Abstract
Background
The structure of the aorta is considered to influence exercise systolic blood pressure (SBP) response, which, in turn, might impact upon adverse outcomes. The current study sought to investigate the relationship of aortic calcification and exercise SBP with adverse outcomes among elderly individuals.
Methods and Results
We retrospectively reviewed 702 elderly individuals (>65 years of age) without obstructive coronary artery disease (CAD; luminal stenosis <50%) who underwent coronary computed tomography (CT) and exercise treadmill testing. ΔSBP stage2 and ΔSBP peak were defined as the difference in systolic blood pressure (SBP) between rest and stage 2 or peak exercise, respectively. Thoracic aortic calcium score (TACS) and coronary artery calcium score (CACS) were measured using CT scanning procedures. The primary endpoints were defined as all‐cause death, admission for heart failure, obstructive CAD requiring coronary intervention, and stroke. In multivariable models, ΔSBP stage2 and ΔSBP peak were positively related with log(TACS+1), even after adjusting for various clinical variables, baseline SBP, and CACS (P<0.001). During a median follow‐up period of 65 months, there were 59 events (8.4%). In a multivariate Cox regression model, independent predictors for all events were age (hazard ratio [HR], 1.12; 95% CI, 1.05–1.19; P<0.001), dyslipidemia (HR, 1.96; 95% CI, 1.14–3.37; P=0.015), and the 4th quartile of TACS (HR, 1.24; 95% CI, 1.03–1.49; P=0.024). Among individual events, the 4th quartile of TACS was the only independent predictor for stroke (HR, 2.15; 95% CI, 1.09–5.13; P=0.044), whereas CACS ≥400 mm3 was an independent predictor for obstructive CAD requiring intervention (HR, 7.04; 95% CI, 1.58–31.36; P=0.010).
Conclusions
Aortic calcification was related to SBP response during exercise and was an independent predictor for outcomes, especially stroke, regardless of resting SBP or CACS.
Keywords: aorta, blood pressure, calcification, exercise
Subject Categories: Computerized Tomography (CT)
Dynamic exercise typically elicits a substantial rise in systolic blood pressure (SBP) without much alteration in diastolic blood pressure (DBP).1 An early elevation in SBP during exercise provides further prognostic information toward cardiovascular outcomes and is known to be an important marker of various cardiovascular diseases, including incident hypertension, myocardial infarction, and stroke, as well as cardiovascular mortality in persons without overt coronary artery disease (CAD).2, 3, 4, 5
Some plausible mechanisms are perhaps responsible, in part, for the observed exaggerated rise in SBP during exercise. Past studies documented that an exaggerated rise in SBP is associated with failure to reduce total peripheral resistance and impaired endothelial vasodilator function.6 An exaggerated exercise SBP response is also associated with other established cardiovascular risk factors, such as insulin resistance, hypercholesterolemia, and carotid atherosclerosis.7, 8, 9 Arterial stiffening has also been proposed as a potential mechanism for an elevated exercise SBP response observed during both peak10 and submaximal exercise intensities.11
The aorta is an active participant of the cardiovascular system, and arterial stiffness has been suggested to be a predictor of cardiovascular morbidity and mortality.12 Aortic calcification has previously been independently associated with arterial stiffening, isolated systolic hypertension, left ventricular (LV) hypertrophy, and diastolic dysfunction in various study populations.13, 14, 15, 16, 17, 18 However, the relationship between exercise SBP and aortic calcification, and how it might adversely impact upon health, has not been fully elucidated, even though a significant interaction might exist between aorta and SBP response during exercise. Computed tomography (CT) is a useful tool for quantifying aortic calcification by aorta calcium scanning,14 and it is possible that calcification of the thoracic aorta assessed by CT scanning may be associated with a higher SBP during exercise and, subsequently, with poor clinical outcomes. In this study, we set out to evaluate the role of aortic calcification on exercise SBP response and how it might impact on mortality and various cardiovascular events among elderly individuals without significant CAD who underwent coronary CT and exercise treadmill testing. Specifically, we hypothesized that aortic calcification would be associated with greater exercise SBP changes and poor clinical outcomes in the elderly.
Methods
Study Population
We retrospectively reviewed 990 elderly individuals (≥65 years of age) who underwent both coronary CT and exercise treadmill testing for evaluation of suspected CAD within 90 days between tests during 2003 through 2009 at Severance Cardiovascular Hospital. We excluded 229 individuals with obstructive CAD on coronary CT (luminal stenosis ≥50%), 31 individuals whose CT scan did not include whole thoracic aorta from aortic arch to diaphragm level, 25 individuals who did not complete 2 stages of the Bruce protocol at treadmill test, and 3 individuals with atrial fibrillation. Patients with obstructive CAD were excluded to abolish the effect of coronary ischemia on exercise blood pressure and capacity. Hence, 702 elderly individuals without obstructive CAD comprised the study population. The median number of days between coronary CT and exercise test was 7 (interquartile range [IQR], 2–14). At the time of index visit, clinical data were collected from routine medical history, physical examination, and laboratory tests. Body mass index (BMI) was calculated from height and weight. Diabetes mellitus was defined as receiving antidiabetic treatments or a fasting plasma glucose of ≥126 mg/dL. Current cigarette smoking was defined as any cigarette smoking in the past month. Hypertension was defined as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg or use of antihypertensive agents. Dyslipidemia was defined as use of cholesterol‐lowering medications or having serum total cholesterol of ≥200 mg/dL. Primary endpoints were defined as all‐cause death, inpatient admissions for heart failure, obstructive CAD requiring coronary artery intervention or bypass surgery, stroke, and a composite of all endpoints. Occurrence of a clinical event was ascertained by review of hospital records and by telephone interview, if necessary. This study was approved by the institutional review board of Yonsei University, Severance Hospital (Seoul, Korea), and all individuals provided informed consent.
