Abstract
Background
The cardiovascular effects of positive airway pressure (PAP) therapy in obstructive sleep apnea (OSA) patients are not clear because of confounding by comorbid conditions.
Methods and Results
Prospective interventional study of PAP therapy and withdrawal. Apnea Hypopnea Index (AHI; events/hour of sleep) was determined from polysomnography. Central aortic blood pressures (BPs), Aortic Augmentation Index (AAIx), and central (PWV c‐f) and peripheral pulse wave (PWV c‐r) velocities were determined by applanation tonometry. Echocardiography and brachial artery reactivity testing were performed at baseline, after 4 and 12 weeks of PAP therapy, and 1 week after PAP withdrawal. The 84 participants were mean (SD) 41.1 (7.6) years old and had 39.8 (24.5) AHI events/hour. After 4 weeks post‐PAP initiation and sustained after 12 weeks, subjects experienced decreases in central systolic BP (P=0.008), diastolic BP, mean BP, AAIx, and PWV c‐r, and brachial artery dilation (all P<0.001), as well as improvements in left ventricular diastolic function and systemic and pulmonary vascular resistance. In adjusted models, PAP use (hours/night) predicted reductions in diastolic BP (β=−0.65 [SE, 0.32] mm Hg/hour; P=0.045), AAIx (β=−0.53 [0.27] %/hour; P=0.049) and PWV c‐r (β=−0.13 [0.05] m·s−1/hour; P=0.007), and improved brachial artery flow‐mediated dilation (β=0.31 [0.14] %/hour use; P=0.015). After 1 week of PAP withdrawal, brachial diameter, diastolic BP, mean BP, AAIx, and heart rate increased (P≤0.05).
Conclusions
PAP therapy reduces arterial tone and improves endothelial and diastolic function in young to middle‐aged adults. This positive effect is observed after 4 weeks and depends on hours of use, but reverts quickly with PAP withdrawal.
Clinical Trial Registration
URL: https://clinicaltrials.gov/. Unique identifier: NCT01317329.
Keywords: echocardiography, endothelial function, obstructive sleep apnea, positive airway pressure ventilation compliance, pulse wave velocity
Subject Categories: Hypertension, Echocardiography, Endothelium/Vascular Type/Nitric Oxide, Compliance/Adherence
Introduction
Moderate‐to‐severe obstructive sleep apnea (OSA) affects ≈13% of men and 6% of women in the United States.1 OSA is characterized by intermittent hypoxia, arousals, and exaggerated ventilatory efforts with excessive negative intrathoracic pressure fluctuations that result in increased sympathetic nerve activity, oxidative stress, inflammation, endothelial dysfunction, vascular stiffness, and abnormal glucose metabolism.2, 3, 4, 5, 6 OSA is associated with systemic hypertension and increased risk for fatal and nonfatal cardiovascular disease (CVD) events.7 Because obesity and insulin resistance tend to accompany OSA as comorbidities, it has been difficult to isolate the independent effect of OSA and its treatments on the development and consequences of hypertension. Several studies have postulated that increased sympathetic tone and endothelial dysfunction cause hypertension in OSA.8, 9, 10
Treatment with positive airway pressure (PAP) may reduce CVD risk11 however, the extent, time course, mechanisms, and durability of its benefit are not well known. These are especially important issues given that the diagnosis of OSA tends to be delayed12 and compliance with PAP therapy can be poor or intermittent, especially in young adults.13, 14, 15 Furthermore, blood pressure (BP) and endothelial function responses to PAP therapy have differed between studies, in part because of variables that may confound vascular responses to OSA and PAP therapy, especially age and preexisting hypertension,16 as well as sex, race/ethnicity,17 obesity, and degree of sleepiness.18 Across these studies, subjects also differed in OSA severity or had variable exposures to PAP; some studies were underpowered. Also, the effects of short‐term PAP withdrawal on cardiovascular measures only have been characterized in a few studies,19, 20 though its effects on sleep architecture and obstructive event rates indicate a rapid return to almost baseline values.21
Our study evaluated the time course and magnitude of PAP therapy effects on arterial stiffness, endothelium‐dependent vasodilation, and cardiac function. In order to avoid the influence of confounding effects of advanced age, prolonged hypertension, and use of PAP, we focused on younger, treatment‐naïve adults (18–50 years old) with moderate‐to‐severe OSA who were normotensive (including only mild hypertensive subjects with well‐controlled hypertension). They were evaluated at baseline and after 4 and 12 weeks of PAP therapy, then again after 1 week of PAP withdrawal. The aims of our study were to: (1) determine the time course and magnitude of effect of PAP therapy on measures of arterial stiffness and tone, endothelial function, and cardiac size and function and (2) assess the effect of PAP withdrawal on these changes.
Methods
Participants
The study was reviewed and approved by the University of Wisconsin Health Sciences Institutional Review Board (Madison, WI); all subjects signed the approved consent form. All subjects were assessed during the morning hours after 10 hours of fasting, refraining from caffeine, smoking, and exercise. Patients from Wisconsin Sleep (a clinical sleep center) that met the inclusion and exclusion criteria were invited to participate after completion of clinically indicated overnight in‐laboratory polysomnography (PSG) or home sleep testing and routine survey questionnaires. We enrolled subjects with an Apnea Hypopnea Index (AHI) of >10 events/hour and an Epworth Sleepiness Scale (ESS) ≥10 or AHI ≥20 events/hour, who were between the ages of 18 and 50 years and naïve of past apnea treatment. Subjects could not have hypertension; however, stable treatment for at least 6 weeks before enrollment was permitted.
Polysomnography
Overnight PSG (n=47 subjects) was performed using Respironics Alice Sleepware (version 5; Respironics, Inc., Murraysville, PA) digital PSG equipment. Recordings were performed according to American Academy of Sleep Medicine (AASM) guidelines and included 6 channels of electroencephalography, electrooculography, submental electromyography (EMG), electrocardiography (ECG), bilateral tibial EMG, respiratory inductance plethysmography, pulse oximetry, and a position sensor. Home sleep testing (n=43 subjects) was performed using the Respironics PDx Portable Sleep System (Respironics, Inc.); channels recorded included nasal cannula, oral thermistor, chest and abdominal effort, pulse oximetry, heart rate, snoring, and position. Sleep recordings, sleep staging, and scoring of associated events were performed according to AASM criteria22 by registered PSG technologists. Physicians certified in sleep medicine reviewed all studies and prepared clinical reports. ESS scores were collected at the time of sleep studies.
