Abstract
Background
Host factors and complications have been associated with higher mortality in infective endocarditis (IE). We sought to develop and validate a model of clinical characteristics to predict 6‐month mortality in IE.
Methods and Results
Using a large multinational prospective registry of definite IE (International Collaboration on Endocarditis [ICE]–Prospective Cohort Study [PCS], 2000–2006, n=4049), a model to predict 6‐month survival was developed by Cox proportional hazards modeling with inverse probability weighting for surgery treatment and was internally validated by the bootstrapping method. This model was externally validated in an independent prospective registry (ICE‐PLUS, 2008–2012, n=1197). The 6‐month mortality was 971 of 4049 (24.0%) in the ICE‐PCS cohort and 342 of 1197 (28.6%) in the ICE‐PLUS cohort. Surgery during the index hospitalization was performed in 48.1% and 54.0% of the cohorts, respectively. In the derivation model, variables related to host factors (age, dialysis), IE characteristics (prosthetic or nosocomial IE, causative organism, left‐sided valve vegetation), and IE complications (severe heart failure, stroke, paravalvular complication, and persistent bacteremia) were independently associated with 6‐month mortality, and surgery was associated with a lower risk of mortality (Harrell's C statistic 0.715). In the validation model, these variables had similar hazard ratios (Harrell's C statistic 0.682), with a similar, independent benefit of surgery (hazard ratio 0.74, 95% CI 0.62–0.89). A simplified risk model was developed by weight adjustment of these variables.
Conclusions
Six‐month mortality after IE is ≈25% and is predicted by host factors, IE characteristics, and IE complications. Surgery during the index hospitalization is associated with lower mortality but is performed less frequently in the highest risk patients. A simplified risk model may be used to identify specific risk subgroups in IE.
Keywords: infection, mortality, prognosis, surgery, valves
Subject Categories: Infectious Endocarditis, Valvular Heart Disease, Mortality/Survival, Clinical Studies
Introduction
Infective endocarditis (IE) remains a serious disease due to its high morbidity and mortality.1 Despite improvements in diagnostic testing, antimicrobial treatment, and surgical intervention, changes in the epidemiology of IE, including the rise of health care–associated infection and Staphylococcus aureus as a virulent causative organism,2, 3 increase the risk of complications and death in the acute phase of IE. Furthermore, survival after hospital discharge may be reduced by late complications and comorbid conditions.4, 5
In patients with active IE, complications such as heart failure, abscess formation, and embolic events portend poor prognosis and are regarded as indications for surgical intervention to improve the chance of survival.6, 7 These complications have been associated with increased in‐hospital mortality, yet their impact on longer term outcome is not well defined. Although previous studies have developed risk models for IE survival, these studies have been limited by retrospective data collection, limited sample size, regional selection bias, and variable adjustment for surgical intervention.8, 9
We hypothesized that specific clinical characteristics of an IE episode, including surgical intervention, would be associated with 6‐month mortality in IE. Using 2 separate prospective multinational registries of definite IE, the objective of this study was to develop and validate a risk model for 6‐month mortality in IE.
Methods
Study Populations and Clinical Data
The study populations for this analysis were obtained from the International Collaboration on Endocarditis (ICE)–Prospective Cohort Study (PCS) and ICE‐PLUS cohorts. These 2 prospective multinational registries collected consecutive cases of definite IE by modified Duke criteria,10 with prespecified definitions of variables, as described previously.3, 11 For the derivation cohort, the ICE‐PCS registry included 5676 patients from 64 centers in 28 countries who were hospitalized between June 2000 and December 2006 for definite IE and who had vital status follow‐up information available at 1 year after discharge. For the validation cohort, the ICE‐PLUS registry included 2124 IE patients from 34 centers in 18 countries who were hospitalized between September 1, 2008, and December 31, 2012, and who had 6‐month vital status follow‐up data (all ICE sites listed in the Appendix). Patients with device‐related IE were excluded from the analyses. The study was approved by the institutional review board or ethics committee at each participating site, according to local standards.
Statistical Analysis
Descriptive statistics
Baseline characteristics and clinical events are presented as medians with 25th and 75th percentiles for continuous variables and frequencies with proportions for categorical variables. Statistical comparisons between groups were made with the Wilcoxon rank sum test for continuous variables and the Fisher exact test for categorical variables.
Propensity score models
A multivariable logistic regression model was fit in the ICE‐PCS and ICE‐PLUS data sets independently to calculate a propensity score (probability) for surgical treatment. The response variable was receipt of surgery during the index hospitalization. The model included variables that were selected a priori by an experienced cardiologist (A.W.) and from practice guidelines6, 7 and previous studies1, 12, 13 because those would be evaluated in the decision to treat IE with surgery. These variables were age >70 years, history of hemodialysis, history of intravenous drug use, time of ≥1 month since first IE manifestation, paravalvular complication, New York Heart Association class 3 or 4 (versus 1 or 2), aortic valve vegetation, new mitral or aortic valve regurgitation, and causative microorganism (S aureus, coagulase‐negative staphylococci, and viridans group streptococci). The predicted probabilities of surgery were calculated and used as inverse probability weights in models predicting outcome. Weights were trimmed at 20 to avoid overly influential observations.
Model Derivation
The primary outcome variable was 6‐month mortality. This information was coded as time‐to‐event data using an indicator of alive and time (in days) from admission to either death or 6 months after discharge for those who were alive at that time. Variables previously shown to be associated with survival in IE were selected a priori and included age in years (as categories ≤45, 46–60, 61–70, and >70), history of hemodialysis, diabetes mellitus, injection drug use, time of >1 month from first IE manifestation to admission, hospital‐acquired infection, prosthetic valve IE, causative microorganism, aortic or mitral valve regurgitation, aortic valve vegetation, mitral valve vegetation, paravalvular complication, heart failure, New York Heart Association class 3 or 4 heart failure, stroke, embolization, persistent bacteremia, and surgery.
The variables included in the final parsimonious derivation model were selected as follows. A Cox proportional hazards model predicting survival at 6 months after discharge was fit in the ICE‐PCS data set, including all of the predictor variables listed above. Surgery was included as a time‐dependent variable. The model was weighted by the inverse probability (propensity) of surgery. This “full” model was fit on 1000 bootstrap samples from the ICE‐PCS data. Variables that were significant at P<0.05 in ≥90% of the bootstrap samples were included in the final model. In addition, several variables were included based on the previously described a priori criteria.
The proportional hazards assumption was evaluated graphically through an analysis of Schoenfeld residuals. These residuals were plotted against time for each variable in the final model and examined for systematic deviations from constancy.
Model Validation
For internal validation, 1000 bootstrap samples were selected from the ICE‐PCS data set. Propensity score for surgery was estimated for each sample and used for inverse weighting in proportional hazards models predicting survival at 6 months after discharge. These models included the same variables as those in the final development model. Harrell's C statistic14 was calculated for each of these samples for comparison to that estimated from the model in the ICE‐PCS data set. Because survival models include time to event as well as event, this index of predictive discrimination includes both outcome and time to outcome in determining concordance. Each event (death) was paired with all other observations, and the pairs were considered concordant (1) for event/nonevent pairs, if the predicted probability of event was higher than the paired nonevent or (2) for event/event pairs, if the predicted probability of event was higher and the time to event was smaller, or the converse. Harrell's C statistic is the proportion of all pairs that were concordant.
