Abstract
Background
Endothelial dysfunction is an early stage of atherosclerosis. Single‐nucleotide polymorphisms (SNPs) have been associated with vascular dysfunction, cardiac events, and coronary artery remodeling. We aimed to detect SNPs associated with endothelial dysfunction and determine whether these associations are sex specific.
Methods and Results
Six hundred forty‐three subjects without significant obstructive coronary artery disease underwent invasive coronary endothelial function assessment. We collected data from 1536 SNPs that had previously been associated with vasoreactivity, angiogenesis, inflammation, artery calcification, atherosclerotic risk factors, insulin resistance, hormone levels, blood coagulability, or with coronary heart disease. Coronary vascular reactivity was assessed by the percent change in coronary artery diameter ≤ −20% after an intracoronary bolus injection of acetylcholine on invasive coronary physiology study. SNPs significantly associated with coronary epicardial endothelial dysfunction were ADORA1,KCNQ1, and DNAJC4 in the whole cohort, LPA, MYBPH, ADORA3, and PON1 in women and KIF6 and NFKB1 in men (P<0.01).
Conclusions
We have identified several significant SNPs that are associated with an increased risk of coronary endothelial dysfunction. These associations appear to be sex specific and may explain gender‐related differences in development of atherosclerosis.
Keywords: acetylcholine, coronary disease, endothelium, genetics
Subject Categories: Functional Genomics, Genetics
Introduction
Coronary endothelial dysfunction (CED) is an early stage in the development of atherosclerosis and is an independent predictor of adverse cardiovascular outcomes.1, 2, 3, 4, 5 Sex has been identified as an independent factor contributing to cardiovascular disease morbidity and mortality and endothelial dysfunction, but the role of CED has not been fully explored.2, 6
Sex plays a role in the development of atherosclerosis, but sex‐specific genetic associations and the relationship with endothelial dysfunction have not been identified.7 Healthy women have been shown to have higher endothelium‐dependent dilation when compared to their male counterparts, and the presence of cardiovascular risk factors has been associated with sex‐specific effects on endothelium‐dependent dilation.8 The Women's Ischemic Syndrome Evaluation study also suggested that sex plays a role in development of atherosclerosis, reporting increased likelihood of diffuse plaque formation in women when compared to men who developed discrete coronary lesions.9 Moreover, men have been shown to have a greater atheroma burden with more eccentric atheroma and diffuse epicardial endothelial dysfunction than women, suggesting increased structural and functional epicardial abnormalities when compared to their female counterparts.10 The reason for this difference is not entirely understood, and may be affected by genetic variability among single‐nucleotide polymorphisms (SNPs) and their association with early atherosclerosis and CED.10 Genetic variations have been thought to play a role in the development of cardiovascular disease, and recent studies have focused on defining the genes that are responsible.11, 12, 13, 14 New loci associated with cardiovascular risk factors, subclinical indexes, and disease end points have provided important insights that shed light on biologic pathways that may be involved in the development of cardiovascular disease. These can plausibly be targeted for prevention and treatment in the future.6 Identification of genotypic predictors of disease may enhance our understanding of molecular mechanisms underlying CED and the development of atherosclerosis. While several studies have focused on determination of genetic associations with cardiovascular disease, few have focused on genetic and sex‐specific associations with endothelial dysfunction, a precursor of cardiovascular disease. We aimed to evaluate this genetic variability and determine the association of sex‐specific SNPs with epicardial CED.
Methods
Patient Population
The study was performed at Mayo Clinic in Rochester, Minnesota. The study protocol was approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from each patient.
As described previously,15 cardiac catheterization and coronary microvascular function testing was performed on 643 patients enrolled between January 1993 and December 2010. The decision to pursue cardiac catheterization and invasive coronary physiology testing was made by the referring cardiologist. Patients presented with chest pain and had clinical indication for routine coronary angiography to rule out underlying coronary artery disease. All patients enrolled in the study had no evidence of obstruction of coronary arteries on coronary angiography. They then underwent a coronary physiology study to evaluate for coronary microvascular dysfunction.
All patients who met criteria to undergo a coronary physiology study were included in the study. Exclusion criteria included unstable angina pectoris, history of uncontrolled systemic hypertension that requires long‐term therapy, valvular heart disease, left ventricular ejection fraction <40%, and/or significant endocrine disorders including diabetes, hepatic, renal, or inflammatory disease.
