Abstract
Object
Choroid plexus tumors (CPTs) are rare intracranial neoplasms that constitute approximately 2%–5% of all pediatric brain tumors. Most of these tumors present with severe hydrocephalus. The optimal perioperative management and oncological care remain a matter of debate. The authors present the epidemiological and clinical features of CPTs from a 20-year single-institutional experience.
Methods
A total of 39 consecutive patients with pathologically proven CPTs (31 choroid plexus papillomas [CPPs] and 8 choroid plexus carcinomas [CPCs]) were included in this series. Patient demographics, clinical presentation, comorbidities, indications for surgery, radiological studies, tumor location, and all operative variables were reviewed for each case. Multivariate regression analysis was performed to identify independent predictors of tumor recurrence and survival.
Results
The overall mean age (± SD) was 13.13 ± 19.59 years (15.27 ± 21.10 years in the CPP group and 3.66 ± 3.59 years in the CPC group). Hydrocephalus was noted at presentation in 34% of patients. The most common presenting symptoms were headache (32%) and nausea/vomiting (26%). Gross-total resection (GTR) was achieved in 86% of CPPs and in 71% of CPCs (p = 0.57). There was 100% survival in patients with CPPs observed at the 5- and 10-year follow-up and 71% survival in patients with CPCs at the 5-year follow-up. In a multivariate regression analysis, a diagnosis of papilloma, preoperative vision changes, or hydrocephalus; right ventricle tumor location; and GTR were all independently associated with a decreased likelihood of tumor recurrence at last follow-up.
Conclusions
The authors’ study suggests that patients with CPCs are more likely to experience local recurrence and metastasis; hence, GTR with chemotherapy and radiotherapy, particularly for CPCs, is pivotal in preventing recurrence and prolonging survival. While GTR was important for local control following resection of CPPs, it had a minimal effect on prolonging survival in this patient cohort.
Keywords: choroid plexus tumor, choroid plexus carcinoma, choroid plexus papilloma, oncology
Choroid plexus tumors are rare intracranial neoplasms of neuroectodermal origin and account for less than 1% of all brain tumors in adults. These tumors constitute approximately 2%–5% of all pediatric brain tumors and are most often seen when patients are younger than 2 years of age. In children, these neoplasms are most often located in the ventricles, although extraventricular sites have been reported in the literature.2,5–7,17,22,23,27 Choroid plexus tumors can be grossly divided into 2 categories: CPPs and CPCs. Choroid plexus papillomas are considered benign lesions (WHO Grade I), and long-term survival is common for patients with these tumors. Choroid plexus carcinomas are typically malignant (WHO Grade III), aggressive lesions that have 5-year survival rates reported to be around 40%.25–31
The majority of patients present with signs of increased intracranial pressure and hydrocephalus. The cause of hydrocephalus remains uncertain and is likely to be related to overproduction of CSF, obstruction of CSF flow, or impaired absorption. Accordingly, postoperative CSF diversion is common in the management of patients with CPPs and CPCs. Gudeman et al.12 reported a resolution of hydrocephalous following tumor resection, suggesting that CSF hypersecretion contributed to the observed ventriculomegaly.
Numerous published studies have suggested that adjuvant therapy, radiation alone, or radiation in combination with chemotherapy decreases the incidence of local recurrence and improves long-term survival. Hashizume et al.14 and Griffin et al.11 showed that patients with residual tumor after surgery responded to irradiation. Similarly, Geerts et al.9 noted that after subtotal resection, radiation therapy (alone or in combination with chemotherapy) offered a survival advantage. Conversely, Wolff and colleagues29 noted that only 8 of 22 patients with CPCs responded to chemotherapy. Hence, the role of adjuvant therapy in the treatment of CPTs, in particular CPCs, remains unclear.
Given the rare nature of these tumors, there are a number of important questions that remain incompletely answered. Few published studies have demonstrated that GTR plays an important role in preventing local recurrence and improving survival for CPPs; however, the role of GTR in preventing recurrence and improving survival is debatable in the CPC literature. Furthermore, the role of supplemental chemotherapy and radiation therapy in the treatment of CPCs is poorly understood.
Given the paucity of published studies reporting long-term outcomes and epidemiological and clinical features of CPTs, our objective was to present our experience with all such neoplasms resected at The Johns Hopkins Hospital between 1988 and 2008.
Methods
Patient Selection
Institutional review board approval was obtained prior to commencement of this study. Thirty-nine consecutive patients with pathologically proven CPTs resected at The Johns Hopkins Hospital between January 1998 and December 2008 were included in this series. There were 31 patients with CPPs and 8 with CPCs. Of the 8 patients with CPCs, 1 was excluded because of lack of sufficient clinical, radiographic, and pathological data, leaving 7 CPCs available for analysis.
Patient demographics, clinical presentation, comorbidities, indications for surgery, radiological studies, tumor location, and all operative variables were reviewed for each case. Additionally, postoperative radiographic findings, recurrence, survival, and the need for adjuvant radiotherapy, alone or in combination with chemotherapy, were also recorded for each case.
