Abstract
Background
Industry‐sponsored clinical trials produce high‐quality data sets that can be used by researchers to generate new knowledge. We assessed the availability of individual participant‐level data (IPD) from large cardiovascular trials conducted by major pharmaceutical companies and compiled a list of available trials.
Methods and Results
We identified all randomized cardiovascular interventional trials registered on ClinicalTrials.gov with >5000 enrollment, sponsored by 1 of the top 20 pharmaceutical companies by 2014 global sales. Availability of IPD for each trial was ascertained by searching each company's website/data‐sharing portal. If availability could not be determined, each company was contacted electronically. Of 60 included trials, IPD are available for 15 trials (25%) consisting of 204 452 patients. IPD are unavailable for 15 trials (25%). Reasons for unavailability were: cosponsor did not agree to make IPD available (4 trials) and trials were not conducted within a specific time (5 trials); for the remaining 6 trials, no specific reason was provided. For 30 trials (50%), availability of IPD could not be definitively determined either because of no response or requirements for a full proposal (23 trials).
Conclusions
IPD from 1 in 4 large cardiovascular trials conducted by major pharmaceutical companies are confirmed available to researchers for secondary research, a valuable opportunity to enhance science. However, IPD from 1 in 4 trials are not available, and data availability could not be definitively determined for half of our sample. For several of these trials, companies require a full proposal to determine availability, making use of the IPD by researchers less certain.
Keywords: data sharing, trials
Subject Categories: Quality and Outcomes, Ethics and Policy
Introduction
Clinical trials are considered the gold standard for evaluating the safety and efficacy of medical therapeutics. The field of cardiovascular disease, in particular, has seen several clinical trials over the years, several of which have been industry sponsored, which inform day‐to‐day practice. The number, size, and quality of these trials have immensely increased over the past 2 decades. Although specifically designed to compare 2 or more interventions, clinical trials also generate high‐quality data that can be subsequently used by investigators to generate new scientific knowledge.1, 2, 3 Furthermore, using these existing trial data sets maximizes the return on the investments of trial participants, investigators, and sponsors.4, 5, 6
Several pharmaceutical and medical device companies are making efforts to share individual participant‐level data (IPD) for research purposes7, 8, 9, 10, 11, 12, 13; however, the actual availability of these data is unknown. The aim of this study was to assess the availability of IPD from large cardiovascular interventional trials conducted by major pharmaceutical companies to external investigators for research. We focused on cardiovascular outcomes trials that enrolled 5000 patients or more and were sponsored by 1 of the 20 largest pharmaceutical companies for 3 reasons: (1) Large trials are among the trials most likely to be practice changing and yield critical insights for medicine, so interest in IPD for purposes of validation and replication research is likely to be high; (2) large trials are among the trials most likely to offer ample opportunity for subanalyses and secondary research questions, so interest in IPD for purposes of secondary research is likely to be high; and (3) the largest pharmaceutical companies both abide by the principles of Pharmaceutical Research and Manufacturers of America (PhRMA), the pharmaceutical manufacturers trade organization that adopted principles for responsible data sharing in July 2013 for all of its member organizations, and are more likely to have the resources necessary to have established mechanisms for responsible data sharing.14 Our purpose was to compile a list of these trials, with the intent to spur interest in their use among the cardiology research community and, in the process, provide insights into the extent to which different companies have made their trial data available and the processes to access these data.
Methods
Study Design and Sample
ClinicalTrials.gov, created as a result of the US Food and Drug Administration Modernization Act of 1997, is a registry of clinical trials information for both federally and privately funded trials. We conducted a search of ClinicalTrials.gov on January 9, 2015 to identify all registered large, randomized, interventional clinical trials, defined as enrollment >5000 patients, that were focused on cardiovascular diseases—defined as all trials within the domain of cardiology as well as diabetes mellitus, obesity, and venous thromboembolism/pulmonary embolism—for which the primary sponsor and responsible party (as identified on ClinicalTrials.gov) was a pharmaceutical company listed in the top 20 pharmaceutical companies by 2014 global sales.15 All trials with a status of “completed,” “terminated,” and “active, not recruiting” were included (for trials that were active, not recruiting, primary results had to have been published).