For external model validation, the variables from the final prediction model derived from the ICE‐PCS data set were fit in a proportional hazards model in the ICE‐PLUS data set. As with the development model, this model was weighted by the inverse probability of surgery from the ICE‐PLUS propensity model. Harrell's C statistic was used to compare the performance of the models in ICE‐PCS and ICE‐PLUS.14
In another evaluation of model performance, a risk score was derived from the parameter estimates in the final Cox proportional hazards model. Each variable in the model was assigned an integer value based on the relative size of the parameter estimates. The risk score was then the sum of the products of the integer value times the variable value, and these were stratified into quintiles. The risk score ranged between 0 and 22, and the risk score ranges in the quintiles were 0 to 6, 7 to 8, 9 to 10, 11 to 16, and 17 to 22. Kaplan–Meier cumulative survival probabilities were plotted by stratum of risk score, and a log‐rank test was used to determine statistical difference among the curves. Receiver operating characteristic analysis was performed for the simplified risk score to determine sensitivity and specificity for predicting 6‐month mortality.
Sensitivity Analysis
To determine whether geographic region confounded the estimation derived from the Cox model in ICE‐PCS, geographic region was added to the final model with the levels Europe, North America, South America, and other. In a second sensitivity analysis, we added the Society of Thoracic Surgeons (STS) IE score15 to the final Cox model in the ICE‐PLUS data set. Finally, to evaluate the sensitivity of the predictive model to variable selection, the bootstrap analysis of the development data set was repeated on the full set of variables selected a priori. The distribution of the Harrell's C values from this set of analyses was compared with those from models with the reduced set of predictors.
Statistical analyses were performed using SAS version 9.4 software (SAS Institute), and plots were generated with S‐plus 8.1 (TIBCO Software Inc).
Results
The overall clinical characteristics of the 2 cohorts are shown in Tables 1 and 2, including stratification by survival to 6 months after hospital discharge. In ICE‐PCS (n=4049), 777 (19.2%) patients died in the hospital and 971 (24.0%) had died at 6‐month follow‐up; in ICE‐PLUS (n=1197), 286 (23.9%) died in the hospital and 342 (28.6%) had died at 6 months. Characteristics strongly associated with 6‐month mortality included hospital‐acquired infection, diabetes mellitus, renal disease, hemodialysis, S aureus infection, heart failure and its severity, persistent bacteremia, and stroke.
Table 1.
Demographic and Clinical Characteristics of IE Patients in the International Collaboration on Endocarditis–Prospective Cohort Study Cohort
| Variable | Overall n=4049 | Alive n=3077 | Died (6 Months) n=971 | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, y (range) | 59 (45–72) | 57 (42–69) | 68 (53–76) | – | <0.001 |
| Male | 2752 (68.1) | 2134 (69.4) | 617 (63.7) | 0.77 (0.66–0.90) | <0.001 |
| First clinical manifestation ≥1 month | 890 (22.9) | 764 (25.8) | 126 (13.6) | 0.45 (0.37–0.56) | <0.001 |
| Prosthetic valve IE | 967 (23.9) | 676 (22.0) | 291 (30.0) | 1.52 (1.29–1.79) | <0.001 |
| Transfer from other hospital | 1780 (44.4) | 1379 (45.3) | 401 (41.7) | 0.86 (0.74–1.00) | 0.049 |
| Hospital acquired | 576 (14.3) | 340 (11.1) | 236 (24.5) | 2.66 (2.15–3.14) | <0.001 |
| Previous IE | 342 (8.5) | 267 (8.7) | 75 (7.8) | 0.88 (0.67–1.16) | 0.39 |
| Diabetes mellitus | 689 (17.3) | 437 (14.4) | 252 (26.5) | 2.14 (1.78–2.56) | <0.001 |
| Moderate or severe renal disease | 357 (19.2) | 188 (13.8) | 169 (34.2) | 3.24 (2.53–4.16) | <0.001 |
| Hemodialysis dependent | 297 (7.5) | 157 (5.2) | 140 (14.6) | 3.14 (2.45–4.02) | <0.001 |
| HIV | 85 (2.1) | 63 (2.1) | 22 (2.3) | 1.12 (0.65–1.85) | 0.70 |
| Injection drug use | 414 (10.4) | 354 (11.6) | 60 (6.3) | 0.52 (0.38–0.69) | <0.001 |
| New moderate or severe aortic or mitral regurgitation | 2387 (59.4) | 1804 (59.1) | 583 (60.6) | 1.07 (0.92–1.24) | 0.41 |
| Aortic valve vegetation | 1644 (41.1) | 1250 (41.1) | 394 (41.3) | 1.01 (0.87–1.17) | 0.94 |
| Mitral valve vegetation | 1763 (44.2) | 1277 (42.0) | 486 (51.1) | 1.44 (1.24–1.67) | <0.001 |
| Staphylococcus aureus | 1218 (30.1) | 808 (26.3) | 409 (42.1) | 2.04 (1.75–2.38) | <0.001 |
| Coagulase‐negative Staphylococcus | 379 (9.4) | 272 (8.8) | 107 (11) | 1.28 (1 .00–1.62) | 0.043 |
| Viridans group streptococcus | 699 (17.3) | 617 (20.1) | 82 (8.4) | 0.37 (0.28–0.47) | <0.001 |
| New or worsening heart failure | 1316 (33.2) | 833 (27.5) | 483 (51.5) | 2.88 (2.40–3.26) | <0.001 |
| NYHA class 3 or 4 | 878 (23.3) | 516 (17.8) | 362 (41.9) | 3.34 (2.82–3.95) | <0.001 |
| Paravalvular complication | 974 (24.4) | 688 (22.6) | 286 (30.0) | 1.47 (1.24–1.73) | <0.001 |
| Intracardiac abscess | 634 (16.0) | 411 (13.6) | 223 (23.8) | 1.99 (1.65–2.40) | <0.001 |
| Persistent bacteremia | 370 (9.3) | 208 (6.9) | 162 (17.2) | 2.81 (2.24–3.52) | <0.001 |
| Stroke | 776 (19.5) | 467 (15.44) | 309 (32.7) | 2.67 (2.25–3.17) | <0.001 |
| Embolization | 1001 (25.3) | 741 (24.55) | 260 (27.8) | 1.18 (1.00–1.40) | 0.048 |
| Surgery this IE episode | 1949 (48.4) | 1574 (51.4) | 375 (38.9) | 0.66 (0.52–0.70) | <0.001 |
HIV indicates human immunodeficiency virus; IE, infective endocarditis; NYHA, New York Heart Association; OR, odds ratio.
Table 2.