Clinical Evaluation
Preoperative evaluation consisted of a history of presenting illness, physical examination, and radiographic studies. Radiographic studies included CT scanning, MRI, or ultrasonography, where appropriate. Additionally, patients with CPCs who only had preoperative CT scans underwent postoperative staging MRI scans of the entire neuraxis.
Histological Examination
Neuropathological review was performed of all available pathological samples. Histological diagnosis was assessed according to the WHO criteria for CPTs. Choroid plexus papillomas were composed of a single layer of cuboidal-to-columnar cells resting on a basement membrane overlying papillary and vascularized connective tissue. Choroid plexus carcinomas were diagnosed when there was evidence of anaplasia, such as increased mitotic activity, nuclear atypia, loss of papillary differentiation, necrosis, or immunoreactivity for the epithelial marker cytokeratin. Based on these criteria, the tumors were classified as benign CPPs (n = 31) or malignant CPCs (n = 8).
Clinical Management
The initial therapy was surgery in all cases, and all patients also underwent postoperative imaging. The extent of surgery was assessed according to the operative chart and the contrast-enhanced postoperative CT or MRI scan. The degree of resection was characterized as GTR, debulking (> 75% reduction in tumor size), or partial resection (25%–75% reduction). In cases in which operative reports and neuroimaging findings differed, the degree of resection was based on imaging results. Additionally, the decision to use neo-adjuvant chemotherapy and/or radiation therapy was made on a case-by-case basis.
Statistical Analysis
Parametric data are given as the mean ± SD and were compared using the Student t-test. Nonparametric data are given as median and IQR and were compared using the Mann-Whitney U-test. Fisher exact analysis was used to preoperatively compare patients with CPPs and CPCs. Univariate analysis (JMP version 6, SAS Institute) was performed to identify independent predictors of tumor recurrence and survival. Variables associated with recurrence or survival (p < 0.15) in univariate analysis were then included in a stepwise multivariate proportional hazards regression (Cox) model. Variables in the multivariate analysis with p < 0.05 were considered significant (JMP version 6). Estimated Kaplan-Meier plots were generated for progression-free and overall survival and were compared using the log-rank test.
Results
Thirty-eight consecutive patients who underwent resection of pathologically proven CPTs were included. The baseline characteristics are given in Tables 1 and 2. Overall, the mean age at presentation was 13.13 ± 19.59 years (15.27 ± 21.10 years [CPP] vs 3.66 ± 3.59 years [CPC], p = 0.04; Table 1). Thirty-eight percent of patients with CPPs and 43% of patients with CPCs (p = 0.99) were younger than 2 years of age. There was no significant sex predilection (p = 0.35); 35% of patients with papillomas and 57% with carcinomas were male (Table 1).
TABLE 1.
Preoperative characteristics and initial presenting symptoms of 38 patients who underwent resection of pathologically proven CPTs
| Cohort |
||||
|---|---|---|---|---|
| Demographic | CPP (n = 31) | CPC (n = 7) | Combined (n = 38) | p Value |
| male patients* | 11 (35) | 4 (57) | 15 (39) | 0.35 |
| age at presentation (yrs) | 0.04 | |||
| median | 5 | 1.83 | 3.54 | |
| IQR | 0.5–19.75 | 0.95–6.64 | 0.5–3.41 | |
| mean duration of symptoms (mos) | 10.39 ± 17.58 | 2.25 ± 0.95 | 8.80 ± 16.17 | 0.04 |
| age at op (yrs) | 0.01 | |||
| median | 10.41 | 3.16 | 7.5 | |
| IQR | 0.79–25.47 | 1.75–6.95 | 0.91–6.41 | |
Presented as the number (%).
TABLE 2.
Preoperative characteristics and initial presenting symptoms of 38 patients who underwent resection of pathologically proven CPTs*
| % Cohort |
||||
|---|---|---|---|---|
| Presenting Symptoms | CPP | CPC | Combined | p Value |
| increased ICP | 6.45 | 14.28 | 7.89 | 0.91 |
| enlarged head size/fontanels | 22.58 | 14.28 | 21.05 | 0.27 |
| papilledema | 9.67 | 14.28 | 10.52 | 0.80 |
| confusion | 0.00 | 14.28 | 2.63 | 0.35 |
| irritability | 9.67 | 14.28 | 10.52 | 0.80 |
| lethargy | 16.12 | 14.28 | 15.78 | 0.44 |
| headaches | 25.80 | 57.14 | 31.57 | 0.82 |
| nausea/vomiting | 16.12 | 71.42 | 26.31 | 0.12 |
| hemiparesis | 6.45 | 0.00 | 5.26 | 0.10 |
| visual field defect | 22.58 | 28.57 | 23.68 | 0.60 |
| seizure | 16.12 | 0.00 | 13.15 | 0.06 |
| ataxia | 16.10 | 14.30 | 15.78 | 0.44 |
| CN palsy | 3.22 | 0.00 | 2.63 | 0.16 |
| hydrocephalus | 35.48 | 28.57 | 34.12 | 0.30 |
| developmental delay | 16.12 | 0.00 | 13.15 | 0.06 |
| problems feeding | 9.67 | 0.00 | 7.89 | 0.08 |
Some patients had multiple symptoms. Abbreviations: CN = cranial nerve; ICP = intracranial pressure.