In March to April 2015, each sponsoring company's website was searched to identify availability of IPD to external investigators. If trial availability could not be determined online, we contacted the sponsoring company electronically. Some pharmaceutical companies provide access to clinical trial data through online platforms, such as ClinicalStudyDataRequest.com16 hosted by Ideapoint Inc. and the Yale University Open Data Access (YODA) Project17 hosted by Yale University (all authors are affiliated with the YODA Project). These portals serve to route data requests to independent review panels that determine access to data. For companies participating in such platforms, contact was made through the online submission form on the portal. One of the companies participating in ClinicalStudyDataRequest.com did not have a mechanism at the start of the study for submitting an inquiry regarding available data, but it was subsequently updated and the request for data availability for this company was submitted on September 27, 2015. The remaining companies were contacted by a standardized e‐mail (Data S1) sent to the address listed as their contact information on their website (Table 1). If an e‐mail was not available, we used the phone number listed. All requests specified the National Clinical Trial (NCT) number of the trial(s) in question and inquired about the availability of IPD from the clinical trial to external investigators for research purposes. Nonrespondents and partial respondents (those for which we received availability information for some trials conducted by the pharmaceutical company but not all) received a follow‐up request 8 weeks after initial contact.
Table 1.
Availability of Individual Participant‐Level Data for Trials Sponsored by the Top 20 Pharmaceutical Companies
| Company | No. Trials That Fit Criteria | No. Trials Available | No. Trials Not Available | No. Trials With Status Unknown | Comments | Contact Information |
|---|---|---|---|---|---|---|
| AbbVie | 1 | 1 | 0 | 0 | Trial available |
URL: http://www.abbvie.com/research-innovation/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-investigators.html
Email: accesstodata@abbvie.com |
| AstraZeneca | 8 | 0 | 0 | 8 | Full proposal required |
URL: www.astrazenecaclinicaltrials.com
Email: ClinicalTrialTransparency@AstraZeneca.com |
| Bayer | 1 | 0 | 1 | 0 | Trial not available; completed before January 2014 | URL: https://clinicalstudydatarequest.com/ |
| Boehringer Ingelheim | 5 | 4 | 0 | 1 | Some trials available | URL: https://clinicalstudydatarequest.com/ |
| Bristol‐Myers Squibb | 6 | 0 | 6 | 0 | Trials not available; no specific reason provided |
URL: http://bmsctdt-public.steeprockinc.com/
Email: clinical.trials@bms.com; drug.information@bms.com |
| Eli Lilly | 3 | 1 | 1 | 1 | Some trials available; collaborator unwilling to share IPD | URL: https://clinicalstudydatarequest.com/ |
| Gilead | 1 | 0 | 0 | 1 | Unknown | URL: http://www.gilead.com/research/clinical-trials (only phone number listed) |
| GlaxoSmithKline | 4 | 4 | 0 | 0 | Trials available | URL: https://clinicalstudydatarequest.com/ |
| Johnson & Johnson | 2 | 0 | 2 | 0 | Trial not available; collaborator unwilling to share IPD | URL: http://yoda.yale.edu/how-request-data |
| Merck | 5 | 4 | 0 | 1 | Some trials available; full proposal required | URL: http://engagezone.merck.com/ds_documentation.php |
| Novartis | 4 | 0 | 0 | 4 | Unknown | URL: https://clinicalstudydatarequest.com/ |
| Novo Nordisk | 1 | 0 | 0 | 1 | Full proposal required |
URL: http://www.novonordisk-trials.com/website/content/how-to-access-clinical-trial-datasets.aspx
Email: irb-secretariat@novonordisk.com; clinicaltrials@novonordisk.com |
| Pfizer | 3 | 0 | 3 | 0 | Trials not available; completed before September 2007 |
URL: https://iirsubmission.pfizer.com
Email: IIR@Pfizer.com; CTD@Pfizer.com |
| Roche | 2 | 0 | 2 | 0 | Trial not available; product was developed in collaboration with another partner and trial was completed less than 18 months ago | URL: https://clinicalstudydatarequest.com/ |
| Sanofi | 13 | 0 | 0 | 13 | Full proposal required | URL: https://clinicalstudydatarequest.com/ |
| Takeda | 1 | 1 | 0 | 0 | Trial available | URL: https://clinicalstudydatarequest.com/ |
| Total | 60 | 15 | 15 | 30 |
IPD indicates individual participant‐level data.
