Abstract
The neuropeptide oxytocin interacts with mesolimbic dopamine neurons to mediate reward associated with filial behaviors, but also other rewarding behaviors such as eating or taking drugs of abuse. Based on its efficacy to decrease intake of other abused substances, oxytocin administration is implicated as a possible treatment for excessive alcohol consumption. We tested this hypothesis by measuring ethanol intake in male Sprague–Dawley rats injected with oxytocin or saline using two different ethanol self-administration paradigms. First, a dose–response curve was constructed for oxytocin inhibition of fluid intake using a modified drinking-in-the-dark model with three bottles containing .05% saccharine, 10% ethanol in saccharine, and 15% ethanol in saccharine. Doses of oxytocin tested were 0.05, 0.1, 0.3, and 0.5 mg/kg (I.P.). Next, rats received 0.3 mg/kg oxytocin preceding operant sessions in which they were trained to lever-press for either plain gelatin or ethanol gelatin in order to compare oxytocin inhibition of ethanol intake versus caloric intake. For the three-bottle choice study, rats consumed significantly less ethanol when treated with the three higher doses of oxytocin on the injection day. In the operant study, 0.3 mg/kg oxytocin significantly decreased ethanol gel consumption to a greater extent than plain gel consumption, both in terms of the amount of gel eaten and calories consumed. These data affirm oxytocin's efficacy for decreasing ethanol intake in rats, and confirm clinical studies suggesting oxytocin as a potential treatment for alcoholism.
Keywords: Addiction, Alcohol, Ethanol, Oxytocin, Rat
Alcohol use and abuse can cause problems for individuals as well as society as a whole (Kivimaki et al., 2014; Luu et al., 2014) and the search for efficacious treatments that reduce ethanol intake and cravings invites novel approaches to possible therapies. The activity of dopaminergic neurons in mesolimbic pathways determines the reward value of both natural and substance-related reinforcers (Gordon et al., 2011) such as sexual activity, pair bonding, and consumption of high caloric foods and drugs of abuse such as ethanol (Chang et al., 2014). The neuropeptide oxytocin impacts upon neurons in the mesolimbic pathway thereby providing the possibility of oxytocin regulation of these behaviors (Shahrokh et al., 2010). Oxytocin has been shown to inhibit heroin and methamphetamine self-administration at doses as low as 0.3 mg/kg (Kovács et al., 1985; Carson et al., 2010). These studies suggest that oxytocin might also have the potential to be a treatment for addictive behaviors including alcoholism.
When administered intranasally, oxytocin decreases both alcohol craving and withdrawal symptoms in alcoholics in an inpatient setting (Pedersen et al., 2013). Additionally, polymorphisms in the oxytocin receptor gene are related to the degree of alcohol-related aggressive behavior (Johansson et al., 2012). Currently, few studies have fully addressed the effects of oxytocin on ethanol intake. In mice, systemic administration of 10 mg/kg oxytocin decreases voluntary ethanol consumption in a stressful environment (Peters et al., 2013). Adolescent administration of 1 mg/kg oxytocin decreases ethanol intake well into adulthood (Bowen et al., 2011). Unfortunately, the higher doses of oxytocin used in these studies may have had sedative or other non-selective effects, which could confound interpretations of the results.
Another important confounding factor to address when assessing oxytocin's ability to regulate ethanol consumption is its effects on food reward and caloric intake. Studies using obese rats have found that oxytocin administration reduces food intake and augments weight loss, even at very low doses (Maejima et al., 2011; Morton et al., 2012). One study in humans found that intranasal oxytocin reduced snack intake, particularly reducing sugary snack intake by 25% (Ott et al., 2013). Other studies indicate that oxytocin activity may be inhibited by sugar consumption in rodents (Mitra et al., 2010). Thus, appropriate controls should be included in any study of the effects of oxytocin on ethanol intake to account for the caloric value of ethanol.
Our experiments utilize two different models of ethanol consumption. In the first experiment, rats were given a three-bottle choice of saccharine, 10% ethanol with saccharine, and 15% ethanol with saccharine using a modified “drinking-in-the-dark” paradigm. In this way, we could assess whether oxytocin inhibited overall ethanol consumption (both concentrations) or selectively inhibited intake of a higher concentration of ethanol. A second group of rats were trained to operantly respond for 10% ethanol-containing gelatin (Peris et al., 2006) with plain gelatin serving as a control for nonselective effects of oxytocin (e.g. sedation and appetite suppression).
