Abstract
Background
The endogenous amino acid homoarginine predicts mortality in cerebro‐ and cardiovascular disease. The objective was to explore whether homoarginine is associated with atrial fibrillation (AF) and outcome in patients with acute chest pain.
Methods and Results
One thousand six hundred forty‐nine patients with acute chest pain were consecutively enrolled in this study, of whom 589 were diagnosed acute coronary syndrome (ACS). On admission, plasma concentrations of homoarginine as well as brain natriuretic peptide (BNP), and high‐sensitivity assayed troponin I (hsTnI) were determined along with electrocardiography (ECG) variables. During a median follow‐up of 183 days, 60 major adverse cardiovascular events (MACEs; 3.8%), including all‐cause death, myocardial infarction, or stroke, were registered in the overall study population and 43 MACEs (7.5%) in the ACS subgroup. Adjusted multivariable Cox regression analyses revealed that an increase of 1 SD of plasma log‐transformed homoarginine (0.37) was associated with a hazard reduction of 26% (hazard ratio [HR], 0.74; 95% CI, 0.57–0.96) for incident MACE and likewise of 35% (HR, 0.65; 95% CI, 0.49–0.88) in ACS patients. In Kaplan–Meier survival curves, homoarginine was predictive for patients with high‐sensitivity assayed troponin I (hsTnI) above 27 ng/L (P<0.05). Last, homoarginine was inversely associated with QTc duration (P<0.001) and prevalent AF (OR, 0.83; 95% CI, 0.71–0.95).
Conclusion
Low plasma homoarginine was identified as a risk marker for incident MACEs in patients with acute chest pain, in particular, in those with elevated hsTnI. Impaired homoarginine was associated with prevalent AF. Further studies are needed to investigate the link to AF and evaluate homoarginine as a therapeutic option for these patients.
Keywords: acute coronary syndrome, atrial fibrillation, homoarginine, l‐arginine:glycine amidinotransferase
Subject Categories: Atrial Fibrillation, Acute Coronary Syndromes, Biomarkers, Endothelium/Vascular Type/Nitric Oxide
Introduction
During the last 5 years, several studies indicated that low concentrations of the naturally occurring amino acid homoarginine predict a risk of adverse cardio‐ and cerebrovascular outcome and of mortality (reviewed in previous works1, 2). Analyses of the associated clinical phenotypes, as well as experimental studies in mice, have proven a mechanistic link of impaired homoarginine and cerebrovascular disease.3 Synthesis of homoarginine in humans is catalyzed by the enzyme, l‐arginine:glycine amidinotransferase (AGAT, EC 2.1.4.1), which is also known as the first and rate‐limiting enzyme of creatine synthesis.1 Because of a structural similarity with l‐arginine, homoarginine is supposed to interfere with the nitric oxide (NO) pathway. Homoarginine is a weak alternative NO synthase substrate, competes with l‐arginine for cellular influx and efflux transporters, and improves l‐arginine availability, inhibiting its metabolism by arginases.4 In line with these mechanistic insights, low homoarginine concentrations have been linked to phenotypes of (sub)clinical atherosclerosis, that is, brachial intima‐media and aortic wall thickness.5, 6 In patients with stable coronary heart disease, endothelial dysfunction and vessel obliteration are the clinical features of a general atherosclerotic phenotype with a subsequent coronary manifestation. Onset of an acute coronary syndrome (ACS) is related to plaque instability and subsequent inflammation, coronary thrombosis, and/or vasospasms. The link between endothelial dysfunction and ACS is thus not direct, but peripheral endothelial function testing predicts cardiovascular outcome in ACS, and subsequent normalization of endothelial function in these patients lowers event rates.7, 8
Atrial fibrillation (AF) is independently associated with an increased risk of incident myocardial infarction (MI).9 Symptoms of AF and ACS as dyspnea and chest discomfort are similar and may thus obscure the presence of acute myocardial ischemia.10 The concomitance of AF in ACS was shown to complicate MI, given that conduction of rapid arrhythmias causes additional impairment of coronary perfusion and left ventricular function.11 Unsurprisingly, patients with ACS and coexisting AF were shown to have worse outcomes.12
To explore whether homoarginine levels are associated with AF and outcome in patients with acute chest pain, plasma homoarginine was measured in 1649 consecutive patients presenting at 3 German chest pain unit centers. Specifically, this study aimed to investigate (1) the associations of homoarginine with cardiovascular outcome and (2) a potential relationship with additional biomarkers and cardiovascular phenotypes.
Methods
Study Protocol
A total of 1818 patients ages 18 to 85 with suspected ACS were enrolled in an observational multicenter cohort at 3 German tertiary care centers between January 2007 and December 2008 (University Medical Center of the Johannes–Gutenberg University Mainz, University Heart Center Hamburg‐Eppendorf, and Federal Armed Forces Hospital Koblenz). Eligible patients were between 18 and 85 years of age. Exclusion criteria were major surgery or trauma within the preceding 4 weeks, pregnancy, intravenous drug misuse, and anemia with hemoglobin level below 10 g/dL. Subjects provided blood samples on admission for biomarker determination in parallel with routine blood draws.13, 14 At the same time point, a 12‐lead electrocardiogram (ECG) was recorded. Medical history was taken, including time of chest pain onset, a physical examination was performed, and questionnaire data on cardiovascular disease risk factors and symptoms were collected based on self‐reported information and medical charts. All patients provided written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committees in Rhineland‐Palatinate and Hamburg. Of 1818 patients initially enrolled in the study, 1649 (66% men) were included in the present analysis based on available follow‐up (n=1785) and homoarginine data (n=1680; overlap exists), of whom 589 (36%) were diagnosed with ACS (n=221 unstable angina pectoris [UAP], n=111 ST‐elevation myocardial infarction [STEMI], and n=257 non‐ST‐elevation myocardial infarction [NSTEMI]).