At each study visit after PAP therapy establishment, compliance information (hours of use, AHI and leak) was downloaded from the patient's PAP equipment. Total hours of PAP use divided by the exact number of days until the study visit (4 or 12 weeks ± 1 week windows) were used to calculate “average hours PAP use/night” as our predictor variable. The sleep specialist that interpreted the initial PSGs and identified possible candidates to be enrolled in the study also indicated whether it would be safe for them to experience a week of withdrawal visit based on minimal and average nocturnal oxygen desaturation. Only subjects approved by the sleep specialist were invited to participate in the optional withdrawal visit to test the reversibility of PAP benefits. A subgroup of 44 subjects agreed to refrain from PAP use for 6.6 (3.0) days; their demographic characteristics were not different from the full study cohort and are described in Table 2.
Arterial Tonometry
Radial pulse wave analysis and pulse wave velocity (PWV) were assessed by arterial tonometry using a Sphygmocor MM3 system (Atcor Medical Inc, Itasca, IL). Radial artery tonometry and calibrated brachial pressures obtained by oscillometry with a Dynamap Pro‐400 (DINAMAP; GE Medical Systems, Milwaukee, WI) were used for pulse wave analysis. From these data, wave reflections, their timing, and Aortic Augmentation Index (AAIx)23 information were obtained. A validated transfer function was used to derive central aortic pressures from radial tonometry tracings as previously described.24 AAIx derived from central aortic pressure and central pulse pressure were calculated, thus providing 2 measures of wave reflections and cardiac load.25 AAIx was corrected for heart rate. PWV, the gold‐standard measure of arterial stiffness, was obtained in 2 arterial territories, central (carotid‐femoral, PWVc‐f) and peripheral (carotid‐radial, PWVc‐r), as markers of early changes in arterial stiffness and vascular tone.26, 27, 28 PWVc‐f was used as a measure of conduit artery passive (capacitative) stiffness. It is dependent mainly on the elastin/collagen composition of the aorta as well as its diameter and wall thickness, whereas PWVc‐r was used as a measure of muscular arterial bed active stiffness. It is dependent on multiple factors, though principally endothelial function and sympathetic resting tone. Stable 10‐second tonometry tracings were obtained where at least 80% of the beats had a detectable upstroke. Only recordings with a mean time SD below 6% and derived PWV SD below 10% were accepted.
Endothelial Function Assessment by Brachial Artery Flow‐Mediated Dilation
Brachial artery imaging was performed with an L12‐3 MHz linear transducer on a CX50 system (Philips Ultrasound, Andover, MA). Flow‐mediated dilation (FMD; %) was measured as previously described29; the diameter of a preselected segment of the brachial artery was measured before and after a hyperemic stimulus provoked by a 5‐minute forearm occlusion at 250 mm Hg. Percentage dilation is a measure of endothelium‐mediated response to increased shear stress. In our laboratory, subjects who underwent repeat FMD scans ≈2 weeks apart had an interscan absolute ∆FMD of only 0.26% (−0.43% to +0.72%; P=0.498); median inter‐reader variability was −0.14% to +0.09%, with correlations of 0.97 to 0.99 (P<0.001).30
Transthoracic Echocardiography
Imaging followed the American Society of Echocardiography (ASE) Guidelines for acquisition, measurement, and interpretation.31, 32 Images were obtained using a Philips CX50 system (Philips Healthcare, Andover, MA) with a 4V1c transducer by a single registered cardiac sonographer. Measurements were performed by an individual blind to the sleep study and PAP compliance data offline in triplicate using a Syngo Workplace (Siemens, Issaquah, WA) workstation by a single registered cardiac sonographer (C.E.K.) and over‐read by a Level III echocardiographer (J.H.S.). Left ventricle (LV) mass was calculated using the two‐dimensional (2D)‐derived ASE corrected cubed formula, at end‐diastole. LV ejection fraction (LVEF) was calculated using the biplane method of disks using a semiautomated border detection algorithm.33 Left atrial (LA) volume was measured using the biplane area‐length method. LV diastolic function LVDF assessments used mitral valve pulsed wave Doppler and Doppler tissue imaging at rest and during the Valsalva maneuver.34 LV outflow tract diameter and pulse wave Doppler were used to calculate stroke volume and cardiac output. The combination of mean brachial arterial pressure (oscillometric technique), right atrial (RA) pressure estimated based on inferior vena cava diameter and respiratory changes, and mean flow (LV outflow tract cardiac output) were used to calculate systemic vascular resistance (SVR).
Right ventricle (RV) end‐diastolic area, RV fractional area change, pulmonary systolic pressure (derived from the peak tricuspid regurgitation Doppler velocity and estimated RA pressure), pulmonary vascular resistance (PVR; 10× tricuspid valve peak regurgitation velocity/time‐velocity integral from the RV outflow tract),35 and RA area36 were measured in triplicate and calculated. Tricuspid annular plane systolic excursion (TAPSE) was obtained using 2D‐guided M‐mode tracings from apical 4 chamber views, maximizing the image resolution and alignment between the cardiac apex and the tricuspid annulus.
The ECG parameters described in this manuscript were obtained from ≥98% of participants; missing data were attributable to subjects with poor acoustic windows or incomplete Doppler signals. Intrareader reproducibility was assessed by Pearson correlation coefficients and coefficients of variation (CVs) using the root mean‐squared approach.37 A random sample of 10 echocardiograms was remeasured by the same reader (C.E.K.); reproducibility was excellent for LV mass (r=0.98; P<0.001; CV=6.1%), LVEF (r=0.83; P=0.003; CV=3.0%), cardiac output (r=0.91; P<0.001; CV=7.9%); LA volume (r=0.92; P<0.001; CV=10.3%), pulmonary artery systolic pressure (r=0.92; P<0.001; CV=7.3%), RV end‐diastolic area (r=0.96; P<0.001; CV=4.8%), RA area (r=0.92; P<0.001; CV=7.2%), PVR (r=0.92; P<0.001; CV=5.4%), and TAPSE (r=0.83; P<0.005; CV=6.0%). These results are within the desirable reproducibility for ECG outcome variables used in clinical trials or observational studies.32, 38, 39
Statistical Analysis
Analyses were performed with SAS software (version 9.2; SAS Institute, Inc, Cary, NC). AHI was parameterized as log10(AHI+1) when analyzed as a continuous variable because of its skewed distribution.40 Data are presented as mean (SD) for continuous variables. For discrete variables, results are presented as counts and percentages. AHI was the main measure of OSA severity. Additional measures of disease severity, such as peak O2 desaturation, overall nocturnal hypoxia, and time with O2 saturation <89%, were tested and displayed similar effects; they are not presented in this article.