Both of these experiments sought to determine oxytocin's ability to decrease voluntary ethanol consumption in rats while not affecting control behaviors or caloric intake. Our ultimate goal is to better characterize oxytocin as a possible treatment for alcohol addiction, as well as further our understanding of how oxytocin regulates reward pathways in the brain.
1. Method
1.1. Subjects
All studies used male Sprague–Dawley rats weighing approximately 300 g at the start of the experiments. Rats were individually housed and kept on a 12/12 h light/dark cycle with ad libitum access to food and water unless otherwise noted. For the three-bottle choice experiment, the lights turned off at 11 AM every day, while for the operant studies the lights were turned off at 6 PM every day. All rats were allowed a one week habituation period to acclimate to the room environment prior to any experimentation. During the habituation period, no injections were given and no ethanol was provided to the rats but they were handled on a daily basis. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee and adhered to the animal welfare guidelines issued by the National Institutes of Health.
1.2. Three-bottle choice: drinking in the dark
Rats initially received 2 days of 24 h access to three bottles containing three different solutions: 0.05% saccharine (Sigma Aldrich), 10% ethanol in 0.05% saccharine, and 15% ethanol in 0.05% saccharine, followed by a 24 h “break period” where one normal water bottle was placed in the cage. This was followed by one week of 2 h daily access to the three bottles to establish baseline consumption levels. The 2-h access period took place approximately 3 h into the rats' dark (active) cycle throughout the experiment. After baseline consumption was established, rats were habituated to receiving an intraperitoneal (I.P.) saline injection of 1 ml per kilogram (kg) body weight, 30 min prior to receiving access to the bottles. These saline injections occurred every day throughout the rest of the procedure except for when oxytocin injections were given. Oxytocin treatment occurred only after animals had habituated to the daily saline injections and ethanol consumption had returned to normal levels.
On experiment days, rats received an I.P. injection of oxytocin 30 min prior to access to the three bottles. Each oxytocin injection day was followed by four consecutive days of saline injections. The rats received a total of four different doses of oxytocin: 0.05, 0.1, 0.3, and 0.5 mg/kg. Doses were calculated based on the weekly body weight of each rat. A Latin Square design was implemented to determine the order of the injection days for each rat to ensure a randomized, non-confounded treatment schedule.
1.3. Operant self-administration of ethanol gel and plain gel
The operant self-administration experiment utilized plain gelatin and 10% ethanol gelatin as a reward. A separate group of rats (N = 12) were initially given 24 h access to ethanol gel (n = 6) or plain gel (n = 6) in 50 ml jars in their home cages for 48 h as a habituation period. Ethanol gelatin was composed of 10% ethanol (w/w), 2.5% gel (Knox brand, Kraft Foods, Northfield, IL), and 10% polycose (Abbot Laboratories, Abbott Park, IL), all by weight in water. Plain gelatin had the same concentrations of gel and polycose, but with an equivalent amount of water in place of ethanol. Two days of 24-h access in the home cage was followed by two days of 6-h access then two days of 3-h access and finally rats were placed on 1-h free access (starting 4 h after light on) until baseline consumption was stabilized. Operant training was then conducted as previously described (Kasper et al., 2013) and these 30-min operant sessions also started 4 h after lights on. Briefly, rats were initially trained to bar press on a fixed ratio (FR) 1 schedule. A small amount of gel was placed on the active lever on the first day of operant responding to train rats to press only the active lever for gelatin delivery. A free delivery of gelatin also occurred at the start of the session to show rats where the gelatin would appear after successful responding. After the first day of operant training, no gel was placed inside the chamber and no free deliveries were given. After ten days of FR1 responding, rats were switched to an FR5 schedule, where they remained for the duration of the experiment. After about two weeks, baseline consumption on an FR5 schedule was stable at 1.2 ± 1.3 g ethanol/kg body wt, at which point rats were habituated to receiving daily I.P. saline injections 30 min prior to the start of the operant session. Once baseline consumption returned to normal levels after habituation to saline injections, rats were given an I.P. injection of 0.3 mg/kg body weight oxytocin or an equivalent volume of saline 30 min prior to the start of the operant session. The dose of 0.3 mg/kg was chosen based on its efficacy in the three-bottle choice study to selectively inhibit ethanol consumption without any trend for affecting saccharine consumption.
