Janus is the ancient Roman god of beginning and transition. He is depicted as having 2 faces that can look back to the past and also into the future. Lindner and colleagues first reported that hemodialysis patients have accelerated atherosclerosis and cautioned that cardiovascular mortality could be the major impediment to the long‐term survival of these patients.1 Indeed, it is now well recognized that patients with decreased glomerular filtration rate (GFR) are at a greater risk for incident myocardial infarction and death from coronary artery disease (CAD) compared with the general population. Emerging literature also suggest that underlying macrovascular disease could also contribute to progression of chronic kidney disease (CKD). Findings from the Atherosclerosis Risk in Communities (ARC) and the Cardiovascular Health Studies (CHS) showed those with a history of cardiovascular disease (CVD) are associated with a faster decline in estimated GFR (eGFR) compared with patients without such history.2 A retrospective cohort study from Canada reported that an interim cardiovascular event was associated with a 4‐ to 5‐fold higher relative risk of subsequent end‐stage renal disease (ESRD).3 The Reasons for Geographical and Racial Difference in Stroke (REGARDS) cohort study showed that patients with CAD have a high prevalence of CKD and these individuals are largely unaware of their kidney disease.4 Thus, there is a bidirectional relationship between CKD and cardiovascular disease (CVD) and the magnitude of the problem is underappreciated.
Growing opulence and urbanization has led to globalization of the epidemic of type 2 diabetes mellitus (T2DM). It is estimated that the number of people with diabetes will reach 300 million by 2025. Diabetes is the most common cause of ESRD in the United States and across the world, accounting for about 45% of new patients initiated on renal replacement therapy. Patients with T2DM have a higher risk of CV mortality than nondiabetic populations.5 It is well recognized that the presence of CKD greatly amplifies the CVD risk associated with T2DM.
In this issue of JAHA, Sabe and associates report that in diabetic patients with CKD and anemia, history of CAD is associated with progression to ESRD, in the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) study participants.6 The TREAT study randomized 4038 patients with diabetes, CKD, and anemia to darbepoetin α or placebo. The study results indicated that the routine use of erythropoietin‐stimulating agents in anemic patients with diabetes and CKD not on dialysis does not reduce renal and cardiovascular events. However, study participants with elevated levels of baseline troponin T and N‐terminal pro‐brain natriuretic peptide were independently associated with a higher risk of ESRD, suggesting that underlying CVD is a risk factor for CKD progression.6
In the present study, Sabe et al noted that those with CAD were less likely to have proteinuria, but the eGFR was not significantly different between those with and without CAD, which could not be explained by use of angiotensin‐converting enzyme inhibitor or angiotensin receptor blockers.7 Traditionally, diabetic nephropathy is described as a chronic progressive disorder that is characterized by microalbuminuria, followed by macroalbuminuria, and hypertension leading to progressive loss of eGFR resulting in ESRD. Recently, several epidemiological studies have shown that frequently T2DM patients with reduced eGFR have no proteinuria.8 The mechanism underlying the progressive GFR decline in nonalbuminuric diabetic nephropathy is not known, but ischemic vascular disease, cholesterol microemboli, interstitial fibrosis, and premature senescence of the diabetic kidney have been proposed as potential causes. It is possible that subjects with CAD also have intrarenal vascular diseases. However, in a study of patients with T2DM and CKD, intrarenal vascular resistance, studied by renal duplex scan, was not different in those with and without proteinuria.9 However, Doppler ultrasound may not be sensitive enough to detect intrarenal atherosclerotic changes.
