Abstract
Background
The efficacy of P2Y12 inhibition for the prevention of cardiovascular events in patients with peripheral arterial disease (PAD) has been established. However, the therapeutic effects on ischemic limb complications are less clear. Accordingly, we aimed to develop a novel murine model of thrombotic hindlimb ischemia to reflect that found in patients with PAD exhibiting ischemic limb symptoms. We further investigated the effects of P2Y12 deficiency and P2Y12 inhibition by prasugrel in this model.
Methods and Results
Thrombus formation induced by application of ferric chloride to the femoral artery resulted in a significant reduction in blood flow in the injured limb. In gait analysis using the CatWalk system, moderate difficulties in grounding and weight bearing of the ischemic limb, including reduction of maximum contact area and stance phase duration and increasing in swing phase duration in the ischemic limb, were observed in this model. Blood flow reduction and gait abnormalities gradually recovered over 21 days to levels present before arterial injury. Compared to wild‐type (WT) mice, significant increases in blood flow and improvement in gait were observed in P2Y12‐deficient mice. In addition, daily oral administration of prasugrel (3 mg/kg per day) to WT mice resulted in significant inhibition of blood flow reduction and gait abnormalities to levels found in P2Y12 deficient mice.
Conclusions
Acute femoral artery thrombosis resulted in hindlimb ischemia and moderate gait abnormalities in mice. In addition, the present study suggests a possible role of P2Y12 in the complications with thrombotic limb ischemia.
Keywords: femoral artery thrombosis, gait abnormalities, limb ischemia, P2Y12, prasugrel
Subject Categories: Atherosclerosis, Peripheral Vascular Disease, Thrombosis, Vascular Disease
Introduction
Patients with peripheral arterial disease (PAD) have an increased risk of cardiovascular events, such as myocardial infarction, ischemic stroke, and vascular death.1, 2 Platelets have been considered to play a central role in the development of arterial thrombosis in patients with systemic atherosclerotic vascular disease, including PAD.3 The benefits of antiplatelet therapy for the secondary prevention of cardiovascular events in patients with PAD have been evaluated, and antiplatelet therapy, notably P2Y12 inhibition, has resulted in reduced risk of major adverse cardiovascular events in these patients.1, 4 On the basis of this evidence, treatments with antiplatelet therapy for the prevention of cardiovascular events in PAD patients have been recommended.5, 6 Treatment for limb complications of PAD, including intermittent claudication, ischemic pain, or ischemic ulceration, is also a clinical goal; however, the efficacy of P2Y12 inhibition on the progression of ischemic symptoms as the primary end point in PAD patients has not been consistent.7, 8, 9, 10, 11, 12 Further studies are therefore required to better understand the role for P2Y12 inhibition in controlling ischemic limb complications in patients with PAD. Prasugrel, a third‐generation thienopyridine antiplatelet agent used clinically to treat patients with acute coronary syndrome who are undergoing percutaneous coronary intervention,13 has been shown to provide effective inhibition of platelet activation and aggregation via the selective inhibition of platelet P2Y12.14, 15 Furthermore, it has also been shown to be more potent and consistent than clopidogrel for P2Y12 inhibition.16 While prasugrel's profile would suggest that it would provide benefit in ischemic limb complications, as well as reducing the risk of cardiovascular events through potent and consistent P2Y12 inhibition, the efficacy of prasugrel in PAD remains to be explored in both nonclinical and clinical studies.
To date, critical limb ischemia (CLI) models have been widely used for nonclinical studies of PAD17, 18, 19; but in the majority of patients with PAD, the disease is at the mild PAD stage, which is asymptomatic or results in intermittent claudication, and only 1% to 3% of patients have CLI.5 It is therefore desirable to have an experimental model of mild PAD reflecting this level of disease in PAD patients and thus allowing for more relevant pharmacological interventions. For the present study, we hypothesized that thrombus formation in the mouse femoral artery might cause acute limb ischemia and moderate walking abnormalities similar to those in patients with mild PAD. Additional limitations of current models relate to the evaluation of gait abnormalities in the hindlimb ischemia models. Evaluation of the walking profile in CLI models has mostly involved the use of a treadmill20, 21; however, this only allows measurement of walking time and walking distance, and it can only be used to evaluate animals able to walk. A more sensitive and reproducible system to evaluate walking function was therefore required. The CatWalk system (Noldus Information Technology, Wageningen, The Netherlands), a video‐based system for automatic gait analysis, allows the determination of a variety of gait parameters from the animal's footprints captured optically.22, 23 This system has been utilized in animal models of neuropathic pain,24 inflammatory pain,25 spinal cord injury,23 Parkinson's disease,26 and cerebral infarction.27 However, to our knowledge, there have been no reports of the CatWalk system being used for evaluating PAD models.