Study Protocol
Vasoactive medications were discontinued for at least 36 hours prior to catheterization. Eating, drinking, or tobacco use was discontinued for at least 12 hours prior to the procedure.15, 16, 17, 18, 19, 20, 21, 22 Diagnostic coronary angiography was performed with a 6F or 7F guiding catheter using a standard femoral percutaneous approach. Nonionic contrast material was used. No nitroglycerin was given prior to the diagnostic procedure. Unfractionated intravenous heparin was administered for an activated clotting time of ≈250 s.
Coronary vascular reactivity was studied using a 0.014‐inch Doppler tipped guidewire (FloWire: Volcano Corp, CA) in the left anterior descending coronary artery.15, 16, 17, 18, 19, 20, 21, 22 Intracoronary infusion of incremental doses of acetylcholine to a maximum tolerable dose (10−6, 10−5, and 10−4 mol/L at 1 mL/min at 3‐minute intervals) was given. Epicardial coronary artery endothelium‐dependent function was calculated as the percent increase in coronary artery diameter, (CAd) in response to acetylcholine. The cut‐off points were derived from the presence or absence of impaired epicardial endothelial function, defined as percent change in CAd (%CAd) to acetylcholine less than −20%.16, 20
Genomic Data and Blood Collection
As previously described,15, 23DNA was extracted from blood using the Mayo Clinic Biospecimens Accessioning and Processing facility. Pico Green analysis was run on all samples to assess quality. Samples were genotyped at the Mayo Genotyping Core facility using an Illumina custom GoldenGate panel (San Diego, CA).24 Per 96‐well plate, there were 85 unique samples, 5 duplicate DNA samples, and 6 quality control CEPH samples. The 1536 tag SNPs represented genes with previously identified associations with coronary vasoreactivity, angiogenesis, inflammation, artery calcification, atherosclerosis risk factors, insulin resistance, female hormones, blood coagulation system, or prevalence of coronary heart disease (CHD). Of these, 242 SNPs were excluded from the analysis due to minor allele frequencies <5%, Hardy Weinberg Equilibrium, P‐values <0.001, or SNP call rates <95% (ie, missing values for at least 5% of the subjects). The majority of SNPs failed because they were monomorphic or had a very low minor allele frequency. Genetic positions were listed in Build 36.
Statistic Methodology
All statistical analysis was performed using PLINK and SAS version 9.3 (SAS Institute Inc., Cary, NC).25 Categorical data were analyzed using the χ2 test and continuous variables were analyzed using two‐sample t test and summarized using mean±SD. Logistic regression was run using the end point of %CAd response to acetylcholine < −20 to test for genetic differences after adjusting for age, sex, diabetes, smoking status, and body mass index, assuming a log‐additive genetic model. Models were run testing for an interaction between sex and each SNP to determine sex‐specific associations.
Results
Baseline Characteristics
Overall, median age was 49.7±11.4 years. Median age of women was 51.4±10.9 years, while it was 46.6 years for men. The majority of patients were of European ancestry (93% white+5.6% unknown and presumed white). Baseline characteristics are summarized in Table 1. Of women enrolled, 58% were postmenopausal. While only 8% of the population had diabetes, hypertension was present in 41%, dyslipidemia in 55%, and 13% were current smokers. Aspirin was used by 50%, 37% used calcium channel blockers, and 39% used lipid lowering drugs. Of women, 27% were using hormone replacement therapy.
Table 1.
Patient Characteristics
| All | Women | Men | P Value | |
|---|---|---|---|---|
| n=643 | n=426 | n=217 | ||
| Age, y | 49.7 (11.4) | 51.4 (10.9) | 46.6 (11.7) | <0.001 |
| Body mass index, kg/m2 | 29.1 (6.2) | 29.2 (6.8) | 28.9 (4.7) | 0.63 |
| Postmenopausal | 243 (58%) | 243 (58%) | — (—) | |
| % change CAd (Ach) | −15.1 (21.2) | −13.6 (20.3) | −17.9 (22.7) | 0.015 |
| Risk factor | ||||
| Diabetes mellitus | 52 (8%) | 28 (7%) | 24 (11%) | 0.049 |
| Hypertension | 264 (41%) | 166 (39%) | 98 (45%) | 0.13 |
| Dyslipidemia | 353 (55%) | 218 (52%) | 135 (62%) | 0.010 |
| Family history | 409 (65%) | 271 (65%) | 138 (66%) | 0.77 |
| Lipoprotein A | 24.0 (31.0) | 23.7 (30.5) | 24.6 (32.3) | 0.76 |
| hsCRP, mg/dL | 2.7 (24.0) | 3.4 (29.5) | 1.4 (3.5) | 0.37 |
| Homocysteine, μmol/L | 8.0 (4.4) | 7.8 (5.0) | 8.4 (2.9) | 0.12 |
| Smoking | <0.001 | |||
| Never | 326 (51%) | 242 (57%) | 84 (39%) | |
| Former | 232 (36%) | 146 (34%) | 86 (40%) | |
| Current | 83 (13%) | 37 (9%) | 46 (21%) | |
| Drugs | ||||
| Aspirin | 326 (51%) | 206 (48%) | 120 (55%) | 0.010 |
| Calcium channel blockers | 239 (37%) | 150 (35%) | 89 (41%) | 0.12 |
| Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker | 104 (16%) | 62 (15%) | 42 (19%) | 0.12 |
| β‐Blocker | 186 (29%) | 128 (30%) | 58 (27%) | 0.38 |
| Diuretics | 106 (16%) | 85 (20%) | 21 (10%) | <0.001 |
| Lipid‐lowering drugs | 248 (39%) | 151 (35%) | 97 (45%) | 0.020 |
| Estrogen replacement therapy | 117 (27%) | 117 (27%) | — (—) | |
Values are given as n (%) or mean (SD). P‐value shows women vs men. Ach, acetylcholine; CAd, coronary artery diameter; hsCRP, high‐sensitivity C‐reactive protein.
