Abstract
Background
Although clear evidence shows that chronic kidney disease is a predictor of cardiovascular events, death, and accelerated coronary artery disease (CAD) progression, it remains unknown whether CAD is a predictor of progression of chronic kidney disease to end‐stage renal disease. We sought to assess whether CAD adds prognostic information to established predictors of progression to dialysis in patients with chronic kidney disease, diabetes, and anemia.
Methods and Results
Using the previously described Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) population, we compared baseline characteristics of patients with and without CAD. Cox proportional hazards models were used to assess the association between CAD and the outcomes of end‐stage renal disease and the composite of death or end‐stage renal disease. Of the 4038 patients, 1791 had a history of known CAD. These patients were older (mean age 70 versus 65 years, P<0.001) and more likely to have other cardiovascular disease. CAD patients were less likely to have marked proteinuria (29% versus 39%, P<0.001), but there was no significant difference in estimated glomerular filtration rate between the 2 groups. After adjusting for age, sex, race, estimated glomerular filtration rate, proteinuria, treatment group, and 14 other renal risk factors, patients with CAD were significantly more likely to progress to end‐stage renal disease (adjusted hazard ratio 1.20 [95% CI 1.01–1.42], P=0.04) and to have the composite of death or end‐stage renal disease (adjusted hazard ratio 1.15 [95% CI 1.01–1.30], P=0.03).
Conclusions
In patients with chronic kidney disease, diabetes, and anemia, a history of CAD is an independent predictor of progression to dialysis. In patients with diabetic nephropathy, a history of CAD contributes important prognostic information to traditional risk factors for worsening renal disease.
Keywords: coronary disease, diabetes mellitus, kidney
Subject Categories: Coronary Artery Disease; Diabetes, Type 2; Nephrology and Kidney
Introduction
Cardiovascular disease is the leading cause of death in patients with type 2 diabetes mellitus (T2DM)1, 2 and in those with chronic kidney disease (CKD).3 T2DM and CKD are known independent risk factors for cardiovascular death, and patients with CKD are more likely to die from cardiovascular causes than to progress to end‐stage renal disease (ESRD).3 The association between kidney disease and poor cardiovascular outcomes has been demonstrated in several populations,4, 5, 6 and a meta‐analysis showed that patients with an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 had higher all‐cause and cardiovascular mortality compared with patients with an eGFR >90 mL/min per 1.73 m2. Furthermore, mortality increased with each incremental decrease in eGFR.4
Diabetes increases the risk of cardiovascular disease including coronary artery disease (CAD), stroke, peripheral artery disease, and heart failure (HF).7 Several studies have demonstrated that having diabetes alone is a cardiovascular risk equivalent in terms of cardiovascular event rates.8, 9 In addition, diabetic patients with evidence of microalbuminuria have a 2‐fold increased risk of cardiovascular death, even in the absence of nephropathy.10 Patients with CKD also have higher rates of stroke, HF, and myocardial infarction3 as well as cardiovascular death independent of diabetes status.11 The National Kidney Foundation task force recognizes that patients with both T2DM and CKD are at the highest risk for the development of cardiovascular disease6 and advocates aggressive cardiovascular risk management of these patients.
Although clear evidence shows that CKD is a predictor of cardiovascular events and death3, 11 and is associated with accelerated CAD progression,12 the reverse has not been examined. Specifically, it is not known whether CAD is an independent predictor of progression of CKD to ESRD in patients with T2DM. We compared renal outcomes and mortality in patients with diabetic nephropathy in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) according to history of known CAD.13
Methods
TREAT was a randomized placebo‐controlled trial of darbepoetin alfa treatment in 4038 patients with CKD (GFR 20–60 mL/min per 1.73 m2), anemia (hemoglobin ≤11.0 g/dL), and T2DM. Patients were excluded if they had a recent cardiovascular event or a history of renal transplantation. Patients were randomized to placebo or darbepoetin with dose adjusted to a hemoglobin level of 13.0 g/dL. Although there was a significant difference in the secondary outcome of stroke, there were no significant differences between the 2 groups in terms of the primary end points: time to the composite of all‐cause mortality, stroke, HF, myocardial infarction, or hospitalization for acute myocardial ischemia or the time to the composite of ESRD or all‐cause mortality.13 This study was approved by an institutional review committee, and the participants gave informed consent to participate in TREAT.
Statistical Analysis
Our primary predictor was a history of known CAD, defined as a self‐reported history of CAD with or without a history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention, as described previously.14 We assessed whether a history of CAD was associated with the risk of ESRD, as defined in TREAT.13 Because patients with CAD have a high propensity for death, we also assessed the association of CAD with the composite end point of ESRD or death. In addition, we examined the association of CAD with cardiovascular and noncardiovascular death. A total of 4038 patients were included in this analysis. Variables that were collected in the baseline case report form were used, excluding those with missing data, resulting in 4010 patients in the renal analysis (model 1) and 3877 patients in the death (all‐cause, cardiovascular, and noncardiovascular) analysis (model 2).
Baseline characteristics were compared for the groups with known CAD and no known CAD. For continuous variables, a Student t test and a Wilcoxon rank sum test were used to compare normal and non‐normal variables, respectively. For categorical variables, a chi‐square test was used.
