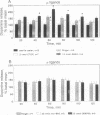
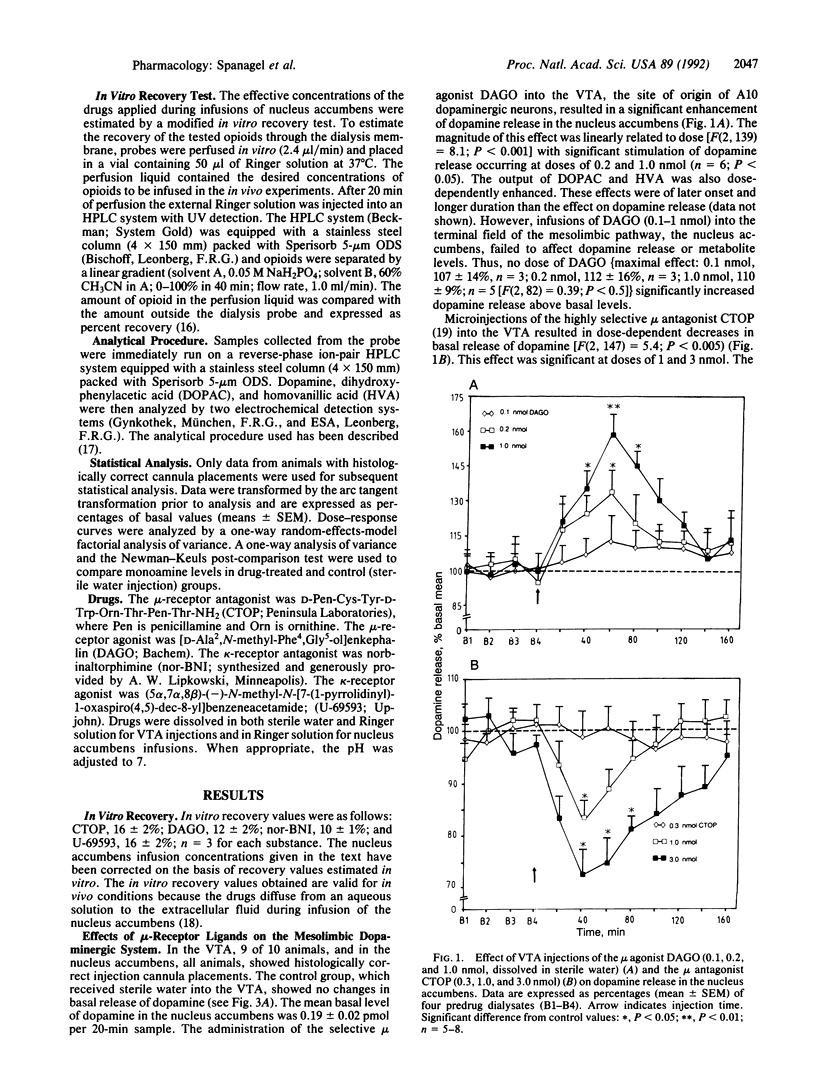
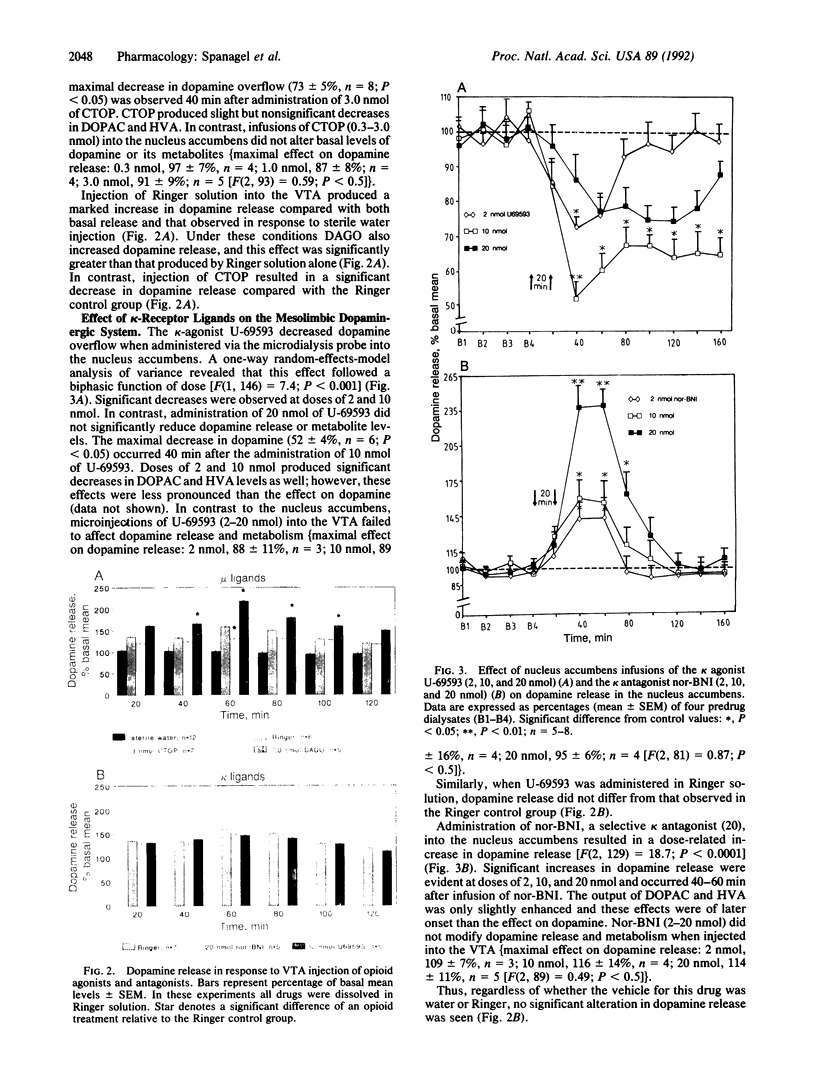
Abstract
The mesolimbic dopaminergic system has been implicated in mediating the motivational effects of opioids and other drugs of abuse. The site of action of opioids within this system and the role of endogenous opioid peptides in modulating dopamine activity therein remain unknown. Employing the technique of in vivo microdialysis and the administration of highly selective opioid ligands, the present study demonstrates the existence of tonically active and functionally opposing mu and kappa opioid systems that regulate dopamine release in the nucleus accumbens, the major terminal area of A10 dopaminergic neurons. Thus, stimulation of mu-type receptors in the ventral tegmental area, the site of origin of A10 dopaminergic neurons, increases dopamine release whereas the selective blockade of this opioid receptor type results in a significant decrease in basal dopamine release. In contrast, stimulation of kappa-type receptors within the nucleus accumbens decreases dopamine release whereas their selective blockade markedly increases basal dopamine release. These data show that tonic activation of mu and kappa receptors is required for the maintenance of basal dopamine release in the nucleus accumbens. In view of the postulated role of the mesolimbic system in the mediation of drug-induced alterations in mood and affect, such findings may have implications for the treatment of opiate dependence and affective disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acquas E., Carboni E., Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol. 1991 Jan 25;193(1):133–134. doi: 10.1016/0014-2999(91)90214-b. [DOI] [PubMed] [Google Scholar]
- Ahtee L., Attila L. M., Carlson K. R. Augmentation of morphine-induced changes in brain monoamine metabolism after chronic naltrexone treatment. J Pharmacol Exp Ther. 1990 Nov;255(2):803–808. [PubMed] [Google Scholar]
- Bals-Kubik R., Herz A., Shippenberg T. S. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98(2):203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Hüttemeier P. C. Microdialysis--theory and application. Prog Neurobiol. 1990;35(3):195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988 Mar;244(3):1067–1080. [PubMed] [Google Scholar]