Definition of Variables
Individuals treated with antihypertensive medication or previously diagnosed as having hypertension were considered to have hypertension. Individuals with total cholesterol >200 mg/dL at admission or with a previous hyperlipidemia diagnosis were regarded as hyperlipidemic. Patients on medication or dietary treatment for diabetes were considered to have diabetes mellitus. Obesity was defined as body mass index (BMI) ≥30 kg/m2. Estimated glomerular filtration rate (eGFR) was computed using the CKD‐EPI formula.15 Previous cardiovascular disease (CVD) and chronic kidney disease (CKD) were self‐reported in a standardized interview. Prevalent AF was diagnosed based upon evaluation of the 12‐lead ECG recordings at admission. QRS and QTc duration were assessed using baseline (at admission) ECG recordings. For QRS analysis, participants were excluded for prevalent AF on ECG; pacemaker in place; ventricular pre‐excitation; second‐ or third‐degree heart block; history of MI or heart failure; and QRS ≥120 ms. For QT analysis, participants were excluded for prevalent AF on ECG; pacemaker in place; QRS ≥120 ms.
Measurement of Plasma Homoarginine and Additional Biomarkers and Laboratory Parameters
Venous blood was collected into EDTA tubes. After centrifugation, EDTA plasma aliquots were stored at −80°C in 2 central laboratories (University Medical Center of the Johannes‐Gutenberg University Mainz and University Heart Center Hamburg‐Eppendorf). For each analyte, all samples were analysed in 1 batch in one of the central laboratories. Plasma homoarginine was determined from frozen EDTA plasma samples with a high throughput mass spectrometric (MS) assay, applying electrospray ionization/liquid chromatography (LC)‐MS/MS.16 Briefly, proteins were precipitated by adding 25 μL of EDTA plasma to 100 μL of internal standard (2.5 μmol/L [13C6]‐homoarginine) dissolved in methanol. Samples were centrifuged, evaporated, and subsequently converted to their butyl ester derivatives using 1 N of butanolic hydrochloric acid. After centrifugation, eluates were dried by heating and redissolved in 100 μL of methanol/water (25:75), containing 0.1% ammonium formate, before measurement. Plates were placed in a CTC PAL autosampler, and 20‐μL aliquots were subjected to further analysis in the MS system (Varian 1200 MS; Agilent Technologies, Santa Clara, CA). The lower limit of quantification for homoarginine was determined to be 0.01 μmol/L. Intra‐ and interassay coefficients of variation were ≤7.5%. l‐arginine, asymmetric dimethylarginine (ADMA), and symmetric dimethylarginine (SDMA) were measured applying LC‐MS/MS,17 as described in detail elsewhere.18 Brain natriuretic peptide (BNP), high‐sensitivity C‐reactive protein (hsCRP), high‐sensitivity assayed troponin I (hsTnI), midregional proadrenomedullin (MR‐proADM), midregional proatrial natriuretic peptide (MR‐proANP), cystatin C, high‐density lipoprotein (HDL) cholesterol, triglycerides, and creatine kinase were measured using dedicated commercial assays, as described elsewhere.14, 19
Mortality and Nonfatal Cardiovascular Event Assessment
Six‐month follow‐up was performed by standardized telephone interview, letter, and review of medical charts. If patients could not be contacted by telephone interview or letter, the general practitioner was contacted. Medical charts were obtained from hospitals and general practitioners. All self‐reported nonfatal strokes and MIs were verified by medical charts. Major adverse cardiovascular events (MACEs), including nonfatal strokes and MIs, fatal strokes and MIs, and all‐cause mortality, were adjudicated by at least 2 cardiologists blinded for homoarginine measurements. Follow‐up information was missing in 2% of enrolled patients.
Statistical Analyses
Participants were divided into 2 groups based on diagnosis of ACS and noncardiac chest pain (NCCP). Categorical variables are reported as proportions, and continuous variables are reported as medians and interquartile ranges [IQRs]. Comparison of 2 groups was performed with Fisher's exact test for binary variables and the Mann–Whitney U test for continuous variables. Three group comparisons of quantitative variables were done with the help of the Kruskal–Wallis test.
Spearman correlations were used to calculate univariate associations between homoarginine and continuous variables. A linear regression with log‐transformed homoarginine as the dependent variable and cardiovascular risk factors as independent variables was performed. The association of homoarginine to QRS and QTc duration was examined linearly regressing log‐homoarginine on each of these ECG variables using different multivariable adjustments. A combined endpoint comprising all‐cause mortality, nonfatal stroke, and nonfatal MI (MACE) was used in all survival analyses. A homoarginine cutoff for 6‐month prediction of MACE was derived by maximizing the sum of 6‐month sensitivity and specificity for MACE. Sensitivity and specificity were computed using the Kaplan–Meier approach described in Heagerty et al.20 Kaplan–Meier survival curves at the median value of homoarginine as well as at the evaluated cutoff for homoarginine were constructed in the overall sample and the subgroup of ACS patients. Statistical comparisons were made using the log‐rank test. Cox proportional hazards modeling was used to calculate hazard ratios (HRs) and 95% CIs using a series of multivariable adjustments. For these analyses, homoarginine was used after being log‐transformed. In model 1, age and sex were included. Model 2 comprised model 1 with additional adjustment for other risk factors similar to the GRACE model applied previously in this cohort.19 Model 3 comprised model 2 with additional adjustment for in‐hospital treatment (percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]). HRs are given for a 1‐SD increase in log‐homoarginine. The proportional hazards assumption was tested and visually assessed using the methods described by Grambsch and Therneau.21 No deviations from this assumption were found. Patients were divided into 2 BNP groups using a cut‐off value of 80 pg/mL as a predictor of the presence of congestive heart failure,22 and the homoarginine cutoff was used to produce survival curves on each BNP group. The log‐rank test was used to test for differences. This was repeated, exchanging BNP by hsTnI, a previously identified biomarker for MI, with a cutoff of 27 ng/L based on the 99th percentile in 5000 individuals of the Gutenberg Health Study.23
The association of AF and homoarginine, which was used log‐transformed, was examined using logistic regression. Three models were considered, the first one was adjusted for age and sex; the second for variables in model 1 plus common cardiovascular risk factors. Model 3 comprised model 2 including adjustment for in‐hospital treatment (PCI or CABG). Statistical analyses were performed using R software (version 3.2.2; R Core Team 2013; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/). All statistical tests reported are 2‐sided, with significance defined by P<0.05.