Findings from observational studies have shown that those surviving an episode of acute kidney injury (AKI) are at risk for CKD. Patients with CAD have high risk for AKI because of advanced age, comorbidities, heart failure, radiocontrast exposure, medication use, and coronary artery bypass surgery. James et al observed a graded increase in risk of ESRD, which varied by the severity of AKI, among patients who underwent coronary angiography and developed AKI.10 In a nationwide cohort of patients who underwent coronary artery bypass grafting, even a small postoperative increase in serum creatinine was associated with an increase in the long‐term risk of ESRD.11 In this study, Sabe et al tested this possibility by including a time‐varying covariate for coronary revascularization in the model and did not find any significant impact on CKD progression, but this secondary analysis of TREAT cannot completely exclude the possibility that AKI could have contributed to the risk of ESRD in this population.7
Atherosclerosis is an indolent, fibroproliferative disease fueled by chronic inflammation. Immune cells dominate the atherosclerotic lesion and exhibit evidence of activation. Findings from the Chronic Renal Insufficiency Cohort study showed that plasma levels of pro‐inflammatory cytokines and positive acute‐phase proteins were higher in subjects with lower levels of kidney function.12 We showed that specific inflammatory biomarkers are associated with cardiac geometry and risk of atrial fibrillation in patients with CKD.13, 14 Potential causes of inflammation in CKD include chronic subclinical infections, volume overload, increased oxidative stress, sympathetic overactivity, poor nutrition, and vitamin D deficiency, which are also risk factors for CVD. Inflammation plays a critical role in the progression of CKD. A variety of cytokines, chemokines, and growth factors act in concert to create an imbalance in matrix formation and degradation, which leads to overall accumulation of extracellular matrix and eventually glomerulosclerosis and interstitial fibrosis. Future studies should examine whether attenuation of inflammation has a salutary effect on the progression of CKD and CVD.
Sabe et al also found that CAD was independently associated with death in TREAT study participants.7 Study of prevalent cohorts of Medicare enrollees from 1996 to 2000 showed that those with CKD are 5 to 10 times more likely to die before reaching ESRD than the non‐CKD group.15 In studies examining the risk of ESRD, death before ESRD prevents ESRD from occurring and is a competing event. The standard Cox model, which fails to adjust for competing risk of death, can overestimate the absolute risk. The present study does not account for death as a competing event in the analysis, which the authors acknowledge in the discussion.
The investigators noted that CAD was associated with death among TREAT study participants only when history of heart failure (HF) was excluded from the model. HF is an important and rapidly growing healthcare problem. It is estimated that about 50% of HF patients die at 4 years and 40% of admitted patients with HF are dead or readmitted within 1 year.16 Epidemiological studies indicate that CKD is present in 35% to 70% of HF patients and is a strong and independent predictor of death. The prevalence of reduced kidney function in patients with HF increases with age, HF severity, and presence of hypertension and diabetes. Other risk factors include anemia, low serum albumin, and uses of renin–angiotensin system inhibitors, aldosterone antagonists, and diuretics. It is possible that the mortality in patients with CAD is mediated through HF.
To summarize, this study highlights the interaction and interdependency of progression of CAD and CVD, and also presents heightened risk for death in patients with CKD. The cause for this close relationship between loss of kidney function and accelerated atherosclerosis could be attributable to a set of distinct as well as shared risk factors (Figure). Framingham score has poor accuracy in predicting incident CHD in patients with CKD, but intensive search for silent heart disease in CKD and incipient CKD in patients with CAD is not practical. It is important to include CKD patients in studies examining CVD to further understand the risk of progression and prognosis of these intertwined disease processes. Laboratory‐based and translational research will enable us to better understand the key factors and molecular pathways mediating the cross‐link between CKD and CVD, and thus lead to target specific interventions.
Figure 1.