In this study, we aimed to develop a novel murine model of thrombotic hindlimb ischemia and to investigate the effects of P2Y12 deficiency and P2Y12 antagonism on blood flow and walking function in the ischemic limb.
Materials and Methods
Materials
Prasugrel hydrochloride (prasugrel) was provided by Ube Industries Ltd. (Ube, Japan). Ferric chloride (FeCl3) was purchased from Sigma‐Aldrich Co. (St. Louis, MO). Gum arabic was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Animals
C57BL/6J mice were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). Mice lacking P2Y12 receptors (P2Y12‐deficient mice) were generated as previously described28 with a mixed C57BL/6‐129 background and backcrossed for 7 generations with C57BL/6J mice. General observation was conducted once daily during the quarantine, acclimatization, and experimental periods with no abnormalities being observed. All animal studies were approved by the Institutional Animal Care and Use Committee (Nissei Bilis Co., Ltd. and Daiichi Sankyo Co., Ltd.).
Drug Administration
Prasugrel was suspended in a 5% solution of gum arabic. Starting 2 days before the arterial injury (Day −2), prasugrel or the vehicle were administered once a day for 23 days to each mouse by oral gavage with a volume of 10 mL/kg. The prasugrel doses used for the repeated administration studies were based on a previous platelet aggregation study.28
FeCl3‐Induced Arterial Thrombosis
On the day of arterial injury (Day 1), nonfasted mice were restrained on a heating pad under 1.5% isoflurane anesthesia. An incision was made above the femoral artery from the inguinal crease, and the left femoral artery was exposed and gently excised from the femoral nerve and vein. Filter paper (1×2 mm, Qualitative filter paper No. 2; Advantec Toyo Kaisha, Ltd., Tokyo, Japan) saturated with 10% FeCl3 was placed on the femoral artery at the midpoint between the lateral circumflex femoral artery branch and popliteal artery branch for 3 minutes at 0.9 to 1.1 hours after administering prasugrel or the vehicle. In the sham group, the femoral artery was treated with distilled water in place of the FeCl3.
Measurement of Blood Flow
Blood flow images were captured of the mice under isoflurane inhalation anesthesia before and 1 hour after the arterial injury or the sham operation on Day 1. On Days 3, 7, and 21, anesthetized mice were laid in a supine position and blood flow images of the hindlimbs were captured with a laser speckle blood flow imager (OZ‐1, Omegawave, Inc., Tokyo, Japan). Using the blood flow images for each animal, the relative blood flow was calculated as the ratio of the injured hindlimb value to the normal hindlimb value.
Morphometric Analysis of the Vessel Lesions
After capturing the blood flow images on Day 1, the anesthetized animals were exsanguinated with perfusion of saline via the left ventricle to an incision in the right atrium. Following euthanasia, perfusion fixation was performed with neutral buffered 10% formalin for 5 to 9 minutes, and then the FeCl3‐injured area of the left femoral artery was excised and fixed by immersion in neutral buffered 10% formalin. Paraffin sections were prepared at 3 positions on the femoral arteries: the midsection of the FeCl3‐injured segment, 100 μm distal from the midsection, and 200 μm distal from the midsection. Elastica van Gieson staining was performed at each position. For each of these Elastica van Gieson–stained sections, image analysis software (WinROOF 2013; Mitani Corporation, Tokyo, Japan) was used to determine the area of the lumen (A), the area surrounded by internal elastic lamina (B), and the area of the thrombus (T). By using each value, lumen stenosis [100×{B−(A−T)}/B] was calculated. Values for the thrombus area and lumen stenosis in the 3 positions were averaged.
Gait Analysis
Gait analysis was performed using the CatWalk system in accordance with the manufacturer's instructions and previously published procedures.22, 23 Briefly, this system consists of an enclosed glass runway with customized lighting. When the animal's paws make contact with the glass runway, light is reflected downwards and the illuminated contact area is recorded with a high‐speed color video camera positioned under the runway. From the position, intensity, duration, and contact area of each paw, the analysis system can calculate multiple gait parameters for the animal.
The gait patterns were investigated on Days −3 (pre), 3, 7, and 21. We analyzed the following 4 parameters: (1) maximum (max) contact area: the surface area contacted by the paw during a stance phase; (2) swing phase duration: the time in seconds in a step cycle during which a paw was not in contact with the glass plate; (3) stance phase duration: the time in seconds in a step cycle during which a paw was in contact with the glass plate; and (4) stride length: the distance between successive placements of the same paw. For each parameter, we calculated as a percentage the ratio of the injured hindlimb value to the contralateral normal hindlimb value.