Results
Description of the Study Sample
The present study included 1649 patients (66% males) with a mean age of 62±13 years. Among all patients included, 589 were diagnosed with ACS (368 acute myocardial infarctions [AMIs] and 221 UAPs) and 1060 with NCCPs. Bivariate analyses among these groups revealed higher prevalence of traditional cardiovascular risk factors (specifically, current smoker, diabetes mellitus, hypertension, or history of CVD) in the ACS subsample (Table 1). In patients diagnosed with ACS, the cardiac biomarkers, BNP, hsTnI, and hsCRP, were significantly increased. Median [IQR] plasma homoarginine was 2.38 [1.86–3.01] μmol/L, with moderately lower levels in patients with AMI (2.34 [1.80–2.92] μmol/L; n=368) compared to patients with UAP (2.40 [1.95–3.05] μmol/L; n=221) or NCCP (2.40 [1.86–3.05] μmol/L; n=1060; P=0.11 for Kruskal–Wallis test). Crude Spearman correlation analyses for circulating homoarginine are summarized in Table S1. Inverse correlations with homoarginine were noticed for age, QTc duration, cystatin C, and HDL cholesterol. Positive correlations were observed with BMI, triglycerides, and circulating l‐arginine concentrations.
Table 1.
Baseline Demographics and Clinical Characteristics of All Patients and for Acute Coronary Syndrome and Noncardiac Chest Pain Subsamples
| All Patients n=1649 | Acute Coronary Syndrome n=589 | Noncardiac Chest Pain n=1060 | P Value | |
|---|---|---|---|---|
| Age, y | 64 [53, 72] | 66 [56, 74] | 61 [50, 70] | <0.001 |
| Sex, men (%) | 1088 (66) | 428 (73) | 660 (62) | <0.001 |
| Body mass index, kg/cm2 | 27.2 [24.8, 30.4] | 27.5 [24.8, 30.4] | 27 [24.7, 30.4] | 0.23 |
| Systolic BP, mm Hg | 140 [129, 160] | 145 [130, 160] | 140 [127, 158] | 0.06 |
| Diastolic BP, mm Hg | 80 [70, 85] | 80 [70, 86] | 78 [70, 85] | 0.08 |
| Current smoker, n (%) | 384 (23) | 157 (27) | 227 (22) | 0.01 |
| Diabetes, n (%) | 253 (16) | 127 (22) | 126 (12) | <0.001 |
| Hypertension, n (%) | 1223 (74) | 473 (80) | 750 (71) | <0.001 |
| Previous MI, n (%) | 370 (23) | 163 (28) | 207 (20) | <0.001 |
| Cerebrovascular disease of stroke, n (%) | 92 (5.7) | 46 (7.9) | 46 (4.4) | <0.01 |
| Atrial fibrillation, n (%) | 288 (18) | 95 (16) | 193 (18) | 0.28 |
| Congestive heart failure, n (%) | 68 (4.3) | 27 (4.9) | 41 (4.0) | 0.44 |
| Medication | ||||
| ß‐Blocker, n (%) | 654 (40) | 256 (44) | 398 (38) | 0.02 |
| ACE inhibitor, n (%) | 574 (35) | 232 (39) | 342 (32) | <0.01 |
| Diuretics, n (%) | 473 (29) | 183 (31) | 290 (27) | 0.11 |
| Statin, n (%) | 517 (31) | 211 (36) | 306 (30) | <0.01 |
| Antiplatelet drug, n (%) | 653 (40) | 275 (47) | 378 (36) | <0.001 |
| QRS duration, ms | 98 [92, 108] | 100 [92, 110] | 98 [92, 108] | 0.10 |
| QTc duration, ms | 433 [414, 454] | 436 [416, 458] | 433 [413, 452] | 0.03 |
| Time of chest pain onset, h | 4.3 [2.0, 13.2] | 4.5 [2.0, 15.4] | 4.2 [2.0, 11.8] | 0.09 |
| PCI or CABG, n (%) | 453 (28) | 453 (77) | 0 (0) | <0.001 |
| Total cholesterol, mg/dL | 195 [165, 227] | 198 [167, 233] | 194 [164, 224] | 0.05 |
| LDL, mg/dL | 116 [89, 145] | 123 [94, 152] | 113 [87, 142] | <0.001 |
| HDL, mg/dL | 48 [40, 59] | 46 [38, 56] | 49 [40, 61] | <0.001 |
| Triglycerides, mg/dL | 119 [76, 188] | 123 [79, 189] | 117 [75, 187] | 0.29 |
| Homoarginine, μmol/L | 2.38 [1.86, 3.01] | 2.37 [1.85, 2.95] | 2.40 [1.86, 3.05] | 0.40 |
| l‐Arginine, μmol/L | 117 [92, 145] | 117 [87, 144] | 118 [94, 146] | 0.07 |
| ADMA, μmol/L | 0.60 [0.52, 0.69] | 0.60 [0.53, 0.69] | 0.60 [0.52, 0.68] | 0.65 |
| SDMA, μmol/L | 0.50 [0.42, 0.60] | 0.50 [0.42, 0.60] | 0.50 [0.42, 0.60] | 0.45 |
| BNP, pg/mL | 34.3 [12.2, 10.3] | 61.3 [21.0, 182] | 26.8 [5.0, 69.0] | <0.001 |
| hsCRP, mg/L | 2.5 [1.3, 5.7] | 2.9 [1.5, 6.6] | 2.3 [1.1, 5.3] | <0.001 |
| hsTnI, ng/L | 7.9 [3.7, 40.1] | 76.8 [13.3, 637] | 4.9 [3.0, 10.5] | <0.001 |
| eGFR, mL/min for 1.73 m2 | 84.3 [69.1, 95.4] | 81.9 [65.8, 93.6] | 85.4 [71.3, 96.9] | <0.001 |
| Cystatin C, mg/L | 0.67 [0.58, 0.79] | 0.70 [0.60, 0.83] | 0.65 [0.56, 0.77] | <0.001 |
Data are presented as median [interquartile range] or n (%), as appropriate. ADMA indicates asymmetric dimethylarginine; BP, blood pressure; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate computed using the CKD‐EPI formula; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; hsTnI, high‐sensitivity assayed troponin I; LDL, low‐density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; SDMA, symmetric dimethylarginine.