Demographic and Clinical Characteristics of IE Patients in the International Collaboration on Endocarditis–PLUS Cohort
| Variable | Overall n=1197 | Alive n=849 | Died (6 Months) n=342 | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, y | 62 (46–73) | 59 (44–71) | 68 (54–76) | – | <0.001 |
| Male | 815 (68.3) | 602 (71.1) | 209 (61.3) | 0.64 (0.49–0.85) | 0.001 |
| First clinical manifestation ≥1 month | 396 (33.6) | 300 (35.8) | 95 (28.4) | 0.71 (0.53–0.94) | 0.017 |
| Prosthetic valve IE | 348 (29.1) | 226 (26.6) | 120 (35.1) | 1.49 (1.13–1.97) | 0.005 |
| Transfer from other hospital | 591 (49.7) | 427 (50.5) | 162 (48.1) | 0.91 (0.70–1.18) | 0.479 |
| Hospital acquired | 193 (16.3) | 101 (12.1) | 91 (26.6) | 2.65 (1.90–3.68) | <0.001 |
| Previous endocarditis | 111 (9.4) | 83 (9.9) | 28 (8.3) | 0.83 (0.51–1.32) | 0.442 |
| Diabetes mellitus | 236 (20.0) | 136 (16.2) | 98 (29.1) | 2.12 (1.55–2.88) | <0.001 |
| Moderate or severe renal disease | 147 (12.5) | 70 (8.3) | 76 (22.9) | 3.27 (2.25–4.73) | <0.001 |
| Hemodialysis dependent | 62 (5.2) | 31 (3.7) | 31 (9.1) | 2.63 (1.52–4.55) | <0.001 |
| HIV | 23 (2.0) | 18 (2.2) | 4 (1.2) | 0.56 (0.14–1.72) | 0.348 |
| Injection drug use | 74 (6.3) | 57 (6.8) | 15 (4.5) | 0.65 (0.34–1.18) | 0.177 |
| New moderate or severe aortic or mitral regurgitation | 675 (58.6) | 485 (59.1) | 187 (57.2) | 0.93 (0.71–1.21) | 0.596 |
| Aortic valve vegetation | 504 (42.6) | 344 (40.9) | 157 (46.7) | 1.27 (0.97–1.65) | 0.078 |
| Mitral valve vegetation | 555 (46.8) | 383 (45.4) | 172 (51.0) | 1.27 (0.97–1.65) | 0.140 |
| Staphylococcus aureus | 279 (23.3) | 167 (19.7) | 110 (32.2) | 1.94 (1.44–2.59) | <0.001 |
| Coagulase‐negative Staphylococcus | 102 (8.5) | 65 (7.7) | 36 (10.5) | 1.42 (0.90–2.21) | 0.109 |
| Viridans group streptococcus | 189 (15.8) | 152 (17.9) | 36 (10.5) | 0.54 (0.36–0.80) | 0.002 |
| New or worsening heart failure | 456 (38.9) | 289 (34.4) | 165 (50.3) | 1.93 (1.47–2.52) | <0.001 |
| NYHA Class 3 or 4 | 72 (6.2) | 38 (4.6) | 34 (10.3) | 2.39 (1.43–3.98) | <0.001 |
| Paravalvular complication | 410 (34.7) | 282 (33.5) | 126 (37.4) | 1.18 (0.90–1.55) | 0.223 |
| Intracardiac abscess | 380 (32.1) | 252 (30.1) | 126 (37.2) | 1.38 (1.04–1.81) | 0.019 |
| Persistent bacteremia | 141 (12.7) | 78 (9.7) | 63 (21.0) | 2.49 (1.70–3.63) | <0.001 |
| Stroke | 260 (22.2) | 152 (18.1) | 106 (32.5) | 2.18 (1.61–2.94) | <0.001 |
| Embolization | 400 (33.8) | 295 (34.8) | 102 (30.7) | 0.83 (0.62–1.10) | 0.193 |
| Surgery for this IE episode | 647 (54.6) | 499 (59.5) | 146 (42.9) | 0.51 (0.39–0.67) | <0.001 |
HIV indicates human immunodeficiency virus; IE, infective endocarditis; NYHA, New York Heart Association; OR, odds ratio.
Surgery was performed in 1949 of 4049 (48.1%) of ICE‐PCS patients and 647 of 1197 (54.0%) of ICE‐PLUS patients. A high percentage of patients who underwent surgery were transferred to the ICE sites (58% in ICE‐PCS and 60% in ICE‐PLUS). Surgery was performed at a median of 7 days (interquartile range 3–17 days) in ICE‐PCS and 6 days (interquartile range 2–16 days) in ICE‐PLUS. Types of valve surgeries performed are shown in Table 3. In‐hospital mortality in patients who underwent surgery was 313 of 1949 (16.1%) in ICE‐PCS and 125 of 647 (19.3%) in ICE‐PLUS; the 6‐month mortality in surgically treated patients was 19.2% in ICE‐PCS and 22.6% in ICE‐PLUS.
Table 3.
Types of Cardiac Surgeries for Infective Endocarditis
| Type of Valve Surgery | ICE‐PCS (n=1807) | ICE‐PLUS (n=621) |
|---|---|---|
| Aortic valve replacement | 778 (43%) | 246 (40%) |
| Mitral valve replacement | 403 (22%) | 136 (22%) |
| Mitral valve repair | 89 (5%) | 37 (6%) |
| Multiple valve replacement/repair | 413 (23%) | 171 (28%) |
ICE indicates International Collaboration on Endocarditis; PCS, Prospective Cohort Study.
The proportional hazards model results are summarized in Table 4. Patient age and complications of IE, particularly advanced heart failure symptoms and stroke, were most strongly associated with mortality; viridans streptococcal infection and surgery were associated with lower mortality. The ICE‐PLUS model included the same variables that were in the ICE‐PCS model; no model reduction was performed. Kaplan–Meier survival curves for the proportional hazards models are shown in Figures 1 and 2. Variables associated with complicated IE were present in higher percentages across the quintiles of risk, including older age, comorbid medical conditions, and IE complications; however, there was an inverse relationship between use of surgery and predicted 6‐month mortality. The direction and strength of the associations were consistent across both IE cohorts. Harrell's C statistic was 0.715 for the derivation model and 0.682 for the validation model.
Table 4.
Proportional Hazards Model Results for the ICE‐PCS Derivation (n=2646) and ICE‐PLUS Validation (n=887) Cohorts and 6‐Month Mortality
| Variable | ICE‐PCS | ICE‐PLUS | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age 46–60 y | 1.51 (1.29–1.78) | <0.0001 | 1.69 (1.28–2.25) | 0.0003 |
| Age 61–70 y | 1.87 (1.58–2.20) | <0.0001 | 1.79 (1.34–2.40) | <0.0001 |
| Age >70 y | 2.90 (2.50–3.37) | <0.0001 | 3.09 (2.40–4.01) | <0.0001 |
| History of dialysis | 2.04 (1.80–2.31) | <0.0001 | 2.08 (1.56–2.73) | <0.0001 |
| Nosocomial IE | 1.47 (1.33–1.63) | <0.0001 | 1.48 (1.23–1.77) | <0.0001 |
| Prosthetic IE | 1.20 (1.09–1.32) | 0.0002 | 1.09 (0.92–1.30) | 0.33 |
| IE symptom onset to admission >30 days | 0.74 (0.65–0.84) | <0.0001 | 1.08 (0.90–1.29) | 0.42 |
| Staphylococcus aureus IE | 1.42 (1.29–1.56) | <0.0001 | 1.55 (1.30–1.86) | <0.0001 |
| Viridans group IE | 0.63 (0.53–0.75) | <0.0001 | 0.80 (0.62–1.02) | 0.08 |
| Aortic vegetation | 1.21 (1.09–1.33) | 0.0002 | 1.21 (1.03–1.42) | 0.02 |
| Mitral vegetation | 1.20 (1.08–1.32) | 0.0004 | 0.99 (0.92–1.02) | 0.63 |
| NYHA class 3 or 4 | 2.19 (2.00–2.39) | <0.0001 | 1.60 (1.20–2.10) | 0.0009 |
| Stroke | 1.76 (1.60–1.94) | <0.0001 | 1.65 (1.39–1.94) | <0.0001 |
| Paravalvular complication | 1.47 (1.34–1.61) | <0.0001 | 1.32 (1.13–1.54) | 0.0005 |
| Persistent bacteremia | 1.53 (1.35–1.72) | <0.0001 | 1.92 (1.56–2.35) | <0.0001 |
| Surgical treatment | 0.66 (0.60–0.74) | <0.0001 | 0.74 (0.62–0.89) | 0.0012 |
| Harrell's C statistic | 0.715 | 0.682 | ||
HR indicates hazard ratio; ICE, International Collaboration on Endocarditis; IE, infective endocarditis; NYHA, New York Heart Association; PCS, Prospective Cohort Study.
