Supplemental Digital Content is available in the text.
Summary:
Surgical decompression of peripheral branches of the trigeminal and occipital nerves has been shown to alleviate migraine symptoms. Site II surgery involves decompression of the zygomaticotemporal branch of the trigeminal nerve by the technique developed by Guyuron. Failure of site II surgery may occur secondary to an inability to recognize a second temporal trigger: site V, the auriculotemporal nerve. A direct approach for site V has been used with no clear description in the literature. Herein, we describe a safe and efficient method for auriculotemporal nerve decompression during the Guyuron endoscopic approach. Close attention to all temporal sites is necessary to avoid potential failure of migraine decompression surgery.
Migraine has classically been described as a disorder that is central in origin; however, recent evidence suggests that compression of peripheral nerves may be important in migraines and in various forms of chronic headache pathogenesis. This reasoning has been supported by electrophysiologic, anatomic, and proteomic studies1–3 and provides a physiologic basis for surgical decompression. Decompression of peripheral branches of the trigeminal and occipital nerves has been shown to improve or eliminate symptoms.4–12
Four primary trigger sites have been described by Guyuron and targeted by migraine surgeons: frontal (site I), temporal—the zygomaticotemporal branch of the trigeminal nerve (ZTBTN)—(site II), rhinogenic (site III), and greater occipital (site IV), as well as several minor sites that have been described, including site V, the auriculotemporal nerve (ATN). Chim et al13 identified 3 points of compression along the ATN: the first 2 correspond to preauricular fascial bands and the third corresponds to the superficial temporal artery (STA). Guyuron et al14 developed the technique for endoscopic decompression of ZTBTN, and recently, it has been recognized that the failure of site II surgery may be because of the lack of identification and decompression of this second temple trigger—ATN compression.
To justify site V surgery, besides history of higher pain in the temple area, point tenderness and response to nerve blocks are utilized. These patients may complain of more superior pain localized in the temple as opposed to diffuse low temporal pain closer to the lateral canthus (site II). In addition, one can perform a Doppler scan of the area and confirm the artery/nerve compression point preoperatively as described by Guyuron et al.15 We find the intraoperative use of Doppler to be particularly helpful. The direct approach for site V has been used with no clear description in the literature of the specific technique yielding the safest and most efficient ATN decompression, commonly performed in conjunction with ZTBTN decompression. Herein, we describe our technique for site V surgery, which can be used during the endoscopic approach for ZTBTN or separately.
METHODS
Preoperative screening consists of history and physical examination, including positive response to nerve blocks and pain and point tenderness above the temporomandibular joint (TMJ) along the main trunk of the temporal artery (preauricular fascial bands, site 5B) or along the compression point corresponding to the anterior branch of the STA (site 5A). Preoperative marking is based on the point of maximal tenderness and Doppler findings to pinpoint vessel location. Tenderness above the TMJ in the periauricular distribution of the ATN should not be confused with TMJ tenderness, which is commonly associated with dental malocclusion, TMJ clicking on examination, and tenderness above the condyle rather than on the TMJ itself.
If ATN surgery is combined with endoscopic ZTBTN surgery, the former is addressed first. The forehead is injected with 1% lidocaine with epinephrine 1/100,000 in non–hair-bearing areas and 1% lidocaine with 0.25% ropivacaine 1/200,000 in hair-bearing areas for adequate hemostasis. It is imperative that before injection, the location of the vessel is identified, because the vasoconstrictive effect of epinephrine may make this difficult. Doppler may also be used after incision, because identification of the vessel may be difficult without excessive dissection, which could result in injury to the surrounding nerves or in difficulty placing Guyuron endoscopic access devices.
Standard 5–7 port incision is designed for site II surgery as described by Guyuron.5,16 After marking with the aid of Doppler, a 1.5-cm lateral incision is made and is extended anteriorly if necessary. Retraction is done with double hooks. Dissection to identify the vessel and nerve should be performed with blunt tip scissors along the direction of the vessel to avoid unnecessary trauma and bleeding. Attention must be paid to the hair follicles during electrocautery dissection, done after incision to avoid a bloody field. (See Video 1, Supplemental Digital Content 1, which demonstrates the preoperative Doppler signaling the course of the superficial temporal artery. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A184.)
Video Graphic 1.