Statistical Analysis
Data are presented as mean±SE. Student t tests were used for the comparisons between the wild‐type (WT) and P2Y12‐deficient mice and between the control and sham groups. A paired t test was used for the comparison of the relative blood flow before and 1 hour after arterial injury. Two‐way ANOVA was used for the comparison among the genotype (WT/P2Y12 deficiency) and the injury (pre/post). Dunnett's test was used for the comparison between the control and all prasugrel groups. In all the analyses, statistical significance was defined as P<0.05. SAS 9.2 for Windows (SAS Institute Inc., Cary, NC) and EXSUS Ver. 7.7.1 (Arm Systex Co., Ltd., Osaka, Japan) were used for the analyses.
Results
Effects of P2Y12 Deficiency on Thrombus Formation and the Blood Flow of the FeCl3‐Injured Hindlimb
Representative blood flow images of the hindlimbs of WT and P2Y12‐deficient mice are shown in Figure 1A. The relative blood flow of the injured hindlimb before/after arterial injury is shown in Figure 1B. There were no differences in the relative blood flow between WT mice and P2Y12‐deficient mice before arterial injury. In the WT mice, a statistically significant reduction in relative blood flow was observed 1 hour after the arterial injury (P=0.0008). In the P2Y12‐deficient mice, the relative blood flow after the arterial injury was not significantly reduced, and it was significantly higher than that of the WT mice (P=0.0018). Typical Elastica van Gieson–stained cross‐sections of the FeCl3‐injured femoral arteries on Day 1 are shown in Figure 1C. In the morphometric analysis, significantly less thrombus area was observed in P2Y12‐deficient mice compared with the WT mice (P=0.0021, Figure 1D). Lumen stenosis was also significantly lower in P2Y12‐deficient mice compared to WT mice (P=0.0041, Figure 1E).
Figure 1.
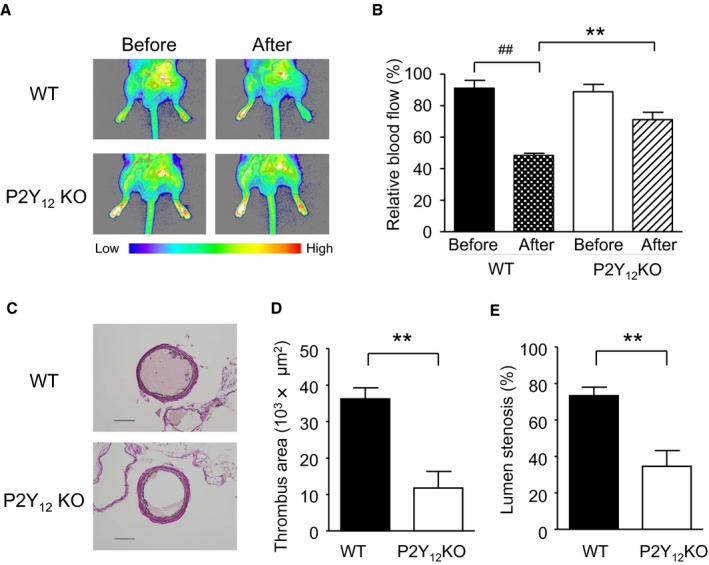
Hindlimb blood flow and pathology in wild‐type (WT) and P2Y12‐deficient mice. Blood flow images of hindlimbs were captured by laser speckle imaging in WT and P2Y12‐deficient (KO) mice. Representative blood flow images and relative blood flow for before and 1 hour after arterial injury are shown in (A) and (B), respectively. The results of 2‐way ANOVA were as follows: Genotype: F (1, 16)=5.88, P=0.0275; Injury: F (1, 16)=52.02, P<0.0001; and Genotype × Injury interaction: F(1, 16)=8.82, P=0.0090. Typical Elastica van Gieson–stained images of the injured arteries are shown in (C). Scale bar, 100 μm. Thrombus area (D) and lumen stenosis (E) were measured morphometrically. Data are presented as mean±SE (n=5). **P<0.01 vs WT mice (Student t test). ## P<0.01 vs before the arterial injury (paired t test).
Effects of Prasugrel on the Blood Flow of the FeCl3‐Injured Hindlimb
Representative hindlimb blood flow images after arterial injury on Day 1 in the sham, control, and prasugrel groups are shown in Figure 2A. The time course of relative blood flow following arterial injury is shown in Figure 2B. Relative blood flow in the sham group ranged from 97.2±3.4% to 105.4±3.1% over the study period. In the control (vehicle) group, relative blood flow of the injured hindlimb was reduced 1 hour after arterial injury on Day 1 and then gradually recovered to pre‐injury levels through Day 21. The reduction of relative blood flow in the injured hindlimb was statistically significant compared to the sham group from Day 1 to Day 21; the values for relative blood flow on Days 1, 3, 7, and 21 were 47.7±1.5% (P<0.0001), 69.9±2.1% (P<0.0001), 90.3±3.8% (P=0.0265), and 90.3±3.4% (P=0.0237), respectively. The lower‐dose prasugrel groups showed similar time courses for the relative blood flow; however, similar to what was seen in the P2Y12‐deficient mice, prasugrel at 3 mg/kg per day significantly ameliorated the reduction of the relative blood flow on Days 1 and 3 compared to the control group. The values of relative blood flow on Days 1, 3, 7, and 21 were 65.0±6.0% (P=0.0051), 89.5±4.5% (P=0.0001), 95.8±3.7% (P=0.6242), and 102.0±3.5% (P=0.0732), respectively.