Figure 1.
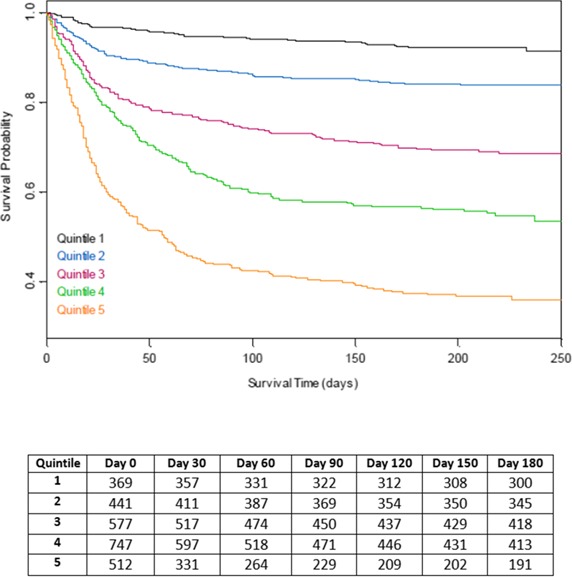
Kaplan–Meier survival probabilities for the International Collaboration on Endocarditis–Prospective Cohort Study derivation cohort by quintiles of simplified risk score.
Figure 2.
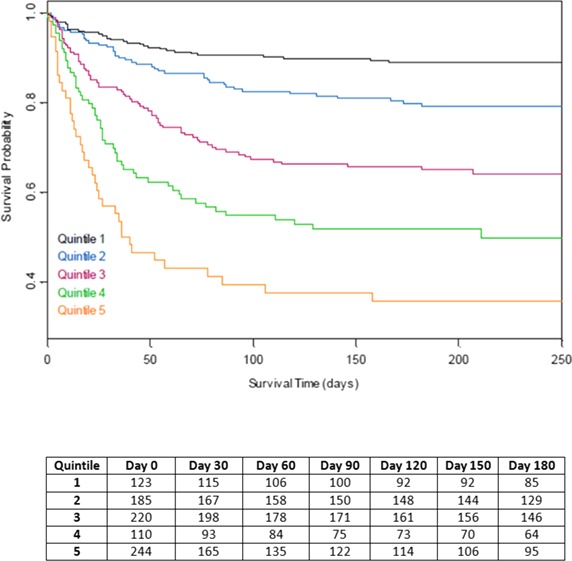
Kaplan–Meier survival probabilities for the International Collaboration on Endocarditis–PLUS validation cohort by quintiles of simplified risk score.
A simplified, weighted risk score was developed (Table 5) and correlated with observed 6‐month mortality (adjusted R 2=0.91 and R 2=0.97 for ICE‐PCS and ICE‐PLUS cohorts, respectively) (Figure 3). Using the simplified risk score, quintiles of risk for 6‐month mortality were calculated, and the clinical characteristics of these subgroups are shown in Table 6. The 6‐month mortality rates for these quintiles are shown in Figure 4. Receiver operating characteristics for the simplified risk score in the ICE‐PCS derivation cohort are shown in Figure 5. A simplified risk score ≥8 had sensitivity 86.7% and specificity 50.8% for predicting overall 6‐month mortality (negative predictive value of 86% and positive predictive value of 39%).
Table 5.
Simplified Risk Score Calculation for 6‐Month Mortality in IE
| Prognostic Variable | Weight |
|---|---|
| Constant | 4 |
| Host factors | |
| Age ≤45 y | 0 |
| Age 46–60 y | 2 |
| Age 61–70 y | 3 |
| Age >70 y | 4 |
| History of dialysis | 3 |
| IE factors | |
| Nosocomial IE | 2 |
| Prosthetic IE | 1 |
| Symptoms >1 month before admission | −1 |
| Staphylococcus aureus | 1 |
| Viridans group streptococci | −2 |
| Aortic vegetation | 1 |
| Mitral vegetation | 1 |
| IE complications | |
| NYHA class 3 or 4 heart failure | 3 |
| Stroke | 2 |
| Paravalvular complication | 2 |
| Persistent bacteremia | 2 |
| Surgical treatment | −2 |
Probability of 6‐month mortality=2.416×score+0.109×score2−4.849. IE indicates infective endocarditis; NYHA, New York Heart Association.
Figure 3.
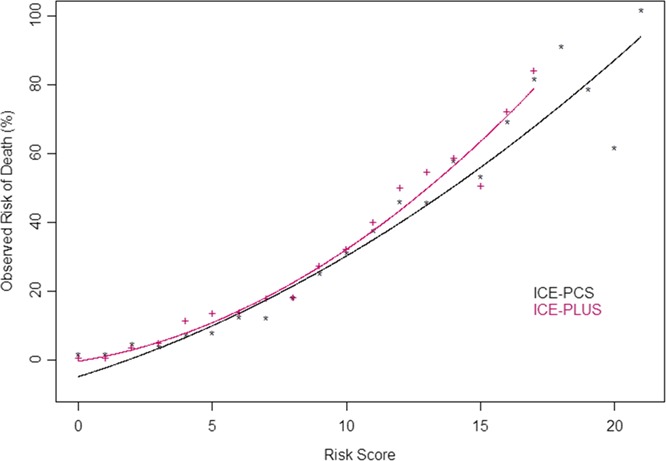
Relationships between simplified infective endocarditis risk scores and observed 6‐month mortality in ICE‐PCS derivation cohort and ICE‐PCS validation cohorts. Adjusted R 2=0.91 and R 2=0.97 for ICE‐PCS and ICE‐PLUS cohorts, respectively. ICE indicates International Collaboration on Endocarditis; PCS, Prospective Cohort Study.
Table 6.