Coronary Epicardial Endothelial Dysfunction
The %CAd induced by acetylcholine was lower in men than in women (Table 1). Diabetes and smoking were significantly higher in patients with abnormal endothelial function when compared to patients with normal endothelial function. Medication was similar between men and women.
Coronary Epicardial Endothelial Dysfunction and SNP Analysis
We identified several SNPs associated with epicardial endothelial dysfunction (Figure). In women (Table 2), rs12038000 was associated with the adenosine A3 receptor gene (ADORA3), rs16851008 with ADORA1, and k1_201395343 and rs16851020 with both ADORA1 and with myosin binding protein H gene (MYBPH). Moreover, rs7767084, rs9365171, rs3798221, rs9364564, rs7453899, rs35600881, rs13202636, and rs1321195 were all associated with LPA, and rs2237583 is associated with paraoxonase 1 gene (PON1). These SNPs were associated with an increased risk of abnormal %CAd induced by acetylcholine (P<0.01).
Figure 1.
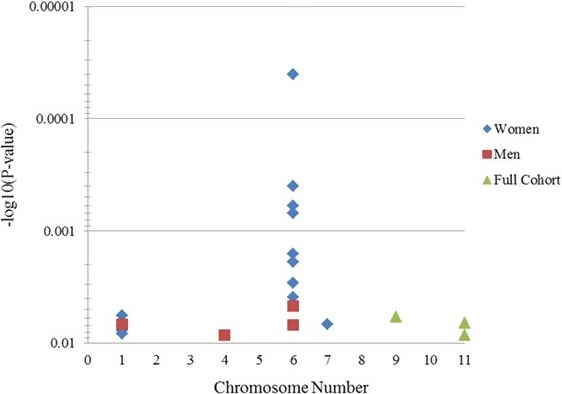
Macrovascular significant SNPs: P values (minus log‐transformed) are shown in a signal intensity (Manhattan) plot relative to their genomic position in macrovascular endothelial function. Each SNP is plotted with respect to its chromosomal location (x axis) and its P value (y axis on the left). The minimum y axis marks the threshold for significance (P=0.01). SNP indicates single‐nucleotide polymorphism.
Table 2.