Cox proportional hazards models were used to examine the relationship between the predictor of interest and the outcomes. We used previously published models for the prediction of renal outcomes15 (model 1) and death16 (model 2), with some revisions. Model 2, which was originally used to predict cardiovascular outcomes and death, included a covariate for coronary heart disease.16 We excluded this covariate from the current analysis because of overlap with our primary predictor. Treatment group (darbepoetin versus placebo) was included in model 2,16 as originally described, and added to model 1.15 CAD was included in all models, and duration of T2DM and systolic blood pressure were added to model 1. The urine protein/creatinine ratio and ferritin were log transformed, and C‐reactive protein was included as a categorical variable in the models.15, 16
Within each model, the statistical strength of the predictive contribution of the covariates to the outcomes of interest was expressed as the chi‐square statistic 2‐sided P value. Stata/SE version 11.1 (StataCorp LP) was used for all analyses.
Results
Of the 4038 patients included in these analyses, 1791 (44%) had known CAD. Baseline characteristics of those with and without known CAD are shown in Table 1. Patients with known CAD were older, more likely to be male, and more likely than the patients without known CAD to be of white race. As expected, patients with known CAD were significantly more likely to have a history of cardiovascular as well as cerebrovascular disease. Patients with known CAD had significantly lower blood pressure (mean systolic blood pressure 134 versus 137 mm Hg; P<0.001), lower hemoglobin A1c (7.2 versus 7.4; P<0.001), and a lower mean low‐density lipoprotein level (86.3 versus 95.4 mg/dL; P<0.001) than the patients without known CAD. They were more likely to be taking an aldosterone receptor antagonist, β‐blocker, or statin but less likely to be on an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker. Patients with known CAD were less likely to have marked proteinuria, defined as a urine protein/creatinine ratio >1 (29% versus 39%; P<0.001), but there was no significant difference in eGFR between the 2 groups (P=0.93). The 2 groups did not differ in terms of treatment with the original study drug, darbepoetin (P=0.16).
Table 1.
Baseline Characteristics of Patients With and Without Known CAD
| Variable | Known CAD (n=1791) | No Known CAD (n=2247) | P Value |
|---|---|---|---|
| Demographics and exam | |||
| Age (y), mean (SD) | 70 (9.0) | 65 (11.0) | <0.001 |
| Male sex, n (%) | 874 (48.8) | 852 (37.9) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 1301 (72.6) | 1269 (56.5) | |
| Black | 292 (16.3) | 523 (23.3) | |
| Other | 198 (11.1) | 455 (20.3) | |
| Smoking history | <0.001 | ||
| Never | 901 (50.3) | 1366 (60.8) | |
| Current | 79 (4.4) | 125 (5.6) | |
| Past | 811 (45.3) | 756 (33.6) | |
| Blood pressure (mm Hg), mean (SD) | |||
| Systolic | 134 (19.0) | 137 (19.0) | <0.001 |
| Diastolic | 71 (11.0) | 73 (11.0) | <0.001 |
| BMI (kg/m2), mean (SD) | 31.4 (6.9) | 31.6 (7.8) | 0.30 |
| Medical history, n (%) | |||
| Cardiovascular disease | |||
| Angina | 664 (37.1) | 82 (3.7) | <0.001 |
| MI | 741 (41.4) | 0 | N/A |
| HF | 962 (53.7) | 385 (17.1) | <0.001 |
| CABG | 571 (31.9) | 0 | N/A |
| PCI | 367 (20.5) | 0 | N/A |
| Valvular heart disease | 243 (13.6) | 105 (4.7) | <0.001 |
| AICD | 53 (3.0) | 4 (0.2) | <0.001 |
| Atrial fibrillation | 309 (17.3) | 116 (5.2) | <0.001 |
| Cerebrovascular disease | |||
| Stroke | 276 (15.4) | 171 (7.6) | <0.001 |
| TIA | 162 (9.0) | 103 (4.6) | <0.001 |
| Peripheral arterial disease | 499 (27.9) | 280 (12.5) | <0.001 |
| History of hypertension | 1698 (94.8) | 2033 (90.5) | <0.001 |
| History of AKI | 218 (12.2) | 177 (7.9) | <0.001 |
| Duration of diabetes (years), median (IQR) | 15.9 (8.6, 22.7) | 15.1 (8.1, 21.1) | 0.001 |
| Laboratory indices | |||
| Ferritin (μg/L), median (IQR) | 132 (68, 253) | 134 (66, 261) | 0.90 |
| Transferrin saturation, mean (SD) | 0.24 (0.10) | 0.24 (0.09) | 0.35 |
| BUN (mg/dL), mean (SD) | 42.9 (17.6) | 42.0 (16.6) | 0.11 |
| eGFR (/10 mL/min/1.73 m2), mean (SD) | 35.2 (11.7) | 35.1 (12.0) | 0.93 |
| Protein/creatinine ratio (g/g), median (IQR) | 0.3 (0.1, 1.3) | 0.5 (0.1, 2.3) | <0.001 |
| Proteinuria (protein/creatinine ratio >1 g/g), n (%) | 523 (29.2) | 874 (38.9) | <0.001 |
| Albumin (g/dL), mean (SD) | 4.0 (0.4) | 3.9 (0.5) | <0.001 |