Figure 2.
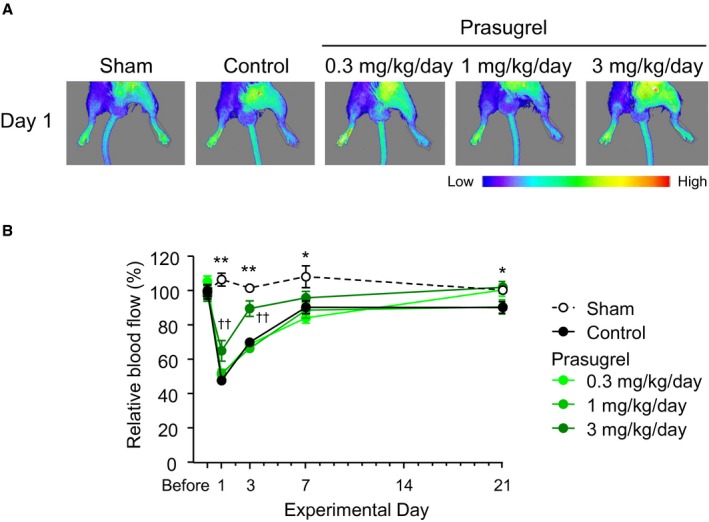
Effects of prasugrel on injured hindlimb blood flow. Representative blood flow images at 1 hour after the arterial injury on Day 1 are shown in (A). Relative blood flow was calculated for each animal at each time point (B). Data are presented as the mean±SE (n=10). Before: before the arterial injury. Day 1: 1 hour after the arterial injury. *P<0.05, **P<0.01 vs control group (Student t test). †† P<0.01 vs control group (Dunnett's test).
Effects of P2Y12 Deficiency and Prasugrel on Gait Parameters of the FeCl3‐Injured Hindlimb
The effects of P2Y12 deficiency on gait abnormalities in this thrombotic hindlimb ischemia model were evaluated 3 days after arterial injury (Day 3). The maximum contact area of the P2Y12‐deficient mice was significantly increased compared to that of the WT mice (P=0.0021); the ischemic‐to‐normal ratios for the maximum contact area were 94.10±15.59% and 31.92±7.64% for the P2Y12‐deficient and WT mice, respectively. The swing phase duration in the P2Y12‐deficient mice was significantly reduced compared to that for the WT mice (P=0.0110); the ischemic‐to‐normal ratios for swing phase duration were 109.96±6.91% and 160.83±16.56% for the P2Y12‐deficient mice and WT mice, respectively. The stance phase duration in P2Y12‐deficient mice was significantly increased compared to that for the WT mice (P=0.0021); the ischemic‐to‐normal ratios for stance phase duration were 97.28±7.20% and 54.99±7.63% for the P2Y12‐deficient and WT mice, respectively. The stride length of the injured limb did not alter in either the P2Y12‐deficient mice or the WT mice.
Next, the effects of prasugrel on gait parameters in this thrombotic hindlimb ischemia model were evaluated on Days −3 (pre), 3, 7, and 21. The time courses in the ratios of gait parameters of the injured hindlimb to normal hindlimb are shown in Figure 3. The gait parameters of the 3 mg/kg per day prasugrel‐treatment group were similar to those of the P2Y12‐deficient mice. Prior to the arterial injury, there were no significant differences for all the gait parameters between the sham group and the control group, and between all prasugrel groups and the control group. The maximum contact area in the control group was significantly reduced on Days 3 and 7 compared to the sham group; the ratios of ischemic hindlimb maximum contact area to normal hindlimb maximum contact area on Days 3 and 7 were 47.65±7.24% (P=0.0002) and 61.24±7.78% (P=0.0002), respectively (Figure 3A). Prasugrel at 3 mg/kg per day significantly reduced the decrease in the maximum contact area on Days 3 and 7 compared to the control group; the ischemic‐to‐normal ratios for the maximum contact area on Days 3 and 7 were 107.23±11.61% (P=0.0004) and 96.61±9.48% (P=0.0083), respectively (Figure 3A). The swing phase duration in the control group was significantly increased on Days 3 and 7 compared to the sham group; the ischemic‐to‐normal ratios for swing phase duration on Days 3 and 7 were 178.28±18.90% (P=0.0024) and 142.51±12.38% (P=0.0035), respectively (Figure 3B). Prasugrel at 3 mg/kg per day significantly prevented the increase in swing phase duration on Days 3 and 7 compared to the control group; the ischemic‐to‐normal ratios for swing phase duration on Days 3 and 7 were 126.10±12.09% (P=0.0231) and 106.06±9.50% (P=0.0400), respectively (Figure 3B). The stance phase duration in the control group was significantly decreased on Days 3 and 7 compared to the sham group; the ischemic‐to‐normal ratios for stance phase duration on Days 3 and 7 were 58.09±7.79% (P=0.0032) and 72.29±6.60% (P=0.0025), respectively (Figure 3C). Prasugrel at 3 mg/kg per day significantly reduced the decrease in stance phase duration on Days 3 and 7 compared to the control group; the ischemic‐to‐normal ratios for stance phase duration on Days 3 and 7 were 103.94±10.06% (P=0.0013) and 100.96±8.89% (P=0.0142), respectively (Figure 3C). In the analysis of stride length, there were no significant differences between the sham group and the control group, or between all prasugrel groups and the control group (Figure 3D).