SNPs Associated With Macrovascular/Epicardial Endothelial Dysfunction Significant Only Among Females
| Chr. | Significant SNP in Females | Position | Gene Region | Risk Allele X/Y | Overall Allele X Frequency | OR Overall | P Value Overall | OR Men | P Value Men | OR Women | P Value Women | P Value SNP×Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs12038000 | 111859362 | ADORA3 | A/G | 0.61 | 1.30 | 0.0357 | 0.93 | 0.7307 | 1.54 | 0.0057a | 0.0708 |
| 1 | rs16851008 | 201387919 | ADORA1 | G/A | 0.87 | 1.33 | 0.1066 | 0.79 | 0.3910 | 1.93 | 0.0077a | 0.0354 |
| 1 | k1_201395343 | 201395343 | ADORA1/MYBPH | A/G | 0.87 | 1.28 | 0.1549 | 0.73 | 0.2538 | 1.94 | 0.0083a | 0.0254 |
| 1 | rs16851020 | 201400275 | MYBPH/ADORA1 | C/A | 0.87 | 1.29 | 0.1502 | 0.70 | 0.2132 | 1.96 | 0.0070a | 0.0182 |
| 6 | rs7767084a | 160882493a | LPA a | G/A | 0.16 | 1.32 | 0.0780 | 0.71 | 0.2483 | 1.73 | 0.0039a | 0.0075a |
| 6 | rs9365171 | 160901726 | LPA | A/C | 0.35 | 1.37 | 0.0118 | 0.99 | 0.9691 | 1.65 | 0.0016a | 0.0571 |
| 6 | rs3798221a | 160918138a | LPA a | T/G | 0.19 | 1.58 | 0.0023 | 0.80 | 0.4244 | 2.08 | 0.00004a | 0.0061a |
| 6 | rs9364564a | 160919030a | LPA a | A/G | 0.18 | 1.50 | 0.0134 | 0.77 | 0.3854 | 2.00 | 0.0004a | 0.0097a |
| 6 | rs7453899 | 160930756 | LPA | T/A | 0.35 | 1.35 | 0.0167 | 1.00 | 1.0000 | 1.60 | 0.0029a | 0.0848 |
| 6 | rs35600881 | 160946754 | LPA | A/G | 0.23 | 1.50 | 0.0046 | 0.95 | 0.8481 | 1.79 | 0.0006a | 0.0487 |
| 6 | rs13202636 | 160949718 | LPA | G/A | 0.23 | 1.49 | 0.0053 | 0.95 | 0.8481 | 1.78 | 0.0007a | 0.0520 |
| 6 | rs1321195 | 161004146 | LPA | T/C | 0.13 | 1.48 | 0.0256 | 0.80 | 0.4877 | 1.90 | 0.0019a | 0.0267 |
| 7 | rs2237583a | 94788113a | PON1 a | G/A | 0.70 | 1.15 | 0.3002 | 0.70 | 0.0898 | 1.63 | 0.0068a | 0.0030a |
OR indicates odds ratio; SNPs, single‐nucleotide polymorphisms.
SNPs both significant in sex and SNP×Sex.
In men (Table 3), we found that rs17511046 was associated with ADORA1, rs3774933 and rs1599961 were associated with nuclear factor of κ light polypeptide gene enhancer in B‐cell 1 gene (NF‐κB1), and rs20456 is associated with both LOC100124373 and KIF6. Also, k6_39474093 is associated with KIF6. These SNPs were associated with increased risk of abnormal coronary artery dilation indicating epicardial endothelial dysfunction (P<0.01). Only ADORA1 and NFKB1 SNPs showed significant differences between men and women. The strongest evidence for differences between men and women was seen in SNPs on LPA and PON1 (interaction P<0.01) (Table 3).
Table 3.
SNPs Associated With Macrovascular/Epicardial Endothelial Dysfunction Significant Only Among Males
| Chr. | Significant SNP in Males | Position | Gene Region | Risk Allele X/Y | Overall Allele X Frequency | OR Overall | P Value Overall | OR Men | P Value Men | OR Women | P Value Women | P Value SNP×Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs17511046a | 201376668a | ADORA1 a | G/A | 0.93 | 1.17 | 0.5066 | 3.88 | 0.0068a | 0.68 | 0.1609 | 0.0034a |
| 4 | rs3774933a | 103645369a | NFKB1 a | G/A | 0.39 | 1.06 | 0.6362 | 1.72 | 0.0085a | 0.80 | 0.1472 | 0.0068a |
| 4 | rs1599961a | 103662599a | NFKB1 a | A/G | 0.39 | 1.06 | 0.6362 | 1.72 | 0.0085a | 0.80 | 0.1472 | 0.0068a |
| 6 | rs20456 | 39432901 | LOC100124373/KIF6 | C/T | 0.43 | 1.31 | 0.0283 | 1.70 | 0.0069a | 1.10 | 0.5601 | 0.0622 |
| 6 | k6_39474093 | 39474093 | KIF6 | T/A | 0.06 | 1.95 | 0.0060 | 4.00 | 0.0047a | 1.44 | 0.2188 | 0.0582 |
OR indicates, odds ratio; SNPs, single‐nucleotide polymorphisms.
SNPs both significant in sex and SNP×Sex.
Comparison of SNPs was also made by stratifying patients into 3 groups: men, premenopausal women, and postmenopausal women, in order to assess the effect of menopause on these findings. We find that certain SNPs remain significantly associated with macrovascular endothelial dysfunction in premenopausal women, with others found to be significant in postmenopausal women (Tables 4 and 5). For example, rs12038000 was associated with the adenosine A3 receptor gene (ADORA3), rs16851008 with ADORA1, and k1_201395343 and rs16851020 with both ADORA1 and with myosin binding protein H gene (MYBPH) in postmenopausal women but not in premenopausal women (Tables S2 and S3). rs2237583 was significantly associated with PON1 in postmenopausal women but not premenopausal women, and rs7767084 was associated with LPA in premenopausal women but not in postmenopausal women. rs9365171, rs3798221, rs9364564, and rs7453899 were associated with LPA in both premenopausal women and postmenopausal women.