Comparison of Clinical Characteristics of IE in the International Collaboration on Endocarditis–PLUS Cohort by Quintiles of Risk for 6‐Month Mortality
| Variable | Quintile of Risk for 6‐Month Mortality | P Value | ||||
|---|---|---|---|---|---|---|
| 1 (n=224) | 2 (n=188) | 3 (n=200) | 4 (n=98) | 5 (n=172) | ||
| Host factors | ||||||
| Age ≤45 y | 58.9 | 16.5 | 11.0 | 6.1 | 1.7 | <0.001 |
| Age 46–60 y | 23.7 | 33.5 | 19.5 | 19.4 | 9.9 | |
| Age 61–70 y | 12.1 | 23.4 | 18.5 | 30.6 | 26.2 | |
| Age >70 y | 5.4 | 26.6 | 51.0 | 43.9 | 62.2 | |
| History of dialysis | 0.4 | 2.1 | 1.5 | 7.1 | 16.3 | <0.001 |
| History of diabetes | 7.1 | 14.9 | 23.0 | 27.6 | 36.0 | <0.001 |
| IE factors | ||||||
| Nosocomial IE | 0.9 | 7.4 | 14.0 | 28.6 | 44.8 | <0.001 |
| Prosthetic IE | 14.3 | 25 | 33.0 | 35.7 | 41.3 | <0.001 |
| Staphylococcus aureus | 12.1 | 18.6 | 19.0 | 24.5 | 42.4 | <0.001 |
| Viridans group streptococci | 36.2 | 19.1 | 12.0 | 6.1 | 1.2 | <0.001 |
| Aortic vegetation | 33.0 | 44.1 | 43.0 | 37.8 | 46.5 | 0.048 |
| Mitral vegetation | 42.9 | 42 | 53.5 | 52.0 | 53.5 | 0.067 |
| IE complications | ||||||
| NYHA class 3 or 4 | 0.0 | 2.7 | 4.5 | 10.2 | 14.5 | <0.001 |
| Stroke | 9.4 | 14.4 | 20.0 | 28.6 | 41.3 | <0.001 |
| Paravalvular complication | 17.0 | 27.7 | 42.0 | 42.9 | 50.6 | <0.001 |
| Persistent bacteremia | 4.0 | 4.8 | 8.0 | 17.3 | 26.7 | <0.001 |
| Surgical treatment | 79.9 | 67.6 | 47.0 | 35.7 | 25.0 | <0.001 |
| 6‐month mortality | 10.3 | 17.0 | 25.5 | 37.8 | 52.9 | <0.001 |
IE indicates infective endocarditis; NYHA, New York Heart Association.
Figure 4.
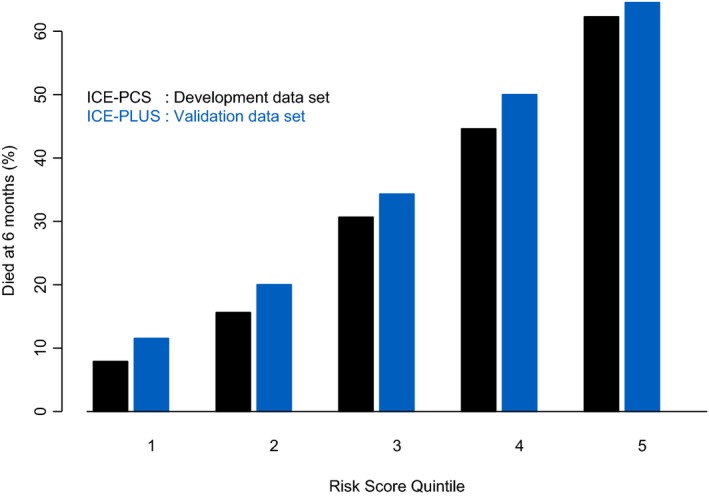
Mortality at 6 months by quintiles of simplified risk score in the development and validation cohorts. ICE indicates International Collaboration on Endocarditis; PCS, Prospective Cohort Study.
Figure 5.
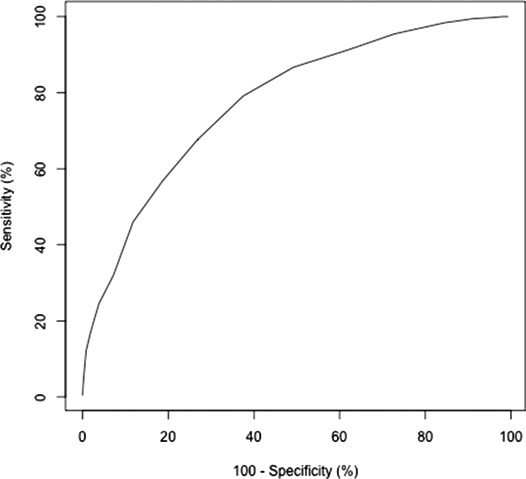
Receiver operating characteristic analysis for simplified risk score in the International Collaboration on Endocarditis–Prospective Cohort Study derivation cohort.
Sensitivity analysis for geographic region was performed in the derivation model for predicting 6‐month mortality. Although geographic regions of North and South Americas were associated with a lower risk of mortality, the variables in the model were unchanged when geographic region was added. Similarly, sensitivity analysis of the validation model found a significant association for Society of Thoracic Surgeons IE score, but other variables including surgery remained statistically associated with mortality. In assessing the effect of variable selection on model fit, there was no important difference in Harrell's C statistic comparing the final derivation model with one including all of the variables selected a priori. The Schoenfeld residuals analysis revealed no violations of the proportional hazards assumption in the final derivation model.
Discussion
IE is often associated with serious complications, such as heart failure from progressive tissue destruction, stroke, and sepsis, yet other host and IE characteristics may also affect longer term survival. We derived and validated a simplified risk score for predicting 6‐month mortality in IE using readily available clinical variables at the time of IE diagnosis. The prognostic significance of these variables on outcome was independent of surgical intervention, which was associated with a lower risk of mortality at 6 months after adjustment for surgical propensity. These independent and weighted risk factors for mortality were developed from a large prospective multinational data set and validated in a separate prospective multinational cohort of IE.
Previous studies have evaluated clinical characteristics associated with mortality in IE, generally focusing on in‐hospital mortality.13 In the ICE‐PCS cohort, in‐hospital mortality was associated with IE complications of prosthetic or mitral valve IE, pulmonary edema, paravalvular complication, and S aureus infection.1 Longer term studies have demonstrated higher mortality rates in IE patients beyond the index hospitalization, predominantly due to noncardiovascular conditions.4, 5 A recent study from Taiwan using administrative claims also found higher risk of cardiovascular events in long‐term follow‐up.5 In our previous study of 1‐year survival after prosthetic valve endocarditis, the rate of mortality remained high for the first 4 months after admission for IE in patients treated with medical therapy alone or in combination with surgery.16
Predictors of intermediate‐term mortality have also been evaluated in other studies of IE. In a retrospective regional study of left‐sided IE, abnormal mental status, comorbid illness, moderate to severe heart failure, microbiologic organism at baseline, and medical therapy were associated with 6‐month mortality in a logistic regression model.8 This model was validated in a split‐sample cohort but was limited to patients with complicated left‐sided IE and did not evaluate surgery as a time‐dependent covariate.8 In a more recent retrospective study of possible or definite IE, Sy et al evaluated the time‐dependent nature of these clinical characteristics during the index hospitalization. Whereas several variables of acute physiology (eg, heart rate, serum creatinine, platelet count) were associated with 6‐month mortality on day 1, only heart failure, comorbid illness, and platelet count at 15 days were associated with this outcome.9 Furthermore, surgery, which was performed in 57 of 192 patients, was not significantly associated with lower 6‐month mortality.9
The present study confirms many of these previous findings and extends the risk association to include a number of other baseline variables (including host factors of age and dialysis) and other IE complications, such as stroke or persistent bacteremia. A simplified weighted ICE prognostic score was developed for clinical use, and these prognostic factors for mortality were categorized into 4 groups of variables: host factors, IE characteristics, complications, and treatment with or without surgery. Many of the variables predictive of 6‐month mortality, ranging from ≈10% to >50% across risk quintiles, have been associated previously with mortality during the index hospitalization,1, 13 and our risk score emphasizes the additive prognostic associations of these clinical characteristics.
We found significant independent associations between mortality and nearly all IE complications that are accepted indications for surgical intervention.6, 17 Importantly, in the validated model using the ICE‐PLUS cohort, all IE complications that predicted mortality occurred before surgery was performed in those patients, illustrating the longer term prognostic significance of these sequelae, even with surgical treatment. The additional strengths of our study that improve the generalizability of the findings include the larger sample sizes in both derivation and validation cohorts; separate derivation and external validation cohorts of definite IE, including different years of data collection; consistency of the results despite differences in the 2 cohorts; and the contemporary, prospective, multinational data collection.