CT Protocol and Image Analysis
Patients were scanned using a 64‐section CT scanner (Sensation 64; Siemens Healthcare, Forchheim, Germany). For calcium scanning, unenhanced CT was performed with prospective electrocardiography (EGC)‐triggered acquisitions in mid‐diastole using 120 to 140 kV with 150 to 220 mAs, depending on the patient's size; 240‐ms exposure time per rotation; 330‐ms gantry rotation time; and 64×0.6 mm slice collimation. Calcium scans were reconstructed at 70% of the R‐R interval using a slice thickness of 3 mm with an increment of 3 mm. Coronary artery and thoracic aorta calcium scoring was performed on reconstructed images. Foci of coronary artery, aortic valve, and thoracic aorta were identified and scored by an experienced technician who was masked to the patient's medical records, using semiautomatic software (Vitrea 2.0; Vital Images, Minnetonka, MN) and verified by imaging cardiologists.19 Lesion‐specific calcium scores were summed across all lesions identified within left main, left anterior descending, left circumflex, and right coronary arteries to provide coronary artery calcium score (CACS). The thoracic aorta calcium score (TACS) included calcium scored in the ascending aorta, aortic arch, and descending aorta to the diaphragm level. An objective volume scoring method included in the system software was determined, which provided a score in cubic millimeters.20
Subsequently, standard CT angiography was performed. Patients without a contraindication to beta‐adrenergic blocking agents and with initial heart rates higher than 65 beats/min received a single oral dose of 40 mg of propranolol hydrochloride 1 hour before coronary CT angiography. Two types of CT system configurations were employed: (1) a 64‐slice CT scanner (Sensation 64; Siemens Medical Solutions, Forchheim, Germany) using retrospective ECG gating with tube current modulation from 2003 through 2009 with the following parameters: (1) rotation time: 330 ms, tube voltage: 100 to 120 keV, tube current: 400 to 800 mA, and pitch factor: 0.2; and (2) a 64‐row CT scanner (LightSpeed VCT XT; GE Healthcare, Milwaukee, WS) using a prospectively ECG‐gated axial technique from 2008 through 2009 with the following parameters: rotation time: 350 ms, tube voltage: 100 to 120 keV, and tube current: 300 to 900 mA. A real‐time bolus‐tracking technique was applied to trigger the initiation of the scan.
Exercise Testing
A symptom‐limited exercise treadmill test was performed according to the Bruce protocol.21 Resting SBP and DBP were measured in the brachial artery in the seated position. During the exercise stress test, heart rhythm, SBP, and DBP were recorded at the end of each stage of exercise, at peak stress, and during recovery (5 minutes after completion of the exercise). ΔSBP was defined as the difference between SBP at rest and exercise (ΔSBPstage2, stage 2 exercise; ΔSBPpeak, peak exercise). Pulse pressure (PP) was defined as the difference between SBP and DBP. ΔPP was defined as the difference in PP between rest and exercise (ΔPPstage2, stage 2 exercise; ΔPPpeak, peak exercise). A 12‐lead ECG was obtained every minute, and a 3‐lead ECG for heart rhythm was monitored continuously. Indications for terminating the exercise test are previously described.22
Statistical Analysis
Distributions of all relevant variables are reported as percentages or as means±SD for normally distributed variables and median (with 25–75%) for non‐normally distributed variables. To minimize skewness, we transformed CACS and TACS before analysis by adding 1 and obtaining the natural logarithm of the value (ie, log[CACS+1] and log[TACS+1]). We evaluated the linear relationships by use of univariate linear regression analysis to determine the independent correlates of exercise ΔSBPstage2, ΔSBPpeak, ΔPPstage2, and ΔPPpeak. We performed a univariate analysis before the multivariate analysis in an effort to ascertain candidate variables, which might be associated with exercise changes of SBP and PP. After that, variables that had a P<0.2 in univariate analysis were retained for subsequent multivariate linear regression,23 with sex, age, and resting heart rate forced entry into all models. Multivariate Cox proportional‐hazards regression analysis was employed to determine independent variables for event‐free survival after acquisition of coronary CT. Kaplan–Meier survival curves were employed to plot all clinical events according to the time to first event. A P<0.05 was considered statistically significant.
Results
Population Characteristics
Demographic characteristics and CT variables of all patients enrolled in this study are shown in Table 1. Mean age was 69±4 years, and there were 298 males (42%). Diabetes, smoking, dyslipidemia, and hypertension were observed in 125 (18%), 95 (14%), 348 (50%), and 392 patients (56%), respectively. Among those enrolled, 269 (38%), 249 (35%), and 177 (25%) patients were receiving beta‐blockers (BBs), calcium‐channel blockers (CCBs), and angiotensin‐converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs). Mean seated SBP and DBP at rest were 125±16 and 77±9 mm Hg, respectively. Median CACS and TACS were 4 (IQR, 0–56) and 442 mm3 (IQR, 125–1235), respectively.
Table 1.
Baseline Characteristics
| Variable | Participants (n=702) |
|---|---|
| Baseline characteristics | |
| Age, y | 69±4 |
| Men, n (%) | 298 (42) |
| Diabetes, n (%) | 125 (18) |
| Smoking, n (%) | 95 (14) |
| Dyslipidemia, n (%) | 348 (50) |
| Hypertension, n (%) | 392 (56) |
| Medications n (%) | |
| Beta‐blocker | 269 (38) |
| Calcium‐channel blocker | 249 (35) |
| ACEi/ARB | 177 (25) |
| BMI, kg/m2 | 24.5±2.7 |
| Total cholesterol/HDL ratio | 3.7±0.9 |
| Resting systolic BP, mm Hg | 125.0±15.7 |
| Resting diastolic BP, mm Hg | 76.5±9.2 |
| Resting PP, mm Hg | 48.2±14.6 |
| Resting heart rate, bpm | 57.9±7.4 |
| CT variables | |
| CACS, mm3 | 4 (0–56) |
| log(CACS+1) | 2.1±2.2 |
| TACS, mm3 | 442 (125–1235) |
| log(TACS+1) | 5.9±1.9 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor antagonist; BMI, body mass index; BP, blood pressure; CACS, coronary artery calcium score; CT, computed tomography; HDL, high‐density lipoprotein; PP, pulse pressure; TACS, thoracic aorta calcium score.