Statistical Analysis
We categorized the trials by availability into 3 categories—available, unavailable, and unknown (for the trials for which availability could not be definitively determined). We reported the categories of availability of trials by proportion for the overall sample and raw numbers for each pharmaceutical company. The process to access IPD was cataloged for trials for which the status was available. Stated reasons for trial unavailability were also reported. The Yale Human Investigation Committee deemed this study exempt from needing institutional review board approval.
Results
We identified 60 trials on ClinicalTrials.gov that met our criteria, which were funded by 16 of the top 20 pharmaceutical companies by 2014 global sales. All the trials in our sample were drug trials given that no device trials met our inclusion criteria. Of these 16 companies, 8 participate in ClinicalStudyDataRequest.com and 1 in the YODA Project. Fourteen of these sixteen companies have made commitments to the Principles for Responsible Clinical Trials Data Sharing set forth by the PhRMA as of November 30, 2015.14 The “year of completion” of the trials that met our criteria was distributed from 2001 to 2014. A histogram of trials by year of completion is shown in Figure.
Figure 1.
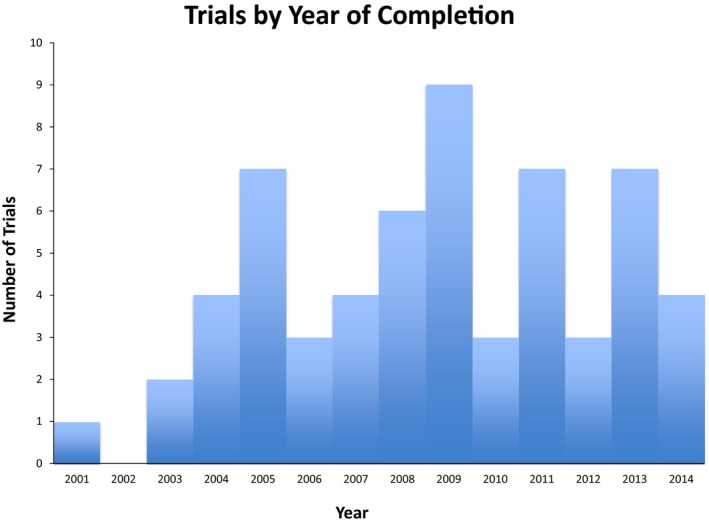
Trials by year of completion. A histogram of the 60 trials that met our search criteria on ClinicalTrials.gov by year of completion.
By searching ClinicalStudyDataRequest.com and individual company websites, IPD from 9 trials (15%) were confirmed to be available. Requests for IPD from the remaining 51 trials were submitted to the corresponding companies. Of these, IPD from 6 (10%) were confirmed to be available. In total, 15 trials (25%) from 6 different companies were available, consisting of 204 452 patients with conditions such as coronary artery disease, acute coronary syndromes, atrial fibrillation, stroke, and obesity (Table 1). A summary of the available trials is shown in Table 2.
Table 2.