Surgery was found to be independently associated with lower 6‐month mortality in our risk models, even after adjustment for operative propensity and risk; however, surgery was performed less frequently in the subgroups of patients with highest risk of 6‐month mortality, illustrating the selection bias for this intervention even in the presence of indications. Our recent study of surgical indications and treatment in the ICE‐PLUS cohort found that approximately one‐quarter of left‐sided IE cases with indications for surgery did not undergo surgery due to operative risk factors, such as sepsis, but that surgery was associated with a survival benefit after adjustment for operative risk.12 The present study highlights the risk of death related to host and IE characteristics even beyond the period of surgical intervention. Multidisciplinary strategies for management of IE cases by cardiologists, infectious disease specialists, and cardiac surgeons have been found to improve survival,18, 19 but additional studies are needed to optimize the use of surgery in higher risk patients.
Our study has several limitations. All participating ICE sites are referral centers with multidisciplinary experience in the management of IE and the availability of cardiac surgery, which may affect the generalizability of the results. Regional differences in IE characteristics and treatment may exist within the cohort, but sensitivity analysis by geographic region did not substantially change the hazard ratios for mortality in the derivation model. The use of surgery was not prespecified by a study protocol, but we have previously found that surgery was performed for guideline‐directed indications6, 7 in the ICE‐PLUS cohort.12 Although treatment selection bias for surgery may influence the results of the present study, surgical intervention was evaluated as a time‐dependent variable with inverse probability adjustment for surgery in both models. Other clinical variables not collected or included in the models may be associated with mortality or may confound the relationships between defined variables and outcome.
In summary, clinical characteristics of the host, pathogen, IE episode, and surgical treatment are independently associated with 6‐month mortality in IE. A simplified prognostic score may be used to identify specific risk subgroups to compare treatment strategies and to optimize the use and timing of surgery.
Appendix
ICE Participating Sites
Last updated: November 19, 2014
Argentina: Liliana Clara, MD, Marisa Sanchez, MD (Hospital Italiano). José Casabé, MD, PhD, Claudia Cortes, MD, (Hospital Universitario de la Fundaciòn Favaloro). Francisco Nacinovich, MD, Pablo Fernandez Oses, MD, Ricardo Ronderos, MD, Adriana Sucari, MD, Jorge Thierer, MD (Instituto Cardiovascular). Javier Altclas, MD, Silvia Kogan, MD (Sanatorio de la Trinidad Mitre). Australia: Denis Spelman, MD (Alfred Hospital). Eugene Athan, MD, Owen Harris, MBBS, (Barwon Health). Karina Kennedy, MBBS, Ren Tan, MBBS (Canberra Hospital). David Gordon, MBBS, PhD, Lito Papanicolas, MBBS (Flinders Medical Centre). Tony Korman, MD, Despina Kotsanas, BSc (Hons) (Southern Health). Robyn Dever, MD, Phillip Jones, MD, Pam Konecny, MD, Richard Lawrence, MD, David Rees, MD, Suzanne Ryan, MHSc (St. George Hospital). Michael P. Feneley, MD, John Harkness, MD, Phillip Jones, MD, Suzanne Ryan, MHSc (St. Vincent's). Phillip Jones, MD, Suzanne Ryan, MHSc (Sutherland). Phillip Jones, MD, Jeffrey Post, MD, Porl Reinbott, Suzanne Ryan, MHSc (The University of New South Wales). Austria: Rainer Gattringer, MD, Franz Wiesbauer, MD (Vienna General Hospital). Brazil: Adriana Ribas Andrade, Ana Cláudia Passos de Brito, Armenio Costa Guimarães, MD (Ana Neri Hospital). Max Grinberg, MD, PhD, Alfredo José Mansur MD, PhD, Rinaldo Focaccia Siciliano, MD, Tania Mara Varejao Strabelli, MD, Marcelo Luiz Campos Vieira, MD (Heart Institute (Incor), University of Sao Paulo Medical School). Regina Aparecida de Medeiros Tranchesi, MD, Marcelo Goulart Paiva, MD (Hospital 9 de Julho). Claudio Querido Fortes, MD, PhD (Hospital Universitario Clementino Fraga Filho/UFRJ). Auristela de Oliveira Ramos, MD (Instituto Dante Pazzanese de Cardiologia). Clara Weksler, MD, Giovanna Ferraiuoli, MD, Wilma Golebiovski, MD, (Instituto Nacional de Cardiologia), Cristiane Lamas, MD, MRCP, PhD (Unigranrio and Instituto Nacional de Cardiologia, Rio de Janeiro). Canada: James A. Karlowsky, MD, Yoav Keynan, MD, Andrew M. Morris, MD, Ethan Rubinstein, MD, LL.B (University of Manitoba). Chile: Sandra Braun Jones, MD, Patricia Garcia, MD (Hospital Clínico Pont. Universidad Católica de Chile). M. Cereceda, MD, Alberto Fica, Rodrigo Montagna Mella, Md (Hospital Clinico Universidad de Chile). Columbia: Ricardo Fernandez, MD, Liliana Franco, MD, Javier Gonzalez, MD, Astrid Natalia Jaramillo, MD (Clinica Cardiovascular Medellín). Croatia: Bruno Barsic, MD, PhD, Suzana Bukovski, MD, PhD Vladimir Krajinovic, MD, Ana Pangercic, MD, Igor Rudez, MD, Josip Vincelj, MD, PhD (University Hospital for Infectious Diseases). Czech Republic: Tomas Freiberger, MD, PhD, (Ceitec, Masaryk University, Brno) Jiri Pol, MD, Barbora Zaloudikova, MSc (Centre for Cardiovascular Surgery and Transplantation). Egypt: Zainab Ashour, MD, Amani El Kholy, MD, Marwa Mishaal, MD, Dina Osama, MD, Hussien Rizk, MD (Cairo University Medical School). France: Neijla Aissa, MD, Corentine Alauzet, MD, Francois Alla, MD, PhD, CHU Catherine Campagnac, RN, Thanh Doco‐Lecompte, MD, Christine Selton‐Suty, MD (CHU Nancy‐Brabois). Jean‐Paul Casalta, MD, Pierre‐Edouard Fournier, MD, Gilbert Habib, MD, Didier Raoult, MD, PhD, Franck Thuny, MD (Faculté de Médecine de Marseille). Francois Delahaye, MD, PhD, Armelle Delahaye, Francois Vandenesch, MD (Hospital Louis Pradel). Erwan Donal, MD, Pierre Yves Donnio, PhD, Erwan Flecher, MD, PhD, Christian Michelet, MD, PhD, Matthieu Revest, MD, Pierre Tattevin, MD, PhD, (Pontchaillou University). Florent Chevalier, MD, Antoine Jeu, MD, Jean Paul Rémadi, MD, Dan Rusinaru, MD, Christophe Tribouilloy, MD, PhD (South Hospital Amiens). Yvette Bernard, MD, Catherine Chirouze, MD, Bruno Hoen, MD, PhD, Joel Leroy, MD, Patrick