| Hemoglobin (g/dL), mean (SD) | 10.4 (1.0) | 10.3 (1.0) | 0.11 |
| Hemoglobin A1c, mean (SD) | 7.2 (1.4) | 7.4 (1.6) | <0.001 |
| Total cholesterol (mg/dL), mean (SD) | 170.6 (52.2) | 182.8 (52.0) | <0.001 |
| LDL (mg/dL), mean (SD) | 86.3 (38.2) | 95.4 (40.7) | <0.001 |
| HDL (mg/dL), mean (SD) | 46.3 (13.8) | 49.8 (15.7) | <0.001 |
| Triglycerides (mg/dL), median (IQR) | 153 (108, 227) | 156 (111, 233) | 0.19 |
| CRP category, n (%) | 0.02 | ||
| ≤3.0 mg/dL | 894 (49.9) | 1218 (54.2) | |
| >3.0 to <6.6 mg/dL | 426 (23.8) | 480 (21.4) | |
| ≥6.6 mg/dL | 471 (26.3) | 549 (24.4) | |
| Medications, n (%) | |||
| Insulin | 896 (50.3) | 1093 (48.6) | 0.38 |
| Oral hypoglycemic (oral antidiabetic agents) | 1002 (55.9) | 1291 (57.5) | 0.34 |
| ACEI or ARB | 1388 (77.5) | 1835 (81.7) | <0.001 |
| Beta blocker | 1131 (63.1) | 859 (38.2) | <0.001 |
| Aldosterone receptor antagonist | 139 (7.8) | 70 (3.1) | <0.001 |
| Statin | 1200 (67.0) | 1164 (51.8) | <0.001 |
| Aspirin | 934 (52.1) | 779 (34.7) | <0.001 |
| Oral iron | 1211 (67.7) | 1388 (61.9) | <0.001 |
| Treatment with darbepoetin, n (%) | 870 (48.6) | 1142 (50.8) | 0.16 |
ACEI indicates angiotensin converting enzyme inhibitor; AICD, automatic implantable cardioverter defibrillator; AKI, acute kidney injury; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure; IQR, interquartile range; LDL, low‐density lipoprotein; MI, myocardial infarction; N/A, not available; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Renal Outcomes
Of the 4038 patients analyzed, 668 (17%) developed ESRD and 1270 (31%) had the composite of death or ESRD. Overall, 293 (16.4%) of the patients with known CAD and 375 (16.7%) of those without known CAD developed ESRD. CAD was not significantly associated with progression to ESRD (unadjusted hazard ratio [HR] 1.03, 95% CI 0.89–1.21) on univariate analysis; however, after adjusting for the covariates in model 1, a history of known CAD was significantly associated with progression to ESRD (adjusted HR 1.20, 95% CI 1.01–1.42) (Table 2). A strong confounder in model 1 was the log urine protein/creatinine ratio, which was significantly higher in the patients without known CAD (Table 1). After adjustment for this covariate alone, CAD emerged as a significant predictor of ESRD (adjusted HR 1.34, P<0.001).
Table 2.
Multivariable Models for Renal Outcomes
| Variable | ESRD | ESRD or Death | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Known CAD | 1.20 (1.01–1.42) | 0.041 | 1.15 (1.01–1.30) | 0.031 |
| Age | 1.00 (0.99–1.00) | 0.432 | 1.02 (1.01–1.02) | <0.001 |
| Male sex | 1.69 (1.44–1.99) | <0.001 | 1.42 (1.26–1.59) | <0.001 |
| Race (referent: white) | ||||
| Black | 1.72 (1.43–2.08) | <0.001 | 1.27 (1.10–1.47) | 0.001 |
| Other | 1.00 (0.81–1.25) | 0.966 | 0.92 (0.77–1.08) | 0.312 |
| BMI, per 10 kg/m2 | 0.81 (0.71–0.92) | 0.001 | 0.80 (0.74–0.88) | <0.001 |
| Insulin use | 1.15 (0.97–1.36) | 0.112 | 1.14 (1.00–1.29) | 0.042 |
| eGFR, per 10 mL/min/1.73 m2 | 0.52 (0.47–0.58) | <0.001 | 0.77 (0.71–0.82) | <0.001 |
| BUN, per 10 mg/dL | 1.12 (1.06–1.17) | <0.001 | 1.08 (1.04–1.13) | <0.001 |
| Log (UPCR) | 1.87 (1.73–2.02) | <0.001 | 1.42 (1.35–1.49) | <0.001 |
| Albumin, per 1 g/dL | 0.70 (0.58–0.84) | <0.001 | 0.61 (0.53–0.70) | <0.001 |
| Prior stroke | 1.17 (0.91–1.50) | 0.211 | 1.30 (1.11–1.53) | 0.002 |
| Prior PAD | 1.10 (0.90–1.35) | 0.355 | 1.13 (0.98–1.30) | 0.084 |
| Prior HF | 1.30 (1.09–1.56) | 0.004 | 1.49 (1.31–1.69) | <0.001 |
| History of arrhythmia | 1.17 (0.93–1.48) | 0.189 | 1.27 (1.09–1.48) | 0.002 |
| Hemoglobin, per 1 g/dL | 0.95 (0.88–1.03) | 0.188 | 0.95 (0.90–1.00) | 0.073 |
| Log (ferritin) | 1.10 (1.01–1.19) | 0.030 | 1.04 (0.98–1.11) | 0.202 |
| CRP (referent: ≤3.0 mg/L) | ||||
| CRP 3.1–6.5 mg/L | 1.13 (0.93–1.37) | 0.229 | 1.16 (1.00–1.34) | 0.044 |
| CRP ≥6.6 mg/L | 1.31 (1.08–1.58) | 0.006 | 1.42 (1.24–1.63) | <0.001 |
| History of acute kidney injury | 1.32 (1.05–1.66) | 0.019 | 1.22 (1.03–1.45) | 0.023 |
| Systolic blood pressure | 1.00 (1.00–1.01) | 0.248 | 1.00 (1.00–1.00) | 0.763 |
| Duration of T2DM | 1.00 (1.00–1.00) | 0.109 | 1.00 (1.00–1.00) | 0.835 |
| Treatment with darbepoetin | 1.08 (0.93–1.26) | 0.321 | 1.12 (1.00–1.25) | 0.044 |
BMI indicates body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HF, heart failure; HR, hazard ratio; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus; UPCR, urine protein/creatinine ratio.