Cardiovascular Trials With Confirmed Availability of Individual Participant‐Level Data
| NCT No. | Study Title | Condition | Intervention(s) | Enrollment | Completion Year | Sponsor | Where to Request |
|---|---|---|---|---|---|---|---|
| NCT00234832 | A Long Term Study of Sibutramine and the Role of Obesity Management in Relation to Cardiovascular Disease in Overweight and Obese Patients (SCOUT) | Obesity |
Drug: Sibutramine Drug: Placebo |
10 777 | 2009 | AbbVie | E‐mail: accesstodata@abbvie.com |
| NCT00153062 | PRoFESS—Prevention Regimen For Effectively Avoiding Second Strokes | Stroke |
Drug: Aggrenox Drug: Clopidogrel placebo Drug: Micardis Drug: Aggrenox placebo Drug: Clopidogrel Drug: Micardis placebo |
20 332 | 2008 | Boehringer Ingelheim | Submit request by https://clinicalstudydatarequest.com/ |
| NCT00262600 | Randomized Evaluation of Long Term Anticoagulant Therapy (RE‐LY) With Dabigatran Etexilate | Atrial Fibrillation |
Drug: Warfarin Drug: Dabigatran dose 1 Drug: Dabigatran dose 2 |
18 113 | 2009 | Boehringer Ingelheim | Submit request via https://clinicalstudydatarequest.com/ |
| NCT00808067 | RELY‐ABLE Long Term Multi‐center Extension of Dabigatran Treatment in Patients With Atrial Fibrillation Who Completed RE‐LY Trial | Atrial Fibrillation |
Drug: Dabigatran dose 1 Drug: Dabigatran dose 2 |
5897 | 2012 | Boehringer Ingelheim | Submit request by https://clinicalstudydatarequest.com/ |
| NCT02181985 | Full Dose Tenecteplase (TNK‐tPA) Together With Heparin Sodium, Full Dose Tenecteplase With Enoxaparin, Half Dose Tenecteplase Together With Abciximab and Heparin Sodium in Patients With Acute Myocardial Infarction (AMI) (ASSENT 3) | ST‐segment elevation Myocardial Infarction |
Drug: Full dose TNK‐tPA Drug: Half dose TNK‐tPA Drug: Heparin Drug: Enoxaparin Drug: Abciximab |
5989 | 2001 | Boehringer Ingelheim | Submit request by https://clinicalstudydatarequest.com/ |
| NCT00190593 | Raloxifene Use for The Heart | Cardiovascular Diseases, Breast Neoplasms |
Drug: Raloxifene Drug: Placebo |
10 000 | 2005 | Eli Lilly | Submit request by https://clinicalstudydatarequest.com/ |
| NCT00064428 | MICHELANGELO OASIS‐6: Fondaparinux in ST Elevation Myocardial Infarction | ST‐segment Elevation Myocardial Infarction |
Drug: Fondaparinux—UFH indicated Other: Control—UFH not indicated Drug: Fondaparinux—UFH not indicated Drug: Control—UFH |
12 092 | 2006 | GlaxoSmithKline | Submit request by https://clinicalstudydatarequest.com/ |
| NCT00139815 | Michelangelo—Oasis 5 | Non ST‐Segment Elevation Acute Coronary Syndrome |
Drug: Fondaparinux Drug: Enoxaparin |
20 078 | 2005 | GlaxoSmithKline | Submit request by https://clinicalstudydatarequest.com/ |
| NCT00799903 | STABILITY: The Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy Trial | Coronary Artery Disease |
Drug: Darapladib Drug: Placebo |
15 828 | 2013 | GlaxoSmithKline | Submit request by https://clinicalstudydatarequest.com/ |
| NCT01000727 | SOLID‐TIMI 52: The Stabilization Of pLaques usIng Darapladib‐Thrombolysis In Myocardial Infarction 52 Trial | Acute Coronary Syndrome |
Drug: Darapladib Drug: Placebo |
13 026 | 2014 | GlaxoSmithKline | Submit request by https://clinicalstudydatarequest.com/ |
| NCT00089895 | EARLY ACS: Early Glycoprotein IIb/IIIa Inhibition in Patients With Non‐ST‐segment Elevation Acute Coronary Syndrome | Non‐ST Segment Elevation Acute Coronary Syndrome |
Drug: Eptifibatide (Integrilin) Drug: Placebo |
9406 | 2008 | Merck | Submit request by http://engagezone.merck.com/ds_documentation.php |
| NCT00202878 | IMPROVE‐IT: Examining Outcomes in Subjects With Acute Coronary Syndrome: Vytorin (Ezetimibe/Simvastatin) vs Simvastatin (P04103) | Acute Coronary Syndrome |
Drug: Ezetimibe/simvastatin Drug: simvastatin |