Figure 3.
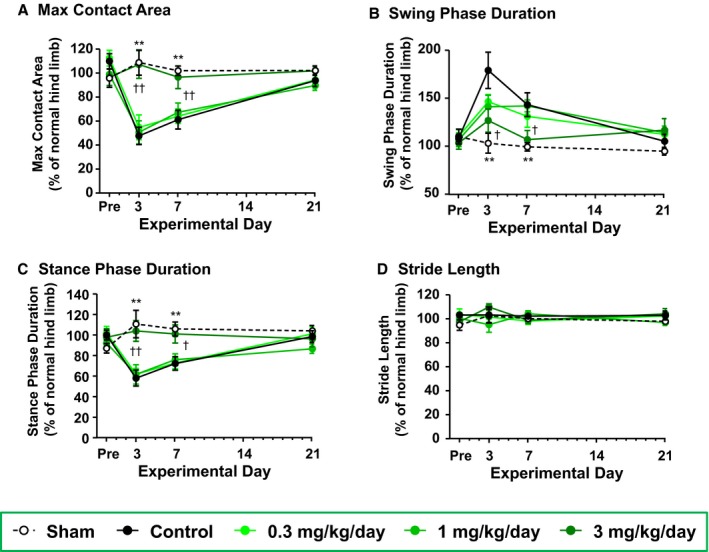
Effects of prasugrel on max contact area (A), swing phase duration (B), stance phase duration (C) and stride length (D) in mice with hindlimb injury. The gait parameters were measured by CatWalk and calculated as ratios (%) of the injured hindlimb value to the normal hindlimb value on Day −3 (pre) and on Days 3, 7, and 21 after the arterial injury. Data are presented as mean±SE (n=10). *P<0.05, **P<0.01 vs control group (Student t test). † P<0.05, †† P<0.01 vs control group (Dunnett's test).
Discussion
The role of the platelet P2Y12 ADP receptor in cardiovascular and peripheral atherothrombosis in patients with PAD and the therapeutic potential of P2Y12 antagonism for disease modification are of clinical interest. In the present study, we examined the effects of P2Y12 deficiency and prasugrel treatment in a new model of thrombotic hindlimb ischemia. Both P2Y12 deficiency and prasugrel administration attenuated blood flow reduction and yielded improvements in gait abnormalities in this model of limb ischemia with walking dysfunction. While P2Y12 antagonists appear to be efficacious in reducing cardiovascular events in patients with PAD, their efficacy in controlling intermittent claudication in patients with PAD is less clear. Ticlopidine, the first‐generation thienopyridyl P2Y12 antagonist, demonstrated beneficial effects on the improvement of limb functions8, 9 and the prevention of vascular complications8, 11 in patients with intermittent claudication. However, other studies reported that ticlopidine and clopidogrel, the second‐generation thienopyridine, had no clear beneficial effects on symptoms in PAD.7, 10, 12 One possible reason for these mixed results is that the antiplatelet effects of ticlopidine and clopidogrel may not have been sufficient to improve the limb ischemia in PAD. Of note, prasugrel has a more potent and consistent P2Y12 inhibitory profile compared to clopidogrel.16 The present study showed a relationship between inhibition of platelet activation via ADP‐P2Y12 signaling and the symptoms in the thrombotic hindlimb ischemia model. Similar data were found in P2Y12 deficient mice. Taken together, these data suggest that prasugrel, by providing more optimal P2Y12 blockade,16 could potentially reduce both cardiovascular and peripheral ischemic events in patients with PAD.