Table 4.
Genes With Significant SNPs in Men for Macrovascular Endothelial Dysfunction
| Gene With Associated Significant SNPs in Men | Significant SNP | SNP×Sex Significant P Values |
|---|---|---|
| ADORA1 a | rs17511046a | 0.0034a |
| KIF6 a | rs20456 | |
| k6_39474093 | ||
| NFKB1 a | rs3774933a | 0.0068a |
| rs1599961a | 0.0068a | |
| LOC100124373 | rs20456 | |
| ADORA1 a | rs17511046a | 0.0034a |
SNPs indicates single‐nucleotide polymorphisms.
SNPs both significant in sex and SNP×Sex.
Table 5.
Genes With Significant SNPs in Women for Macrovascular Endothelial Dysfunction
| Gene With Associated Significant SNPs in Women | Significant SNP | SNP×Sex Significant P Values |
|---|---|---|
| ADORA1 | rs16851008 | |
| k1_201395343 | ||
| rs16851020 | ||
| LPA | rs7767084a | 0.0075a |
| rs9365171 | ||
| rs3798221a | 0.0061a | |
| rs9364564a | 0.0097a | |
| rs7453899 | ||
| rs36500881 | ||
| rs13202636 | ||
| rs1321195 | ||
| MYBPH | k1_201395343 | |
| rs16851020 | ||
| ADORA3 | rs12038000 |
SNPs indicates single‐nucleotide polymorphisms.
SNPs both significant in sex and SNP×Sex.
Discussion
The current study demonstrates sex‐specific differences in SNPs and overlap region in some genes associated with epicardial CED in humans. These observations may explain differences in the propensity for development of early atherosclerosis between men and women and may have potential sex‐specific therapeutic implications.
Genetic Associations in Women
In women, variations within ADORA3, ADORA1, MYBPH, LPA, and PON1 genes were associated with epicardial CED. This is a plausible association that plays a crucial role in vascular homeostasis, which may be regulated by sex hormones, especially estrogen.26 Estrogen contributes to regulation of vascular tone, modulates recruitment of circulating cells, effects platelet function, and plays a role in processes responsible for vascular repair.26 Gene variants in women modulate the function of estrogen, its receptors, and are implicated in aspects of cardiovascular inflammation, platelet function, and vascular repair.26, 27
Moreover, risk associated with LPA, PON1, and KCNQ1 variants affecting CED may be mediated through dysfunction in lipid metabolism. LPA risk alleles correlate with high plasma lipoprotein (a), which is associated with atherosclerotic vascular disease leading to CHD and may be thrombogenic.28 It inhibits fibrinolysis, accumulates in the vascular wall in atherosclerotic lesions, and may proliferate in human smooth muscle cells.28, 29 PON1 exerts anti‐atherogenic effects by protecting low‐density lipoproteins against oxidation, which has been implicated in oxidative stress and coronary spasm.29 Thus, PON1 is associated with ox‐low‐density lipoprotein, which is associated with CED.30 KCNQ1 SNPs are associated with type 2 diabetes mellitus via a reduction in insulin secretion and higher fasting glucose. This may affect lipid metabolism in patients with type 2 diabetes and CHD.31
Adenosine plays an important role in cardiac reperfusion response to ischemia.32 Variants in adenosine receptor genes including ADORA3 and ADORA1 may predict cardiac response to ischemia or injury.32 Upregulation of adenosine A3 receptors mRNA upon preconditioning is sex specific and depends on a woman's menstrual cycle.33 In this study, the gene region of A1 receptors was alike in both women and men. Our study suggests that sex‐specific SNPs on ADORA3 relate to epicardial CED and that ADORA1 polymorphisms play a sex‐specific role in endothelial function, depending on the variation of the SNP.
Myosin binding protein H (MYPH) was also associated with epicardial CED in women. It is known that MYPH is expressed in ventricular Purkinje cells,34 but how MYBPH gene mediates coronary epicardial endothelial function has yet to be elucidated. While little is known about MYPH and its association with coronary epicardial endothelial function, awareness of various genetic polymorphisms associated with epicardial CED is important since further studies on these genes may implicate a mechanism explaining this association.