In a multivariable linear regression model, log‐homoarginine was independently and positively associated with male sex, BMI, systolic blood pressure (BP), and eGFR. Negative associations were observed with congestive heart failure, current smoking, and age. No significant association was observed with HDL cholesterol, diabetes mellitus, and previous MI (Table 2).
Table 2.
Linear Regression Modeling for Homoarginine
| Variable | ß‐Estimate (SE) | P Value |
|---|---|---|
| Age, y | −0.004 (0.001) | <0.001 |
| Sex, male | 0.122 (0.022) | <0.001 |
| BMI, kg/m2 | 0.013 (0.002) | <0.001 |
| Systolic BP, mm Hg | 0.001 (0.000) | 0.004 |
| Current smoking | −0.104 (0.025) | <0.001 |
| Diabetes mellitus | −0.001 (0.029) | 0.98 |
| Congestive HF | −0.159 (0.049) | 0.001 |
| Previous MI | −0.029 (0.025) | 0.24 |
| HDL cholesterol, mg/dL | −0.001 (0.001) | 0.09 |
| eGFR, mL/min for 1.73 m2 | 0.002 (0.001) | <0.01 |
For the continuous variables, ß‐estimates are shown for a 1‐unit change in the variable. Homoarginine was log‐transformed. BMI indicates body mass index; BP, blood pressure; eGFR, glomerular filtration rate estimated using the CKD‐EPI formula; HDL, high‐density lipoprotein; HF, heart failure; MI, myocardial infarction.
Homoarginine and Major Adverse Outcomes in the Study Sample
During a median follow‐up of 183 days, a total of 60 MACEs (3.8%), including 19 deaths, 10 nonfatal strokes, and 31 nonfatal MIs, were observed. Circulating homoarginine concentrations were significantly higher in participants without incident MACE as compared with those who experienced an event during the follow‐up period (2.39 [1.87–3.01] vs 2.09 [1.48–2.77] μmol/L; P=0.008). In the overall study population, Kaplan–Meier analysis revealed that cumulative incidence of MACE was significantly higher in individuals with homoarginine at or below the median (2.38 μmol/L; 4.7%) compared with homoarginine above the median (2.9%; P<0.05; Figure 1A). A similar association was observed in the subgroup of diagnosed ACS (43 MACE [7.5%], including 13 deaths, 4 nonfatal strokes, and 26 nonfatal MIs; P<0.005; Figure 1B). In adjusted Cox regression analyses, an increase of 1 SD log‐homoarginine was associated with a hazard reduction of 26% for incident MACE (model 3; Table 3). Similar results were observed in the subgroup of ACS patients; hazard reduction related to an increase of homoarginine was 35% in the adjusted model (model 3; Table 3). The predictive cut‐off value for homoarginine for MACE in this study population was determined to be 1.41 (95% CI, 1.25, 2.77) μmol/L, with a sensitivity of 25% and a selectivity of 91%. Using this cutoff, Kaplan–Meier analysis showed a higher cumulative incidence of MACE in individuals with homoarginine at or below the cutoff (1.41 μmol/L; 10.7%) compared with homoarginine above the cutoff (3.1%; P<0.001, log‐rank test; Figure S1A). Similar results were obtained in the ACS subgroup (23% vs 6.0%; P<0.001, log‐rank test; Figure S1B). Further subgroup analyses revealed that homoarginine below the cutoff predicted MACE in patients with low and high BNP equally (P<0.05; Figure 2A). Of note, homoarginine was only predictive in patients with elevated hsTnI (hsTnI >27 ng/L; P<0.01; Figure 2B).
Figure 1.
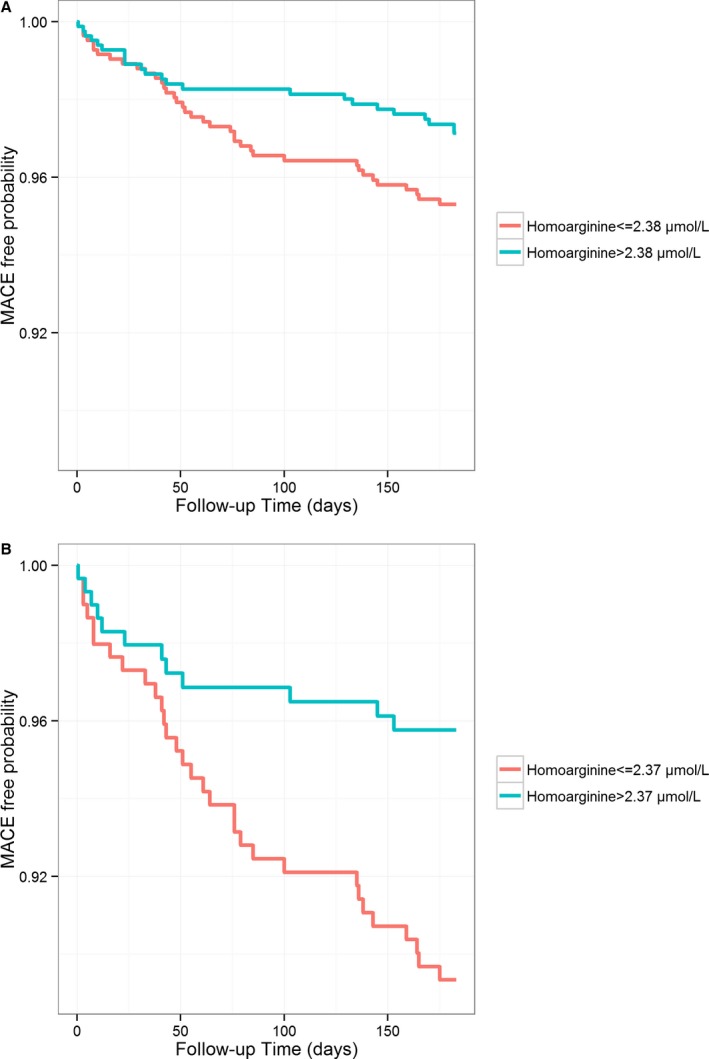
Unadjusted failure curves for incidence of the combined endpoint comprising all‐cause mortality, nonfatal stroke, and nonfatal myocardial infarction according to homoarginine median (A) in the overall study population (2.38 μmol/L; n=1649; P=0.047, log‐rank test) and (B) in the ACS subsample (2.37 μmol/L; n=589; P<0.005, log‐rank test). ACS indicates acute coronary syndrome; MACE, major adverse cardiovascular event.