To date, PAD/CLI models such as multivessel ligation, vessel excision, and lauric acid injection have been widely used in nonclinical studies of PAD.17, 18, 19 Previous studies with these CLI models have reported improvements in blood flow, walking function, and/or gangrene of the ischemic limb, in response to a variety of antiplatelet agents such as thromboxane A2 receptor antagonist,29 5‐HT2A receptor antagonists,30, 31 phosphodiesterase 3 inhibitors,20, 21 and P2Y12 antagonists.19, 32 However, in PAD patients, the complications of CLI are typically defined as severe rest pain and ischemic skin lesions,33, 34 and many of the CLI animal models report severe necrosis at the periphery of the ischemic limb, presumably due to severe occlusion of the proximal arteries.19, 35, 36 Approximately 1% to 3% of PAD patients are clinically classified as having CLI, whereas 30% to 50% experience intermittent claudication and 50% to 70% are asymptomatic, with the latter group being classified as having “mild” PAD.5 Thus, the symptoms of the existing animal models of PAD/CLI seem to be too severe compared to the symptoms experienced by the vast majority of PAD patients, and a PAD model that more closely reflects the clinical pathology of mild PAD is desirable. In the present study, we examined thrombotic hindlimb ischemia induced by the application of FeCl3 to the femoral artery. We determined in this model that blood flow reduction and gait disturbances in the injured limb appeared to be secondary to platelet thrombus‐induced vessel occlusion at the discrete site of FeCl3 application, and no evidence of limb necrosis was found. While beyond the scope of the current study, some of the effects seen in this model may reflect neovascularization processes, including vasculogenesis, arteriogenesis, and angiogenesis, which would require further studies to evaluate. Alternatively, in contrast to the continuous recurrent leg muscle pain experienced by patients with mild PAD, our murine model of thrombotic hindlimb ischemia showed transient limb ischemia and walking dysfunction associated with an acute femoral artery thrombosis. However, limb ischemia without severe limb lesions observed in our model might be considered to reflect the situation in patients with mild PAD.5 Furthermore, the impact of P2Y12 deficiency or pharmacological antagonism in the present model were similar to clinical observations on the efficacy of ticlopidine in patients with intermittent claudication.8, 9 Therefore, we believe our model mimics at least acute limb ischemia in patients with mild PAD. To our knowledge, the present study is the first to evaluate a thrombotic hindlimb ischemia model as a model of mild PAD.
The results of gait analysis using the CatWalk system showed that animals subject to our thrombotic hindlimb injury were ambulatory but had moderate difficulties in grounding and bearing weight on the injured limb. In previous studies, treadmill testing has been widely used for the evaluation of walking profiles in CLI animal models20, 21 and demonstrated the difficulty of spontaneous walking due to the severity of the limb injury. Thus, forced walking on the treadmill was commonly used for functional evaluation in these animal models of CLI. The CatWalk system allows for the detection of subtle changes in natural walking.22, 23 Such profiles proved of interest in gait evaluation in the current model. Intermittent claudication in patients with PAD is caused by the ischemic pain from insufficient blood flow in the iliac and femoral arteries.37 If ischemic pain occurs in the hindlimb, grounding and weight bearing of the limb would be difficult. Our thrombotic hindlimb ischemia model showed a decrease in contact area with the floor and contact duration for the ischemic limb. In particular, maximum contact area and stance phase duration of the ischemic limb were reduced, and swing phase duration of the ischemic limb was increased, while stride length of the ischemic limb did not alter in our model. Vrinten and colleagues showed that paw placement‐related parameters of the injured limb such as contact area and contact intensity were reduced in their chronic constriction injury model, a neuropathic pain model.24 Their model also showed prolongation of swing phase duration and shortening of stance phase duration in the injured limb.24 Parvathy and colleagues evaluated gait disturbances in an inflammatory pain model.25 Similar to the neuropathic pain model, grounding and weight bearing of the injured limb were reduced in their complete Freund's adjuvant–induced arthritis model. However, the stride length of the injured limb did not change significantly compared to that of the normal limb in their model.25 Thus, the changes in gait in our model were similar to those in these 2 pain models. Analgesic treatment could be a possible future approach to determine whether the gait abnormalities in the present thrombotic limb ischemia model are caused by pain. Alternatively, it could be argued that the changes in gait are caused by acute femoral nerve injury due to the FeCl3 application. However, to the best of our knowledge, prasugrel shows selective P2Y12 inhibition and has no direct neuroprotective effect. Thus, prasugrel is likely to inhibit the gait disturbances via the inhibition of thrombotic ischemia. Our results demonstrated the usefulness of using the CatWalk system for the study of moderate gait abnormalities associated with limb ischemia.
In the present study, we used laser speckle blood flow imaging, which is a noninvasive blood flow measurement technique utilizing principles similar to those of laser Doppler imaging,38 to evaluate hindlimb ischemia. However, laser speckle imaging captures blood flow images in a much shorter time but has relatively low spacial resolution compared with laser Doppler imaging. Since there are no reports that compare blood flow in the murine hindlimb by each technique, further study is required to determine the differences, if any, in results obtained by each technique.