Plesiat, MD (University Medical Center of Besançon). Germany: Christoph Naber, MD, PhD, Carl Neuerburg (Universitaetskliniken Bergmannsheil Bochum). Bahram Mazaheri, PhD, Christoph Naber, MD, PhD, Carl Neuerburg (University Essen). Greece: Sophia Athanasia, Ioannis Deliolanis, Helen Giamarellou, MD, PhD, Tsaganos Thomas, MD, Efthymia Giannitsioti, MD (Attikon University General Hospital). Elena Mylona, MD, Olga Paniara, MD, PhD, Konstantinos Papanicolaou, MD, John Pyros, MD, Athanasios Skoutelis, MD, PhD (Evangelismos General Hospital of Athens). Elena Mylona, MD, Olga Paniara, MD, PhD, Konstantinos Papanikolaou, MD, John Pyros, MD, Athanasios Skoutelis, MD, PhD (Evangelismos General Hospital of Athens). India: Gautam Sharma, MD (All India Institute of Medical Sciences). Johnson Francis, MD, DM, Lathi Nair, MD, DM Vinod Thomas, MD, DM, Krishnan Venugopal, MD, DM (Medical College Calicut). Ireland: Margaret M. Hannan, MB, BCh BAO, MSc, John P. Hurley, MB, BCh (Mater Hospitals). Israel: Amos Cahan, MD, Dan Gilon, MD, Sarah Israel, MD, Maya Korem, MD, Jacob Strahilevitz, MD (Hadassah‐Hebrew University).Ethan Rubinstein, MD, LL.B, Jacob Strahilevitz, MD (Tel Aviv University School of Medicine). Italy: Emanuele Durante‐Mangoni, MD, PhD, Irene Mattucci, MD, Daniela Pinto, MD, Federica Agrusta, MD, Alessandra Senese, MD, Enrico Ragone, MD, PhD, Riccardo Utili, MD, PhD (II Università di Napoli). Enrico Cecchi, MD, Francesco De Rosa, MD, Davide Forno, MD, Massimo Imazio, MD, Rita Trinchero, MD (Maria Vittoria Hospital). Paolo Grossi, MD, PhD, Mariangela Lattanzio, MD, Antonio Toniolo, MD (Ospedale di Circolo Varese). Antonio Goglio, MD, Annibale Raglio, MD, DTM&H, Veronica Ravasio, MD, Marco Rizzi, MD, Fredy Suter, MD (Ospedali Riuniti di Bergamo). Giampiero Carosi, MD, Silvia Magri, MD, Liana Signorini, MD (Spedali Civili – Università di Brescia). Lebanon: Zeina Kanafani, MD, MS, Souha S.Kanj, MD, Ahmad Sharif‐Yakan, MD (American University of Beirut Medical Center). Malaysia: Imran Abidin, MD (University of Malaya Medical Center). Syahidah Syed Tamin, MD (National Heart Institute). Mexico: Eduardo Rivera Martínez, MD, Gabriel Israel Soto Nieto, MD (Instituto Nacional de Cardiología Ignacio Chávez). Netherlands: Jan T.M. van der Meer, MD, PhD (University of Amsterdam). New Zealand: Stephen Chambers, MD, MSc (University of Otago), David Holland, MB, ChB, PhD (Middlemore Hospital), Arthur Morris, MD (Diagnostic Medlab), Nigel Raymond, MB, ChB (Wellington Hospital), Kerry Read, MB, ChB (North Shore Hospital). David R. Murdoch, MD, MSc, DTM&H (University of Otago). Romania: Stefan Dragulescu, MD, PhD, Adina Ionac, MD, PhD, Cristian Mornos, MD (Victor Babes University of Medicine and Pharmacy). Russia: O.M. Butkevich, PhD (Learning‐Scientific Centre of Medical Centre of Russian Presidential Affairs Government Medical Centre of Russian). Natalia Chipigina, PhD, Ozerecky Kirill, MD, Kulichenko Vadim, Tatiana Vinogradova, MD, PhD (Russian Medical State University). Saudi Arabia: Jameela Edathodu, MBBS, Magid Halim, MBBS (King Faisal Specialist Hospital & Research Center). Singapore: Yee‐Yun Liew, Ru‐San Tan, MBBS (National Heart Centre). Slovenia: Tatjana Lejko‐Zupanc, MD, PhD, Mateja Logar, MD, PhD, Manica Mueller‐Premru, MD, PhD (Medical Center Ljublijana). South Africa: Patrick Commerford, MD, Anita Commerford, MD, Eduan Deetlefs, MD, Cass Hansa, MD, Mpiko Ntsekhe, MD (University of Cape Town and Groote Schuur Hospital). Spain: Manuel Almela, MD, Yolanda Armero, MD, Manuel Azqueta, MD, Ximena Castañeda, MD, Carlos Cervera, MD, PhD, MD, PhD, Carlos Falces, MD, PhD, Cristina Garcia‐de‐la‐Maria, PhD, Guillermina Fita, MD, Jose M. Gatell, MD, PhD, Magda Heras, MD, PhD Jaime Llopis, MD, PhD, Francesc Marco, MD, PhD, Carlos A. Mestres, MD, PhD, José M. Miró, MD, PhD, Asuncion Moreno, MD, PhD, Salvador Ninot, MD, Carlos Paré, MD, PhD Juan M. Pericas, MD, Jose Ramirez, MD, PhD, Irene Rovira, MD, Marta Sitges, MD, PhD (Hospital Clinic – IDIBAPS. University of Barcelona, Barcelona, Spain). University of Barcelona, Barcelona, Spain). Ignasi Anguera, MD, PhD, Bernat Font, MD, Joan Raimon Guma, MD (Hospitál de Sabadell). Javier Bermejo, Emilio Bouza, MD, PhD, Miguel Angel Garcia Fernández, MD, Victor Gonzalez‐Ramallo, MD, Mercedes Marín, MD, Patricia Muñoz, MD, PhD, Miguel Pedromingo, MD, Jorge Roda, Marta Rodríguez‐Créixems, MD, PhD, Jorge Solis, MD (Hospital General Universitario Gregorio Marañón). Benito Almirante, MD, Nuria Fernandez‐Hidalgo, MD, Pilar Tornos, MD (Hospital Universitari Vall d'Hebron). Arístides de Alarcón, Ricardo Parra (Hospital Universitario Virgen del Rocío). Sweden: Eric Alestig, MD, Magnus Johansson, MD, PhD, Lars Olaison, MD, PhD, Ulrika Snygg‐Martin, MD (Sahlgrenska Universitetssjukhuset/Östra). Thailand: Orathai Pachirat, MD, Pimchitra Pachirat, MD, Burabha Pussadhamma, MD, Vichai Senthong, MD (Khon Kaen University). United Kingdom: Anna Casey, MBBS, Tom Elliott, PhD, DSc, Peter Lambert, BSc, PhD, DSc, Richard Watkin, MBBS (Queen Elizabeth Hospital). Christina Eyton, John L. Klein, MD (St. Thomas’ Hospital). United States of America: Suzanne Bradley, MD, Carol Kauffman, MD (Ann Arbor VA Medical Center). Roger Bedimo, MD, MS (Dallas VA Medical Center). Vivian H. Chu, MD, MHS, G. Ralph Corey, MD, Anna Lisa Crowley, MD, MHS, Pamela Douglas, MD, Laura Drew, RN, BSN, Vance G. Fowler, MD, MHS, Thomas Holland, MD, Tahaniyat Lalani, MBBS, MHS, Daniel Mudrick, MD, Zaniab Samad, MD, MHS, Daniel Sexton, MD, Martin Stryjewski, MD, MHS, Andrew Wang, MD, Christopher W. Woods, MD, MPH (Duke University Medical Center). Stamatios Lerakis, MD (Emory University). Robert Cantey, MD, Lisa Steed, PhD, Dannah Wray, MD, MHS (Medical University of South Carolina). Stuart A. Dickerman, MD (New York University Medical Center). Hector Bonilla, MD, Joseph DiPersio, MD, PhD, Sara‐Jane Salstrom, RN (Summa Health System). John Baddley, MD, Mukesh Patel, MD (University of Alabama at Birmingham). Gail Peterson, MD, Amy Stancoven, MD (UT‐Southwestern Medical Center). Donald Levine, MD, Jonathan Riddle, Michael Rybak, PharmD, MPH (Wayne State University). Christopher H. Cabell, MD, MHS (Quintiles).