18 141 | 2014 | Merck | Submit request by http://engagezone.merck.com/ds_documentation.php |
| NCT00526474 | Trial to Assess the Effects of Vorapaxar (SCH 530348; MK‐5348) in Preventing Heart Attack and Stroke in Patients With Atherosclerosis (TRA 2°P—TIMI 50) (P04737) | Atherosclerosis |
Drug: Vorapaxar Drug: Placebo |
26 449 | 2011 | Merck | Submit request by http://engagezone.merck.com/ds_documentation.php |
| NCT00527943 | Trial to Assess the Effects of Vorapaxar (SCH 530348; MK‐5348) in Preventing Heart Attack and Stroke in Particpants With Acute Coronary Syndrome (TRA•CER) (Study P04736) | Non‐ST Segment Elevation Acute Coronary Syndrome |
Drug: Vorapaxar Drug: Placebo |
12 944 | 2011 | Merck | Submit request by http://engagezone.merck.com/ds_documentation.php |
| NCT00968708 | Cardiovascular Outcomes Study of Alogliptin in Patients With Type 2 Diabetes and Acute Coronary Syndrome (EXAMINE) | Diabetes Mellitus Type 2, Acute Coronary Syndrome |
Drug: Alogliptin Drug: Placebo |
5380 | 2013 | Takeda | Submit request by https://clinicalstudydatarequest.com/ |
NCT indicates National Clinical Trial; UFH, unfractionated heparin.
Fifteen trials (25%) with 160 122 patients were confirmed to be unavailable (Table 1). Reasons for trials to be unavailable included whether the trial was completed within a specified time period (5 trials sponsored by 3 companies; less than 18 months ago [1 trial], before January 1, 2014 [1 trial], before September 2007 [3 trials]), or the product was developed in collaboration with another pharmaceutical manufacturer, or partner, who has not agreed to share IPD (4 trials). One company that had funded 6 trials did not provide a reason as to why the data were unavailable.
For 30 trials (50%), with 338 424 patients availability of IPD could not be definitively determined (Table 1). Four companies that sponsored a total of 23 (38%) of the trials included in our sample required a full research proposal to be submitted before determining availability of the data to an external researcher. Some of the companies that did not respond, or who require a full research proposal before making a determination, list criteria for available trial data on their website. Based on these criteria, we estimate that IPD from 7 more clinical trials may be available; however, the sponsoring company did not confirm their availability.
Discussion
In our study of 60 large interventional cardiovascular clinical trials sponsored by the 20 largest pharmaceutical companies, we found that IPD from 15 trials, or one quarter, are available to external researchers upon request. These data are a valuable resource for enhancing scientific study, and their availability advances the research enterprise. However, notably, despite apparent openness to data sharing, the actual availability of IPD is low. IPD for 15 other trials in our study, one quarter of our sample, are not available, whereas the availability of IPD to external researchers could not be definitively determined for half of our sample; for several trials, the companies required a full proposal before determining availability of the data, making use of the IPD by researchers less certain.
Clinical trial data are the highest quality data that the research enterprise generates. The past decade has seen increasing societal pressure to make clinical trial data available, both to enhance transparency and accelerate the generation of new knowledge. The wheels have now turned beyond whether to share data, to establishing robust mechanisms to share data. The adoption of common data sharing principles by PhRMA and the European Federation of Pharmaceutical Industries and Associations (EFPIA),18 and the recent Institute of Medicine report, which provides guidance on sharing data,19 have been seminal events in this journey.