SBP Changes During Exercise Test
Because there are no accepted thresholds or cut‐off points for TACS risk categories, we arbitrarily stratified TACS into quartiles to compare BP changes during exercise. Cutoffs for TACS quartiles were 0 to 125 (first quartile), 125 to 442 (second quartile), 442 to 1235 (third quartile), and >1235 (fourth quartile). Trend of changes in ΔSBP and ΔPP during exercise test and at peak exercise according to quartiles of TACS values are displayed in Table 2. ΔSBP and ΔPP were not significantly different among the quartiles of TACS at stage 1 exercise. However, ΔSBP and ΔPP were significantly higher during stage 2 and peak exercises and during the recovery phase for the fourth quartile as compared with the lowest quartile (all P<0.05).
Table 2.
Change in Systolic Blood Pressure and Pulse Pressure According to Quartiles of TACS
| Variable | TACS Quartiles | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | P for Trend | 1st vs 2nd | 1st vs 3rd | 1st vs 4th | |
| ΔSBP | ||||||||
| Stage 1 | 12.9±21.4 | 11.2±20.4 | 14.0±24.7 | 12.4±21.5 | 0.864 | 0.864 | 0.644 | 0.824 |
| Stage 2 | 24.3±22.4 | 24.3±23.8 | 26.2±25.9 | 30.8±25.0 | 0.101 | 0.987 | 0.471 | 0.011 |
| Peak exercise | 51.2±21.6 | 52.1±24.1 | 52.2±22.0 | 56.8±21.7 | 0.027 | 0.713 | 0.677 | 0.017 |
| Recovery | 20.5±20.8 | 23.5±20.4 | 21.7±20.2 | 26.0±20.8 | 0.039 | 0.183 | 0.595 | 0.016 |
| ΔPP | ||||||||
| Stage 1 | 18.4±21.3 | 17.3±19.6 | 18.3±23.9 | 18.7±22.3 | 0.809 | 0.603 | 0.948 | 0.908 |
| Stage 2 | 30.7±21.6 | 29.3±20.4 | 30.2±23.8 | 37.4±25.8 | 0.013 | 0.546 | 0.859 | 0.012 |
| Peak exercise | 50.6±20.9 | 51.1±22.7 | 50.1±23.0 | 58.0±22.9 | 0.006 | 0.838 | 0.813 | 0.002 |
| Recovery | 22.0±19.2 | 24.4±19.7 | 22.5±19.2 | 28.7±20.2 | 0.008 | 0.244 | 0.813 | 0.002 |
ΔPP indicates change in pulse pressure during exercise; ΔSBP, change in systolic blood pressure during exercise; TACS, thoracic aorta calcium score.
Relationship Between Aortic Calcification and Blood Pressure Response During Exercise Testing
Mean SBP, DBP, and PP during the second stage of exercise were 152±27, 72±13, and 80±25 mm Hg, respectively. ΔSBPstage2 and ΔPPstage2 were 31.8±23.1 and 27.3±23.9 mm Hg, respectively. ΔSBPpeak and ΔPPpeak were 53.4±22.3 and 52.4±22.6 mm Hg, respectively. The relationship of standard risk factors and aortic calcification to resting and ΔSBPstage2 or ΔSBPpeak are summarized in Table 3. Multivariate regression analysis revealed that log(TACS+1) was independently related to resting SBP, even after adjusting for sex, age, resting heart rate, diabetes, and hypertension (P=0.048). log(TACS+1) was also related to ΔSBPstage2 or ΔSBPpeak, after adjusting for sex, age, resting SBP, resting DBP, resting heart rate, other comorbidities, and log(CACS+1) (P=0.001 and 0.010, respectively). No significant associations were observed between log(TACS+1) and ΔDBP both at rest and at stage 2 of exercise. The relationship between standard risk factors and aortic calcification to resting and ΔPPstage2 or ΔPPpeak is shown in Table 4. Similar to SBP, log(TACS+1) was independently associated to resting PP, after adjusting for sex, age, resting heart rate, diabetics, and log(CACS+1) (P=0.049). log(TACS+1) also demonstrated a relationship with ΔPPstage2 and ΔPPpeak, even after adjusting sex, age, resting heart rate, other comorbidities, and log(CACS+1) (all P=0.004).
Table 3.