Estrogen likely plays a significant role in explaining these findings and may partially explain the sex‐specific differences. Several genetic variations were noted in postmenopausal women but not premenopausal women. On the other hand, several genetic variations do not appear to be associated with menopausal status, and rather simply female sex. Additional investigation to further define the association with menopause is necessary.
Genetic Associations in Men
In men, genetic variations within ADORA1, NFKB1, LOC100124373, and KIF 6 genes are associated with epicardial CED. Gene variants related with the activity of nitric oxide synthase itself, angiogenesis, and inflammation modify CED in men in our study. These SNPs in men may mediate testosterone or its receptors and have adverse effects on cardiovascular morbidity and mortality.35
Nuclear factor‐κB denotes a family of transcription factors involved in both pro‐inflammatory and anti‐inflammatory processes in atherogenesis.36 Polymorphisms in NFKB1 promoter are associated with an increased risk of CHD and heart failure.36 Inflammatory mechanisms affect all phases of coronary artery disease. Errors in genes encoding inflammatory or anti‐inflammatory molecules are candidates for increasing risk of developing complications from coronary artery disease.36 There are sex‐specific variations in NF‐κB activity, which may play a role in development of CED and atherosclerosis.37, 38
It is important to note the overlap in gene regions between women and men in our study. We found overlap of gene region in ADORA1 and KIF6 without duplication of SNPs. The kinesin family member 6 gene encodes an intracellular protein, which transports cellular cargo and is expressed in coronary endothelial cells.39 The association between KIF6 polymorphism and increased risk of CHD in male patients has been previously described.40 Arg719 allele of KIF6 was associated with increased risk of CHD and myocardial infarction in populations of healthy women with low prevalence of CHD.40 KIF6 polymorphisms may be involved in interrupting intracellular transport in endothelial cells in women or men depending on the SNPs predisposing to development of CED and CHD.
Limitations
Our study has several inherent limitations. First, this is a cross‐sectional study in a unique patient population with early coronary atherosclerosis as defined by endothelial dysfunction. Further studies are needed to determine why a significant variant might have an effect in males and not in females with similar cardiovascular risk factors.
While most SNPs that we identified as significantly associated with early coronary atherosclerosis characterized by CED lie within currently presumed noncoding intronic sequences, there are several potential mechanisms that could explain their association. These SNPs may be in linkage disequilibrium with promoter SNPs that have not yet been identified or that were not genotyped in this study.41 Furthermore, these intronic SNPs may have promoter functions that have not yet been identified, and intronic variants may potentially affect receptor function through alternative splicing mechanisms41 SNPs in the 5′ upstream region could play a significant role affecting gene transcription.
Additionally, the number of tests performed is another limitation of the study. We have performed a large number of tests and it is possible that these results are significant purely by chance. Further studies are necessary to confirm these findings.
Conclusions
The current study reports for the first time the association between sex‐specific gene variants and physiological functional abnormalities related to early coronary atherosclerosis in humans characterized by epicardial endothelial dysfunction in coronary arteries. The study may help explain sex‐specific differences in development of coronary endothelial function and atherosclerosis.
Sources of Funding
This work was supported by the National Institutes of Health (NIH Grant HL‐92954 and AG‐31750 to A. Lerman) and P30CA15083 (Mayo Clinic Cancer Center) and CTSA Grant Number KL2 RR024151 to P. J. M. Best. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. S. Yoshino was supported by Fukuda Foundation for Medical Technology, Japan.
Disclosures
None.