In conclusion, acute femoral artery thrombosis resulted in hindlimb ischemia and moderate gait abnormalities in a murine model. Our data further suggest a possible role of P2Y12 in the complications with thrombotic limb ischemia and the therapeutic potential of P2Y12 antagonism in PAD.
Disclosures
Jakubowski is an employee and minor shareholder of Eli Lilly and Company. Other authors are employees of Daiichi Sankyo Co., Ltd.
Acknowledgments
We would like to thank Eriko Iwashita and Nissei Bilis Co., Ltd. for their expert technical contributions.
(J Am Heart Assoc. 2016;5:e002889 doi: 10.1161/JAHA.115.002889)
References
- 1. CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 2. Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Röther J, Salette G, Goto S, Smith SC Jr, Liau CS, Wilson PW, Steg PG; REduction of Atherothrombosis for Continued Health Registry Investigators . Three‐year follow‐up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pineda JR, Kim ES, Osinbowale OO. Impact of pharmacologic interventions on peripheral artery disease. Prog Cardiovasc Dis. 2015;57:510–520. [DOI] [PubMed] [Google Scholar]
- 4. Antithrombotic Trialists' Collaboration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group , Bell K, Caporusso J, Durand‐Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E III, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33:S1–S75. [DOI] [PubMed] [Google Scholar]
- 6. Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, Golzarian J, Gornik HL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE; American College of Cardiology Foundation Task Force; American Heart Association Task Force . Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1555–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aukland A, Hurlow RA, George AJ, Stuart J. Platelet inhibition with Ticlopidine in atherosclerotic intermittent claudication. J Clin Pathol. 1982;35:740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arcan JC, Blanchard J, Boissel JP, Destors JM, Panak E. Multicenter double‐blind study of ticlopidine in the treatment of intermittent claudication and the prevention of its complications. Angiology. 1988;39:802–811. [DOI] [PubMed] [Google Scholar]
- 9. Balsano F, Coccheri S, Libretti A, Nenci GG, Catalano M, Fortunato G, Grasselli S, Violi F, Hellemans H, Vanhove P. Ticlopidine in the treatment of intermittent claudication: a 21‐month double‐blind trial. J Lab Clin Med. 1989;114:84–91. [PubMed] [Google Scholar]
- 10. Fagher B. Long‐term effects of ticlopidine on lower limb blood flow, ankle/brachial index and symptoms in peripheral arteriosclerosis. A double‐blind study. The STIMS Group in Lund. Swedish Ticlopidine Multicenter Study. Angiology. 1994;45:777–788. [DOI] [PubMed] [Google Scholar]
- 11. Bergqvist D, Almgren B, Dickinson JP. Reduction of requirement for leg vascular surgery during long‐term treatment of claudicant patients with ticlopidine: results from the Swedish Ticlopidine Multicentre Study (STIMS). Eur J Vasc Endovasc Surg. 1995;10:69–76. [DOI] [PubMed] [Google Scholar]
- 12. Singer E, Imfeld S, Staub D, Hoffmann U, Buschmann I, Labs KH, Jaeger KA. Effect of aspirin versus clopidogrel on walking exercise performance in intermittent claudication—a double‐blind randomized multicenter trial. J Am Heart Assoc. 2012;1:51–56 doi: 10.1161/JAHA.111.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Zidar JP; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–1959. [DOI] [PubMed] [Google Scholar]
- 14. Niitsu Y, Jakubowski JA, Sugidachi A, Asai F. Pharmacology of CS‐747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost. 2005;31:184–194. [DOI] [PubMed] [Google Scholar]
- 15. Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25:357–374. [DOI] [PubMed] [Google Scholar]
- 16. Dobesh PP. Pharmacokinetics and pharmacodynamics of prasugrel, a thienopyridine P2Y12 inhibitor. Pharmacotherapy. 2009;29:1089–1102. [DOI] [PubMed] [Google Scholar]
- 17. Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind‐limb ischemia. Nat Protoc. 2009;4:1737–1746. [DOI] [PubMed] [Google Scholar]
- 18. Niiyama H, Huang NF, Rollins MD, Cooke JP. Murine model of hindlimb ischemia. J Vis Exp. 1035;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashida S, Ishihara M, Ogawa H, Abiko Y. Protective effect of ticlopidine on experimentally induced peripheral arterial occlusive disease in rats. Thromb Res. 1980;18:55–67. [DOI] [PubMed] [Google Scholar]