Clinical Features
Manifestations of hydrocephalus and intracranial hypertension were the most common presenting symptoms. Thirteen patients (34%) in our combined cohort presented with hydrocephalus (11 [35%] of 31 patients with CPPs vs 2 [29%] of 7 patients with CPCs). Twelve patients (32%) in our combined cohort presented with headache as the primary complaint, or as a part of the syndrome complex (8 with CPPs [26%] vs 4 with CPCs [57%]). Approximately one-quarter of patients in both groups presented with vision changes. The initial presenting symptoms are detailed in Table 2.
Method of Tumor Detection
Overall, the majority of tumors were detected via CT scanning or MRI. The typical appearance of the tumor on neuroradiological examinations was that of a hyperdense, contrast-enhancing lesion developing in the ventricles close to the choroid plexus. Seventy-four percent of patients with CPPs underwent preoperative MRI that was used for detection compared with 14% of patients with CPCs (p < 0.01) (Figs. 1 and 2). Preoperative CT scanning of the head was performed in 45% of patients with CPPs and in 71% of patients with CPCs (p = 0.21). A CPP was detected in 1 patient in utero on prenatal ultrasonography. Of the 8 patients with CPCs, 6 underwent postoperative staged MRI of the entire neuraxis. This included 5 of the 6 patients who had undergone only preoperative CT scanning of the head. Four of the 6 patients did not have distant metastasis, while 1 had diffuse leptomeningeal spread to the cervical and thoracic spine and the other had a dural-based metastasis to the right CPA. The 2 patients who did not undergo neuraxis imaging were lost to follow-up very shortly after resection.
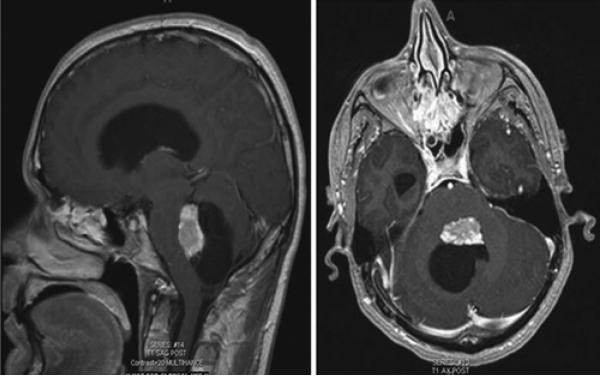
Fig. 1.
Preoperative axial (left) and coronal (right) contrast-enhanced T1-weighted MRI studies showing a typical CPP arising in the posterior horn of the left lateral ventricle.
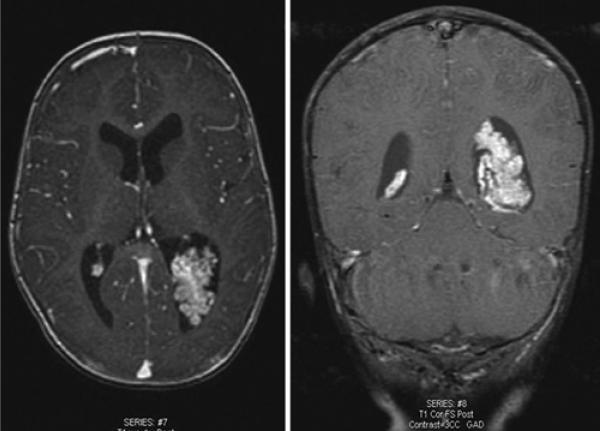
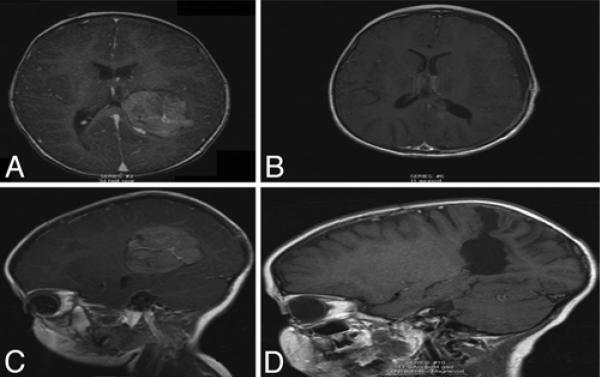
Fig. 2.
Magnetic resonance images obtained in a 4-year-old girl, showing a representative CPC. A: Preoperative axial T1-weighted image demonstrating a CPC in the posterior horn of the left lateral ventricle. B: Postoperative axial T1-weighted image. C: Preoperative sagittal T1-weighted image showing a large carcinoma in the lateral ventricle. D: Complete excision was achieved. Postoperatively, the patient did not require VP shunt placement.