Supporting information
Table S1. Patient Characteristics
Table S2. SNPs Associated With Macrovascular/Epicardial Endothelial Dysfunction Significant Only Among Females
Table S3. SNPs Associated With Macrovascular/Epicardial Endothelial Dysfunction Significant Only Among Males
(J Am Heart Assoc. 2016;5:e002544 doi: 10.1161/JAHA.115.002544)
References
- 1. Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–2782b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang J, Bingaman S, Huxley VH. Intrinsic sex‐specific differences in microvascular endothelial cell phosphodiesterases. Am J Physiol Heart Circ Physiol. 2010;298:H1146–H1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruggiero D, Paolillo S, Ratta GD, Mariniello A, Formisano T, Pellegrino AM, Filardi PP. [Endothelial function as a marker of pre‐clinical atherosclerosis: assessment techniques and clinical implications]. Monaldi Arch Chest Dis. 2013;80:106–110. [DOI] [PubMed] [Google Scholar]
- 4. Ghanavatian S, Diep LM, Barany P, Heimburger O, Seeberger A, Stenvinkel P, Rohani M, Agewall S. Subclinical atherosclerosis, endothelial function, and serum inflammatory markers in chronic kidney disease stages 3 to 4. Angiology. 2014;65:443–449. [DOI] [PubMed] [Google Scholar]
- 5. Garcia MM, Lima PR, Correia LC. Prognostic value of endothelial function in patients with atherosclerosis: systematic review. Arq Bras Cardiol. 2012;99:857–865. [DOI] [PubMed] [Google Scholar]
- 6. O'Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med. 2011;365:2098–2109. [DOI] [PubMed] [Google Scholar]
- 7. Spence JD, Pilote L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis. 2015;241:208–210. [DOI] [PubMed] [Google Scholar]
- 8. Brar V, Gill S, Cardillo C, Tesauro M, Panza JA, Campia U. Sex‐specific effects of cardiovascular risk factors on endothelium‐dependent dilation and endothelin activity in middle‐aged women and men. PLoS One. 2015;10:e0121810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender‐based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–S29. [DOI] [PubMed] [Google Scholar]
- 10. Han SH, Bae JH, Holmes DR Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–1369. [DOI] [PubMed] [Google Scholar]
- 11. Smolkova B, Bonassi S, Buocikova V, Dusinska M, Horska A, Kuba D, Dzupinkova Z, Raslova K, Gasparovic J, Sliz I, Ceppi M, Vohnout B, Wsolova L, Volkovova K. Genetic determinants of quantitative traits associated with cardiovascular disease risk. Mutat Res. 2015;778:18–25. [DOI] [PubMed] [Google Scholar]
- 12. Rankinen T, Sarzynski MA, Ghosh S, Bouchard C. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circ Res. 2015;116:909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puckelwartz MJ, McNally EM. Genetic profiling for risk reduction in human cardiovascular disease. Genes (Basel). 2014;5:214–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muir AR, Menown IB. Genetic biomarkers in cardiovascular disease. Biomark Med. 2013;7:497–499. [DOI] [PubMed] [Google Scholar]
- 15. Yoshino S, Cilluffo R, Best PJ, Atkinson EJ, Aoki T, Cunningham JM, de Andrade M, Choi BJ, Lerman LO, Lerman A. Single nucleotide polymorphisms associated with abnormal coronary microvascular function. Coron Artery Dis. 2014;25:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 17. Widmer RJ, Flammer AJ, Herrmann J, Rodriguez‐Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;304:H393–H397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. [DOI] [PubMed] [Google Scholar]
- 19. Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol. 2000;35:1654–1660. [DOI] [PubMed] [Google Scholar]
- 20. Al Suwaidi J, Higano ST, Holmes DR Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. [DOI] [PubMed] [Google Scholar]
- 21. Nishimura RA, Lerman A, Chesebro JH, Ilstrup DM, Hodge DO, Higano ST, Holmes DR Jr, Tajik AJ. Epicardial vasomotor responses to acetylcholine are not predicted by coronary atherosclerosis as assessed by intracoronary ultrasound. J Am Coll Cardiol. 1995;26:41–49. [DOI] [PubMed] [Google Scholar]
- 22. Lerman A, Holmes DR Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431. [DOI] [PubMed] [Google Scholar]
- 23. Lu C, Gao Y, Zhou H, Tian H. The relationships between PON1 activity as well as oxLDL levels and coronary artery lesions in CHD patients with diabetes mellitus or impaired fasting glucose. Coron Artery Dis. 2008;19:565–573. [DOI] [PubMed] [Google Scholar]
- 24. Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost‐effective approach to high‐throughput genotyping. Biotechniques. 2002;suppl:56–58, 60‐1. [PubMed] [Google Scholar]
- 25. Purcell S, Neale B, Todd‐Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole‐genome association and population‐based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium‐derived hyperpolarizing factor. J Endocrinol. 2008;197:447–462. [DOI] [PubMed] [Google Scholar]