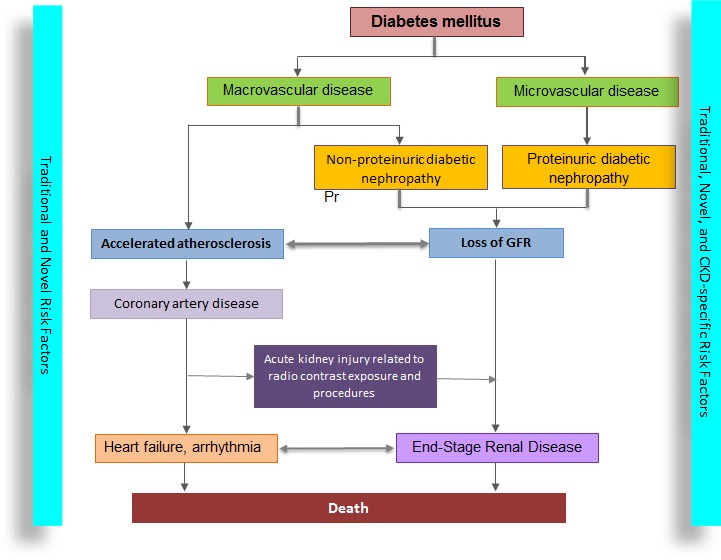
Microvascular and macrovascular diseases in patients with diabetes lead to loss of kidney function through proteinuric and nonproteinuric kidney disease. There is a bidirectional relationship between loss of glomerular filtration rate and progression of atherosclerosis. In addition to CKD‐specific risk factors, these patients have an increased burden of traditional and novel risk factors for CVD, which accelerates the progression of atherosclerosis. The association between coronary artery disease and progression of CKD could be related to acute KI (due to exposure to radiocontrast and coronary artery bypass surgery) and heart failure. CKD indicates chronic kidney disease; CVD, cardiovascular disease; GFR, glomerular filtration rate; KI, kidney injury.
Sources of Funding
Raj is supported by National Institutes of Health grants 1R01DK073665‐01A1, 1U01DK099924‐01, and 1U01DK099914‐01.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. [DOI] [PubMed] [Google Scholar]
- 2. Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167:1130–1136. [DOI] [PubMed] [Google Scholar]
- 3. Sud M, Tangri N, Pintilie M, Levey AS, Naimark D. Risk of end‐stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation. 2014;130:458–465. [DOI] [PubMed] [Google Scholar]
- 4. McClellan WM, Newsome BB, McClure LA, Cushman M, Howard G, Audhya P, Abramson JL, Warnock DG. Chronic kidney disease is often unrecognized among patients with coronary heart disease: the REGARDS Cohort Study. Am J Nephrol. 2009;29:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruno G, Merletti F, Bargero G, Novelli G, Melis D, Soddu A, Perotto M, Pagano G, Cavallo‐Perin P. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia. 2007;50:941–948. [DOI] [PubMed] [Google Scholar]
- 6. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. [DOI] [PubMed] [Google Scholar]
- 7. Sabe M, Claggett B, Burdmann E, Desai A, Ivanovich P, Lewalramani R, Lewis E, McMurray J, Olson K, Parfrey P, Solomon S, Pfeffer M. Coronary artery disease is a predictor of progression to dialysis in patients with chronic kidney disease, type 2 diabetes, and anemia: an analysis of the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). J Am Heart Assoc. 2016;5:e002850. doi: 10.1161/JAHA.115.002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, Hojs R, Abbate M, de Vries AP. Non‐proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:382–391. [DOI] [PubMed] [Google Scholar]
- 9. MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, Matthews PG, Thomas MC, Power DA, Jerums G. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care. 2006;29:1560–1566. [DOI] [PubMed] [Google Scholar]
- 10. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. [DOI] [PubMed] [Google Scholar]
- 11. Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long‐term risk of end‐stage renal disease. Circulation. 2014;130:2005–2011. [DOI] [PubMed] [Google Scholar]
- 12. Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS. Association between albuminuria, kidney function, and inflammatory biomarker profile. Clin J Am Soc Nephrol. 2012;7:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amdur RL, Mukherjee M, Go A, Barrows IR, Ramezani A, Shoji J, Reilly MP, Gnanaraj J, Deo R, Roas S, Keane M, Master S, Teal V, Soliman EZ, Yang P, Feldman H, Kusek JW, Tracy CM, Raj DS. Interleukin‐6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC Study. PLoS One. 2016;11:e0148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta J, Dominic EA, Fink JC, Ojo AO, Barrows IR, Reilly MP, Townsend RR, Joffe MM, Rosas SE, Wolman M, Patel SS, Keane MG, Feldman HI, Kusek JW, Raj DS. Association between inflammation and cardiac geometry in chronic kidney disease: findings from the CRIC Study. PLoS One. 2015;10:e0124772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;87:S24–S31. [DOI] [PubMed] [Google Scholar]
- 16. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]