The primary predictor, PAP compliance, was expressed in average hours of use during the observational period (hour/day). We also tested PAP effects in 2 categorical groups: average PAP use <4 or ≥4 hours/night as usually presented in the literature. Because the results were very similar, results using the continuous variable are presented.
Paired t tests were used to compare outcome measures between baseline and after 4 and 12 weeks of PAP treatment. We additionally performed paired t test comparisons between 12 weeks of PAP treatment and after 1 week of PAP withdrawal. Multivariable linear regression models were used to estimate associations between PAP use and changes in cardiovascular outcomes at each follow‐up visit. Final models included age, sex, body mass index (BMI), systolic BP, antihypertensive medication use, log10(AHI+1), and PAP compliance. Models that predicted FMD changes were also adjusted for baseline brachial artery diameter variations between visits. A “time” by PAP use interaction term was tested to assess whether treatment effect differed over time. It was found to be a nonsignificant predictor, indicating that there were no differences in PAP effect between 4 and 12 weeks of therapy. Therefore, week 4 and week 12 changes in outcome variables were combined with “time” as a covariate and analyzed as a multivariate regression model with repeated measures with a compound symmetry covariance structure.
P values <0.05 were considered statistically significant for main‐effects variables; P values <0.01 were considered statistically significant for interaction effects.
Results
Subjects
Overnight sleep testing was performed at subjects’ homes using a type III home sleep testing device (N=43) or in a clinical sleep laboratory using full PSG (N=47) for a final enrollment of 90 subjects. One subject was disqualified after rereviewing the PSG because of AHI <10 events/hour at baseline. Of the remaining 89 enrolled, 84 subjects returned for either both visits (n=78 subjects), week 4 only (n=4), or week 12 only (n=2). Figure 1 shows a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (www.consort-statement.org) for enrollment, retention, and analysis. Demographic characteristics are presented in Table 1. Subjects were (mean [SD]) 41.1 (SD=7.6) years old, 77% were male, 89% white with a BMI of 35.4 (7.4) kg/m2, mean AHI of 39.8 (24.5) events/hour, and Epworth score of 9.8 (4.9) with 45.2% having a score above 10. Systolic and diastolic BP were 127 (11) and 77 (8) mm Hg, respectively. Only 20% of subjects were on antihypertensive medications. All were well controlled: 13% on monotherapy and 7% on 2 or 3 antihypertensive medications. Residual AHI was 3.42 (2.89) and 2.97 (2.27) events/hour after 4 and 12 weeks of treatment, respectively. As summarized in a recent meta‐analysis41 and observed in this study, PAP compliance after 12 weeks was associated with a modest increase in BMI (+0.25 [SE, 0.95] kg/m2; P=0.021) compared to baseline. Change in BMI was independently predicted by baseline BMI (P=0.003) in a model adjusted for age, sex, BP medications, and baseline systolic BP.
Figure 1.
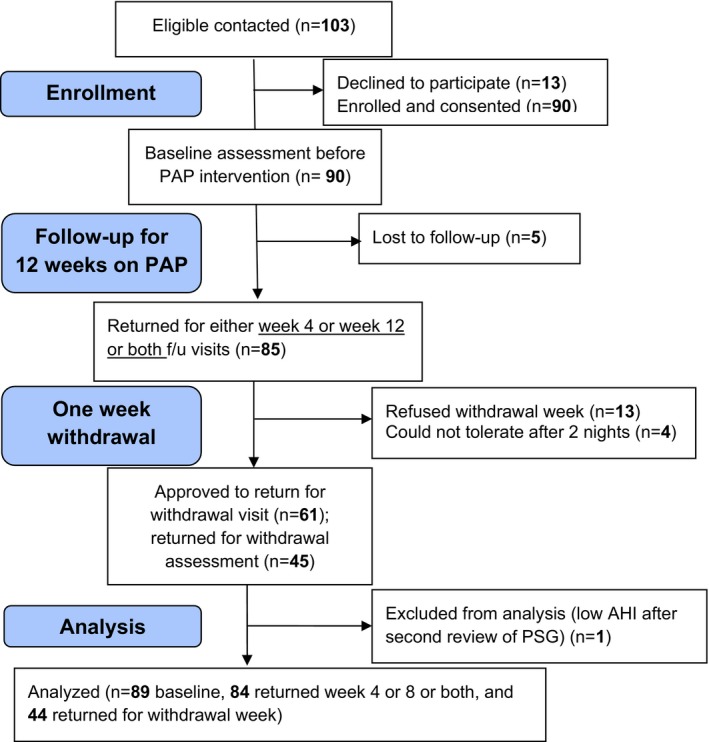
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for enrollment, intervention, follow‐up, and data analysis. AHI indicates Apnea‐hypopnea Index; PAP, positive airway pressure; PSG, .
Table 1.
Baseline Characteristics
| Variable (N=84) | Mean | SD |
|---|---|---|
| Age, y | 41.1 | 7.6 |
| Sex (% male) | 77 | |
| Body mass index, kg/m2 | 35.4 | 7.4 |
| Race (% white) | 89 | |
| AHI, events/hr | 39.8 | 24.5 |
| BP‐lowering medication use (%) | 20 | |
| Log10 (AHI+1) | 1.54 | 0.23 |
| Mean SpO2 saturation (%) | 92.9 | 1.7 |
| Percentage of sleep time with SpO2 saturation <89% (%) | 18.0 | 22.7 |
| Epworth score | 9.8 | 4.9 |
| BPs | ||
| Central systolic, mm Hg | 115.0 | 9.7 |
| Brachial systolic, mm Hg | 127.5 | 11.8 |
| Diastolic, mm Hg | 76.6 | 7.95 |
| Mean, mm Hg | 94.6 | 7.6 |
| Heart rate, beats/min | 63.7 | 9.1 |
| PWVc‐f, m/sec | 6.6 | 1.0 |
| PWVc‐r, m/sec | 8.3 | 1.2 |
| Augmentation indexHR corrected (%) | 15.1 | 9.7 |
| Maximum relative FMD (%) | 4.40 | 2.89 |
| Maximum absolute FMD, mm | 0.19 | 0.11 |
| Brachial artery diameter, mm | 4.38 | 0.64 |
| Left ventricular mass indexed, g/m2 | 73.5 | 12.9 |
| Left ventricular ejection fraction (%) | 59.1 | 4.1 |
| Diastolic function, % | ||
| 1=normal | 56.0 | |
| 2=abnormal relaxation | 9.5 | |
| 3=pseudo normal | 34.5 | |
| Left atrial volume indexed, mL/m2 | 27.2 | 5.2 |
| TAPSE, mm | 22.4 | 3.1 |
| Right ventricular end‐diastolic area, cm2 | 20.2 | 4.3 |
| Pulmonary artery systolic pressure, mm Hg | 27.2 | 5.5 |
| Cardiac output, L/min | 5.8 | 1.2 |
| Systemic vascular resistance, dynes×s/cm5 | 1251 | 268 |
| Pulmonary vascular resistance (Woods Units) | 1.70 | 0.30 |
AHI indicates Apnea Hypopnea Index; BP, blood pressure; FMD, flow‐mediated vasodilation; PWVc‐f, carotid‐femoral pulse wave velocity; PWVc‐r, carotid‐radial pulse wave velocity; TAPSE, tricuspid annular plane systolic excursion.