Relationship of Standard Risk Factors and Aortic Calcification to Resting and Change in Systolic Blood Pressure During Exercise
| Variable | Resting SBP | ΔSBPstage2 | ΔSBPpeak | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | |
| Male sex | 0.042 | 0.270 | 0.044 | 0.321 | −0.139 | <0.001 | −0.092 | 0.043 | 0.150 | <0.001 | 0.149 | 0.001 |
| Age | 0.097 | 0.011 | 0.041 | 0.403 | −0.032 | 0.423 | −0.124 | 0.009 | −0.077 | 0.042 | −0.085 | 0.070 |
| Resting SBP | — | — | — | — | −0.071 | 0.070 | −0.044 | 0.401 | −0.151 | <0.001 | −0.165 | 0.001 |
| Resting DBP | — | — | — | — | −0.114 | 0.004 | −0.096 | 0.056 | −0.104 | 0.006 | −0.041 | 0.417 |
| Resting heart rate | 0.203 | <0.001 | 0.193 | <0.001 | 0.024 | 0.606 | 0.010 | 0.821 | −0.029 | 0.514 | 0.027 | 0.548 |
| Diabetes | 0.079 | 0.038 | 0.011 | 0.869 | 0.037 | 0.352 | — | — | 0.085 | 0.025 | 0.113 | 0.013 |
| Hypertension | 0.091 | 0.017 | 0.058 | 0.207 | 0.079 | 0.047 | 0.115 | 0.012 | 0.031 | 0.415 | — | — |
| Dyslipidemia | −0.008 | 0.839 | 0.002 | — | −0.024 | 0.550 | — | — | −0.084 | 0.027 | −0.048 | 0.283 |
| Use of BB | −0.007 | 0.865 | — | — | −0.091 | 0.020 | −0.113 | 0.012 | −0.002 | 0.950 | — | — |
| Use of CCB | −0.012 | 0.747 | — | — | 0.053 | 0.272 | — | — | −0.018 | 0.641 | — | — |
| Use of ACEi/ARB | 0.084 | 0.033 | 0.033 | 0.479 | 0.035 | 0.377 | — | — | 0.005 | 0.905 | — | — |
| BMI | 0.013 | 0.738 | — | — | 0.060 | 0.128 | — | — | 0.050 | 0.190 | 0.049 | 0.266 |
| Smoking | −0.034 | 0.365 | — | — | 0.040 | 0.312 | — | — | 0.045 | 0.240 | — | — |
| Total cholesterol/HDL | 0.055 | 0.188 | — | — | 0.034 | 0.435 | — | — | −0.035 | 0.402 | — | — |
| log(CACS+1) | 0.110 | 0.004 | 0.074 | 0.138 | −0.002 | 0.954 | −0.077 | 0.126 | 0.020 | 0.595 | −0.084 | 0.087 |
| log(TACS+1) | 0.149 | <0.001 | 0.096 | 0.048 | 0.132 | 0.001 | 0.175 | 0.001 | 0.071 | 0.060 | 0.100 | 0.010 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; BMI, body mass index; CACS, coronary artery calcium score; CCB, calcium‐channel blocker; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; SBP, systolic blood pressure; TACS, thoracic aorta calcium score; ΔSBPpeak, change of systolic blood pressure during peak exercise; ΔSBPstage2, change of systolic blood pressure during stage 2 exercise.
Table 4.
Relationship of Standard Risk Factors and Aortic Calcification to Resting and Change in Pulse Pressure During Exercise
| Variable | Resting PP | ΔPPstage2 | ΔPPpeak | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | β | P Value | |
| Male sex | 0.011 | 0.771 | 0.021 | 0.643 | −0.086 | 0.030 | −0.125 | 0.060 | 0.098 | 0.010 | 0.056 | 0.217 |
| Age | 0.151 | <0.001 | 0.079 | 0.087 | −0.027 | 0.492 | −0.081 | 0.094 | −0.066 | 0.085 | −0.065 | 0.175 |
| Resting PP | — | — | — | — | −0.033 | 0.411 | — | — | −0.110 | 0.004 | −0.121 | 0.010 |
| Resting heart rate | 0.152 | 0.001 | 0.135 | 0.003 | 0.028 | 0.553 | −0.003 | 0.952 | −0.005 | 0.914 | 0.007 | 0.877 |
| Diabetes | 0.157 | <0.001 | 0.102 | 0.023 | 0.076 | 0.056 | 0.080 | 0.088 | 0.117 | 0.002 | 0.138 | 0.003 |
| Hypertension | 0.061 | 0.112 | — | — | 0.042 | 0.300 | — | — | 0.018 | 0.647 | — | — |
| Dyslipidemia | 0.021 | 0.579 | — | — | −0.050 | 0.209 | — | — | −0.069 | 0.072 | −0.021 | 0.642 |
| Use of BB | −0.035 | 0.363 | — | — | −0.085 | 0.038 | −0.121 | 0.009 | −0.022 | 0.570 | — | — |
| Use of CCB | 0.012 | 0.753 | — | — | 0.032 | 0.426 | — | — | −0.019 | 0.621 | — | — |
| Use of ACEi/ARB | 0.098 | 0.011 | 0.038 | 0.393 | 0.044 | 0.274 | — | — | 0.041 | 0.290 | — | — |
| BMI | 0.003 | 0.942 | — | — | 0.074 | 0.063 | 0.125 | 0.007 | 0.053 | 0.164 | 0.047 | 0.304 |
| Smoking | −0.035 | 0.351 | — | — | 0.024 | 0.541 | — | — | 0.033 | 0.391 | — | — |
| Total cholesterol/HDL | 0.046 | 0.265 | — | — | 0.002 | 0.959 | — | — | −0.045 | 0.280 | — | — |
| log(CACS+1) | 0.108 | 0.005 | 0.070 | 0.153 | 0.034 | 0.397 | −0.030 | 0.566 | 0.018 | 0.640 | −0.101 | 0.055 |
| log(TACS+1) | 0.173 | <0.001 | 0.097 | 0.049 | 0.105 | 0.008 | 0.128 | 0.004 | 0.086 | 0.023 | 0.144 | 0.004 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; BMI, body mass index; CACS, coronary artery calcium score; CCB, calcium‐channel blocker; HDL, high‐density lipoprotein; PP, pulse pressure; TACS, thoracic aorta calcium score; ΔPPpeak, change of pulse pressure during peak exercise; ΔPPstage2, change of pulse pressure during stage 2 exercise.
Predictors for Primary Endpoint
During a median follow‐up period of 65 months (IQR, 19–90), there were 59 events (8.4%), including 11 all‐cause deaths (1.6%), 13 inpatient admissions for heart failures (1.9%), 11 CADs requiring coronary intervention (1.6%), and 24 strokes (3.4%). TACS was higher in patients with events as compared with those without an event (2628±1952 vs 1742±995 mm3; P=0.008). However, no significant differences in ΔSBPstage2 (26.6±23.8 vs 23.6±29.6 mm Hg; P=0.455) or ΔSBPpeak (53.5±21.9 vs 48.9±26.8 mm Hg; P=0.208) were observed in patients with versus without events. Table 5 reports the multivariate analysis of variables as potential predictors of all endpoints in the studied population. In univariate analysis, age and 4th quartile of TACS were associated with all events (P<0.001 and =0.001, respectively). CACS over 400 mm3 demonstrated a borderline statistical significance (P=0.059). In multivariate analysis, age (hazard ratio [HR], 1.12; 95% CI, 1.05–1.19; P<0.001), dyslipidemia (HR, 1.96; 95% CI, 1.14–3.37; P=0.05), and the 4th quartile of TACS (HR, 1.24; 95% CI, 1.03–1.49; P=0.024) were independent predictors for all‐cause events, even after adjusting for sex and CACS. Among individual events, the 4th quartile of TACS was the only independent predictor for stroke (HR, 2.15; 95% CI, 1.09–5.13; P=0.044), whereas CACS ≥400 mm3 was independently associated with obstructive CAD requiring intervention (HR, 7.04; 95% CI, 1.58–31.36; P=0.010). Although the 4th quartile of TACS was a predictor for heart failure admission in univariate analysis, in multivariate analysis adjusting for age, sex, dyslipidemia, resting heart rate, and CACS, it demonstrated a nonsignificant relationship with heart failure admission (P=0.119).