We were able to confirm that IPD from 15 large industry‐sponsored clinical trials were available to external researchers upon request. These 15 trials together consist of over 200 000 patients with varied clinical parameters and outcomes. The patient populations in these trials are varied and include coronary artery disease, acute coronary syndromes, atrial fibrillation, stroke, and obesity. The ready availability of these databases presents a tremendous opportunity for researchers, including young investigators such as residents and postdoctoral researchers, to make use of these rich data sets in their projects.
On the other hand, one quarter of the trials were confirmed by the companies to be unavailable, and for half of the trials availability could not be definitively ascertained. Some common reasons for unavailability were encountered. Some companies are only sharing data for trials that were completed after January 1, 2014, which coincides with the date of implementation of the commitments to the Principles for Responsible Clinical Trial Data Sharing of PhRMA and EFPIA member companies (18). On the other hand, some companies reported that they were unable to share data for recently concluded trials (<18 months), which indicates that curating the data and establishing systems for sharing data with external researchers may be a protracted process with room for improvement. Also, some trials that were conducted in collaboration with another pharmaceutical manufacturer or partner (although only 1 company was the primary sponsor and responsible party listed on ClinicalTrials.gov) were unavailable because the partner entity had not agreed to share data. This suggests that for such collaborative trials, data‐sharing policies need to be discussed by all partnering organizations and codified at the blueprint stage of the trial.
Of the trials for which availability could not be definitively determined (Table 1), some companies requested a full research proposal before determining availability of IPD for their trials. Although it is reasonable that a full proposal needs to be reviewed before releasing data, the requirements for a full proposal before knowing that the data are available can act as a potential barrier to accessing these data sets. Given that developing and drafting an entire research proposal, as any researcher would agree, is a major endeavor, applicants are more likely to draft a full proposal when they are certain that the data are indeed available. No response was received from 2 companies regarding the availability of IPD, which indicates that either the contact information is inaccurate or a streamlined process needs to be established in these companies to respond to communications from researchers.
Limitations
Our study was limited to large cardiovascular trials sponsored by the 20 largest pharmaceutical companies and thus is not designed to be representative of the extent of data sharing efforts from the companies for all their trials. Hence, Table 1 should not be seen as a report card of their overall data‐sharing efforts. Although we firmly believe that results from all clinical trials should be made available in a timely fashion, we focused this analysis on large cardiovascular trials sponsored by major pharmaceutical manufacturers in order to examine data‐sharing practices for well‐recognized, high‐impact clinical trials sponsored by manufacturers that abide by PhRMA's principles for responsible data sharing.14 Furthermore, our efforts to determine IPD availability for trials were limited to an online search, online request through designated portals, and contact by e‐mail. One could argue that our method of determining availability of IPD could have been more robust and some of the “unknown” trials may indeed be available, but our inability to verify their availability through the above means is representative of an average researcher's efforts to obtain these data, and thus our results constitute a pragmatic assessment of trial availability.
Conclusions
Despite the positive momentum in recent years in regard to data sharing by industry, IPD from only 1 in 4 large cardiovascular trials included in this study could be confirmed to be available for request by external researchers, and data from 1 in 4 trials are confirmed unavailable. In addition, for several trials, companies require a full proposal before confirming data availability, which poses a barrier to accessing these data. These findings highlight barriers that still exist to access IPD from industry‐sponsored trials. Nevertheless, the 15 trials that are available represent an ideal opportunity for further investigation and represent a contribution by these companies to the common scientific good.
Disclosures
Drs Desai, Ross, and Krumholz and Ms Ritchie receive research support from Janssen, the Pharmaceutical Companies of Johnson & Johnson, to develop methods to promote data sharing. Drs Desai, Ross, and Krumholz receive research support from the Centers for Medicare and Medicaid Services to develop and maintain hospital performance measures that are used for public reporting. Dr Ross is a member of a scientific advisory board for FAIR Health, Inc. Dr Krumholz chairs a scientific advisory board for United Healthcare. Dr Murugiah has nothing to disclose.
Supporting information
Data S1. Standard Data Request Email Sent to Companies.
Acknowledgments
The authors thank Tiffany Chang, MPH, for help in researching and identifying trial availability. Chang was paid for this work as a student employee at the Yale–New Haven Hospital Center for Outcomes Research and Evaluation.