ESRD or death occurred in 634 (35%) of patients with known CAD and 636 (28%) of those without known CAD, corresponding to a significant increase in risk for the CAD patients of either progressing to ESRD or dying (unadjusted HR 1.32, 95% CI 1.18–1.47) (Figure). After adjusting for the covariates in model 1, a history of known CAD remained a significant predictor of the composite renal outcome (adjusted HR 1.15, 95% CI 1.01–1.30) (Table 3).
Figure 1.
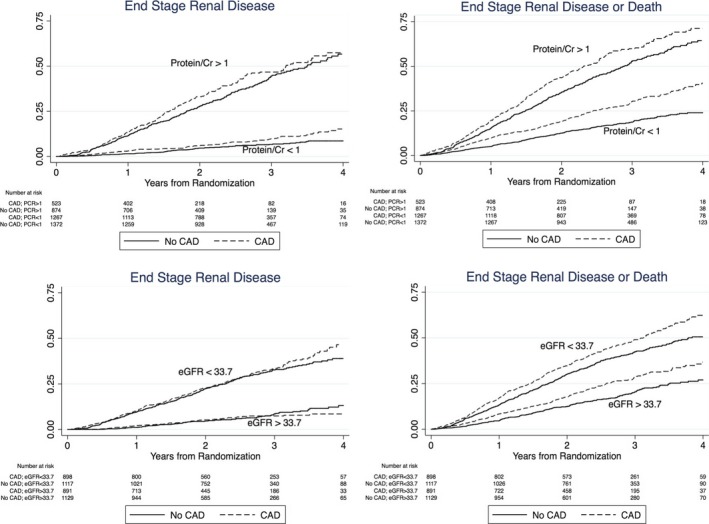
The association between CAD, proteinuria, eGFR and renal outcomes. A history of known CAD contributes to proteinuria and eGFR as a risk factor for progression to ESRD and ESRD or death. Proteinuria is represented as a categorical variable divided at a PCR of 1. eGFR is represented as a categorical variable divided at the median, 33.8 mL/min per 1.73 m2. An interaction term for CAD and proteinuria was added to the final renal multivariable model and found to be nonsignificant for the outcomes of ESRD (P=0.23) and ESRD or death (P=0.10). Similarly, the interaction terms for eGFR and CAD were found to be nonsignificant in the final multivariable model for both outcomes (ESRD, P=0.44; ESRD or death, P=0.59). CAD indicates coronary artery disease; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; PCR, protein/creatinine ratio.
Table 3.
Association Between Known CAD and Outcomes
| Outcome | Participants With Event, n (%), Incidence Rate Per 100 Person‐Years | Unadjusted HR (95% CI) P Value | Adjusted HR (95% CI) P Value | |
|---|---|---|---|---|
| Known CAD (n=1791) | No Known CAD (n=2247) | |||
| Model 1a | ||||
| ESRD |
293 (16.4%) 7.5/100 |
375 (16.7%) 7.3/100 |
1.03 (0.89–1.21) 0.66 |
1.20 (1.01–1.42) 0.04 |
| Death or ESRD |
634 (35.4%) 15.9/100 |
636 (28.3%) 12.1/100 |
1.32 (1.18–1.47) <0.001 |
1.15 (1.01–1.30) 0.03 |
| Model 2b | ||||
| All‐cause death |
444 (24.8%) 10.3/100 |
363 (16.2%) 6.4/100 |
1.61 (1.40–1.85) 0.001 |
1.02 (0.87–1.20) 0.78 |
| Noncardiovascular death |
143 (8.0%) 3.3/100 |
155 (6.9%) 2.7/100 |
1.21 (0.96–1.52) 0.10 |
0.77 (0.60–1.00) 0.046 |
| Cardiovascular death |
301 (16.8%) 7.0/100 |
208 (9.3%) 3.7/100 |
1.91 (1.60–2.28) <0.001 |
1.21 (0.99–1.48) 0.06 |
CAD indicates coronary artery disease; ESRD, end‐stage renal disease; HR, hazard ratio.
Model 1 covariates: age, sex, race, body mass index, insulin use, estimated glomerular filtration rate, blood urea nitrogen, log urine protein/creatinine ratio, albumin, history of stroke, history of peripheral artery disease, history of heart failure, arrhythmia, hemoglobin, log ferritin, C‐reactive protein, history of acute renal failure, duration of diabetes, systolic blood pressure, and treatment with darbepoetin (renal model,15 plus duration of type 2 diabetes mellitus, systolic blood pressure, and treatment with darbepoetin).
Model 2 covariates: age, race, sex, history of heart failure, log urine protein/creatinine ratio, C‐reactive protein, abnormal ECG, serum albumin, arrhythmia, hemoglobin A1c, reticulocytes, blood urea nitrogen, insulin use, cerebrovascular disease, loop diuretics, hemoglobin level, and treatment with darbepoetin (cardiovascular model).14
All‐Cause, Cardiovascular, and Noncardiovascular Death
CAD was found to be a significant predictor of all‐cause death (unadjusted HR 1.61, 95% CI 1.40–1.85), which occurred in 444 (25%) of the patients with known CAD and 363 (16%) of those without known CAD. After adjusting for the variables in model 2, CAD was no longer a significant independent predictor of mortality (adjusted HR 1.02, 95% CI 0.87–1.20). A history of HF appeared to be a strong confounder in this model. With exclusion of this single covariate from the model, CAD remained a significant predictor of all‐cause death (adjusted HR 1.18, P=0.035). Similarly, CAD was a significant predictor of cardiovascular death (unadjusted HR 1.91, 95% CI 1.60–2.28); however, it became nonsignificant after adjustment using model 2 (adjusted HR 1.21, 95% CI 0.99–1.48). Again, a history of HF was a strong confounder in this model.