We also investigated whether the subgroup of subjects that returned after 1 week of PAP withdrawal (n=44) had different baseline characteristics from the ones that attended any on‐treatment visits. The only statistically significant differences we found between the 2 groups were baseline AHI and TAPSE; both were higher in the subjects who did not return (48.1 [29.8] vs 32.3 [15.4] events/hour; P=0.025; and 23.3 [2.7] vs 21.6 [3.3] mm; P=0.008, respectively; Table 2).
Table 2.
Comparison of Baseline Characteristics by Withdrawal Visit Participation
| Participated in Withdrawal Visit | No | Yes | P Value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| N | 40 | 44 | |
| Age, y | 41.4 (7.6) | 40.8 (7.6) | 0.622 |
| Body mass index, kg/m2 | 36.2 (7.0) | 32.8 (7.7) | 0.221 |
| Apnea hypopnea index, events/hr | 48.1 (29.8) | 32.3 (15.4) | 0.025 |
| Blood pressure | |||
| Central systolic, mm Hg | 114.5 (10.4) | 116.1 (9.1) | 0.327 |
| Brachial systolic, mm Hg | 126.3 (12.4) | 128.7 (11.2) | 0.272 |
| Diastolic, mm Hg | 77.2 (7.7) | 76.1 (8.2) | 0.498 |
| Mean, mm Hg | 94.4 (7.8) | 94.7 (7.5) | 0.868 |
| Heart rate, beats/min | 63.2 (9.2) | 64.2 (9.1) | 0.522 |
| PWVc‐f, m/sec | 6.7 (1.0) | 6.4 (1.0) | 0.390 |
| PWVc‐r, m/sec | 8.4 (1.1) | 8.3 (1.2) | 0.669 |
| AAIxHR corrected (%) | 14.6 (9.4) | 15.6 (10.1) | 0.709 |
| Maximum relative FMD (%) | 4.7 (3.0) | 4.2 (2.8) | 0.551 |
| Brachial artery diameter, mm | 4.4 (0.7) | 4.4 (0.6) | 0.907 |
| Left ventricular mass, g | 171.2 (40.4) | 160.9 (37.9) | 0.132 |
| Left ventricular ejection fraction (%) | 59.3 (4.2) | 58.9 (4.0) | 0.539 |
| Diastolic function (grade) | 1.7 (0.9) | 1.9 (0.9) | 0.286 |
| E/e’ ratio | 8.4 (1.9) | 8.5 (1.8) | 0.925 |
| Left atrial volume, mL | 61.3 (14.9) | 60.9 (13.1) | 0.929 |
| TAPSE, mm | 23.3 (2.7) | 21.6 (3.3) | 0.008 |
| Right ventricular end‐diastolic area, cm2 | 20.6 | 19.9 | 0.482 |
| Pulmonary artery systolic pressure, mm Hg | 27.6 (4.8) | 26.7 (6.0) | 0.436 |
| Systemic vascular resistance, dynes×s/cm5 | 1223.6 (237) | 1275.4 (293) | 0.579 |
AAIx indicates Aortic Augmentation Index; FMD, flow‐mediated vasodilation; PWVc‐f, carotid‐femoral pulse wave velocity; PWVc‐r, carotid‐radial pulse wave velocity; TAPSE, tricuspid annular plane systolic excursion.
PAP Effects on Arterial Stiffness
After 4 and 12 weeks of PAP treatment, we observed significant reductions in BPs (Table 3). Brachial diastolic and mean BPs decreased by 4.6 (6.6) and 3.7 (6.1) mm Hg, respectively (P≤0.001). Accordingly, central systolic BP was 2.6 (8.5) mm Hg lower after 4 weeks of PAP (P=0.008) and 2.9 (8.2) mm Hg lower after 12 weeks of PAP (P=0.003). AAIx also significantly declined at 4 weeks (P<0.001) and remained lower at 12 weeks (P<0.001). PWVc‐r was significantly lower than at baseline after both 4 (P<0.001) and 12 (P=0.003) weeks, though reductions in PWVc‐f did not reach statistical significance. In multivariate analysis, both reduction in diastolic BP (β=−0.65 [0.32] mm Hg/hours per night; P=0.045) and AAIx (β=−0.53 [0.27] %/hours/night; P=0.049) were independently predicted by PAP use (Table 4). After a week of PAP withdrawal, diastolic BP, mean BP, central systolic BP, and AAIx all started to reverse toward baseline values with worsening outcomes as compared to week 12 values (all P<0.01).
Table 3.