Anatomical Location
The locations of the lesions are summarized in Table 3. Most CPPs and CPCs were located supratentorially; 29% of CPPs were located in the left lateral ventricle, 26% were in the right lateral ventricle, and 10% were in the third ventricle. Approximately one-third of the lesions were located in the fourth ventricle, and 1 lesion was in the CPA. For CPCs, there was a predilection for the left lateral ventricle (71%), while the remaining tumors (29%) were in the right lateral ventricle. No posterior fossa CPCs were observed. The mean age of patients with fourth ventricle CPPs was found to be significantly higher than those with lesions in the lateral ventricles (31.49 ± 23.89 years vs 5.85 ± 11.23 years, p < 0.005). Figure 3 shows a representative MRI scan of a CPP in the fourth ventricle from this study.
TABLE 3.
Locations of the CPTs*
| % Cohort |
||||
|---|---|---|---|---|
| Location | CPP | CPC | Combined | p Value |
| rt lat ventricle | 25.80 | 28.57 | 26.13 | 0.89 |
| It lat ventricle | 29.03 | 71.42 | 36.84 | 0.06 |
| 3rd ventricle | 9.68 | 0.00 | 7.89 | 0.08 |
| 4th ventricle | 35.48 | 0.00 | 28.94 | 3e-04 |
| CPA | 3.22 | 0.00 | 2.63 | 0.32 |
| other† | rt frontal lobe, lumbosacral spine | rt & lt frontal lobe, lt temporal & occipital lobe, thalamus | ||
Most CPPs and CPCs were located in the supratentorial compartment. Approximately one-third were in the fourth ventricle and 1 was in the CPA.
In the CPP cohort, one patient had metastatic seeding to the lumbosacral spine. In the CPC cohort, lesions were located in the left temporooccipital region in one case, and they involved the thalamus with caudal migration into the quadrigeminal cistern in another case.
Fig. 3.
Representative sagittal (left) and axial (right) T1-weighted contrast-enhanced MRI studies of a CPP arising in the fourth ventricle.
Extent of Resection
Gross-total resection was attempted in every patient. There was no statistically significant difference between the GTR rates of CPPs and CPCs (p = 0.44). Gross-total resection was achieved in 86% of CPPs after the initial operation compared with 71% of CPCs (p = 0.57). The incidence of tumor recurrence was significantly higher in the CPC cohort: 1 patient with a CPP had tumor recurrence, and 4 patients with CPCs experienced recurrence (p < 0.003). Additionally, 2 patients with fourth ventricle CPPs had metastatic seeding to the lumbosacral spine, and 3 of the 7 patients with CPCs experienced metastasis to the neuraxis (p < 0.05). Figure 4 demonstrates the Kaplan-Meier progression-free survival of CPPs and CPCs. These differences were statistically significant (p < 0.01). One patient in each group underwent reoperation for further resection.
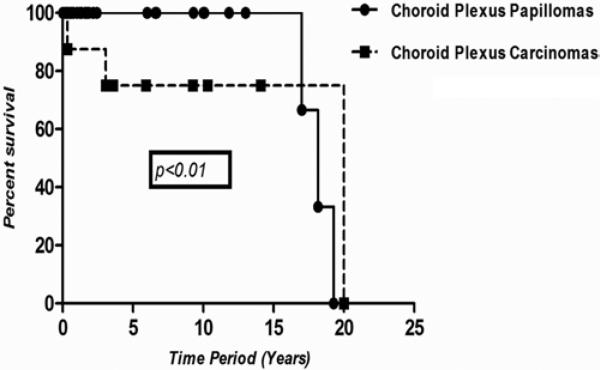
Fig. 4.
Estimated Kaplan-Meier plot depicting survival after resection of CPPs and CPCs. Only patients with observation times > 0 were included. The median and IQR long-term follow-up of patients with CPPs was 21 months (IQR 4.5–104 months), while the median long-term follow-up of patients with CPCs was 71 months (IQR 40–117 months). Five- and 10-year survival for patients with CPPs was 100% compared with a 5-year survival of 71% for those with CPCs (5 of 7). The difference in survival for patients with CPPs versus CPCs was statistically significant (p < 0.01, log-rank test).
Independent Predictors of Tumor Recurrence
Univariate and multivariate analyses were performed to determine independent predictors of tumor recurrence. After univariate analysis, a diagnosis of CPP, preoperative vision changes, preoperative hydrocephalus, right ventricle location, and GTR were all associated with a decreased risk of recurrence at last follow-up. Furthermore, the presence of metastatic lesions was more likely to be a predictor of recurrence at last follow-up. When included in a multivariate stepwise regression model, a diagnosis of CPP (relative risk 0.04 [95% CI 0.0012–0.38], p < 0.003) and GTR (relative risk 0.07 [0.03–0.58], p = 0.01) remained independently associated with decreased tumor recurrence.