Sensitivity Analysis: Angina
To assess whether our results changed significantly with inclusion of these patients, we reanalyzed the models after including 82 patients from TREAT who gave a self‐reported history of angina without a history of CAD, myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. Inclusion of these patients slightly strengthened the relationship between CAD and progression to ESRD (adjusted HR 1.22, 95% CI 1.03–1.45) and the renal composite outcome (adjusted HR 1.17, 95% CI 1.03–1.32) (Tables 4 and 5).
Table 4.
Multivariable Model for Renal Outcomes After Including Patients With Angina
| Variable | ESRD Model | ESRD or Death | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Known CAD | 1.22 (1.03–1.45) | 0.023 | 1.17 (1.03–1.32) | 0.017 |
| Age | 1.00 (0.99–1.00) | 0.411 | 1.02 (1.01–1.02) | <0.001 |
| Male sex | 1.69 (1.44–1.99) | <0.001 | 1.41 (1.26–1.59) | <0.001 |
| Race (referent: white) | ||||
| Black | 1.72 (1.43–2.08) | <0.001 | 1.27 (1.10–1.47) | 0.001 |
| Other | 1.01 (0.81–1.25) | 0.0 | 0.92 (0.78–1.09) | 0.318 |
| BMI, per 10 kg/m2 | 0.81 (0.71–0.92) | 0.001 | 0.80 (0.74–0.88) | <0.001 |
| Insulin use | 1.15 (0.97–1.36) | 0.117 | 1.14 (1.00–1.29) | 0.043 |
| eGFR, per 10 mL/min/1.73 m2 | 0.52 (0.47–0.59) | <0.001 | 0.77 (0.71–0.82) | <0.001 |
| BUN, per 10 mg/dL | 1.12 (1.06–1.17) | <0.001 | 1.09 (1.05–1.13) | <0.001 |
| Log (UPCR) | 1.87 (1.73–2.02) | <0.001 | 1.42 (1.35–1.49) | <0.001 |
| Albumin, per 1 g/dL | 0.70 (0.58–0.84) | <0.001 | 0.61 (0.53–0.70) | <0.001 |
| Prior stroke | 1.16 (0.91–1.49) | 0.231 | 1.30 (1.10–1.53) | 0.002 |
| Prior PAD | 1.10 (0.90–1.34) | 0.368 | 1.13 (0.98–1.30) | 0.085 |
| Prior HF | 1.29 (1.08–1.54) | 0.005 | 1.48 (1.30–1.68) | <0.001 |
| History of arrhythmia | 1.17 (0.92–1.47) | 0.198 | 1.27 (1.09–1.47) | 0.002 |
| Hemoglobin, per 1 g/dL | 0.95 (0.88–1.03) | 0.190 | 0.95 (0.9–1.01) | 0.074 |
| Log (ferritin) | 1.10 (1.01–1.20) | 0.029 | 1.04 (0.98–1.11) | 0.195 |
| C‐reactive protein (referent: ≤3.0 mg/L) | ||||
| CRP 3.1–6.5 mg/L | 1.13 (0.93–1.37) | 0.232 | 1.16 (1.00–1.34) | 0.044 |
| CRP ≥6.6 mg/L | 1.31 (1.08–1.59) | 0.006 | 1.42 (1.24–1.63) | <0.001 |
| History of AKI | 1.31 (1.04–1.65) | 0.021 | 1.22 (1.03–1.44) | 0.024 |
| Systolic blood pressure | 1.00 (1.00–1.01) | 0.252 | 1.00 (1.00–1.00) | 0.758 |
| Duration of T2DM | 1.00 (1.00–1.00) | 0.113 | 1.00 (1.00–1.00) | 0.855 |
| Treatment with darbepoetin | 1.08 (0.92–1.26) | 0.340 | 1.12 (1.00–1.25) | 0.048 |
AKI indicates acute kidney injury; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HF, heart failure; HR, hazard ratio; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus; UPCR, urine protein/creatinine ratio.
Table 5.
Association Between Known CAD and Outcomes After Including Patients With Angina
| Outcome | Participants With Event, n (%), Incidence Rate Per 100 Person‐Years | Unadjusted Model HR (CAD vs No CVD), 95% CI, P Value | Adjusted Model HR (CAD vs No CVD), 95% CI, P Value | |
|---|---|---|---|---|
| Known CAD (n=1873) | No Known CAD (n=2165) | |||
| Model 1a | ||||
| ESRD |
308 (16.4%) 7.5/100 |
360 (16.6%) 7.3/100 |
1.03 (0.89–1.20) 0.67 |
1.22 (1.03–1.45) 0.02 |
| Renal composite |
662 (35.3%) 15.8/100 |
608 (28.1%) 12.1/100 |
1.31 (1.17–1.46) <0.001 |
1.17 (1.03–1.32) 0.02 |
| Model 2b | ||||
| All‐cause death |
466 (24.9%) 10.3/100 |
341 (15.8%) 6.3/100 |
1.65 (1.43–1.89) 0.001 |
1.07 (0.92–1.26) 0.38 |
| Noncardiovascular death |
150 (8.0%) 3.3/100 |
148 (6.8%) 2.7/100 |
1.22 (0.97–1.53) 0.09 |
0.79 (0.62–1.02) 0.08 |
| Cardiovascular death |
316 (16.9%) 7.0/100 |
193 (8.9%) 3.6/100 |
1.98 (1.65–2.36) <0.001 |
1.29 (1.06–1.59) 0.01 |
With inclusion of these 82 patients, CAD was no longer significantly associated with a decreased risk of noncardiovascular death (adjusted HR 0.79, 95% CI 0.62–1.02); however, addition of these patients strengthened the relationship between CAD and cardiovascular death, ESRD, and the composite renal outcome. CAD indicates coronary artery disease; ESRD, end‐stage renal disease; HR, hazard ratio.