Changes From Baseline on Positive Airway Pressure Therapy and Its After Withdrawal
| Variable Changes | Week 4—Baseline | Week 12—Baseline | Week 13a to Week 12 | |||
|---|---|---|---|---|---|---|
| Change Mean (SD) | P Value | Change Mean (SD) | P Value | Change Mean (SD) | P Value | |
| N | 82 | 80 | 44 | |||
| Body mass index, kg/m2 | 0.16 (0.69) | 0.039 | 0.25 (0.95) | 0.021 | −0.03 (0.49) | 0.70 |
| Log10 (AHI+1) | −0.49 (0.33) | <0.001 | −0.53 (0.34) | <0.001 | n/a | n/a |
| Blood pressures | ||||||
| Central systolic, mm Hg | −2.6 (8.5)b | 0.008b | −2.9 (8.2)b | 0.003b | 0.97 (5.4) | 0.25 |
| Brachial systolic, mm Hg | −0.4 (10.9) | 0.76 | −0.3 (9.8) | 0.79 | −0.4 (7.5) | 0.71 |
| Diastolic, mm Hg | −4.6 (6.6)b | <0.001b | −3.9 (6.1)b | <0.001b | 1.8 (4.5)b | 0.010b |
| Mean, mm Hg | −3.7 (6.1)b | <0.001b | −3.6 (5.9)b | <0.001b | 1.9 (4.0)b | 0.003b |
| Heart rate, beats/min | 0.8 (7.9) | 0.38 | 0.6 (6.7) | 0.42 | 2.1 (6.7)b | 0.045b |
| PWVc‐f, m/sec | −0.16 (0.68) | 0.054 | −0.09 (0.90) | 0.44 | −0.01 (0.74) | 0.964 |
| PWVc‐r, m/sec | −0.35 (0.85)b | <0.001b | −0.35 (1.00)b | <0.003b | 0.23 (0.87) | 0.10 |
| AAIxHR corrected (%) | −2.81 (5.24)b | <0.001b | −3.73 (5.13)b | <0.001b | 2.92 (4.69)b | <0.001b |
| Maximum relative FMD (%) | 0.42 (2.64) | 0.16 | 0.37 (2.70) | 0.22 | −0.07 (2.36) | 0.85 |
| Brachial artery diameter, mm | 0.09 (0.17)b | <0.001b | 0.13 (0.19)b | <0.001b | −0.04 (0.11)b | 0.02b |
| Left ventricular mass index, g/m2 | 0.2 (6.3) | 0.75 | −1.4 (6.7) | 0.057 | 0.52 (6.18) | 0.83 |
| Left ventricular ejection fraction (%) | 0.1 (3.8) | 0.814 | 0.7 (4.2) | 0.14 | −0.02 (4.52) | 0.98 |
| Diastolic function (grade) | 0.25 (0.85)b | 0.010b | 0.49 (0.97)b | <0.001b | −0.18 (0.72) | 0.10 |
| E/e’ ratio | −0.12 (1.60) | 0.54 | −0.15 (1.63) | 0.40 | −0.15 (1.72) | 0.56 |
| Left atrial volume indexed, mL/m2 | 0.30 (2.79) | 0.34 | 0.12 (2.80) | 0.70 | 0.10 (0.22) | 0.85 |
| TAPSE, mm | 0.27 (2.76) | ns | 0.91 (2.99)b | 0.008b | 0.02 (0.32) | ns |
| Right ventricular end‐diastolic area, cm2 | 0.07 (2.86) | ns | 0.56 (2.95) | ns | 0.08 (0.25) | ns |
| Pulmonary artery systolic pressure, mm Hg | −0.86 (5.58) | ns | −0.65 (5.31) | ns | 1.50 (5.76) | 0.09 |
| Cardiac output, L/min | 0.05 (0.83) | 0.62 | 0.12 (0.81) | 0.18 | 0.04 (0.78) | 0.77 |
| Systemic vascular resistance, dynes×s/cm5 | −56.6 (194)b | 0.011b | −73.0 (162)b | <0.001b | 1.97 (161) | 0.94 |
| Pulmonary vascular resistance (Woods Units) | −0.09 (0.31)b | 0.017b | −0.10 (0.30)b | 0.004b | 0.05 (0.27) | 0.20 |
AAIx indicates Aortic Augmentation Index; AHI, Apnea Hypopnea Index; FMD, flow‐mediated vasodilation; PWVc‐f, carotid‐femoral pulse wave velocity; PWVc‐r, carotid‐radial pulse wave velocity; TAPSE, tricuspid annular plane systolic excursion.
Week 13=1 week of withdrawal from positive airway pressure (PAP).
P<0.05.
Table 4.
Effects of Baseline AHI Severity and PAP Use on Cardiovascular Outcomes—Main Effects Models
| Outcome Changes | Baseline Log10(AHI+1) | PAP Use (Hours/Night) | ||
|---|---|---|---|---|
| Beta (SE) | P Value | Beta (SE) | P Value | |
| Brachial systolic BP, mm Hg | −3.80 (3.15) | 0.230 | 0.66 (0.46) | 0.150 |
| Diastolic BP, mm Hg | −3.61 (2.23) | 0.107 | −0.65 (0.32)a | 0.045a |
| Mean pressure, mm Hg | −3.00 (1.99) | 0.134 | −0.36 (0.29) | 0.214 |
| Central systolic BP, mm Hg | −4.00 (2.53) | 0.115 | 0.10 (0.37) | 0.782 |
| Body mass index, kg/m2 | 0.68 (0.28)a | 0.017a | 0.03 (0.04) | 0.399 |
| Brachial artery diameter, mm | 0.17 (0.06)a | 0.008a | 0.01 (0.01) | 0.326 |
| Maximum relative FMD (%) | −1.41 (0.97) | 0.150 | 0.34 (0.14)a | 0.015a |
| Maximum absolute FMD, mm | −0.07 (0.04) | 0.085 | 0.02 (0.01)a | 0.007a |
| HR‐corrected augmentation index (%) | −3.25 (1.87) | 0.084 | −0.53 (0.27)a | 0.049a |
| PWVc‐f (femoral), m/sec | −0.23 (0.31) | 0.460 | −0.01 (0.04) | 0.860 |
| PWVc‐r (carotid), m/sec | −0.90 (0.32)a | 0.006a | −0.13 (0.05)a | 0.007a |
| LV ejection fraction (%) | 3.03 (1.41)a | 0.034a | −0.04 (0.20) | 0.845 |
| PA systolic pressure, mm Hg | 5.45 (1.88)a | 0.004a | −0.05 (0.27) | 0.847 |
| Cardiac output, L/min | 0.81 (0.29)a | 0.006a | 0.00 (0.04) | 0.913 |
| SVR (SD) | −0.79 (0.24)a | 0.001a | −0.01 (0.03) | 0.690 |
| Diastolic function (grade) | −1.03 (0.31)a | 0.001a | 0.10 (0.05)a | 0.028a |
Both predictor variables included simultaneously in the models. Output reflects repeated measures modeling. Models adjusted for age, sex, BMI, baseline SBP, and baseline BP medications. FMD models included brachial artery diameter as a covariate. Indexed measures did not include AHI indicates apnea hypopnea index; BMI as a covariate. BMI, body mass index; BP, blood pressure; FMD, flow‐mediated vasodilation; LV, left ventricle; PAP, positive airway pressure; PWVc‐f, carotid‐femoral pulse wave velocity; PWVc‐r, carotid‐radial pulse wave velocity; SE, standard error; SVR, systemic vascular resistance.