Table 5.
Cox Proportional Hazards Regression for Predictors of Outcomes
| Outcome | Predictor | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| All events | Age | 1.14 (1.08–1.22) | <0.001 | 1.12 (1.05–1.19) | <0.001 |
| Male sex | 1.20 (0.72–2.01) | 0.488 | 1.06 (0.62–1.81) | 0.826 | |
| Hypertension | 1.15 (0.68–0.95) | 0.603 | — | — | |
| Diabetes | 0.82 (0.41–1.61) | 0.557 | — | — | |
| Dyslipidemia | 1.60 (0.95–2.70) | 0.081 | 1.96 (1.14–3.37) | 0.015 | |
| Resting heart rate | 1.01 (0.97–1.05) | 0.722 | — | ||
| BMI | 0.97 (0.88–1.06) | 0.461 | — | ||
| Smoking | 1.19 (0.58–2.42) | 0.635 | — | ||
| CACS ≥400 mm3 | 2.26 (0.97–5.27) | 0.059 | 1.83 (0.75–4.48) | 0.184 | |
| TACS 4th vs 1st to 3rd quartiles | 2.41 (1.44–4.04) | 0.001 | 1.24 (1.03–1.49) | 0.024 | |
| All‐cause death | Age | 1.27 (1.12–1.44) | <0.001 | 1.26 (1.09–1.46) | 0.002 |
| Male sex | 1.08 (0.33–3.53) | 0.904 | 0.77 (0.22–2.78) | 0.694 | |
| Hypertension | 1.02 (0.31–3.33) | 0.980 | — | — | |
| Diabetes | 0.38 (0.05–2.99) | 0.360 | — | — | |
| Dyslipidemia | 0.96 (0.29–3.16) | 0.949 | — | — | |
| Resting heart rate | 0.93 (0.83–0.13) | 0.155 | 0.96 (0.85–1.08) | 0.468 | |
| BMI | 0.69 (0.55–0.86) | 0.001 | 0.66 (0.50–0.87) | 0.003 | |
| Smoking | 0.66 (0.08–5.16) | 0.692 | — | — | |
| CACS ≥400 mm3 | 0.99 (0.21–4.59) | 0.991 | 0.47 (0.65–1.74) | 0.814 | |
| TACS 4th vs 1st to 3rd quartiles | 1.67 (0.49–5.72) | 0.412 | 0.93 (0.25–3.49) | 0.908 | |
| Heart failure | Age | 1.16 (1.02–1.32) | 0.029 | 1.08 (0.88–1.33) | 0.483 |
| Male sex | 1.71 (0.54–5.40) | 0.360 | 1.89 (0.33–10.69) | 0.473 | |
| Hypertension | 0.61 (0.19–1.91) | 0.392 | — | — | |
| Diabetes | 1.34 (0.36–4.94) | 0.665 | — | — | |
| Dyslipidemia | 2.32 (0.70–7.72) | 0.170 | 3.63 (0.62–21.28) | 0.152 | |
| Resting heart rate | 1.04 (0.99–1.09) | 0.155 | 1.03 (0.15–7.25) | 0.957 | |
| BMI | 0.98 (0.80–1.20) | 0.848 | — | — | |
| Smoking | 2.18 (0.59–8.04) | 0.244 | — | — | |
| CACS ≥400 mm3 | 1.80 (0.23–13.96) | 0.574 | 1.06 (0.15–7.25) | 0.957 | |
| TACS 4th vs 1st to 3rd quartiles | 3.20 (1.03–9.93) | 0.044 | 4.13 (0.69–24.62) | 0.119 | |
| Obstructive CAD | Age | 1.06 (0.91–1.23) | 0.450 | 1.03 (0.88–1.21) | 0.707 |
| Male sex | 1.56 (0.48–5.13) | 0.460 | 0.04 (0.29–3.77) | 0.956 | |
| Hypertension | 0.69 (0.21–2.26) | 0.537 | — | — | |
| Diabetes | 2.31 (0.68–7.89) | 0.183 | 0.58 (0.17–1.94) | 0.373 | |
| Dyslipidemia | 1.29 (0.39–4.22) | 0.678 | — | — | |
| Resting heart rate | 0.99 (0.89–1.10) | 0.825 | — | — | |
| BMI | 1.02 (0.83–1.26) | 0.826 | — | — | |
| Smoking | 1.40 (0.32–6.48) | 0.667 | — | — | |
| CACS ≥400 mm3 | 7.12 (1.89–26.87) | 0.004 | 7.04 (1.58–31.36) | 0.010 | |
| TACS 4th vs 1st to 3rd quartiles | 1.67 (0.49–5.70) | 0.414 | 1.45 (0.39–5.39) | 0.583 | |
| Stroke | Age | 1.12 (1.02–1.23) | 0.014 | 1.10 (0.99–1.22) | 0.076 |
| Male sex | 0.935 (0.42–2.17) | 0.871 | 0.80 (0.34–1.89) | 0.613 | |
| Hypertension | 2.35 (0.93–5.97) | 0.072 | 0.33 (0.76–5.04) | 0.139 | |
| Diabetes | 0.35 (0.08–1.49) | 0.155 | 1.96 (0.76–5.04) | 0.163 | |
| Dyslipidemia | 1.87 (0.82–4.30) | 0.139 | 1.83 (0.77–4.37) | 0.171 | |
| Resting heart rate | 1.02 (0.97–1.07) | 0.402 | — | — | |
| BMI | 1.07 (0.94–1.23) | 0.312 | — | — | |
| Smoking | 0.92 (0.28–3.10) | 0.896 | — | — | |
| CACS ≥400 mm3 | 1.733 (0.41–7.38) | 0.457 | 1.89 (0.42–8.56) | 0.410 | |
| TACS 4th vs 1st to 3rd quartiles | 2.89 (1.30–6.42) | 0.009 | 2.15 (1.09–5.13) | 0.044 | |
BMI indicates body mass index; CACS, coronary artery calcium score; CAD, coronary artery disease; CI, confidential interval; HDL, high‐density lipoprotein; HR, hazard ratio; TACS, thoracic aorta calcium score.