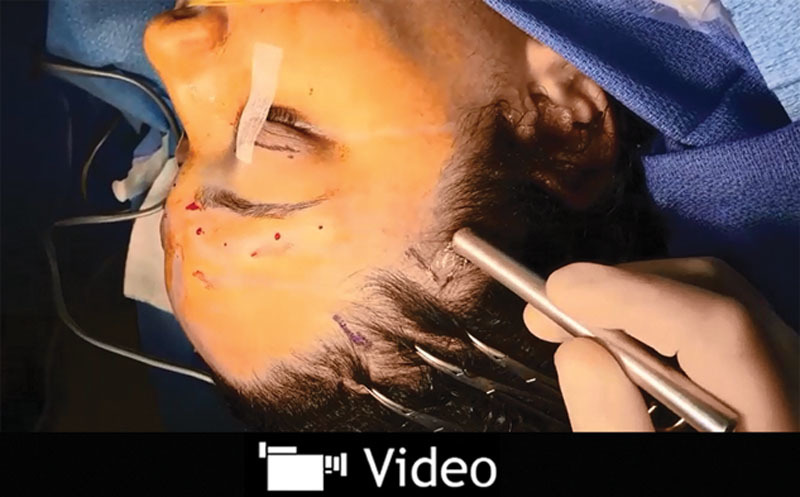
Preoperative Doppler signaling the course of the superficial temporal artery. Note the incision locations: the lateral most incision where the superficial temporal artery compresses the auriculotemporal nerve is roughly 9 to 11 cm from midline. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A184.
Use of Doppler during surgery is often required to minimize unnecessary dissection in search of the vessel. (See Video 2, Supplemental Digital Content 2, which demonstrates the intraoperative Doppler signal once the incision is made to identify the artery to allow for proper decompression of the auriculotemporal nerve. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A185.) The vessel and nerve are commonly found in the superficial layers and along the superficial temporal fascia (Fig. 1). Use of blunt-tip bipolar and regular suction is enough to safely ligate the vessel and nerve after identification. Sharp-tip bipolar at this level will result in cauterization that is not sufficient for the caliber of the vessel and will cause bleeding after the bipolar is separated from the artery. Although the area of dissection is far cephalad to the temporal branch of the facial nerve, caution should be exercised. The patient is not paralyzed. Pediatric patients or those with a short forehead or anterior hairline are at risk for a transmitted cautery wave injury to the facial nerve.
Video Graphic 2.
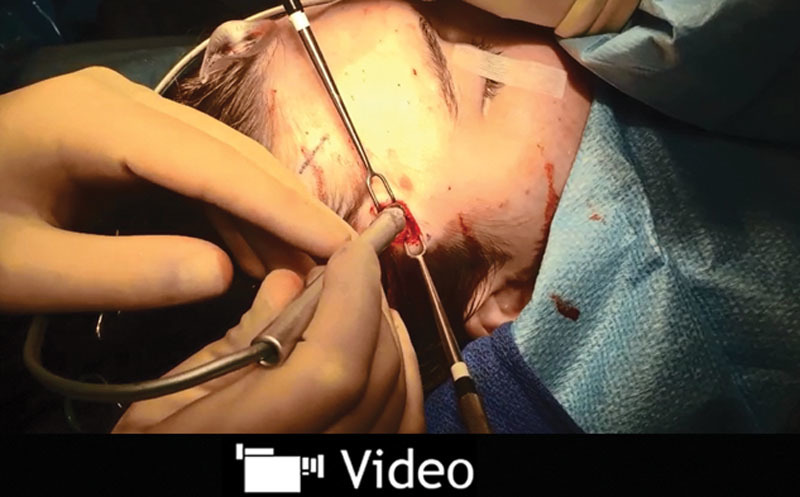
Intraoperative Doppler signal once the incision is made to identify the artery to allow for proper decompression of the auriculotemporal nerve. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A185.
Fig. 1.
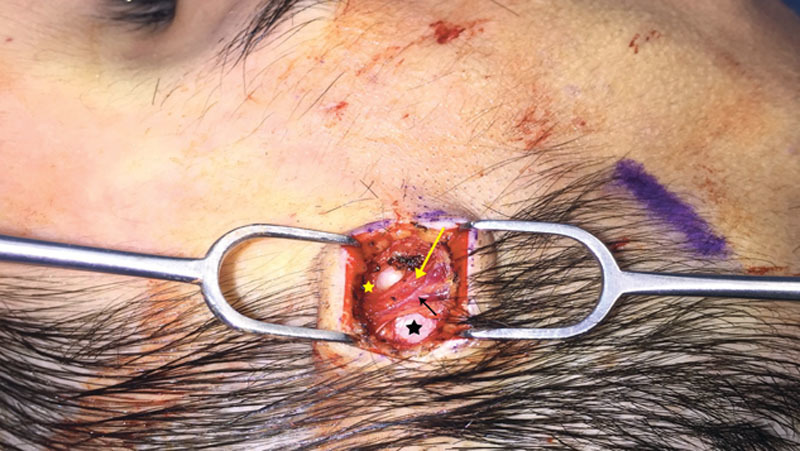
Relationship of the auriculotemporal nerve (yellow arrow) with the superficial temporal artery (black arrow). Note that the artery and nerve are found superficial to the deep temporal fascia (black star) in the same plane as the superficial temporal fascia (yellow star).