Short-Term Outcomes
There were no perioperative deaths. Postoperative morbidity is summarized in Table 4. The most common complications in patients with CPPs were postoperative vision changes in 5 patients, new onset seizures in 4 patients, unilateral weakness in 3 patients, and wound infection requiring surgical debridement and wound revision in 1 patient. There was 1 case of a pulmonary embolism. Postoperatively, in the CPC cohort, 1 patient developed a subdural hematoma that required drainage. The need for CSF diversion was common in patients with both CPPs and CPCs. Seven patients with CPPs (26%) required CSF diversion (6 with a VP shunt and 1 who underwent endoscopic third ventriculostomy), and 2 patients with CPCs (25%) required VP shunts. Five patients with CPPs that needed shunts required revisions. Neither of the patients with CPCs in whom shunts were placed required revision.
TABLE 4.
Postoperative complications after resection of CPTs*
| No. of Patients (%) |
||||
|---|---|---|---|---|
| Complication | CPP | CPC | Combined Cohort | p Value |
| vision change | 5 (16.0) | 0 | 5 (13.00) | 0.02 |
| seizure | 4 (12.9) | 0 | 4 (10.53) | 0.04 |
| wound infection | 1 (3.22) | 0 | 1 (2.63) | 0.32 |
| surgical site hematoma | 0 | 0 | 0 | 0.00 |
| DVT | 0 | 0 | 0 | 0.00 |
| PE | 1 (3.22) | 0 | 1 (2.63) | 0.33 |
The incidence of complications appeared to be higher in the CPP cohort than in the CPC cohort. Abbreviations: DVT = deep venous thrombosis; PE = pulmonary embolism.
Long-Term Outcomes
The median long-term follow-up of patients with CPPs was 21 months (IQR 4.5–104 months), while the median long-term follow-up of patients with CPCs was 71 months (IQR 40–117 months). The 5- and 10-year survival for patients with CPPs was 100%, whereas the 5-year survival for patients with CPCs was 71% (5 of 7 patients). However, in 1 of the 2 patients with CPCs who died, the death was due to an unrelated adrenal malignancy 37 months after resection of the CPC. Figure 4 shows the Kaplan-Meier survival curves for both CPPs and CPCs, with CPPs having a statistically significant improvement in overall survival (p = 0.02). One patient with a CPP and 3 patients with CPCs received adjuvant radiation therapy. The patient with a CPP who received radiation therapy also received adjuvant chemotherapy. Six of the 7 patients with CPCs received additional chemotherapy. There was no statistically significant difference in recurrence (p = 0.40) and/or survival (p = 0.60) between patients who received radiation, alone or in combination with chemotherapy, and those who did not.
Discussion
Our 20-year longitudinal cohort study of all patients treated with CPTs at The Johns Hopkins Hospital between 1988 and 2008 demonstrates that CPCs were 20-fold more likely to locally recur and metastasize; hence, GTR, especially in this cohort, was pivotal in preventing recurrence and prolonging survival. The current study demonstrates that there was no statistically significant difference in recurrence (p = 0.40) and/or survival (p = 0.60) between patients who received radiation therapy or chemotherapy and those who did not; hence, the role of adjuvant therapy in the management of CPTs, in particular CPCs, remains unclear.
Hydrocephalus remains one of the main presenting features of CPTs and is responsible for several problems that adversely influence outcome. The underlying patho-physiological characteristics of hydrocephalus in this setting have been a subject of considerable speculation. Although overproduction of CSF is a major contributing factor, in certain cases, the obstruction of CSF pathways may be the underlying mechanism.2–4,7,13,18,20,21,23 Eisenberg and colleagues6 noted that CPTs produced amounts of CSF that were well in excess of the average level of 450 ml over a 24-hour period. Pencalet and colleagues25 reported a case in which a plexus tumor produced 800 ml of CSF in a 24-hour period. Ghatak and McWhorter10 reported similar findings. The resolution of hydrocephalus after complete tumor resection was the basis for postulating that CSF oversecretion was responsible for the associated hydrocephalus; however, it is now clear that complete resection of CPTs does not always obviate the need for CSF diversion. Pencalet and colleagues25 noted that 9 children in their series required ventricular shunts several months after tumor removal. Similar findings have been reported in other studies.4,7,15 Analogous to these aforementioned studies, we observed a resolution of hydrocephalus after tumor resection in the majority of patients (> 78%); however, 9 patients required postoperative shunt treatment several months after their index operations (7 in the CPP group [23%] vs 2 in the CPC group [28%], p = 0.67). Seven patients with CPPs required CSF diversion (6 with VP shunts and 1 who underwent endoscopic third ventriculostomy), and 2 patients with CPCs required VP shunts. While GTR obviated the need for ventricular shunting in the majority of patients in our series, there were no variables that independently predicted which patients might require permanent shunts. It is likely that other factors, such as meningitis, postoperative changes, metastasis along CSF pathways, or ventricular blood, contribute to the need for permanent CSF diversion.