Figure 1 demonstrates Kaplan–Meier curves showing differences in all event‐free survival rates according to quartiles of TACS. Event‐free survival rate was significantly lower for individuals within the 4th quartile of TACS when compared with the lower quartiles (log‐rank, P=0.002). Figure 2 displays Kaplan–Meier curves for incident stroke and obstructive CAD requiring intervention according to quartiles of TACS or CACS. Incidence of stroke was higher in patients belonging to the 4th quartile of TACS as compared with the lower quartiles (log‐rank, P=0.009), whereas incidence of obstructive CAD requiring intervention was higher in patients with CACS ≥400 mm3 as compared with those whose CACS was <400 mm3 (log‐rank, P=0.004).
Figure 1.
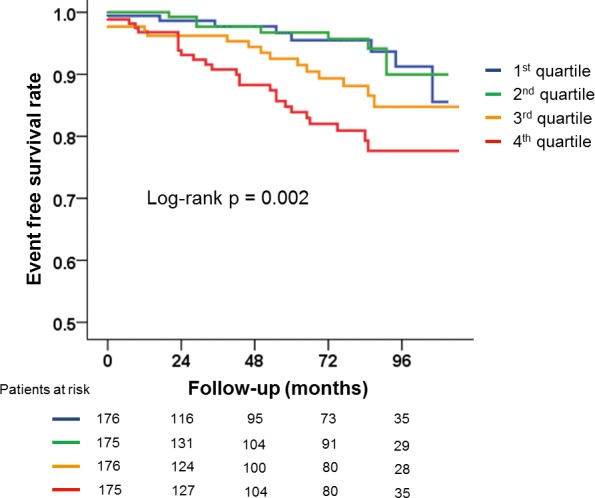
Kaplan–Meier curves displaying the differences in all event‐free survival rates according to thoracic aorta calcium score quartiles.
Figure 2.
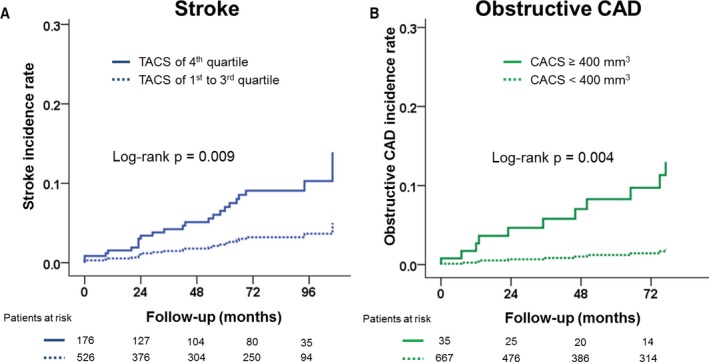
Kaplan–Meier curves displaying the differences in (A) stroke rates and (B) obstructive coronary artery disease according to quartiles of thoracic aorta calcium score (TACS) or coronary artery calcium score (CACS).
Discussion
In the current study, the high presence of aortic calcification was positively correlated with an SBP raise and PP widening during exercise. Foremost, these relationships remained significant after controlling for potential confounders, including CACS and resting blood pressure. In addition, aortic calcification was also related with adverse cardiovascular events, especially stroke, whereas CACS only predicted obstructive CAD events. Quantitative assessment of aortic calcification by use of TACS was feasible, associated with exercise SBP, and proved a useful surrogate for long‐term clinical outcomes other than obstructive CAD, in elderly individuals without obstructive CAD determined by coronary CT angiography, regardless of CACS.
Aortic Calcification in Populations Without Obstructive CAD
Aortic calcification has been reported to be associated with isolated systolic hypertension in healthy individuals13 and suggested to be a potential reason for developing arterial stiffening.24 Aortic calcification and progressive stiffening of the large arteries with advancing age results in a widening of PP and leads to development of isolated systolic hypertension in the elderly.25, 26 Arterial stiffening is one of the suggested mechanisms for a rise in exercise SBP. We previously demonstrated that heavy aortic calcification was associated with arterial stiffening and also resulted in LV hypertrophy and diastolic dysfunction in elderly male patients with hypertension, most likely owing to increased LV afterload associated with heavily calcified aortic wall and decreased aortic compliance.24 In the present study, we also verified that aortic calcification, which might be a surrogate for arterial stiffness, could predict exercise SBP response and cardiovascular outcomes in the elderly who are prone to aortic calcification, irrespective of coronary atherosclerosis.
The pathology of aortic calcification differs to that of coronary artery calcification, which is associated with CAD. Aortic calcification reflects calcification in both the intimal and medial tunica layers of the artery, in contrast to coronary artery calcification, which generally occurs in the intimal layer of an atherosclerotic plaque.27 Medial calcification is not associated with atherosclerotic plaque, but is strongly associated with aging, diabetes, and end‐stage renal disease.28 Interestingly, in the current study, TACS and CACS demonstrated different prognostic roles in the elderly without obstructive CAD. TACS predicted adverse cardiac events other than CAD, whereas CACS mainly predicted significant obstructive CAD. Therefore, aortic calcification as represented by TACS might not always reflect a sole indicator for atherosclerosis, which is in contrast to CACS that is often associated with atherosclerotic plaque on coronary arteries.