Table 3.
Multivariable‐Adjusted HRs for Homoarginine for Major Adverse Events
| Modela | HR [95% CI] | Events (n) |
|---|---|---|
| All participants | ||
| 1 (n=1649) | 0.77 [0.60–0.98] | 60 |
| 2 (n=1471) | 0.75 [0.58–0.97] | 56 |
| 3 (n=1471) | 0.74 [0.57–0.96] | 56 |
| Acute coronary syndrome | ||
| 1 (n=589) | 0.61 [0.46–0.82] | 43 |
| 2 (n=533) | 0.66 [0.50–0.88] | 41 |
| 3 (n=533) | 0.65 [0.49–0.88] | 41 |
Data are presented for 1‐SD increase in log‐homoarginine (0.37). CABG indicates coronary artery bypass grafting; EPV, event per variable; HR, hazard ratio; PCI, percutaneous coronary intervention.
Model 1 adjusted for age and sex; model 2 adjusted for variables in model 1 plus body mass index, current smoking, diabetes mellitus, hypertension, and hyperlipidemia; model 3 adjusted for variables in model 2 plus in hospital treatment (PCI or CABG).
Figure 2.
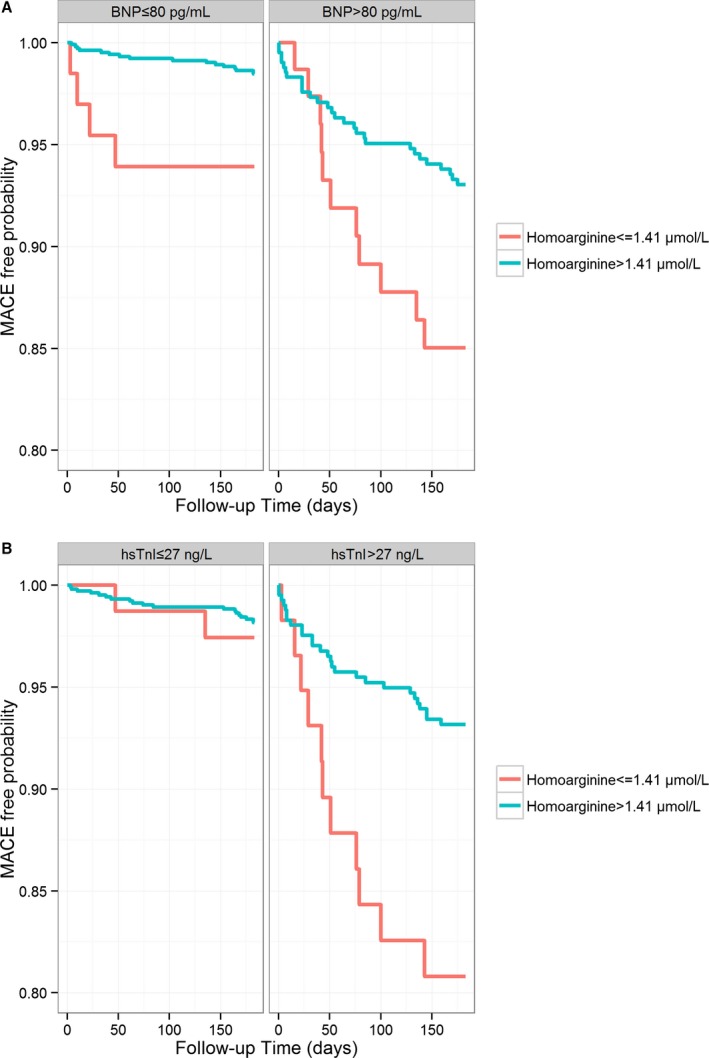
Unadjusted failure curves for incidence of the combined endpoint comprising all‐cause mortality, nonfatal stroke, and nonfatal myocardial infarction according to homoarginine cutoff (1.41 μmol/L) and stratified by (A) BNP (cutoff, 80 pg/mL; n=1127 to 488; P<0.01 and <0.05, log‐rank test) or (B) hsTnI (cutoff, 27 ng/L; n=1131 to 465; P=0.64 and <0.01, log‐rank test). BNP indicates brain natriuretic peptide; hsTnI, high‐sensitivity assayed troponin I; MACE, major adverse cardiovascular event.
Homoarginine and Cardiovascular Phenotypes
The strongest correlations of circulating homoarginine with cardiac biomarkers were observed with MR‐proADM, BNP, and MR‐proANP. Further correlations were observed with creatine kinase and hsTnI (Table 4). In age‐ and sex‐adjusted linear regression analyses, log‐homoarginine was inversely associated with QTc duration (P<0.001; model 1; Table 5). QTc duration remained significantly associated with circulating homoarginine after further adjustment for BMI, current smoking, diabetes mellitus, hypertension, hyperlipidemia, and in‐hospital treatment (P<0.001; model 3; Table 5). In 288 patients suffering from AF, significantly more patients had circulating homoarginine concentrations below/at the median compared to above homoarginine median (n=168 vs 120; P=0.0023, Fisher's exact test; all participants). Similar associations were observed in the ACS subgroup. Adjusted logistic regression analyses showed for an increase of 1 SD log‐homoarginine an odds ratio (OR) reduction for prevalent AF by 17% (model 3; Table 6).
Table 4.