Choroid plexus papillomas were more likely than CPCs to undergo imaging using MRI technology (72% vs 29%, p < 0.01), most likely because all but 1 patient with a CPC in this study was treated prior to 2000 when MRI technology was less readily available. Other authors have reported that most CPTs occur in the lateral ventricles, which was also seen in this study.30 Fifty-five percent of CPPs and 100% of CPCs were located in the lateral ventricles. There were no fourth ventricle CPCs; however, 35% of papillomas were located in the fourth ventricle. As Wolff and colleagues29 also reported, the age at diagnosis of patients with CPCs in the lateral ventricles (3.6 years) was significantly younger (p < 0.001) than those with fourth ventricle lesions (34.8 years).
Numerous studies have shown a positive association between GTR and favorable outcomes. Wrede and colleagues31 published a comprehensive analysis examining 857 patients with CPTs previously reported in the literature. The authors noted that 80% of patients with CPPs had complete excision of their tumor compared with only 40% of patients with CPCs. They reported mean 2-year survival rates for patients with CPPs and CPCs of 73% ± 5% and 42% ± 5%, respectively. Packer et al.24 reported that GTR for CPCs offered the best likelihood for success. The authors found that 80% of patients who underwent GTR remained disease free, while 83% of patients who underwent a subtotal resection experienced a relapse. Similarly, Wolff and colleagues29 noted that GTR was the most important treatment variable influencing long-term survival for patients with CPTs. In their meta-analysis, the authors reported 5- and 10-year survival for patients with CPPs of 81% and 77%, respectively, compared with only 41% and 35%, respectively, for patients with CPCs. In our series, 86% of patients with CPPs and 71% of patients with CPCs underwent GTR of their tumors. Carcinomas were much more likely to recur locally and metastasize. Four of 7 patients with carcinomas experienced a recurrence, and only 1 of 31 patients with papillomas had recurrence (p = 0.003). The 5- and 10-year survival for patients with CPPs was 100%, whereas the 5-year survival for patients with CPCs was 71%. In 1 of the 2 patients with CPCs who died, the death was due to an unrelated adrenal malignancy 37 months after resection of the CPC. Log-rank analysis demonstrated statistically significant improved survival in patients with CPPs (p = 0.02).
One possible reason for the improved survival rates in patients with CPCs in this series as opposed to prior studies is the fact that 71% of patients underwent GTR. We found that after multivariate analysis examining for independent predictors of tumor recurrence, subtotal resection was associated with a greater than 10-fold risk of recurrence (p = 0.01). Additionally, a diagnosis of CPC was associated with a greater than 20-fold increased risk of recurrence (p = 0.003). Therefore, GTR, especially of CPCs, is likely pivotal in preventing recurrence and prolonging survival. While it appears that GTR of CPPs was critical for local disease control, there was no statistical difference in survival at 5 years between patients who had undergone a GTR and those who had undergone subtotal resection (p = 0.68). These survival rates are favorable when compared with those reported in the literature. In 1998, Pencalet and colleagues25 examined 38 consecutive CPTs resected at Hôpital Universitaire Necker-Enfants malades, Paris, and found 5-year survival rates of 100% and 40% for patients with CPPs and CPCs, respectively. McEvoy et al.21 reported their 20-year experience with treating CPTs and observed a 5-year survival rates of 100% for patients with CPPs and 11% for those with CPCs.
Choroid plexus papillomas are relatively benign lesions; as such, complete excision is curative in most cases. The situation is different for CPCs, which are malignant lesions with a high propensity for recurrence. While GTR has been demonstrated to have a favorable impact on survival in this cohort, GTR for CPCs is achieved only in a few cases described in the literature (< 50%).3–5,7,13,15,16 The role of adjuvant therapy, particularly after GTR, remains unclear. Fitzpatrick et al.8 reported that radiotherapy, alone or in combination with chemotherapy, offered a survival advantage after subtotal resection. Several other studies have corroborated these findings.1,19 Nevertheless, Wolff at al.29 reported a poor response to chemotherapy in 8 of 22 patients with carcinomas. Radiotherapy can be effective against recurrence in older children, although it has limited utility in the majority of cases because of the young age of the patients and the size of the field to be irradiated.5 In our series, there was no statistically significant difference in recurrence (p = 0.40) and/or survival (p = 0.60) between patients who received radiation therapy, alone or in combination with chemotherapy, and those who did not. Although several studies have reported that radiation therapy and chemotherapy prolonged survival and improved outcomes, we were unable to corroborate such findings in this series.
One of the challenges in treating patients with CPTs is histological classification. Choroid plexus tumors are graded from I to III as follows: papilloma (WHO Grade I), atypical papilloma (WHO Grade II), and carcinoma (WHO Grade III). The defining features of malignancy include increased mitotic activity, brain invasion, necrosis, and hypercellularity. However, even Grade I lesions can exhibit some level of mitotic activity and degeneration, which are typical of CPCs. In fact, there are reports of the pathological grade being altered when samples are sent to a centralized reference center.3 All samples in this manuscript were reviewed by a certified neuropathologist, but regardless, a possible drawback to a single-center study is that there could be an institutional bias toward grading these tumors that may impact our conclusions.