The deep temporal fascia is then identified with the Guyuron-described dissection and placement of Guyuron Endoscopic Assist Device (EAD) ports. Because the incision is at times extended to identify the temporal artery, the EAD may have to be fixed in place with two 4-0 nylon sutures to prevent dislodgment when using the endoscope.
If one is concerned about an unusually anterior temporal artery causing compression and pain (scars beyond the hairline or proximity to the temporal branch of the facial nerve), ligation of the main trunk of the ATN in the preauricular area is chosen. The area of maximal tenderness above the TMJ, which hosts the main trunk of the ATN, is accessed, and the vessel and nerve are ligated in this area. A 1.5-cm incision is made 0.5 cm in front of the tragus and above the TMJ area with the aid of Doppler. The main trunk of the ATN is identified first, and the vessel is then located in the deeper plane, commonly associated with another small nerve branch. (See Video 3, Supplemental Digital Content 3, which displays the preauricular incision showing the superficial temporal artery and the main trunk of the auriculotemporal nerve. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A186.) Caution should be used to avoid injury to the facial nerve, which is deep to the dissection, and to the commonly visualized vein, which is in a more superficial plane.
Video Graphic 3.
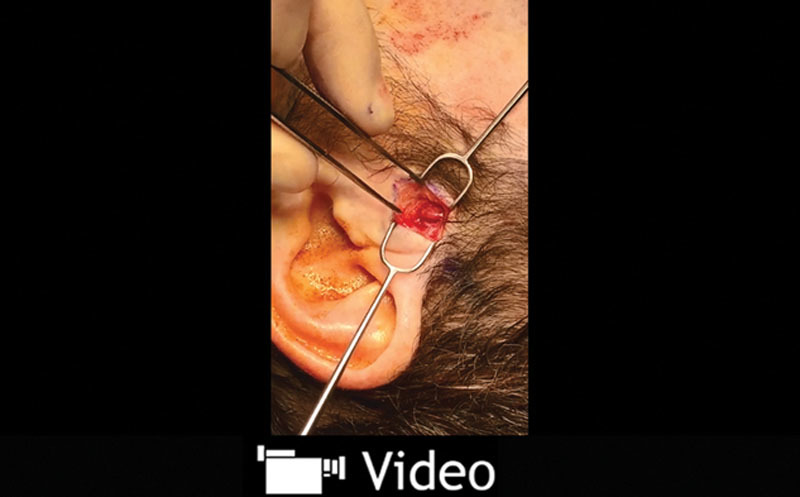
Preauricular incision showing the superficial temporal artery and the main trunk of the auriculotemporal nerve. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A186.
Even with vessel ligation in this area, ATN decompression and STA ligation in the most lateral port should be done, if possible, because of collateral flow that may still exist. If both areas are to be decompressed, lateral port access and decompression are performed first to ensure better visualization of the artery.
Both techniques can be easily combined with decompression of the ZTBTN, each adding 10 to 20 minutes of dissection time. All incisions are closed using 5-0 and 4-0 Monocryl with running locking 5-0 fast suture. Postoperative use of silicone gel will help maintain an aesthetically pleasing scar.
RESULTS
We reviewed 30 patients who underwent this technique with no intraoperative or postoperative complication. The vessel and area of maximal tenderness corresponded to the lateral port with 0.5-cm variation (10–11 cm from midline) in 25 cases. In 2 cases, this point did not correlate with the hair line, one being 2 cm anterior and another being 1.5 cm posterior. In the former case, 5B only surgery was performed. Five cases underwent combined 5A and 5B surgery.
DISCUSSION
Here, we describe a safe and efficient technique for decompression of the ATN that may be easily combined with ZTBTN decompression. Long-term follow-up is needed to compare this technique with the use of a separate incision for decompressing–avulsing the ATN at the lateral port level, as well as comparison with a combined decompression of the ATN with ligation of the superficial temporal artery (TA) at the proximal origin (5B) and 5A surgery. The outcome of the site V decompression will be analyzed in prospective and retrospective trials conducted in the future.
Although preoperative markings in the area of maximal tenderness are important, the use of intraoperative Doppler for identification of the TA during 5A surgery is paramount. The point of crossing with the anterior branch of the temporal artery has been described by Janis et al17; however, its location may be slightly variable in relation to the port incisions. The vessel, which closely corresponds to the nerve and point of maximal tenderness, is usually more anterior to this incision, and the port incision must often be extended up to 0.5 cm for better visualization. Intraoperative use of Doppler here prevents excessive anterior dissection, which can place the temporal branch of the facial nerve at risk and disrupts the fascial planes, causing difficulty later during the endoscopic procedure.