- Dilts R. P., Kalivas P. W. Autoradiographic localization of delta opioid receptors within the mesocorticolimbic dopamine system using radioiodinated [2-D-penicillamine, 5-D-penicillamine]enkephalin (125I-DPDPE). Synapse. 1990;6(2):121–132. doi: 10.1002/syn.890060203. [DOI] [PubMed] [Google Scholar]
- French E. D., Ceci A. Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons. Neurosci Lett. 1990 Nov 13;119(2):159–162. doi: 10.1016/0304-3940(90)90823-r. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Opioid peptides endorphins in pituitary and brain. Science. 1976 Sep 17;193(4258):1081–1086. doi: 10.1126/science.959823. [DOI] [PubMed] [Google Scholar]
- Hakan R. L., Henriksen S. J. Opiate influences on nucleus accumbens neuronal electrophysiology: dopamine and non-dopamine mechanisms. J Neurosci. 1989 Oct;9(10):3538–3546. doi: 10.1523/JNEUROSCI.09-10-03538.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins K. N., Knapp R. J., Lui G. K., Gulya K., Kazmierski W., Wan Y. P., Pelton J. T., Hruby V. J., Yamamura H. I. [3H]-[H-D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2] ([3H]CTOP), a potent and highly selective peptide for mu opioid receptors in rat brain. J Pharmacol Exp Ther. 1989 Jan;248(1):73–80. [PubMed] [Google Scholar]
- Heijna M. H., Padt M., Hogenboom F., Portoghese P. S., Mulder A. H., Schoffelmeer A. N. Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol. 1990 Jun 8;181(3):267–278. doi: 10.1016/0014-2999(90)90088-n. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Duffy P., Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990 May;253(2):858–866. [PubMed] [Google Scholar]
- Kalivas P. W., Widerlöv E., Stanley D., Breese G., Prange A. J., Jr Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther. 1983 Oct;227(1):229–237. [PubMed] [Google Scholar]
- Kendrick K. M. Microdialysis measurement of in vivo neuropeptide release. J Neurosci Methods. 1990 Sep;34(1-3):35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988 Jul;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Millan M. J., Herz A. Antagonist-induced opioid receptor up-regulation. II. Regionally specific modulation of mu, delta and kappa binding sites in rat brain revealed by quantitative autoradiography. J Pharmacol Exp Ther. 1988 Nov;247(2):729–736. [PubMed] [Google Scholar]
- Mucha R. F., Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86(3):274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Mucha R. F., Millan M. J., Herz A. Aversive properties of naloxone in non-dependent (naive) rats may involve blockade of central beta-endorphin. Psychopharmacology (Berl) 1985;86(3):281–285. doi: 10.1007/BF00432214. [DOI] [PubMed] [Google Scholar]