Model 1 covariates: age, sex, race, body mass index, insulin use, estimated glomerular filtration rate, blood urea nitrogen, log urine protein/creatinine ratio, albumin, history of stroke, history of peripheral artery disease, history of heart failure, arrhythmia, hemoglobin, log ferritin, C‐reactive protein, history of acute renal failure, duration of diabetes, systolic blood pressure, and treatment with darbepoetin (renal model,15 plus duration of type 2 diabetes mellitus, systolic blood pressure, and treatment with darbepoetin).
Model 2 covariates: age, race, sex, history of heart failure, log urine protein/creatinine ratio, C‐reactive protein, abnormal ECG, serum albumin, arrhythmia, hemoglobin A1c, reticulocytes, blood urea nitrogen, insulin use, cerebrovascular disease, loop diuretics, hemoglobin level, and treatment with darbepoetin (cardiovascular model).14
Discussion
We found that in patients with CKD, T2DM, and anemia, the presence of known CAD was independently predictive of progression of CKD to ESRD. Contrary to previous studies which suggest an association between proteinuria and CAD,17, 18 in our study patients with CAD had significantly less proteinuria than those without CAD. It is not clear why these patients had less proteinuria despite taking fewer angiotensin receptor blockers or angiotensin‐converting enzyme inhibitors. One possibility is that the patients with CAD were more likely to be of white race, and proteinuria occurs more frequently in non‐whites.19, 20 Another reason may be that the patients with CAD were on more aldosterone receptor antagonists, which have been shown to be associated with a decrease in proteinuria.21 Despite having less proteinuria, the patients with CAD had a higher propensity for progression to dialysis after adjusting for risk factors for dialysis including proteinuria and eGFR. These results suggest that in patients with CKD, a history of CAD can augment the prognostic information provided by traditional risk factors for renal disease progression.
According to the US Renal Data System 2013 Annual Data Report, the incidence of ESRD in 2011 was 357 per 1 million population, and >100 000 patients started therapy for ESRD in 2011 alone, representing a 1.5% increase from the previous year.22 Patients on hemodialysis have twice the mortality rate of the general population with diabetes, CAD, cancer, HF, or stroke and 10 times the mortality rate of similarly aged patients without CKD.22 Overall, 40% of patients with T2DM develop CKD, and T2DM is the most frequent cause of ESRD.23 In addition, evidence shows that anemia is a risk factor that may contribute to the progression of diabetic nephropathy to ESRD.24 Because progression of renal injury in patients with CKD with or without diabetes is quite variable, early identification of risk factors that contribute to renal loss can be important for risk stratification and treatment.23, 25
Vascular calcification has been implicated as the pathophysiological link between CKD and CAD,26 and some studies have suggested that coronary artery calcium scores are associated with increasing severity of kidney disease. The Chronic Renal Insufficiency Cohort study demonstrated a graded association between coronary artery calcium and the severity of CKD after adjusting for traditional risk factors.27 Furthermore, coronary artery calcium scores >100 were significantly more common in patients with CKD and diabetes than in those with CKD alone.27 Garland et al prospectively followed 125 predialysis patients for decline in kidney function and found that, compared with a score of 0, a coronary artery calcium score of 100 to 399 was associated with 7.4 increased odds of a decline in kidney function, and a score >400 was associated with 8.8 increased odds of kidney function decline at 1 year of follow‐up.28 Nevertheless, it is not clear whether vascular calcification is a cause of worsening renal function.
There are a few possible explanations for the observed association between baseline CAD and subsequently worsening renal disease in patients with diabetes. The progression of diabetic nephropathy is marked by accumulation of extracellular matrix, tubulointersitial fibrosis, tubular arteriosclerosis, and abnormal podocyte morphology,29 and inflammation may play an important role. Sun et al found in an animal model that coronary artery stenosis increased renal oxidative stress, fibrosis, inflammation, tubular injury, and microvasculature remodeling.30 Circulating matrix metalloproteinases, which may contribute to renal fibrosis, are elevated in CAD and have been implicated as a poor prognostic indicator in these patients.31, 32 Hsu et al showed that in patients with CAD, circulating matrix metalloproteinases are independently associated with renal disease progression.33 This association was seen in nondiabetic patients but also may be an important link in the diabetic population. Bleyer et al suggested an association between atherosclerosis and progression of renal disease using carotid intimal thickness.34 It is possible that patients with extrarenal atherosclerosis may have renal vasculature arteriosclerosis causing worsening renal disease.