ICE Coordinating Center: Khaula Baloch, MPH, Vivian H. Chu, MD, MHS, G. Ralph Corey, MD, Christy C. Dixon, Vance G. Fowler Jr, MD, MHS, Tina Harding, RN, BSN, Marian Jones‐Richmond, Lawrence P. Park, PhD, Bob Sanderford, Judy Stafford, MS.
ICE Publications Committee: Kevin Anstrom, PhD, Eugene Athan, MD, Arnold S. Bayer, MD, Christopher H. Cabell, MD, MHS, Vivian H. Chu, MD, MHS, G. Ralph Corey, MD, Vance G. Fowler, Jr, MD, MHS, Bruno Hoen, MD, PhD, A. W. Karchmer, MD, José M. Miró, MD, PhD, David R. Murdoch, MD, MSc, DTM&H, Daniel J. Sexton, MD, Andrew Wang, MD.
ICE Steering Committee: Arnold S. Bayer, MD, Christopher H. Cabell, MD, MHS, Vivian Chu, MD, MHS. G. Ralph Corey, D, David T. Durack, MD, D Phil, Susannah Eykyn, MD, Vance G. Fowler, Jr, MD, MHS, Bruno Hoen, MD, PhD, José M. Miró, MD, PhD, Phillipe Moreillon, MD, PhD, Lars Olaison, MD, PhD, Didier Raoult, MD, PhD, Ethan Rubinstein, MD, LLB, Daniel J. Sexton, MD.
Sources of Funding
Dr Wang was funded in part by American Heart Association Mid‐Atlantic Affiliate Grant in Aid #12GRNT12030071.
Disclosures
Dr Chu received a research grant from Merck Co. Dr Wang has received research grants from Edwards Lifesciences; Abbott Vascular; Gilead Sciences; Myokardia; and reviewed a legal case of possible endocarditis.
(J Am Heart Assoc. 2016;5:e003016 doi: 10.1161/JAHA.115.003016)
References
- 1. Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis‐Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benito N, Miro JM, de Lazzari E, Cabell CH, del Rio A, Altclas J, Commerford P, Delahaye F, Dragulescu S, Giamarellou H, Habib G, Kamarulzaman A, Kumar AS, Nacinovich FM, Suter F, Tribouilloy C, Venugopal K, Moreno A, Fowler VG Jr. Health care‐associated native valve endocarditis: importance of non‐nosocomial acquisition. Ann Intern Med. 2009;150:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021. [DOI] [PubMed] [Google Scholar]
- 4. Thuny F, Giorgi R, Habachi R, Ansaldi S, Le Dolley Y, Casalta JP, Avierinos JF, Riberi A, Renard S, Collart F, Raoult D, Habib G. Excess mortality and morbidity in patients surviving infective endocarditis. Am Heart J. 2012;164:94–101. [DOI] [PubMed] [Google Scholar]
- 5. Shih CJ, Chu H, Chao PW, Lee YJ, Kuo SC, Li SY, Tarng DC, Yang CY, Yang WC, Ou SM, Chen YT. Long‐term clinical outcome of major adverse cardiac events in survivors of infectious endocarditis: a nationwide population‐based study. Circulation. 2014;130:1684–1691. [DOI] [PubMed] [Google Scholar]
- 6. Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Muller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–2413. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 8. Hasbun R, Vikram HR, Barakat LA, Buenconsejo J, Quagliarello VJ. Complicated left‐sided native valve endocarditis in adults: risk classification for mortality. JAMA. 2003;289:1933–1940. [DOI] [PubMed] [Google Scholar]
- 9. Sy RW, Chawantanpipat C, Richmond DR, Kritharides L. Development and validation of a time‐dependent risk model for predicting mortality in infective endocarditis. Eur Heart J. 2011;32:2016–2026. [DOI] [PubMed] [Google Scholar]
- 10. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 11. Lalani T, Cabell CH, Benjamin DK, Lasca O, Naber C, Fowler VG Jr, Corey GR, Chu VH, Fenely M, Pachirat O, Tan RS, Watkin R, Ionac A, Moreno A, Mestres CA, Casabe J, Chipigina N, Eisen DP, Spelman D, Delahaye F, Peterson G, Olaison L, Wang A; International Collaboration on Endocarditis‐Prospective Cohort Study I . Analysis of the impact of early surgery on in‐hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment‐selection bias. Circulation. 2010;121:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu VH, Park LP, Athan E, Delahaye F, Freiberger T, Lamas C, Miro JM, Mudrick DW, Strahilevitz J, Tribouilloy C, Durante‐Mangoni E, Pericas JM, Fernandez‐Hidalgo N, Nacinovich F, Rizk H, Krajinovic V, Giannitsioti E, Hurley JP, Hannan MM, Wang A; International Collaboration on Endocarditis I . Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation. 2015;131:131–140. [DOI] [PubMed] [Google Scholar]
- 13. Chu VH, Cabell CH, Benjamin DK Jr, Kuniholm EF, Fowler VG Jr, Engemann J, Sexton DJ, Corey GR, Wang A. Early predictors of in‐hospital death in infective endocarditis. Circulation. 2004;109:1745–1749. [DOI] [PubMed] [Google Scholar]
- 14. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 15. Gaca JG, Sheng S, Daneshmand MA, O'Brien S, Rankin JS, Brennan JM, Hughes GC, Glower DD, Gammie JS, Smith PK. Outcomes for endocarditis surgery in North America: a simplified risk scoring system. J Thorac Cardiovasc Surg. 2011;141:98–106.e1–2. [DOI] [PubMed] [Google Scholar]
- 16. Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante‐Mangoni E, Fowler VG Jr, Gordon D, Grossi P, Hannan M, Hoen B, Munoz P, Rizk H, Kanj SS, Selton‐Suty C, Sexton DJ, Spelman D, Ravasio V, Tripodi MF, Wang A. In‐hospital and 1‐year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013;173:1495–1504. [DOI] [PubMed] [Google Scholar]
- 17. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; Members AATF . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 18. Botelho‐Nevers E, Thuny F, Casalta JP, Richet H, Gouriet F, Collart F, Riberi A, Habib G, Raoult D. Dramatic reduction in infective endocarditis‐related mortality with a management‐based approach. Arch Intern Med. 2009;169:1290–1298. [DOI] [PubMed] [Google Scholar]
- 19. Chirillo F, Scotton P, Rocco F, Rigoli R, Pedrocco A, Martire P, Daniotti A, Minniti G, Polesel E, Olivari Z. Management strategies and outcome for prosthetic valve endocarditis. Am J Cardiol. 2013;112:1177–1181. [DOI] [PubMed] [Google Scholar]