Spearman Correlations Between Homoarginine and Cardiac and Noncardiac Biomarkers in Patients With Acute Chest Pain
| Variables | ρ | P Value |
|---|---|---|
| BNP | −0.23 | <0.001 |
| hsTnI | −0.11 | <0.001 |
| MR‐proANP | −0.23 | <0.001 |
| MR‐proADM | −0.29 | <0.001 |
| Myoglobin | −0.05 | 0.068 |
| Creatine kinase | 0.13 | <0.001 |
| Creatine kinase‐MB | 0.03 | 0.26 |
BNP indicates brain natriuretic peptide; hsTnI, high‐sensitivity assayed troponin I; MR‐proADM, midregional proadrenomedullin; MR‐proANP, midregional proatrial natriuretic peptide.
Table 5.
Homoarginine and Cardiac Phenotypes in Patients With Acute Chest Pain
| Modela | ß‐Estimate (SE) | P Value |
|---|---|---|
| QRS duration | ||
| 1 (n=1635) | −0.0008 (0.0004) | 0.07 |
| 2 (n=1462) | −0.0012 (0.0004) | <0.01 |
| 3 (n=1462) | −0.0012 (0.0004) | <0.01 |
| QTc duration | ||
| 1 (n=1635) | −0.0012 (0.0003) | <0.001 |
| 2 (n=1462) | −0.0012 (0.0003) | <0.001 |
| 3 (n=1462) | −0.0012 (0.0003) | <0.001 |
ß‐estimates are shown for a 1‐unit change in the variable. CABG indicates coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Model 1 adjusted for age and sex; model 2 adjusted for variables in model 1 plus body mass index, current smoking, diabetes mellitus, hypertension, and hyperlipidemia; model 3 adjusted for variables in model 2 plus in hospital treatment (PCI or CABG). Homoarginine was log‐transformed.
Table 6.
Multivariable‐Adjusted ORs for Atrial Fibrillation in Patients With Acute Chest Pain
| Modela | OR [95% CI] | Events (n) |
|---|---|---|
| All participants | ||
| 1 (n=1635) | 0.85 [0.75–0.98] | 288 |
| 2 (n=1462) | 0.83 [0.71–0.95] | 262 |
| 3 (n=1462) | 0.83 [0.71–0.95] | 262 |
Data are presented for 1‐SD increase in log‐homoarginine (0.37). CABG indicates coronary artery bypass grafting; OR, odds ratio; PCI, percutaneous coronary intervention.
Model 1 adjusted for age and sex; model 2 adjusted for variables in model 1 plus body mass index, current smoking, diabetes mellitus, hypertension, and hyperlipidemia; model 3 adjusted for variables in model 2 plus in hospital treatment (PCI or CABG).
Discussion
There are several novel findings from this study. First, low homoarginine concentrations predict cardiovascular outcome in patients with acute chest pain, in particular in those with elevated hsTnI. Second, homoarginine levels are correlated to biomarkers representing cardiovascular function (MR‐proADM, BNP, and MR‐proANP). Last, homoarginine is inversely associated with QTc duration as well as prevalent AF.
Low circulating concentrations of the endogenous amino acid homoarginine have been reported to be a prognostic marker for mortality and cardiovascular events in a variety of diseases, among them heart failure, stroke, and CKD.1, 2 In the present study sample of patients with acute chest pain, median plasma homoarginine was 2.38 [1.86–3.01] μmol/L. Although median homoarginine concentrations were not significantly different between ACS and NCCP patients, the predictive value of low homoarginine on incident MACE—in the overall study population as well as in the subsample of ACS patients—was evident. It is generally accepted that atherosclerosis plays a pivotal role in pathogenesis of ACS.7, 8 In line with this, Valtonen et al. found a positive correlation between homoarginine and endothelium‐dependent brachial artery flow mediated dilation.24 Supporting the association between homoarginine and endothelial function, low circulating homoarginine has recently been linked to phenotypes of (sub)clinical atherosclerosis, that is, brachial intima‐media and aortic wall thickness.5, 6 Vascular atherosclerosis triggers myocardial stress and may, by this end, directly activate the cardiac neurohormonal system.25 We and others found a strong association of low homoarginine concentrations and increased levels of BNP.26, 27, 28, 29 In our study, homoarginine was not only correlated with parameters of myocardial stress (MR‐proADM, BNP, and MR‐proANP), but also with markers of coronary ischemia (creatine kinase, hsTnI). New onset of an acute event occurs upon vessel obliteration and subsequent myocardial necrosis. Of note, the study population investigated here has already been used to identify the predictive value of hsTnI, MR‐proADM, and MR‐proANP for cardiovascular outcomes.13, 19, 23 We therefore determined a cut‐off value for homoarginine for prediction of MACE and evaluated this cutoff in the context of the predefined cutoffs for those already‐established biomarkers, BNP and hsTnI. Only in those patients with elevated hsTnI (cutoff 27 ng/L)23, low homoarginine predicted future cardiovascular events in our study population (Figure 2B). This observation may underline the relevance of homoarginine for patients at higher risk for secondary events. Compared to hsTnI, a valuable marker in the acute phase of coronary ischemia, homoarginine may provide additional prognostic information for short‐ and long‐term outcome. Whereas the present study focused on patients with acute chest pain that were followed up for 183 days, a study including patients routinely referred to coronary angiography and followed for 9.9 years identified circulating homoarginine as a predictor for all‐cause and cardiovascular mortality.30
Of note, homoarginine showed an inverse association with QTc duration. Patients with QTc prolongation are known to experience more likely an ACS.31 In the MERLIN–TIMI 36 study, an abnormal QTc interval was associated with a 2‐fold increased risk of sudden cardiac death in patients with non‐ST elevation ACS.32 So far, nothing is known about homoarginine in AF. In the present study, a statistically significant association of homoarginine with prevalence of AF was detected. Patients suffering from AF were shown to have significantly lower homoarginine concentrations. AF is an important comorbidity in ACS. Most recent studies have demonstrated that AF is associated with a higher risk of subsequent cardiovascular events33 and mortality12 in ACS. Thus, both ECG variables (ie, QTc prolongation) and AF are predictors of adverse outcomes in patients with ACS. Our data showed an association of homoarginine with QTc duration and prevalence of AF. Whether homoarginine is lower because of prevalence of the disease and ECG abnormalities or precedes these electrocardiographic changes cannot be answered from the current analyses. Our data should be viewed as hypothesis generating and thus be interpreted with caution. At this point, further experimental studies are necessary to elucidate a potential causal link between homoarginine and electrocardiographic changes. Whether targeting the homoarginine/AGAT pathway in patients with prevalent AF or QTc prolongation may improve outcome, as shown, for example, for ranolazine,34 needs further investigation.