In the unusual case that ATN decompression is needed without ZTBTN decompression, the orientation of the cephalic decompression incision can be varied, especially if this point is more in the hairline. However, we believe that the anteroposterior Guyuron port incision results in the best aesthetic outcomes and efficiency, especially when combined with site II surgery.
In theory, ligation of the TA and avulsion of the ATN more proximally in the periauricular area alone can be effective, and one may consider this in cases of isolated tenderness in this area. However, if tenderness is present in the 5A site, every effort should be made to address both areas to avoid collateral flow to the distal portion of the artery, resulting in recurrence of symptoms.
CONCLUSIONS
Routine close attention to all 3 sites (2, 5A, and 5B) is necessary to avoid potential failure of migraine nerve decompression surgery. This stepwise method for ATN decompression during the Guyuron endoscopic approach has shown to be a time-efficient and safe technique.
Supplementary Material
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Schueler M, Messlinger K, Dux M, et al. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Schueler M, Neuhuber WL, De Col R, et al. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache. 2014;54:996–1009. doi: 10.1111/head.12371. [DOI] [PubMed] [Google Scholar]
- 3.Guyuron B, Yohannes E, Miller R, et al. Electron microscopic and proteomic comparison of terminal branches of the trigeminal nerve in patients with and without migraine headaches. Plast Reconstr Surg. 2014;134:796e–805e. doi: 10.1097/PRS.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyuron B, Varghai A, Michelow BJ, et al. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106:429–434; discussion 435–437. doi: 10.1097/00006534-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Guyuron B, Tucker T, Davis J. Surgical treatment of migraine headaches. Plast Reconstr Surg. 2002;109:2183–2189. doi: 10.1097/00006534-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Dirnberger F, Becker K. Surgical treatment of migraine headaches by corrugator muscle resection. Plast Reconstr Surg. 2004;114:652–657; discussion 658. doi: 10.1097/01.prs.0000131906.27281.17. [DOI] [PubMed] [Google Scholar]
- 7.Guyuron B, Kriegler JS, Davis J, et al. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005;115:1–9. [PubMed] [Google Scholar]
- 8.Poggi JT, Grizzell BE, Helmer SD. Confirmation of surgical decompression to relieve migraine headaches. Plast Reconstr Surg. 2008;122:115–122; discussion 123. doi: 10.1097/PRS.0b013e31817742da. [DOI] [PubMed] [Google Scholar]
- 9.Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. doi: 10.1097/PRS.0b013e3181adcf6a. [DOI] [PubMed] [Google Scholar]
- 10.Guyuron B, Kriegler JS, Davis J, et al. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;127:603–608. doi: 10.1097/PRS.0b013e3181fed456. [DOI] [PubMed] [Google Scholar]
- 11.Janis JE, Barker JC, Javadi C, et al. A review of current evidence in the surgical treatment of migraine headaches. Plast Reconstr Surg. 2014;134(4 suppl 2):131S–41S. doi: 10.1097/PRS.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 12.Ducic I, Hartmann EC, Larson EE. Indications and outcomes for surgical treatment of patients with chronic migraine headaches caused by occipital neuralgia. Plast Reconstr Surg. 2009;123:1453–1461. doi: 10.1097/PRS.0b013e3181a0720e. [DOI] [PubMed] [Google Scholar]
- 13.Chim H, Okada HC, Brown MS, et al. The auriculotemporal nerve in etiology of migraine headaches: compression points and anatomical variations. Plast Reconstr Surg. 2012;130:336–341. doi: 10.1097/PRS.0b013e3182589dd5. [DOI] [PubMed] [Google Scholar]
- 14.Guyuron B, Totonchi A, Moore J, et al. In: 8th Annual Surgical Treatment of Migraine Headaches Symposium, Case Western Reserve University. Cleveland, Ohio: 2012. Surgical treatment of migraine headaches. [Google Scholar]
- 15.Guyuron B, Riazi H, Long T, et al. Use of a Doppler signal to confirm migraine headache trigger sites. Plast Reconstr Surg. 2015;135:1109–1112. doi: 10.1097/PRS.0000000000001102. [DOI] [PubMed] [Google Scholar]
- 16.Achauer B. Plastic Surgery: Indications, Operations, and Outcomes. St. Louis, MO: Mosby; 2000. [Google Scholar]
- 17.Janis JE, Hatef DA, Ducic I, et al. Anatomy of the auriculotemporal nerve: variations in its relationship to the superficial temporal artery and implications for the treatment of migraine headaches. Plast Reconstr Surg. 2010;125:1422–1428. doi: 10.1097/PRS.0b013e3181d4fb05. [DOI] [PubMed] [Google Scholar]