Clinical factors should also be considered in the observed relationship between CAD and renal decline. HF may contribute to renal progression in patients with CAD, and although we found that CAD was independently associated with worsening renal function independent of a history of HF, it is not known whether these patients had subsequent episodes of HF after the baseline assessment. Another consideration is that patients with CAD may have a greater risk of progressive renal injury related to contrast administration from invasive angiography; however, we tested this hypothesis by including a time‐varying covariate for coronary revascularization in the models and found that coronary revascularization was not significantly associated with renal disease progression and had a negligible effect on the relationship between known CAD and renal disease progression.
In terms of mortality, we found that known CAD was not associated with all‐cause or cardiovascular death independent of a history of HF, suggesting that death caused by CAD in patients with CKD may be predominantly influenced by a history of HF. In contrast, CAD is predictive of noncardiovascular death after adjustment for HF. Bauters et al corroborated the importance of HF by showing that the strongest predictor of mortality in patients with stable CAD is a history of HF hospitalization.35 Furthermore, Parfrey et al found that ischemic heart disease was not a predictor of mortality in dialysis patients independent of HF.36
This study has some limitations. This is a secondary analysis of TREAT, a randomized controlled trial of darbepoetin treatment in patients with anemia, T2DM, and CKD that included patients with eGFR of 20 to 60 mL/min per 1.73 m2. It is possible that our results would change if we included patients with a broader range of eGFRs. In addition, we found known CAD to be predictive of renal events in patients with T2DM, CKD, and anemia, but these findings may not be generalizable to CKD patients without T2DM and anemia. Furthermore, the definition of ESRD in this study was based on the subjective decision of the clinician and not necessarily on the eGFR or rate of deterioration of eGFR.
Another potential limitation is that because CAD was self‐reported, there may be patients with underlying CAD (eg, asymptomatic myocardial ischemia) who were misclassified as having no CAD. This issue of possible misclassification was partially addressed in TREAT because the investigators individually reviewed all forms and patients were reclassified if discrepancies were found, such as a patient reporting a history of coronary artery bypass grafting but no history of CAD. Nevertheless, 3% of patients without self‐reported CAD underwent cardiac revascularization during the follow‐up period of TREAT. Although this finding may suggest misclassification of some of the non‐CAD patients, it may also be consistent with previous data showing that even CKD patients with normal initial coronary angiography might have higher rates of acute myocardial infarction during 3‐year follow‐up, suggesting accelerated coronary atherosclerosis in these patients.37 Patients who reported angina but not CAD were not included as CAD patients in TREAT; therefore, we conducted a sensitivity analysis of our results by including those patients who reported angina without CAD and found that the association between CAD and renal outcomes and cardiovascular death was actually strengthened by including these patients. Another important limitation of this study is that patients were censored at the time of death, which is a competing risk, in the model assessing the outcome of ESRD alone. Finally, although an association between peripheral artery disease and worsening renal function was demonstrated previously,38 we did not find peripheral artery disease to be an independent predictor of ESRD. Since less than 25% of the TREAT cohort had a history of peripheral artery disease, this study may not have been powered to detect a significant association.
In this study of patients with CKD and T2DM, we found that a history of known CAD was an independent predictor of ESRD and the composite of death or ESRD. Because CAD is a known predictor of death, we also assessed the association between CAD and ESRD alone and found that known CAD was an independent risk factor for the progression of CKD to ESRD in this study population. Consequently, patients with diabetic nephropathy and CAD may be a particularly high‐risk group not only in terms of cardiovascular disease but also in terms of renal disease progression. Consequently, CAD should be considered an important factor in the risk stratification of these patients. A continued cooperative effort among cardiologists and nephrologists will be necessary to prevent further disease progression of these interrelated organ systems and, ultimately, to optimize the care of these complicated patients.
Disclosures
Sabe: none; Claggett: none; Burdmann: Research Grant: Amgen, Roche. Consultant/Advisory Board: Amgen, Sigma Pharma; Desai: Research support: Amgen; Ivanovich: Consultant/Advisory Board: Amgen, Baxter, Physician Software Systems, LLC, Reata; Kewalramani: Employment: Amgen. Ownership Interest: Amgen; Lewis: Research Grant: Amgen; McMurray: none; Olson: Employment: Amgen. Ownership Interest: Amgen; Parfrey: Honoraria: Amgen. Consultant/Advisory Board: Amgen; Solomon: Research Grant: Amgen. Consultant/Advisory Board: Amgen; Pfeffer: Research Grant: Amgen, Celladon, Novartis, Sanofi Aventis; Ownership Interest: coinventor on a patent for the use of inhibitors of the renin‐angiotensin system in selected survivors of myocardial infarction, his share is transferred to charity. Consultant/Advisory Board: Aastrom, Abbott Vascular, Amgen, Bristol‐Myers Squibb, Cerenis, Concert, Fibrogen, Genzyme, GlaxoSmithKline, Hamilton Health Sciences, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, Serono, Servier, Teva, University of Oxford.