The limitations inherent in our study have implications for its interpretation. First, the rarity of CPTs makes them extremely challenging to study. Despite having one of the largest series of patients with CPTs in the literature, our study is inherently limited in its power because it comprises only 38 patients. In particular, the role of adjuvant therapy in the treatment of CPTs remains an extremely important unanswered question. Additionally, caution must be used in inferring direct causal relationships given the retrospective nature of the study design. Furthermore, these results are limited to a single institution, making the results challenging to generalize. Despite these limitations, the current study demonstrates that resection for CPPs is usually curative, and maximum resection offers the best chance for long-term survival in patients with CPCs.
Conclusions
Our study demonstrates that patients with CPCs are more likely to experience local recurrence and metastasis; hence, GTR is pivotal in preventing recurrence and prolonging survival in this patient group. While GTR was important for local control after resection of CPPs, it had minimal effect in prolonging survival in this patient cohort.
Abbreviations used in this paper
- CPA
cerebellopontine angle
- CPC
choroid plexus carcinoma
- CPP
choroid plexus papilloma
- CPT
choroid plexus tumor
- GTR
gross-total resection
- IQR
interquartile range
- VP
ventriculoperitoneal shunt
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Bettegowda, Mehta, Weingart, Carson, Jallo, Ahn. Acquisition of data: Bettegowda, Mehta, Chaichana, Weingart. Analysis and interpretation of data: Bettegowda, Mehta, Chaichana, Weingart, Carson, Jallo, Ahn. Drafting the article: Adogwa, Bettegowda, Mehta, Chaichana, Weingart, Jallo, Ahn. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Adogwa. Statistical analysis: Adogwa, Bettegowda, Mehta. Administrative/technical/material support: Carson, Ahn. Study supervision: all authors.
References
- 1.Allen J, Wisoff J, Helson L, Pearce J, Arenson E. Choroid plexus carcinoma—responses to chemotherapy alone in newly diagnosed young children. J Neurooncol. 1992;12:69–74. doi: 10.1007/BF00172458. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa M, Rebelo O, Barbosa P, Lacerda A, Fernandes R. Choroid plexus tumours: a surgically treated series. Neurocirugia (Astur) 2001;12:7–16. doi: 10.1016/s1130-1473(01)70712-9. [DOI] [PubMed] [Google Scholar]
- 3.Berger C, Thiesse P, Lellouch-Tubiana A, Kalifa C, Pierre-Kahn A, Bouffet E. Choroid plexus carcinomas in childhood: clinical features and prognostic factors. Neurosurgery. 1998;42:470–475. doi: 10.1097/00006123-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Boyd MC, Steinbok P. Choroid plexus tumors: problems in diagnosis and management. J Neurosurg. 1987;66:800–805. doi: 10.3171/jns.1987.66.6.0800. [DOI] [PubMed] [Google Scholar]
- 5.Chow E, Reardon DA, Shah AB, Jenkins JJ, Langston J, Heideman RL, et al. Pediatric choroid plexus neoplasms. Int J Radiat Oncol Biol Phys. 1999;44:249–254. doi: 10.1016/s0360-3016(98)00560-4. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg HM, McComb JG, Lorenzo AV. Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J Neurosurg. 1974;40:381–385. doi: 10.3171/jns.1974.40.3.0381. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbogen RG, Winston KR, Kupsky WJ. Tumors of the choroid plexus in children. Neurosurgery. 1989;25:327–335. doi: 10.1097/00006123-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick LK, Aronson LJ, Cohen KJ. Is there a requirement for adjuvant therapy for choroid plexus carcinoma that has been completely resected? J Neurooncol. 2002;57:123–126. doi: 10.1023/a:1015773624733. [DOI] [PubMed] [Google Scholar]
- 9.Geerts Y, Gabreëls F, Lippens R, Merx H, Wesseling P. Choroid plexus carcinoma: a report of two cases and review of the literature. Neuropediatrics. 1996;27:143–148. doi: 10.1055/s-2007-973765. [DOI] [PubMed] [Google Scholar]
- 10.Ghatak NR, McWhorter JM. Ultrastructural evidence for CSF production by a choroid plexus papilloma. J Neurosurg. 1976;45:409–415. doi: 10.3171/jns.1976.45.4.0409. [DOI] [PubMed] [Google Scholar]
- 11.Griffin BR, Stewart GR, Berger MS, Geyer JR, O'Dell M, Rostad S. Choroid plexus carcinoma of the fourth ventricle. Report of a case in an infant. Pediatr Neurosci. 1988;14:134–139. doi: 10.1159/000120378. [DOI] [PubMed] [Google Scholar]
