, Garassino M, Melotti B, Morabito A, Santini D, Brunsvig P, Flotten O, Ahn M-J, Lee DH, Lee J-S, Carcereny E, Felip E, Insa A, Yang JC-H, Tsai C-M, Arkenau H-T, Grummet S, Middleton G, Aggarwal C, Balmanoukian AS, Bessudo A, Blumenschein GR, Jr., Dronca R, Eder JP, Garon EB, Gandhi L, Goldman J, Gubens M, Hager S, Hamid O, Hellmann MD, Horn L, Johnson E, O'Day S, Patnaik A, Ramalingam S, Rizvi NA, Ross H, Socinski M. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 67.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N Y ) 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet P-J, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet Oncology. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, Brahmer JR, Carvajal RD, Hammers HJ, Puzanov I, Hodi FS, Kluger HM, Topalian SL, Pardoll DM, Wigginton JM, Kollia GD, Gupta A, McDonald D, Sankar V, Sosman JA, Atkins MB. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2013–2020. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 73.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 74.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, Kimura R, Tsai JM, Manglik A, Kruse AC, Gambhir SS, Weissman IL, Ring AM. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E6506–6514. doi: 10.1073/pnas.1519623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VLS, Sznol M, Rimm DL, Chen L, Jilaveanu LB. Characterization of PD-L1 Expression and Associated T-cell Infiltrates in Metastatic Melanoma Samples from Variable Anatomic Sites. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3052–3060. doi: 10.1158/1078-0432.CCR-14-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, BalSs J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmuller SB, Okun J, Stevanovic S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, Platten M. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 82.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Boegel S, Schrors B, Vascotto F, Castle JC, Tadmor AD, Schoenberger SP, Huber C, Tureci O, Sahin U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran E, Turcotte S, Gros A, Robbins PF, Lu Y-C, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science (New York, N Y ) 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linnemann C, van Buuren MM, Bies L, Verdegaal EME, Schotte R, Calis JJA, Behjati S, Velds A, Hilkmann H, Atmioui DE, Visser M, Stratton MR, Haanen JBAG, Spits H, van der Burg SH, Schumacher TNM. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nature medicine. 2015;21:81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 85.Robbins PF, Lu Y-C, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, Samuels Y, Rosenberg SA. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature medicine. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie W-R, Hildebrand WH, Mardis ER, Linette GP. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science (New York, N Y ) 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer discovery. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber W-J, Mulder GE, Toebes M, Vesely MD, Lam SSK, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee H-G, Melief CJM, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale A-L, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Imielinsk M, Jager N, Jones DTW, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt ANJ, Valdes-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, I. Australian Pancreatic Cancer Genome. I. B. C. Consortium, I. M.-S. Consortium. PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR, Claviez A, Rosenwald A, Rosenwald A, Borkhardt A, Brors B, Radlwimmer B, Lawerenz C, Lopez C, Langenberger D, Karsch D, Lenze D, Kube D, Leich E, Richter G, Korbel J, Hoell J, Eils J, Hezaveh K, Trumper L, Rosolowski M, Weniger M, Rohde M, Kreuz M, Loeffler M, Schilhabel M, Dreyling M, Hansmann M-L, Hummel M, Szczepanowski M, Ammerpohl O, Stadler PF, Moller P, Kuppers R, Haas S, Eberth S, Schreiber S, Bernhart SH, Hoffmann S, Radomski S, Kostezka U, Klapper W, Sotiriou C, Larsimont D, Vincent D, Maetens M, Mariani O, Sieuwerts AM, Martens JWM, Jonasson JG, Treilleux I, Thomas E, Mac Grogan G, Mannina C, Arnould L, Burillier L, Merlin J-L, Lefebvre M, Bibeau F, Massemin B, Penault-Llorca F, Lopez Q, Mathieu M-C, Lonning PE, Schlooz-Vries M, Tol J, van Laarhoven H, Sweep F, Bult P. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, N Y ) 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 92.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 94.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, Tarkowski R, Dou Y, Cho K, Hensley-Alford S, Munkarah A, Liu R, Zou W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagarsheth N, Peng D, Kryczek I, Wu K, Li W, Zhao E, Zhao L, Wei S, Frankel T, Vatan L, Szeliga W, Dou Y, Owens S, Marquez V, Tao K, Huang E, Wang G, Zou W. PRC2 epigenetically silences Th1-type chemokines to suppress effector T cell trafficking in colon cancer. Cancer Res. 2015 Nov 13; doi: 10.1158/0008-5472.CAN-15-1938. pii: canres.1938.2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nature reviews. Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther P-L, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (New York, N Y ) 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai R-M, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (New York, N Y ) 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre M-L, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (New York, N Y ) 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther P-L, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, N Y ) 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Laboratory investigation; a journal of technical methods and pathology. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A, 3rd, Diaz E, LaFrance LV, Mellinger M, Duquenne C, Tian X, Kruger RG, McHugh CF, Brandt M, Miller WH, Dhanak D, Verma SK, Tummino PJ, Creasy CL. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 105.Li H, Chiappinelli KB, Guzzetta AA, Easwaran H, Yen R-WC, Vatapalli R, Topper MJ, Luo J, Connolly RM, Azad NS, Stearns V, Pardoll DM, Davidson N, Jones PA, Slamon DJ, Baylin SB, Zahnow CA, Ahuja N. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5:587–598. doi: 10.18632/oncotarget.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, Vancriekinge W, Demeyer T, Du Z, Parsana P, Rodgers K, Yen R-W, Zahnow CA, Taube JM, Brahmer JR, Tykodi SS, Easton K, Carvajal RD, Jones PA, Laird PW, Weisenberger DJ, Tsai S, Juergens RA, Topalian SL, Rudin CM, Brock MV, Pardoll D, Baylin SB. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woloszynska-Read A, Mhawech-Fauceglia P, Yu J, Odunsi K, Karpf AR. Intertumor and intratumor NY-ESO-1 expression heterogeneity is associated with promoter-specific and global DNA methylation status in ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:3283–3290. doi: 10.1158/1078-0432.CCR-07-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L, Amoozgar Z, Huang J, Saleh MH, Xing D, Orsulic S, Goldberg MS. Decitabine Enhances Lymphocyte Migration and Function and Synergizes with CTLA-4 Blockade in a Murine Ovarian Cancer Model. Cancer Immunol Res. 2015;3:1030–1041. doi: 10.1158/2326-6066.CIR-15-0073. [DOI] [PubMed] [Google Scholar]
- 109.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K-I, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. The Journal of clinical investigation. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beane JD, Lee G, Zheng Z, Mendel M, Abate-Daga D, Bharathan M, Black M, Gandhi N, Yu Z, Chandran S, Giedlin M, Ando D, Miller J, Paschon D, Guschin D, Rebar EJ, Reik A, Holmes MC, Gregory PD, Restifo NP, Rosenberg SA, Morgan RA, Feldman SA. Clinical Scale Zinc Finger Nuclease-mediated Gene Editing of PD-1 in Tumor Infiltrating Lymphocytes for the Treatment of Metastatic Melanoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23:1380–1390. doi: 10.1038/mt.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nature reviews Drug discovery. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 112.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O'Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Byrd JC, Kipps TJ, Flinn IW, Cooper M, Odenike O, Bendiske J, Rediske J, Bilic S, Dey J, Baeck J, O'Brien S. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53:2136–2142. doi: 10.3109/10428194.2012.681655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nature medicine. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 115.Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunological reviews. 2011;244:218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 117.Chang C-H, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJW, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ho P-C, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui Y-C, Cui G, Micevic G, Perales JC, Kleinstein SH, Abel ED, Insogna KL, Feske S, Locasale JW, Bosenberg MW, Rathmell JC, Kaech SM. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 121.Menetrier-Caux C, Curiel T, Faget J, Manuel M, Caux C, Zou W. Targeting regulatory T cells. Target Oncol. 2012 doi: 10.1007/s11523-012-0208-y. [DOI] [PubMed] [Google Scholar]
- 122.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. mproved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen T-T, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei S, Shreiner AB, Takeshita N, Chen L, Zou W, Chang AE. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7-H1/PD-1 axis and transforming growth factor beta. Cancer Res. 2008;68:5432–5438. doi: 10.1158/0008-5472.CAN-07-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, Fujimoto K, Yamamoto K, Miyamoto T, Uike N, Tanimoto M, Tsukasaki K, Ishizawa K, Suzumiya J, Inagaki H, Tamura K, Akinaga S, Tomonaga M, Ueda R. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 127.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 128.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 130.Maj T, Wei S, Welling T, Zou W. T cells and costimulation in cancer. Cancer journal. 2013;19:473–482. doi: 10.1097/PPO.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 131.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, Regulatory T Cells, and Patient Outcome in Human Ovarian Carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 132.Sun T-W, Gao Q, Qiu S-J, Zhou J, Wang X-Y, Yi Y, Shi J-Y, Xu Y-F, Shi Y-H, Song K, Xiao Y-S, Fan J. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer immunology, immunotherapy : CII. 2012;61:2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H, Nakajima Y. Clinical importance of B7-H3 expression in human pancreatic cancer. British journal of cancer. 2009;101:1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, Liu Y, Chen L. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. Journal of immunology (Baltimore, Md : 1950) 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 135.Rahbar R, Lin A, Ghazarian M, Yau H-L, Paramathas S, Lang PA, Schildknecht A, Elford AR, Garcia-Batres C, Martin B, Berman HK, Leong WL, McCready DR, Reedijk M, Done SJ, Miller N, Youngson B, Suh W-K, Mak TW, Ohashi PS. B7-H4 expression by nonhematopoietic cells in the tumor microenvironment promotes antitumor immunity. Cancer Immunol Res. 2015;3:184–195. doi: 10.1158/2326-6066.CIR-14-0113. [DOI] [PubMed] [Google Scholar]
- 136.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu L-F, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. The Journal of experimental medicine. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ, Chen L. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. The Journal of clinical investigation. 2014;124:1966–1975. doi: 10.1172/JCI74589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flies DB, Higuchi T, Chen L. Mechanistic Assessment of PD-1H Coinhibitory Receptor-Induced T Cell Tolerance to Allogeneic Antigens. Journal of immunology (Baltimore, Md : 1950) 2015;194:5294–5304. doi: 10.4049/jimmunol.1402648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 141.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 142.Chauvin J-M, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen T.-h. T., Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. The Journal of clinical investigation. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. Journal of immunology (Baltimore, Md : 1950) 2010;184:3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosenberg SA. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 2014;11:630–632. doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Jr., Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 150.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, Li W, Wu G, Ren J, Wang Z, Zou W, Wang L. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. OncoImmunology. doi: 10.1080/2162402X.2015.1074374. DOI:10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reis E Sousa C. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cellular & molecular immunology. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 155.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid Dendritic Cells Induce CD8+ Regulatory T Cells In Human Ovarian Carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 156.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano J-P, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers M-C, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nature medicine. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 157.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C, Chaput N, Zitvogel L. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011;71:661–665. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]
- 158.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu Y-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, Liu Y, Tang J, Wang S, Fu YX. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei S, Egenti MU, Teitz-Tennenbaum S, Zou W, Chang AE. Effects of tumor irradiation on host T-regulatory cells and systemic immunity in the context of adoptive T-cell therapy in mice. J Immunother. 2013;36:124–132. doi: 10.1097/CJI.0b013e31828298e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 165.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 166.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 168.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, Jamieson C, Kane CJ, Klatte T, Birner P, Kenner L, Karin M. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan P-Y, Chen S-H. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 171.Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 172.Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, Deen K, Carpenter C, Benson P, Ho TH, Pandite L, de Souza P, Powles T, Motzer RJ. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
- 174.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W. IL-17(+) regulatory T cells in the microenvironments of chronic inflammation and cancer. Journal of immunology. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 175.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. The Journal of clinical investigation. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, Wu Z, Gonen M, Mulvey LA, Bessarabova MO, Huh SJ, Silver SJ, Kim SY, Park SY, Lee HE, Anderson KS, Richardson AL, Nikolskaya T, Nikolsky Y, Liu XS, Root DE, Hahn WC, Frank DA, Polyak K. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. The Journal of clinical investigation. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. The Journal of clinical investigation. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]