- Osborne P. G., O'Connor W. T., Ungerstedt U. Effect of varying the ionic concentration of a microdialysis perfusate on basal striatal dopamine levels in awake rats. J Neurochem. 1991 Feb;56(2):452–456. doi: 10.1111/j.1471-4159.1991.tb08171.x. [DOI] [PubMed] [Google Scholar]
- Petit F., Hamon M., Fournie-Zaluski M. C., Roques B. P., Glowinski J. Further evidence for a role of delta-opiate receptors in the presynaptic regulation of newly synthesized dopamine release. Eur J Pharmacol. 1986 Jul 15;126(1-2):1–9. doi: 10.1016/0014-2999(86)90731-4. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S., Lipkowski A. W., Takemori A. E. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987 Mar 30;40(13):1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippenberg T. S. Identification of the opioid receptor types mediating beta-endorphin-induced alterations in dopamine release in the nucleus accumbens. Eur J Pharmacol. 1990 Nov 6;190(1-2):177–184. doi: 10.1016/0014-2999(90)94124-g. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippenberg T. S. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990 Nov;55(5):1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spyraki C., Fibiger H. C., Phillips A. G. Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology (Berl) 1983;79(2-3):278–283. doi: 10.1007/BF00427827. [DOI] [PubMed] [Google Scholar]
- Stinus L., Koob G. F., Ling N., Bloom F. E., Le Moal M. Locomotor activation induced by infusion of endorphins into the ventral tegmental area: evidence for opiate-dopamine interactions. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2323–2327. doi: 10.1073/pnas.77.4.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori A. E., Ho B. Y., Naeseth J. S., Portoghese P. S. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988 Jul;246(1):255–258. [PubMed] [Google Scholar]
- Watson S. J., Akil H., Richard C. W., 3rd, Barchas J. D. Evidence for two separate opiate peptide neuronal systems. Nature. 1978 Sep 21;275(5677):226–228. doi: 10.1038/275226a0. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Rompre P. P. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. M., Leander J. D. Selective opioid receptor agonists and antagonists: research tools and potential therapeutic agents. J Med Chem. 1990 Mar;33(3):895–902. doi: 10.1021/jm00165a002. [DOI] [PubMed] [Google Scholar]