Aortic Calcification, Exercise Blood Pressure, and Adverse Outcomes
In the present study, heavy aortic calcification (eg, 4th quartile of TACS) was related with composite of adverse outcomes, including all‐cause death, stroke, heart failure, and ischemic heart disease during median follow‐up of 65 months, although the studied population constituted individuals without significant CAD at study entrance. Interestingly, stroke was the most frequent adverse event of all endpoints. It has been reported that descending thoracic aorta calcification was associated with incidence of stroke, and it has been proposed that thoracic aortic calcification is a surrogate marker for generalized atherosclerosis whereby plaques in the aorta itself might be a potential stroke origin.29
In addition, we may add that increased SBP might further provoke stroke and adverse events in individuals with a heavy calcium deposit. In a population‐based study, Kurl et al.4 reported an increased risk of stroke in subjects with increased SBP, and suggested that an increase in the risk of endothelial injury from high exercise SBP, as well as underlying arteriosclerotic disease or structural vascular changes, may reflect some of the plausible mechanisms involved. The association between ΔSBP and aortic calcification was similarly demonstrated during both submaximal and maximal exercise. Yet, ΔSBP and ΔPP during exercise did not predict clinical outcomes in the current study population. This discrepancy might be, in part, attributed to the fact that the population examined in this study was elderly, and most patients were only capable of completing the exercise test at a low‐intensity workload. Further still, most of the enrolled population was prescribed antihypertensive medication, including BB and CCB, which could have also altered the SBP response to exercise.
Although not statistically significant in multivariate analysis, TACS was associated with incidence of heart failure admission during follow‐up in univariate analysis—an observation that perhaps warrants further study, given that heart failure has been reported to be associated with arterial stiffening in the elderly.30 Albeit, because our data did not include the measurement of arterial stiffening, further larger studies are clearly required to assess the association, if any, between aortic calcification, arterial stiffening, and incidence of heart failure. However, we might speculate that aortic calcification represents not only atherosclerotic burden, but also resultant vascular structural changes, which can cause stiffening of the aorta, a rise in exercise SBP, and may exacerbate poor clinical outcomes, such as heart failure and stoke. The role of anticalcification strategies as a novel therapeutic approach for exaggerated SBP response and heavy aortic calcification may warrant further investigation.
CT as a Screening Test Using Aortic Calcium Scoring
In current practice, the use of noncontrast CT of the chest is rapidly emerging as a screening tool for identifying asymptomatic patients at high risk of CAD, as well as for lung cancer screening.31 Coronary CT angiography is gaining further consideration as a gatekeeper or useful alternative for invasive coronary angiography.32 Although information regarding thoracic aortic calcification can also be assessed from CT of the thoracic area, it does not currently have a role in clinical decision making. Notably, it has been previously reported that TACS does not further improve prediction of events over and above CACS.33 Albeit, the current study findings indicate that the measurement of TACS, rather than CACS, might reflect a novel surrogate for detection of vulnerable aortas that may provoke a raised SBP during exercise and possibly lead to poor clinical outcomes in elderly individuals other than obstructive CAD. Therefore, calculation of TACS in addition to coronary CT angiography, and not CACS, might prove more beneficial for screening populations who are at high risk for future cardiovascular disease, especially among elderly individuals without obstructive CAD. Though clearly, further studies are needed to test this speculation.
Study Limitations
The current study findings were based on a retrospective analysis, which may have inferred some biases. Despite this, we carefully reviewed all medical records, along with CT images, in an effort to avoid any of the possible biases that may have arisen. It remains uncertain precisely how much of the calcification detected in the aorta reflects calcified atherosclerotic plaque or nonatherosclerotic plaque, given that intimal calcification cannot be differentiated from medial calcification through CT scanning. Also, considering that our findings are based upon an observational cohort of elderly patients (≥65 years) without obstructive CAD, these observations may not be generalizable to other populations, and therefore our results should be interpreted with caution. Considering ΔSBP was not statistically different between individuals with and without an event in the current study, it is not clear whether a rise in exercise SBP mainly mediated poor clinical outcomes in the background of high TACS. However, one of the possibilities why TACS displayed better prediction of outcomes than ΔSBP would be attributed to the fact that there was relatively more variance in ΔSBP than in TACS measurement. Arterial stiffness was not measured, and therefore further studies with concurrent measures of arterial stiffness, aortic calcification, and exercise blood pressure are warranted to support our hypothesis. In addition, forthcoming studies with larger populations are required, given that we examined multiple endpoints, which can cause numerous issues attributed to multiple testing, and may heighten the probability of a type 1 error. Given that fitness is one of the most important factors for influencing SBP change during exercise, it would also be beneficial to perform the latter analysis while controlling for physical fitness, although because of the retrospective nature of the current study, it was beyond the scope of our investigation to obtain additional data regarding patients’ fitness. Despite this, resting heart rate is a well‐known surrogate of cardiorespiratory fitness and has been utilized in several previous studies.34, 35 Moreover, TACS demonstrated an independent association with resting and exercise SBP, even after controlling for resting heart rate. Hence, we speculate that presence of an association between TACS and SBP might persist even after adjusting for physical fitness. Though clearly, additional studies with better‐defined measures of physical fitness are needed to reinforce this contention.
Conclusion
In this study, presence of aortic calcification was related to elevated SBP during exercise and was a robust predictor of clinical outcomes in elderly individuals without obstructive CAD. Though additional studies are needed to confirm these associations, measurement of TACS from coronary CT scanning may prove useful for predicting exercise SBP response and subsequent adverse clinical outcomes, especially stroke, at least in elderly individuals without obstructive CAD.