P<0.05.
In multivariate analysis, reduction in PWVc‐r was independently associated with log10(AHI+1) and PAP use (hours/night; both P<0.007; Table 4). Figure 2 shows changes in PWVc‐r by PAP compliance categorized by use <4 or ≥4 hours/night at each study visit, where the significant effects of PAP use and withdrawal are demonstrated (P<0.0003 for 4‐ and 12‐week visits). In models adjusting for PAP use, OSA severity (measured by log10[AHI+1]), and their interaction, none of the interaction terms were statistically significant predictors of treatment effect reversal, possibly attributable to a smaller sample size and being underpowered to detect such differences.
Figure 2.
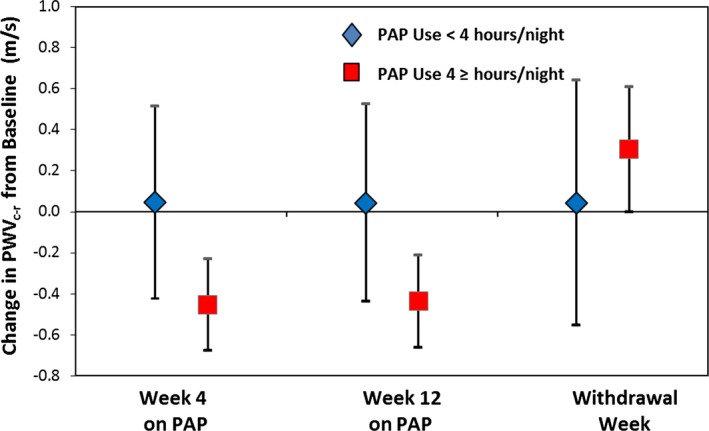
Changes in carotid‐to‐radial pulse wave velocity with positive airway pressure therapy. Changes between baseline PWV c‐r and different study visits, separated by PAP compliance. Noncompliant=used PAP therapy <4 hours a night (blue diamonds). Compliant=used PAP therapy ≥4 hours/night (red boxes). PAP indicates positive airway pressure; PWV, pulse wave velocity.
PAP Effects on Endothelial Function
Resting brachial artery diameter increased after 4 weeks and remained larger than baseline after 12 weeks (P<0.001; Table 3). In the multivariate models, PAP (average daily hours of use during the observational period) for weeks 4 and 12 independently predicted a significant improvement in both relative (β=0.34 [0.14] %/hour use; P=0.015) and absolute (β=0.02 [0.01] mm/hour use; P=0.007) FMD. Baseline AHI, but not PAP use, independently predicted change in resting brachial diameter post‐treatment (β=0.17 [0.06] mm change/log10[AHI+1]; P=0.008). Compared to week 12 visit values, we detected significant reduction in BA diameter after 1 week of PAP withdrawal (P=0.02), but the reduction in FMD did not reach statistical significance (Table 3).
PAP Effects on Cardiac Function and Hemodynamics
All subjects had normal RV and LV systolic function at entry (Table 1), but 44% had some degree of diastolic dysfunction at baseline. Cardiac chamber sizes, LV mass, and systolic function did not change significantly with treatment, whereas diastolic function, SVR, PVR, and TAPSE improved rapidly after 4 and 12 weeks (Table 3). After 1 week of PAP withdrawal, there was a trend toward an increase in pulmonary artery systolic pressure (P=0.09).
In multivariate analysis, the only ECG parameter predicted by PAP compliance was improvement in diastolic function category (β=0.10 [0.05]; P=0.028; Figure 3. In this model, baseline OSA severity was inversely related to diastolic function improvement (Table 4).
Figure 3.
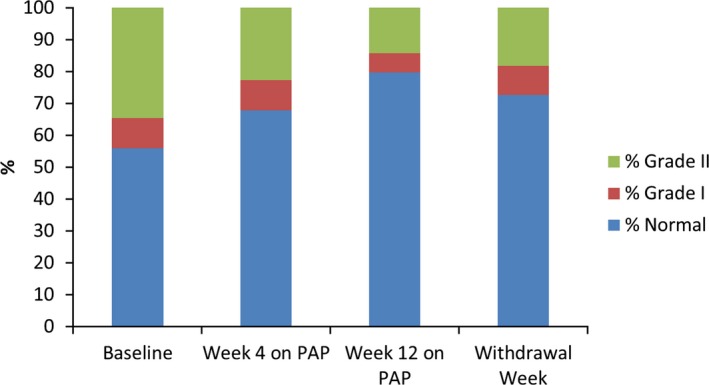
Changes in diastolic function category with positive airway pressure therapy treatment and withdrawal. Stacked bars represent the percentage distribution of diastolic function categories by study visit. Percentage of study subjects with normal function (blue), progressively increased to week 4, and continued to week 12 of PAP use, but declined after 1 week of withdrawal. Percentage of subjects with diastolic dysfunction (green and red) decreased during treatment. PAP indicates positive airway pressure.
Discussion
Although previous analyses have addressed some aspects of the effects of PAP on measures of cardiac and vascular function,16, 42 to the best of our knowledge, our results are the first to identify early changes in vascular stiffness, endothelial function, and cardiac hemodynamics in young individuals with moderate‐to‐severe OSA and identify their changes in response to PAP treatment and its withdrawal. A major strength of our study is the robustness and consistency of our data, as well as the use of objective PAP compliance information. We found that in young to middle‐aged adults with normal BPs, PAP therapy rapidly improved central and peripheral BPs, endothelial function, and LVDF. These changes likely reflect changes in sympathetic tone and endothelial nitric oxide variability. Lower peripheral PWV and central AAIx, both reduce afterload—a stimulus for future cardiac remodeling—leading to improved diastolic function.
Some previous studies have shown that PAP therapy was associated with lower BPs in individuals with prehypertension9 or clinical hypertension,43, 44 although results were inconsistent,45, 46 likely attributable to the effects of confounding variables, such as age, severity and duration of OSA, severity of hypertension, and other factors that affect OSA severity and arterial injury as discussed in the Introduction.