- 20. Ishiwata N, Noguchi K, Kawanishi M, Asakura Y, Hori M, Mitani A, Ito Y, Takahashi K, Nishiyama H, Shudo N, Takahashi S, Takahashi K, Tsuruzoe N, Nakaike S. NT‐702 (parogrelil hydrochloride, NM‐702), a novel and potent phosphodiesterase inhibitor, improves reduced walking distance and lowered hindlimb plantar surface temperature in a rat experimental intermittent claudication model. Life Sci. 2007;81:970–978. [DOI] [PubMed] [Google Scholar]
- 21. Sasaki Y, Suzuki H, Itoh S, Yoshida H, Kondo S, Inoue K, Tanabe S. K‐134, a phosphodiesterase 3 inhibitor, improves gait disturbance and hindlimb blood flow impairment in rat peripheral artery disease models. Eur J Pharmacol. 2012;689:132–138. [DOI] [PubMed] [Google Scholar]
- 22. Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18:187–201. [DOI] [PubMed] [Google Scholar]
- 23. Hamers FP, Koopmans GC, Joosten EA. CatWalk‐assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23:537–548. [DOI] [PubMed] [Google Scholar]
- 24. Vrinten DH, Hamers FF. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102:203–209. [DOI] [PubMed] [Google Scholar]
- 25. Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund's adjuvant‐induced arthritis using the CatWalk system. BMC Musculoskelet Disord. 2013;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou M, Zhang W, Chang J, Wang J, Zheng W, Yang Y, Wen P, Li M, Xiao H. Gait analysis in three different 6‐hydroxydopamine rat models of Parkinson's disease. Neurosci Lett. 2015;584:184–189. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, Liu J. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow Metab. 2008;28:1936–1950. [DOI] [PubMed] [Google Scholar]
- 28. Hashimoto M, Sugidachi A, Isobe T, Niitsu Y, Ogawa T, Jakubowski JA, Asai F. The influence of P2Y12 receptor deficiency on the platelet inhibitory activities of prasugrel in a mouse model: evidence for specific inhibition of P2Y12 receptors by prasugrel. Biochem Pharmacol. 2007;74:1003–1009. [DOI] [PubMed] [Google Scholar]
- 29. Shirakura S, Higo K, Takeda M, Karasawa A. Antithrombotic effects of KW‐3635, a thromboxane A2‐receptor antagonist, in guinea pigs. Jpn J Pharmacol. 1994;65:93–98. [DOI] [PubMed] [Google Scholar]
- 30. Hara H, Shimada H, Kitajima A, Tamao Y. Effect of (+/‐)‐2‐(dimethylamino)‐1‐[[o‐(m‐methoxyphenethyl)phenoxy] methyl]ethyl hydrogen succinate on experimental models of peripheral obstructive disease. Arzneimittelforschung. 1991;41:616–620. [PubMed] [Google Scholar]
- 31. Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Asai F. Effects of R‐102444, an orally active 5‐HT2A receptor antagonist, in rat models of peripheral vascular disease. Vascul Pharmacol. 2004;41:7–13. [DOI] [PubMed] [Google Scholar]
- 32. Ogawa T, Hashimoto M, Niitsu Y, Jakubowski JA, Tani Y, Otsuguro K, Asai F, Sugidachi A. Effects of prasugrel, a novel P2Y(12) inhibitor, in rat models of cerebral and peripheral artery occlusive diseases. Eur J Pharmacol. 2009;612:29–34. [DOI] [PubMed] [Google Scholar]
- 33. Lau JF, Weinberg MD, Olin JW. Peripheral artery disease. Part 1: clinical evaluation and noninvasive diagnosis. Nat Rev Cardiol. 2011;8:405–418. [DOI] [PubMed] [Google Scholar]
- 34. Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G; Society for Vascular Surgery Lower Extremity Guidelines Committee . The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220–234.e1‐2. [DOI] [PubMed] [Google Scholar]
- 35. Tongers J, Knapp JM, Korf M, Kempf T, Limbourg A, Limbourg FP, Li Z, Fraccarollo D, Bauersachs J, Han X, Drexler H, Fiedler B, Wollert KC. Haeme oxygenase promotes progenitor cell mobilization, neovascularization, and functional recovery after critical hindlimb ischaemia in mice. Cardiovasc Res. 2008;78:294–300. [DOI] [PubMed] [Google Scholar]
- 36. Yoshida H, Itoh S, Hara T, Sasaki Y, Kondo S, Nakagawa T, Asanuma A, Tanabe S. A phosphodiesterase 3 inhibitor, K‐134, improves hindlimb skeletal muscle circulation in rat models of peripheral arterial disease. Atherosclerosis. 2012;221:84–90. [DOI] [PubMed] [Google Scholar]
- 37. Doggrell SA. Pharmacotherapy of intermittent claudication. Expert Opin Pharmacother. 2001;2:1725–1736. [DOI] [PubMed] [Google Scholar]
- 38. Briers D, Duncan DD, Hirst E, Kirkpatrick SJ, Larsson M, Steenbergen W, Stromberg T, Thompson OB. Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 2013;18:066018. [DOI] [PubMed] [Google Scholar]