Study Limitations
As a qualitative hypothesis‐generating study, the hypothesis is based on interview data of the research participants. Even though our data might not be representative for other patients with acute chest pain, the validity and generalizability of the study has been previously shown for the diagnostic value of hsTnI and the prognostic value of MR‐proADM and MR‐proANP.13, 19 Furthermore, the cut‐off value evaluated here for homoarginine (1.41 μmol/L) shows low sensitivity (25%) and might therefore not be generally applicable to other collectives. Further investigations in larger study samples are needed to formulate a cut‐off value with higher sensitivity and selectivity values. Another limitation of our study is that coronary angiography data are not available for the entire study population. Furthermore, the overall event rate is relatively low and no incident AF was documented.
Clinical Implications
Homoarginine is an endogenous compound related to QTc duration and AF, implicating a potential role in risk assessment of supraventricular and ventricular arrhythmias. In addition, homoarginine is associated with parameters of cardiac function and coronary ischemia. It is directly related to incidence of MACE and may thus prospectively play a role in the prognosis of ACS. In an advantage to other markers (eg, hsTnI), homoarginine is targetable by oral supplementation.
Conclusions
In summary, the current findings identified homoarginine as a biomarker for MACE in patients with acute chest pain, in particular in those with elevated hsTnI and as a novel correlate for prevalent AF. Low homoarginine is therefore not only a predictor for adverse outcome in patients with acute chest pain, but might also be a new target in these patients, that is, by homoarginine supplementation or AGAT activation.
Sources of Funding
The study was supported by a research grant provided by the Fachbereich Medizin der Universität Hamburg (Forschungsförderungsfond NWF 13/02) to Dr Atzler, the Deutsche Stiftung für Herzforschung (German Heart Research Foundation F/12/08) to Dr Schwedhelm, and an Else Kröner Memorial Stipendium from the Else Kröner‐Fresenius‐Stiftung to Dr Choe. Dr Atzler acknowledges the support of the European Union under a Marie Curie Intra‐European Fellowship for Career Development. Dr Baum is supported by the Clinical Scientist program of the University Medical Center Hamburg‐Eppendorf.
Disclosures
None.
Supporting information
Table S1. Cross‐Sectional Correlation Analyses of Homoarginine
Figure S1. Unadjusted failure curves for incidence of the combined endpoint comprising all‐cause mortality, nonfatal stroke, and nonfatal myocardial infarction according to homoarginine cutoff (1.41 μmol/L) (A) in the overall study population (n=1649; P<0.001 log‐rank test) and (B) in the ACS subsample (n=589; P<0.001, log‐rank test). ACS indicates acute coronary syndrome.
Acknowledgments
We gratefully thank Mariola Kastner and Anna Steenpaß for their excellent technical assistance.
(J Am Heart Assoc. 2016;5:e002565 doi: 10.1161/JAHA.115.002565)
References
- 1. Atzler D, Schwedhelm E, Choe CU. L‐homoarginine and cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2015;18:83–88. [DOI] [PubMed] [Google Scholar]
- 2. Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, Hartaigh BÓ, Lang F, Alesutan I, Voelkl J, März W, Tomaschitz A. Homoarginine in the renal and cardiovascular systems. Amino Acids. 2015;47:1703–1713. [DOI] [PubMed] [Google Scholar]
- 3. Choe CU, Atzler D, Wild PS, Carter AM, Böger RH, Ojeda F, Simova O, Stockebrand M, Lackner K, Nabuurs C, Marescau B, Streichert T, Müller C, Lüneburg N, De Deyn PP, Benndorf RA, Baldus S, Gerloff C, Blankenberg S, Heerschap A, Grant PJ, Magnus T, Zeller T, Isbrandt D, Schwedhelm E. Homoarginine levels are regulated by L‐arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation. 2013;128:1451–1461. [DOI] [PubMed] [Google Scholar]
- 4. Bretscher LE, Li H, Poulos TL, Griffith OW. Structural characterization and kinetics of nitric‐oxide synthase inhibition by novel N5‐(iminoalkyl)‐ and N5‐(iminoalkenyl)‐ornithines. J Biol Chem. 2003;278:46789–46797. [DOI] [PubMed] [Google Scholar]
- 5. Hafner F, Kieninger A, Meinitzer A, Gary T, Froehlich H, Haas E, Hackl G, Eller P, Brodmann M, Seinost G. Endothelial dysfunction and brachial intima‐media thickness: long term cardiovascular risk with claudication related to peripheral arterial disease: a prospective analysis. PLoS One. 2014;9:e93357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atzler D, Gore MO, Ayers CR, Choe CU, Böger RH, de Lemos JA, McGuire DK, Schwedhelm E. Homoarginine and cardiovascular outcome in the population‐based Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2014;34:2501–2507. [DOI] [PubMed] [Google Scholar]
- 7. Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–1932. [DOI] [PubMed] [Google Scholar]
- 8. Careri G, Nerla R, Di Monaco A, Russo G, Stazi A, Villano A, Sestito A, Lanza GA, Crea F. Clinical correlates and prognostic value of flow mediated dilation in patients with non‐ST segment elevation acute coronary syndromes. Am J Cardiol. 2013;111:51–57. [DOI] [PubMed] [Google Scholar]
- 9. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atzema CL, Lam K, Young C, Kester‐Greene N. Patients with atrial fibrillation and an alternative primary diagnosis in the emergency department: a description of their characteristics and outcomes. Acad Emerg Med. 2013;20:193–199. [DOI] [PubMed] [Google Scholar]
- 11. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. [DOI] [PubMed] [Google Scholar]