Reductions in AAIx and PWVc‐r with PAP therapy indicate better “active control of arterial stiffness” by improved endothelial function and sympathetic tone.47 Previous PAP intervention studies that described improvements in arterial stiffness using the brachial‐to‐ankle PWV as a measure of large artery stiffness might have been detecting reductions in peripheral arterial tone and improved endothelial function without true changes in elastic properties of large arteries.48 Our study, by design, was able to differentiate between central and peripheral changes of PWV, showing normal (ie, age‐appropriate) PWVc‐f values at baseline with no significant effects of PAP use or withdrawal on central PWV, consistent with normal BPs in our study group, whereas early changes in peripheral PWV were noted and treatment effects were predicted independently by baseline OSA severity and PAP compliance. Additionally, we detected a significant improvement in diastolic function with PAP use. We postulate that early treatment of OSA can delay the development of vascular damage that leads to systemic hypertension and adverse cardiac remodeling. Our findings are novel, but in general agree with those of Shantsila et al., who found that PAP use for 26 weeks lowered arterial elastance index.49
We did not detect significant changes in cardiac chamber size or LV mass50 that others have described, likely because our subjects were healthy at baseline and the time course of our intervention and its withdrawal were not long enough to see much remodeling besides change in diastolic function, in response to the changes in arterial stiffness and endothelial function we observed. The fact that we were able to detect rapid changes in peripheral and central BPs and withdrawal effects from stopping PAP is consistent with other investigators20, 47 and emphasizes the need for continuous PAP treatment by demonstrating rapid reversal of hemodynamic and vascular improvements observed with PAP after as short a time as 1 week.
Limitations
Our study lacked a control group; each individual served as his or her own control. When studying subjects with sleep apnea, sham preparations and blinding are challenging given that subjects may be able to detect subtherapeutic PAP settings. In addition, a blinded sham intervention was considered too high risk by our institutional review board given the severity of OSA in our subjects. PAP withdrawal, in a subgroup that was similar to our overall treatment group, demonstrated the effects of PAP withdrawal that emphasize the robustness of the observed effects of the PAP intervention as well as the risk of patient noncompliance. Our relatively short (3 month) study length was by design and based on previous publications that were able to demonstrate significant changes in a similar period, while avoiding potentially confounding changes in risk factors or therapies. Additionally, a shorter study length was thought to provide a better retention rate, but may explain why some factors previously shown to change with PAP therapy, such as LV mass, did not change in our study. By design, we studied a healthy and relatively young OSA population to avoid confounders; the beneficial effects observed in our cohort may not be generalizable to OSA patients with more cardiovascular disease risk factors or who are older. Although we had significant representation of races and both sexes, we did not have adequate power to evaluate subgroups.
Conclusions
PAP therapy and compliance improves arterial stiffness, endothelial function, and cardiac hemodynamics, likely by reducing arterial tone, wave reflections, and BP. Our results highlight the need for future longitudinal studies that assess the effects of early detection and treatment of moderate‐to‐severe OSA before the development of clinical hypertension to evaluate whether PAP compliance can prevent the future cardiovascular complications associated with the disease.
Sources of Funding
Dr Korcarz and Ms Barnet were supported by grant K23 HL094760. Instrumentation was provided by NIH grant S10 OD010569. The funding sources played no role in the design and conduct of the study; no role in the collection, management, analysis, or interpretation of the data; and no role in the preparation, review, or approval of the manuscript.
Disclosures
The authors have no potential conflicts of interest related to financial interests, activities, relationships, or affiliations. This was not an industry‐supported study. Dr Benca has served as a consultant to Janssen, Jazz, and Merck and has received grant support from Merck.
Acknowledgments
The authors thank the University of Wisconsin Atherosclerosis Imaging Research Program and Wisconsin Sleep staff for their valuable assistance.
C.E.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions: Conception and design: C.E.K., J.H.S., R.B. Acquisition of data: C.E.K., R.B. Analysis and interpretation of data: C.E.K., J.H.B., J.H.S. Drafting of manuscript: C.E.K., J.H.B., J.H.S., R.B. Critical revision of manuscript for important intellectual content: All authors. Statistical analysis: C.E.K., J.H.B. Administrative and technical support: C.E.K. Obtaining funding: C.E.K., J.H.S.
(J Am Heart Assoc. 2016;5:e002930 doi: 10.1161/JAHA.115.002930)
Presented as an abstract at the American College of Cardiology 65th Annual Scientific Session, April 2‐4, 2016, in Chicago, IL.
References
- 1. Peppard PE, Young T, Barnet JH, Palta M, Hagen E, Hla KM. Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim NH, Cho NH, Yun CH, Lee SK, Yoon DW, Cho HJ, Ahn JH, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Shin C. Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care. 2013;36:3909–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korcarz CE, Stein JH, Peppard PE, Young TB, Barnet JH, Nieto FJ. Combined effects of sleep disordered breathing and metabolic syndrome on endothelial function: the Wisconsin Sleep Cohort Study. Sleep. 2014;37:1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. Wis Med J. 2009;108:263–265. [PMC free article] [PubMed] [Google Scholar]
- 5. Baessler A, Nadeem R, Harvey M, Madbouly E, Younus A, Sajid H, Naseem J, Asif A, Bawaadam H. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—a meta‐analysis. J Inflamm (Lond). 2013;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R. Serum inflammatory markers in obstructive sleep apnea: a meta‐analysis. J Clin Sleep Med. 2013;9:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young T, Finn L, Peppard P, Szklo‐Coxe M, Austin D, Nieto J, Stubbs R, Hla K. Sleep disordered breathing and mortality: eighteen‐year follow‐up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8. Drager LF, Diegues‐Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, Marcondes B, Giorgi DMA, Lorenzi G, Krieger EM. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–254. [DOI] [PubMed] [Google Scholar]
- 9. Drager LF, Pedrosa RP, Diniz PM, Diegues‐Silva L, Marcondes B, Couto RB, Giorgi DM, Krieger EM, Lorenzi‐Filho G. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–555. [DOI] [PubMed] [Google Scholar]
- 10. Amra B, Karbasi E, Hashemi M, Hoffmann‐Castendiek B, Golshan M. Endothelial dysfunction in patients with obstructive sleep apnoea independent of metabolic syndrome. Ann Acad Med Singapore. 2009;38:461–464. [PubMed] [Google Scholar]