- 12.Gudeman SK, Sullivan HG, Rosner MJ, Becker DP. Surgical removal of bilateral papillomas of the choroid plexus of the lateral ventricles with resolution of hydrocephalus. Case report. J Neurosurg. 1979;50:677–681. doi: 10.3171/jns.1979.50.5.0677. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N. Choroid plexus tumors in children. Neurosurg Clin N Am. 2003;14:621–631. doi: 10.1016/s1042-3680(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 14.Hashizume A, Kodama Y, Hotta T, Yuki K, Taniguchi E, Eguchi K, et al. Choroid plexus carcinoma in the lateral ventricle—case report. Neurol Med Chir (Tokyo) 1995;35:742–744. doi: 10.2176/nmc.35.742. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins JC., III Treatment of choroid plexus papillomas in children: a brief analysis of twenty years’ experience. Neuro-surgery. 1980;6:380–384. [PubMed] [Google Scholar]
- 16.Israel Z, Lossos A, Ashkenazi E, Soffer D, Umansky F. Germinoma and choroid plexus papilloma coexisting in the fourth ventricle. Acta Neurochir (Wien) 1996;138:1252–1253. doi: 10.1007/BF01809757. [DOI] [PubMed] [Google Scholar]
- 17.Kimura M, Takayasu M, Suzuki Y, Negoro M, Nagasaka T, Nakashima N, et al. Primary choroid plexus papilloma located in the suprasellar region: case report. Neurosurgery. 1992;31:563–566. doi: 10.1227/00006123-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Lena G, Genitori L, Molina J, Legatte JR, Choux M. Choroid plexus tumours in children. Review of 24 cases. Acta Neurochir (Wien) 1990;106:68–72. doi: 10.1007/BF01809335. [DOI] [PubMed] [Google Scholar]
- 19.Maria BL, Graham ML, Strauss LC, Wharam MD. Response of a recurrent choroid plexus tumor to combination chemotherapy. J Neurooncol. 1985;3:259–262. doi: 10.1007/BF00165187. [DOI] [PubMed] [Google Scholar]
- 20.Mazloom A, Wolff JE, Paulino AC. The impact of radiotherapy fields in the treatment of patients with choroid plexus carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:79–84. doi: 10.1016/j.ijrobp.2009.07.1701. [DOI] [PubMed] [Google Scholar]
- 21.McEvoy AW, Harding BN, Phipps KP, Ellison DW, Elsmore AJ, Thompson D, et al. Management of choroid plexus tumours in children: 20 years experience at a single neurosurgical centre. Pediatr Neurosurg. 2000;32:192–199. doi: 10.1159/000028933. [DOI] [PubMed] [Google Scholar]
- 22.Mehta VA, Bettegowda C, Singer HS, Ahn ES. Medullary cistern choroid plexus papilloma. Childs Nerv Syst. 2010;26:1825–1829. doi: 10.1007/s00381-010-1259-y. [DOI] [PubMed] [Google Scholar]
- 23.Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293–295. doi: 10.1002/(sici)1096-911x(199710)29:4<293::aid-mpo10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 24.Packer RJ, Perilongo G, Johnson D, Sutton LN, Vezina G, Zimmerman RA, et al. Choroid plexus carcinoma of childhood. Cancer. 1992;69:580–585. doi: 10.1002/1097-0142(19920115)69:2<580::aid-cncr2820690250>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S, et al. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998;88:521–528. doi: 10.3171/jns.1998.88.3.0521. [DOI] [PubMed] [Google Scholar]
- 26.Sharma R, Rout D, Gupta AK, Radhakrishnan VV. Choroid plexus papillomas. Br J Neurosurg. 1994;8:169–177. doi: 10.3109/02688699409027963. [DOI] [PubMed] [Google Scholar]
- 27.Steven DA, McGinn GJ, McClarty BM. A choroid plexus papilloma arising from an incidental pineal cyst. AJNR Am J Neuroradiol. 1996;17:939–942. [PMC free article] [PubMed] [Google Scholar]
- 28.Strojan P, Popović M, Surlan K, Jereb B. Choroid plexus tumors: a review of 28-year experience. Neoplasma. 2004;51:306–312. [PubMed] [Google Scholar]
- 29.Wolff JE, Sajedi M, Brant R, Coppes MJ, Egeler RM. Choroid plexus tumours. Br J Cancer. 2002;87:1086–1091. doi: 10.1038/sj.bjc.6600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrede B, Liu P, Ater J, Wolff JE. Second surgery and the prognosis of choroid plexus carcinoma—results of a meta-analysis of individual cases. Anticancer Res. 2005;25(6C):4429–4433. [PubMed] [Google Scholar]
- 31.Wrede B, Liu P, Wolff JE. Chemotherapy improves the survival of patients with choroid plexus carcinoma: a meta-analysis of individual cases with choroid plexus tumors. J Neurooncol. 2007;85:345–351. doi: 10.1007/s11060-007-9428-x. [DOI] [PubMed] [Google Scholar]