- 12. Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. [DOI] [PubMed] [Google Scholar]
- 13. Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth‐Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. [DOI] [PubMed] [Google Scholar]
- 14. Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth‐Zotz S, Warnholtz A, Giannitsis E, Möckel M, Bickel C, Peetz D, Lackner K, Baldus S, Münzel T, Blankenberg S. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atzler D, Mieth M, Maas R, Böger RH, Schwedhelm E. Stable isotope dilution assay for liquid chromatography‐tandem mass spectrometric determination of L‐homoarginine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2294–2298. [DOI] [PubMed] [Google Scholar]
- 17. Gore MO, Lüneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, Atzler D, de Lemos JA, Grant PJ, McGuire DK, Böger RH. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:2682–2688. [DOI] [PubMed] [Google Scholar]
- 18. Schwedhelm E, Maas R, Tan‐Andresen J, Schulze F, Riederer U, Böger RH. High‐throughput liquid chromatographic‐tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:211–219. [DOI] [PubMed] [Google Scholar]
- 19. Tzikas S, Keller T, Ojeda FM, Zeller T, Wild PS, Lubos E, Kunde J, Baldus S, Bickel C, Lackner KJ, Münzel TF, Blankenberg S. MR‐proANP and MR‐proADM for risk stratification of patients with acute chest pain. Heart. 2013;99:388–395. [DOI] [PubMed] [Google Scholar]
- 20. Heagerty PJ, Lumley T, Pepe MS. Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 21. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 22. Dao Q, Krishnaswamy P, Kazanegra R, Harrison A, Amirnovin R, Lenert L, Clopton P, Alberto J, Hlavin P, Maisel AS. Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting. J Am Coll Cardiol. 2001;37:379–385. [DOI] [PubMed] [Google Scholar]
- 23. Zeller T, Ojeda F, Brunner FJ, Peitsmeyer P, Munzel T, Binder H, Pfeiffer N, Michal M, Wild PS, Blankenberg S, Lackner KJ. High‐sensitivity cardiac troponin I in the general population—defining reference populations for the determination of the 99th percentile in the Gutenberg Health Study. Clin Chem Lab Med. 2015;53:699–706. [DOI] [PubMed] [Google Scholar]
- 24. Valtonen P, Laitinen T, Lyyra‐Laitinen T, Raitakari OT, Juonala M, Viikari JS, Heiskanen N, Vanninen E, Punnonen K, Heinonen S. Serum L‐homoarginine concentration is elevated during normal pregnancy and is related to flow‐mediated vasodilatation. Circ J. 2008;72:1879–1884. [DOI] [PubMed] [Google Scholar]
- 25. Abdullah SM, Khera A, Das SR, Stanek HG, Canham RM, Chung AK, Morrow DA, Drazner MH, McGuire DK, de Lemos JA. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n‐terminal pro‐brain natriuretic peptide in a multiethnic population‐based sample (the Dallas Heart Study). Am J Cardiol. 2005;96:1284–1289. [DOI] [PubMed] [Google Scholar]
- 26. Atzler D, Rosenberg M, Anderssohn M, Choe CU, Lutz M, Zugck C, Böger RH, Frey N, Schwedhelm E. Homoarginine‐an independent marker of mortality in heart failure. Int J Cardiol. 2013;168:4907–4909. [DOI] [PubMed] [Google Scholar]
- 27. Drechsler C, Meinitzer A, Pilz S, Krane V, Tomaschitz A, Ritz E, März W, Wanner C. Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail. 2011;13:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pilz S, Edelmann F, Meinitzer A, Gelbrich G, Döner U, Düngen HD, Tomaschitz A, Kienreich K, Gaksch M, Duvinage A, Stahrenberg R, Kunde J, Schmidt A, März W, Wachter R, Pieske B. Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J Cardiac Fail. 2014;20:923–930. [DOI] [PubMed] [Google Scholar]
- 29. Pilz S, Teerlink T, Scheffer PG, Meinitzer A, Rutters F, Tomaschitz A, Drechsler C, Kienreich K, Nijpels G, Stehouwer CD, März W, Dekker JM. Homoarginine and mortality in an older population: the Hoorn study. Eur J Clin Invest. 2014;44:200–208. [DOI] [PubMed] [Google Scholar]
- 30. Pilz S, Meinitzer A, Tomaschitz A, Drechsler C, Ritz E, Krane V, Wanner C, Boehm BO, März W. Low homoarginine concentration is a novel risk factor for heart disease. Heart. 2011;97:1222–1227. [DOI] [PubMed] [Google Scholar]
- 31. de Venecia TA, Lu MY, Nwakile CC, Figueredo VM. Utility of the QT interval in predicting outcomes in patients presenting to the emergency department with chest pain. Coron Artery Dis. 2015;26:422–424. [DOI] [PubMed] [Google Scholar]
- 32. Karwatowska‐Prokopczuk E, Wang W, Cheng ML, Zeng D, Schwartz PJ, Belardinelli L. The risk of sudden cardiac death in patients with non‐ST elevation acute coronary syndrome and prolonged QTc interval: effect of ranolazine. Europace. 2013;15:429–436. [DOI] [PubMed] [Google Scholar]
- 33. Chen SY, Crivera C, Stokes M, Boulanger L, Schein J. Outcomes associated with comorbid atrial fibrillation and heart failure in Medicare beneficiaries with acute coronary syndrome. BMC Health Serv Res. 2014;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scirica BM, Belardinelli L, Chaitman BR, Waks JW, Volo S, Karwatowska‐Prokopczuk E, Murphy SA, Cheng ML, Braunwald E, Morrow DA. Effect of ranolazine on atrial fibrillation in patients with non‐ST elevation acute coronary syndromes: observations from the MERLIN‐TIMI 36 trial. Europace. 2015;17:32–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cross‐Sectional Correlation Analyses of Homoarginine
Figure S1. Unadjusted failure curves for incidence of the combined endpoint comprising all‐cause mortality, nonfatal stroke, and nonfatal myocardial infarction according to homoarginine cutoff (1.41 μmol/L) (A) in the overall study population (n=1649; P<0.001 log‐rank test) and (B) in the ACS subsample (n=589; P<0.001, log‐rank test). ACS indicates acute coronary syndrome.