- 11. Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 12. Fuhrman C, Fleury B, Nguyên X‐L, Delmas M‐C. Symptoms of sleep apnea syndrome: high prevalence and underdiagnosis in the French population. Sleep Med. 2012;13:852–858. [DOI] [PubMed] [Google Scholar]
- 13. Alajmi M, Mulgrew A, Fox J, Davidson W, Schulzer M, Mak E, Ryan C, Fleetham J, Choi P, Ayas N. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta‐analysis of randomized controlled trials. Lung. 2007;185:67–72. [DOI] [PubMed] [Google Scholar]
- 14. Popescu G, Latham M, Allgar V, Elliott M. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2 week trial to identify factors associated with long term use. Thorax. 2001;56:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Zeller M, Severo M, Santos AC, Drummond M. 5‐Years APAP adherence in OSA patients—do first impressions matter? Respir Med. 2013;107:2046–2052. [DOI] [PubMed] [Google Scholar]
- 16. Börgel J, Sanner BM, Keskin F, Bittlinsky A, Bartels NK, Büchner N, Huesing A, Rump LC, Mügge A. Obstructive sleep apnea and blood pressure. Interaction between the blood pressure‐lowering effects of positive airway pressure therapy and antihypertensive drugs. Am J Hypertens. 2004;17:1081–1087. [DOI] [PubMed] [Google Scholar]
- 17. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/Ethnic differences in sleep disturbances: the Multi‐Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robinson G, Smith D, Langford B, Davies R, Stradling J. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–1235. [DOI] [PubMed] [Google Scholar]
- 19. Rossi VA, Stoewhas AC, Camen G, Steffel J, Bloch KE, Stradling JR, Kohler M. The effects of continuous positive airway pressure therapy withdrawal on cardiac repolarization: data from a randomized controlled trial. Eur Heart J. 2012;33:2206–2212. [DOI] [PubMed] [Google Scholar]
- 20. Kohler M, Stoewhas A‐C, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–1199. [DOI] [PubMed] [Google Scholar]
- 21. Young LR, Taxin ZH, Norman RG, Walsleben JA, Rapoport DM, Ayappa I. Response to CPAP withdrawal in patients with mild versus severe obstructive sleep apnea/hypopnea syndrome. Sleep. 2013;36:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iber C, Ancoli‐Israel S, Chesson AL, Quan SF. for the American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: Am Academy of Sleep Medicine; 2007. [Google Scholar]
- 23. Agabiti‐Rosei E, Mancia G, O'Rourke M, Roman M, Safar M, Smulyan H, Wang J, Wilkinson I, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy a consensus document. Hypertension. 2007;50:154–160. [DOI] [PubMed] [Google Scholar]
- 24. Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. [DOI] [PubMed] [Google Scholar]
- 25. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H, Non‐invasive EN. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 26. Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. [DOI] [PubMed] [Google Scholar]
- 27. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 28. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D, Vallance P, Vita J, Vogel R; Force IBART . Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 30. Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dubé MP, Fichtenbaum CJ, Gerschenson M, Mitchell CK, Murphy RL, Squires K, Stein JH; Team AsS . Endothelial function in human immunodeficiency virus‐infected antiretroviral‐naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) study 5152s. J Am Coll Cardiol. 2008;52:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 32. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing G . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 33. Comaniciu D, Zhou X, Krishnan S. Robust real‐time myocardial border tracking for echocardiography: an information fusion approach. IEEE Trans Med Imaging. 2004;23:849–860. [DOI] [PubMed] [Google Scholar]
- 34. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 35. Abbas A, Fortuin F, Schiller N, Appleton C, Moreno C, Lester S. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. [DOI] [PubMed] [Google Scholar]
- 36. Horton K, Meece R, Hill J. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22:776–792. [DOI] [PubMed] [Google Scholar]
- 37. Bland J, Altman D. Measurement error and correlation coefficients. BMJ. 1996;313:41–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ; Echocardiography ASo . American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. [DOI] [PubMed] [Google Scholar]
- 39. Tamborini G, Pepi M, Galli CA, Maltagliati A, Celeste F, Muratori M, Rezvanieh S, Veglia F. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int J Cardiol. 2007;115:86–89. [DOI] [PubMed] [Google Scholar]
- 40. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 41. Drager LF, Brunoni AR, Jenner R, Lorenzi‐Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta‐analysis of randomised trials. Thorax. 2015;70:258–264. [DOI] [PubMed] [Google Scholar]
- 42. Alchanatis M, Paradellis G, Pini H, Tourkohoriti G, Jordanoglou J. Left ventricular function in patients with obstructive sleep apnoea syndrome before and after treatment with nasal continuous positive airway pressure. Respiration. 2000;67:367–371. [DOI] [PubMed] [Google Scholar]
- 43. Duran‐Cantolla J, Aizpuru F, Montserrat JM, Ballester E, Teran‐Santos J, Aguirregomoscorta JI, Gonzalez M, Lloberes P, Masa JF, De La Pena M, Carrizo S, Mayos M, Barbe F. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. [DOI] [PubMed] [Google Scholar]
- 44. Martinez‐Garcia MA, Capote F, Campos‐Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, Masa JF, Gonzalez M, Sacristan L, Barbe F, Duran‐Cantolla J, Aizpuru F, Manas E, Barreiro B, Mosteiro M, Cebrian JJ, de la Pena M, Garcia‐Rio F, Maimo A, Zapater J, Hernandez C, Grau SanMarti N, Montserrat JM. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. J Am Med Assoc. 2013;310:2407–2415. [DOI] [PubMed] [Google Scholar]
- 45. Hermida RC, Zamarron C, Ayala DE, Calvo C. Effect of continuous positive airway pressure on ambulatory blood pressure in patients with obstructive sleep apnoea. Blood Press Monit. 2004;9:193–202. [DOI] [PubMed] [Google Scholar]
- 46. Muxfeldt ES, Margallo V, Costa LM, Guimaraes G, Cavalcante AH, Azevedo JC, de Souza F, Cardoso CR, Salles GF. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65:736–742. [DOI] [PubMed] [Google Scholar]
- 47. Phillips CL, Yee B, Yang Q, Villaneuva AT, Hedner J, Berend N, Grunstein R. Effects of continuous positive airway pressure treatment and withdrawal in patients with obstructive sleep apnea on arterial stiffness and central BP. Chest. 2008;134:94–100. [DOI] [PubMed] [Google Scholar]
- 48. Kitahara Y, Hattori N, Yokoyama A, Nakajima M, Kohno N. Effect of CPAP on brachial‐ankle pulse wave velocity in patients with OSAHS: an open‐labelled study. Respir Med. 2006;100:2160–2169. [DOI] [PubMed] [Google Scholar]
- 49. Shantsila A, Shantsila E, Butt M, Khair OA, Dwivedi G, Lip GY. Ventricular‐arterial coupling in obstructive sleep apnea. J Am Soc Hypertens. 2014;8:624–629. [DOI] [PubMed] [Google Scholar]
- 50. Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, Francis A, Bohonis S, Zeglinski M, Kirkpatrick ID, Sharma S, Jassal DS. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. [DOI] [PubMed] [Google Scholar]