- 27. Miller VM, Best PJ. Implications for reproductive medicine: sex differences in cardiovascular disease. Sex Reprod Menopause. 2011;9:21–28. [PMC free article] [PubMed] [Google Scholar]
- 28. Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, Jones GT, van Rij AM, Eapen DJ, Baas AF, Tregouet DA, Morange PE, Emmerich J, Lindblad B, Gottsater A, Kiemeny LA, Lindholt JS, Sakalihasan N, Ferrell RE, Carey DJ, Elmore JR, Tsao PS, Grarup N, Jorgensen T, Witte DR, Hansen T, Pedersen O, Pola R, Gaetani E, Magnadottir HB, Wijmenga C, Tromp G, Ronkainen A, Ruigrok YM, Blankensteijn JD, Mueller T, Wells PS, Corral J, Soria JM, Souto JC, Peden JF, Jalilzadeh S, Mayosi BM, Keavney B, Strawbridge RJ, Sabater‐Lleal M, Gertow K, Baldassarre D, Nyyssonen K, Rauramaa R, Smit AJ, Mannarino E, Giral P, Tremoli E, de Faire U, Humphries SE, Hamsten A, Haraldsdottir V, Olafsson I, Magnusson MK, Samani NJ, Levey AI, Markus HS, Kostulas K, Dichgans M, Berger K, Kuhlenbaumer G, Ringelstein EB, Stoll M, Seedorf U, Rothwell PM, Powell JT, Kuivaniemi H, Onundarson PT, Valdimarsson E, Matthiasson SE, Gudbjartsson DF, Thorgeirsson G, Quyyumi AA, Watkins H, Farrall M, Thorsteinsdottir U, Stefansson K. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–729. [DOI] [PubMed] [Google Scholar]
- 29. Ito T, Yasue H, Yoshimura M, Nakamura S, Nakayama M, Shimasaki Y, Harada E, Mizuno Y, Kawano H, Ogawa H. Paraoxonase gene Gln192Arg (Q192R) polymorphism is associated with coronary artery spasm. Hum Genet. 2002;110:89–94. [DOI] [PubMed] [Google Scholar]
- 30. Lavi S, McConnell JP, Lavi R, Barsness GW, Rihal CS, Novak GD, Lerman LO, Lerman A. Association between the paraoxonase‐1 192Q>R allelic variant and coronary endothelial dysfunction in patients with early coronary artery disease. Mayo Clin Proc. 2008;83:158–164. [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, Yin Q, Ma G, Qian Q. KCNQ1 gene polymorphisms are associated with lipid parameters in a Chinese Han population. Cardiovasc Diabetol. 2010;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang Z, Diamond MA, Chen JM, Holly TA, Bonow RO, Dasgupta A, Hyslop T, Purzycki A, Wagner J, McNamara DM, Kukulski T, Wos S, Velazquez EJ, Ardlie K, Feldman AM. Polymorphisms in adenosine receptor genes are associated with infarct size in patients with ischemic cardiomyopathy. Clin Pharmacol Ther. 2007;82:435–440. [DOI] [PubMed] [Google Scholar]
- 33. von Arnim CA, Etrich SM, Timmler M, Riepe MW. Gender‐dependent hypoxic tolerance mediated via gender‐specific mechanisms. J Neurosci Res. 2002;68:84–88. [DOI] [PubMed] [Google Scholar]
- 34. Schiaffino S. Protean patterns of gene expression in the heart conduction system. Circ Res. 1997;80:749–750. [DOI] [PubMed] [Google Scholar]
- 35. Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res. 2011;90:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santos DG, Resende MF, Mill JG, Mansur AJ, Krieger JE, Pereira AC. Nuclear factor (NF) kappaB polymorphism is associated with heart function in patients with heart failure. BMC Med Genet. 2010;11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dale E, Davis M, Faustman DL. A role for transcription factor NF‐kappaB in autoimmunity: possible interactions of genes, sex, and the immune response. Adv Physiol Educ. 2006;30:152–158. [DOI] [PubMed] [Google Scholar]
- 38. Vina J, Gambini J, Lopez‐Grueso R, Abdelaziz KM, Jove M, Borras C. Females live longer than males: role of oxidative stress. Curr Pharm Des. 2011;17:3959–3965. [DOI] [PubMed] [Google Scholar]
- 39. Povel CM, Boer JM, Onland‐Moret NC, Dolle ME, Feskens EJ, van der Schouw YT. Single nucleotide polymorphisms (SNPs) involved in insulin resistance, weight regulation, lipid metabolism and inflammation in relation to metabolic syndrome: an epidemiological study. Cardiovasc Diabetol. 2012;11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng P, Lian J, Huang RS, Xu L, Huang Y, Ba Y, Yang X, Huang X, Dong C, Zhang L, Ye M, Zhou J, Duan S. Meta‐analyses of KIF6 Trp719Arg in coronary heart disease and statin therapeutic effect. PLoS One. 2012;7:e50126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Zheng Y, Zhang W, Yu H, Lou K, Zhang Y, Qin Q, Zhao B, Yang Y, Hui R. Polymorphisms of KDR gene are associated with coronary heart disease. J Am Coll Cardiol. 2007;50:760–767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics
Table S2. SNPs Associated With Macrovascular/Epicardial Endothelial Dysfunction Significant Only Among Females
Table S3. SNPs Associated With Macrovascular/Epicardial Endothelial Dysfunction Significant Only Among Males