Sources of Funding
This research was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (MSIP; No. 2012027176).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003131 doi: 10.1161/JAHA.115.003131)
References
- 1. McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34:754–759. [DOI] [PubMed] [Google Scholar]
- 2. Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high‐normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high‐normal blood pressure. J Am Coll Cardiol. 2000;36:1626–1631. [DOI] [PubMed] [Google Scholar]
- 3. Laukkanen JA, Kurl S, Salonen R, Lakka TA, Rauramaa R, Salonen JT. Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle‐aged men. Hypertension. 2004;44:820–825. [DOI] [PubMed] [Google Scholar]
- 4. Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041. [DOI] [PubMed] [Google Scholar]
- 5. Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise‐induced systemic hypertension in healthy subjects. Am J Cardiol. 1999;83:371–375. [DOI] [PubMed] [Google Scholar]
- 6. Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, DeRegis JR, Shapiro EP, Ouyang P. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320. [DOI] [PubMed] [Google Scholar]
- 7. Fossum E, Hoieggen A, Moan A, Rostrup M, Kjeldsen SE. Insulin sensitivity is related to physical fitness and exercise blood pressure to structural vascular properties in young men. Hypertension. 1999;33:781–786. [DOI] [PubMed] [Google Scholar]
- 8. Vacanti LJ, Negrao CE, Sposito AC. Hypercholesterolaemia is associated with an exaggerated elevation in blood pressure during exercise in very elderly subjects. Age Ageing. 2005;34:182–184. [DOI] [PubMed] [Google Scholar]
- 9. Mancini GB, Dahlof B, Diez J. Surrogate markers for cardiovascular disease: structural markers. Circulation. 2004;109:IV22–IV30. [DOI] [PubMed] [Google Scholar]
- 10. Tsiachris D, Tsioufis C, Dimitriadis K, Kokkinos P, Faselis C, Tousoulis D, Michaelides A, Papademetriou V, Stefanadis C. Relationship of ambulatory arterial stiffness index with blood pressure response to exercise in the early stages of hypertension. Blood Press Monit. 2010;15:132–138. [DOI] [PubMed] [Google Scholar]
- 11. Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O'Donnell CJ, Mitchell GF, Vasan RS. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–682. [DOI] [PubMed] [Google Scholar]
- 13. McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki‐Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. [DOI] [PubMed] [Google Scholar]
- 14. Eisen A, Tenenbaum A, Koren‐Morag N, Tanne D, Shemesh J, Imazio M, Fisman EZ, Motro M, Schwammenthal E, Adler Y. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–1334. [DOI] [PubMed] [Google Scholar]
- 15. Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y. Presence of abdominal aortic calcification is significantly associated with all‐cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–425. [DOI] [PubMed] [Google Scholar]
- 16. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end‐stage renal disease. Hypertension. 2001;38:938–942. [DOI] [PubMed] [Google Scholar]
- 17. Rodondi N, Taylor BC, Bauer DC, Lui LY, Vogt MT, Fink HA, Browner WS, Cummings SR, Ensrud KE. Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med. 2007;261:238–244. [DOI] [PubMed] [Google Scholar]
- 18. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. [DOI] [PubMed] [Google Scholar]
- 19. Budoff MJ, Achenbach S, Fayad Z, Berman DS, Poon M, Taylor AJ, Uretsky BF, Williams KA. Task Force 12: training in advanced cardiovascular imaging (computed tomography): endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Atherosclerosis Imaging and Prevention, and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2006;47:915–920. [DOI] [PubMed] [Google Scholar]
- 20. Cho IJ, Chang HJ, Park HB, Heo R, Shin S, Shim CY, Hong GR, Chung N. Aortic calcification is associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction in elderly male patients with hypertension. J Hypertens. 2015;21:21. [DOI] [PubMed] [Google Scholar]
- 21. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40:1531–1540. [DOI] [PubMed] [Google Scholar]
- 22. Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD Jr, Winters WL, Yanowitz FG, Ritchie JL, Gibbons RJ, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Lewis RP, O'Rourke RA, Ryan TJ. ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 1997;30:260–311. [DOI] [PubMed] [Google Scholar]
- 23. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. [DOI] [PubMed] [Google Scholar]
- 24. Cho IJ, Chang HJ, Park HB, Heo R, Shin S, Shim CY, Hong GR, Chung N. Aortic calcification is associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction in elderly male patients with hypertension. J Hypertens. 2015;33:1633–1641. [DOI] [PubMed] [Google Scholar]
- 25. Franklin SS, Gustin W IV, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 26. McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 27. Post W, Bielak LF, Ryan KA, Cheng YC, Shen H, Rumberger JA, Sheedy PF II, Shuldiner AR, Peyser PA, Mitchell BD. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation. 2007;115:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. [DOI] [PubMed] [Google Scholar]
- 29. Hermann DM, Lehmann N, Gronewold J, Bauer M, Mahabadi AA, Weimar C, Berger K, Moebus S, Jockel KH, Erbel R, Kalsch H. Thoracic aortic calcification is associated with incident stroke in the general population in addition to established risk factors. Eur Heart J Cardiovasc Imaging. 2015;16:684–690. [DOI] [PubMed] [Google Scholar]
- 30. Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. [DOI] [PubMed] [Google Scholar]
- 31. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC Jr. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. [DOI] [PubMed] [Google Scholar]
- 32. Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, Govas N, Galbraith PD, Dennie C, Beanlands RS. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging. 2009;2:16–23. [DOI] [PubMed] [Google Scholar]
- 33. Wong ND, Gransar H, Shaw L, Polk D, Moon JH, Miranda‐Peats R, Hayes SW, Thomson LE, Rozanski A, Friedman JD, Berman DS. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:319–326. [DOI] [PubMed] [Google Scholar]
- 34. Black A, Murray L, Cardwell C, Smith GD, McCarron P. Secular trends in heart rate in young adults, 1949 to 2004: analyses of cross sectional studies. Heart. 2006;92:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jurca R, Jackson AS, LaMonte MJ, Morrow JR Jr, Blair SN, Wareham NJ, Haskell WL, van Mechelen W, Church TS, Jakicic JM, Laukkanen R. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med. 2005;29:185–193. [DOI] [PubMed] [Google Scholar]