(J Am Heart Assoc. 2016;5:e003307 doi: 10.1161/JAHA.116.003307)
References
- 1. Pena JM, Aspberg S, MacFadyen J, Glynn RJ, Solomon DH, Ridker PM. Statin therapy and risk of fracture: results from the JUPITER randomized clinical trial. JAMA Intern Med. 2015;175:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer DE, Hellkamp AS, Yuan Z, Lokhnygina Y, Patel MR, Piccini JP, Hankey GJ, Breithardt G, Halperin JL, Becker RC, Hacke W, Nessel CC, Mahaffey KW, Fox KA, Califf RM, Investigators RA. Alternative calculations of individual patient time in therapeutic range while taking warfarin: results from the ROCKET AF trial. J Am Heart Assoc. 2015;4:e001349 doi: 10.1161/JAHA.114.001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weimar C, Cotton D, Sha N, Sacco RL, Bath PM, Weber R, Diener HC. Discontinuation of antiplatelet study medication and risk of recurrent stroke and cardiovascular events: results from the PRoFESS study. Cerebrovasc Dis. 2013;35:538–543. [DOI] [PubMed] [Google Scholar]
- 4. Ross JS, Krumholz HM. Ushering in a new era of open science through data sharing: the wall must come down. JAMA. 2013;309:1355–1356. [DOI] [PubMed] [Google Scholar]
- 5. Krumholz HM, Peterson ED. Open access to clinical trials data. JAMA. 2014;312:1002–1003. [DOI] [PubMed] [Google Scholar]
- 6. Ross JS, Lehman R, Gross CP. The importance of clinical trial data sharing: toward more open science. Circ Cardiovasc Qual Outcomes. 2012;5:238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bayer healthcare extends access to clinical trial data. 2014. Leverkusen, Germany: Bayer News, [Google Scholar]
- 8. Boehringer Ingelheim will make detailed clinical trial data available to the scientific community. 2014. Ingelheim, Germany: Boehringer Ingelhein Press Release. [Google Scholar]
- 9. Bristol‐Myers Squibb expands access to clinical trial data through collaboration with academic research institute. 2014. New York: Bristol‐Myers Squibb Press Releases. [Google Scholar]
- 10. GSK gives update on plans to share detailed clinical trial data as part of its commitment to transparency. 2013. London, UK: GlaxoSmithKline Press Release. [Google Scholar]
- 11. Johnson & Johnson announces clinical trial data sharing agreement with Yale School of Medicine. 2014. New Brunswick: Johnson & Johnson New Releases. [Google Scholar]
- 12. Lilly announces increased access to clinical trials data for qualified researchers. 2014. Indianapolis: Lilly Newsroom. [Google Scholar]
- 13. Krumholz HM, Gross CP, Blount KL, Ritchie JD, Hodshon B, Lehman R, Ross JS. Sea change in open science and data sharing: leadership by industry. Circ Cardiovasc Qual Outcomes. 2014;7:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pharmaceutical Research and Manufacturers of America (PhRMA) . Principles for responsible clinical trial data sharing—certifications. Washington, DC: Pharmaceutical Research and Manufacturers of America (PhRMA); 2015. [Google Scholar]
- 15. Top 25 pharma companies by global sales. PMLive. 2015.
- 16. ClinicalStudyDataRequest . Study sponsors. Ideapoint Inc. 2013.
- 17. The YODA Project . Submit inquiry. Yale University. 2014.
- 18. PhRMA and EFPIA . Principles for responsible clinical trial data sharing: our commitment to patients and researchers. 2013. Brussels; Washington, DC: European Federation of Pharmaceutical Industries and Associations (EFPIA) and the Pharmaceutical Research and Manufacturers of America (PhRMA). [Google Scholar]
- 19. Institute of Medicine . Sharing clinical trial data: maximizing benefits, minimizing risk. 2015. Washington, DC: Institute of Medicine. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Standard Data Request Email Sent to Companies.