(J Am Heart Assoc. 2016;5:e002850 doi: 10.1161/JAHA.115.002850)
References
- 1. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. [DOI] [PubMed] [Google Scholar]
- 2. Cho E, Rimm EB, Stampfer MJ, Willett WC, Hu FB. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol. 2002;40:954–960. [DOI] [PubMed] [Google Scholar]
- 3. Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013;15:340. [DOI] [PubMed] [Google Scholar]
- 4. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 6. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42:1050–1065. [DOI] [PubMed] [Google Scholar]
- 7. Boccara F, Cohen A. Interplay of diabetes and coronary heart disease on cardiovascular mortality. Heart. 2004;90:1371–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker A, Bos G, de Vegt F, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Cardiovascular events in type 2 diabetes: comparison with nondiabetic individuals without and with prior cardiovascular disease. 10‐year follow‐up of the HOORN Study. Eur Heart J. 2003;24:1406–1413. [DOI] [PubMed] [Google Scholar]
- 9. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta‐analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 11. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 12. Cai Q, Mukku VK, Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. A trial of darbepoetin ALFA in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. [DOI] [PubMed] [Google Scholar]
- 14. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill J, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, Uno H. Baseline characteristics in the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am J Kidney Dis. 2009;54:59–69. [DOI] [PubMed] [Google Scholar]
- 15. Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, Levey AS, Lewis EF, McMurray JJ, Parving HH, Solomon SD, Pfeffer MA. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011;58:717–728. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJ, Uno H, Jarolim P, Desai AS, de Zeeuw D, Eckardt KU, Ivanovich P, Levey AS, Lewis EF, McGill JB, Parfrey P, Parving HH, Toto RM, Solomon SD, Pfeffer MA. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the trial to reduce cardiovascular events with aranesp (darbepoetin‐ALFA) therapy (TREAT). Am Heart J. 2011;162:748–755.e743. [DOI] [PubMed] [Google Scholar]
- 17. Cho I, Min HS, Chun EJ, Park SK, Choi Y, Blumenthal RS, Rivera JJ, Nasir K, Kim YJ, Sohn DW, Oh BH, Park YB, Chang HJ. Coronary atherosclerosis detected by coronary CT angiography in asymptomatic subjects with early chronic kidney disease. Atherosclerosis. 2010;208:406–411. [DOI] [PubMed] [Google Scholar]
- 18. Sukhija R, Aronow WS, Kakar P, Garza L, Sachdeva R, Sinha A, Mehta JL. Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol. 2006;98:279–281. [DOI] [PubMed] [Google Scholar]
- 19. Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end‐stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. [DOI] [PubMed] [Google Scholar]
- 20. Bhalla V, Zhao B, Azar KM, Wang EJ, Choi S, Wong EC, Fortmann SP, Palaniappan LP. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care. 2013;36:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bianchi S, Bigazzi R, Campese VM. Long‐term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. [DOI] [PubMed] [Google Scholar]
- 22. Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L. US renal data system 2013 annual data report. Am J Kidney Dis. 2014;63:A7. [DOI] [PubMed] [Google Scholar]
- 23. Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–1605. [DOI] [PubMed] [Google Scholar]
- 24. Ueda H, Ishimura E, Shoji T, Emoto M, Morioka T, Matsumoto N, Fukumoto S, Miki T, Inaba M, Nishizawa Y. Factors affecting progression of renal failure in patients with type 2 diabetes. Diabetes Care. 2003;26:1530–1534. [DOI] [PubMed] [Google Scholar]
- 25. McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol. 2003;14:S65–S70. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura S, Ishibashi‐Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Budoff MJ, Rader DJ, Reilly MP, Mohler ER III, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (chronic renal insufficiency cohort) study. Am J Kidney Dis. 2011;58:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garland JS, Holden RM, Hopman WM, Gill SS, Nolan RL, Morton AR. Body mass index, coronary artery calcification, and kidney function decline in stage 3 to 5 chronic kidney disease patients. J Ren Nutr. 2013;23:4–11. [DOI] [PubMed] [Google Scholar]
- 29. Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun D, Eirin A, Zhu XY, Zhang X, Crane JA, Woollard JR, Lerman A, Lerman LO. Experimental coronary artery stenosis accelerates kidney damage in renovascular hypertensive swine. Kidney Int. 2015;87:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu TC, Leu HB, Lin WT, Lin CP, Lin SJ, Chen JW. Plasma matrix metalloproteinase‐3 level is an independent prognostic factor in stable coronary artery disease. Eur J Clin Invest. 2005;35:537–545. [DOI] [PubMed] [Google Scholar]
- 32. Ye ZX, Leu HB, Wu TC, Lin SJ, Chen JW. Baseline serum matrix metalloproteinase‐9 level predicts long‐term prognosis after coronary revascularizations in stable coronary artery disease. Clin Biochem. 2008;41:292–298. [DOI] [PubMed] [Google Scholar]
- 33. Hsu TW, Kuo KL, Hung SC, Huang PH, Chen JW, Tarng DC. Progression of kidney disease in non‐diabetic patients with coronary artery disease: predictive role of circulating matrix metalloproteinase‐2, ‐3, and ‐9. PLoS One. 2013;8:e70132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG. Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int. 2000;57:2072–2079. [DOI] [PubMed] [Google Scholar]
- 35. Bauters C, Deneve M, Tricot O, Meurice T, Lamblin N. Prognosis of patients with stable coronary artery disease (from the CORONOR study). Am J Cardiol. 2014;113:1142–1145.24507170 [Google Scholar]
- 36. Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE. Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int. 1996;49:1428–1434. [DOI] [PubMed] [Google Scholar]
- 37. Zebrack JS, Anderson JL, Beddhu S, Horne BD, Bair TL, Cheung A, Muhlestein JB. Do associations with C‐reactive protein and extent of coronary artery disease account for the increased cardiovascular risk of renal insufficiency? J Am Coll Cardiol. 2003;42:57–63. [DOI] [PubMed] [Google Scholar]
- 38. O'Hare AM, Rodriguez RA, Bacchetti P. Low ankle‐brachial index associated with rise in creatinine level over time: results from the Atherosclerosis Risk in Communities Study. Arch Intern Med. 2005;165:1481–1485. [DOI] [PubMed] [Google Scholar]