The operant chambers and infusion pumps were located inside sound attenuating isolation cubicles (Coulbourn Instruments, Allentown, PA). Operant chambers consisted of a grid floor, solid walls, and measured 50.8 cm W × 25.4 cm D × 30.5 cm H. Two levers symmetrically protruded from either side of the same wall with a drinking chamber between them. A spout protruded into the drinking chamber and was positioned behind an optical lickometer that recorded each time a rat licked the drinking spout. The spout was connected to a 20 ml syringe filled with gelatin fitted into a syringe pump located outside the operant chamber but within the cubicle. Successful completion of the FR5 contingency resulted in 0.2 g gel delivery. Gel delivery and behavioral data collection were controlled by Graphic State 5.2 operating software (Coulbourn Instruments, Allentown, PA). Pumps were calibrated on a regular basis to ensure accurate gelatin delivery; and, gelatin consumption was confirmed after each operant session by checking the drinking chamber for unconsumed gelatin. Any spillage that occurred was collected in a removable tray under the lickometer, weighed to the nearest 0.01 g and subtracted from the daily total. Spillage was extremely rare (mostly occurring during the first few operant sessions) and did not occur for any animal on any oxytocin treatment day or any of the pre-or post-treatment days included in the analyses.
1.4. Drugs
Oxytocin (Sigma Aldrich) was dissolved in 0.9% saline and administered as an intraperitoneal injection at different concentrations as previously listed. Vehicle injections consisted of 0.9% saline given at the same volume of injection as the oxytocin dose.
1.5. Statistical analysis
For the three-bottle choice experiment, saccharin intake was expressed as ml of fluid per kg of body weight and ethanol intake was expressed as grams of ethanol per kg of body weight. Data were analyzed using a 3 × 4 repeated measures ANOVA regarding treatment (pre-injection, injection, and post-injection) and dose of oxytocin administered (0.05, 0.1, 0.3, and 0.5 mg/kg) as repeated variables. The ANOVA program was provided by SPSS software (IBM Software, Armonk, New York). The pre-injection and post-injection ethanol consumption data for each dose was based on a four day average, respectively.
Gel consumption data was expressed as grams of gel per kg of body weight, with ethanol consumption being expressed as grams of ethanol per kg of body weight (equal to 10% of gel consumed). For analyzing caloric intake, gel consumption was expressed as calories per gram of gel consumed. The 10% ethanol gel contained 1.1 cal/g and the plain gel contained 0.38 cal/g. All data were analyzed by three-way mixed factor ANOVA; main factors included gel type (between group), drug treatment and days (repeated measures). For analysis of a single operant session by minutes, data were analyzed by three-way mixed factor ANOVA with the main factors being gel type (between groups), drug treatment and 3-min intervals (repeated measure). In all cases, the accepted level of significance was p < 0.05.
2. Results
2.1. Three-bottle choice
Total baseline ethanol intake after saline injections (Fig. 1, pre- and post-injection) was 1.6 ± 0.2 g/kg ethanol with 0.6 ± 0.1 g/kg provided by the 10% ethanol solution and 1.0 ± 0.1 g/kg ethanol provided by the 15% solution. Since oxytocin inhibited intake of both ethanol concentrations equally (data not shown), Fig. 1 shows the effect of oxytocin (0.05 mg/kg, 0.1 mg/kg, 0.3 mg/kg, and 0.5 mg/kg) on total ethanol consumption during three-bottle choice sessions. A two-way ANOVA revealed a significant effect of treatment on ethanol consumption (F(2,6) = 29.88; p < 0.005), demonstrating a decrease in total ethanol intake of about 40% on days when oxytocin was administered. Pairwise comparisons of treatment groups demonstrated significant decreases in ethanol intake after oxytocin injection relative to pre-injection and post-injection (after saline injections) for the three higher doses of oxytocin with no difference in the inhibition caused by these three doses.
Fig. 1.
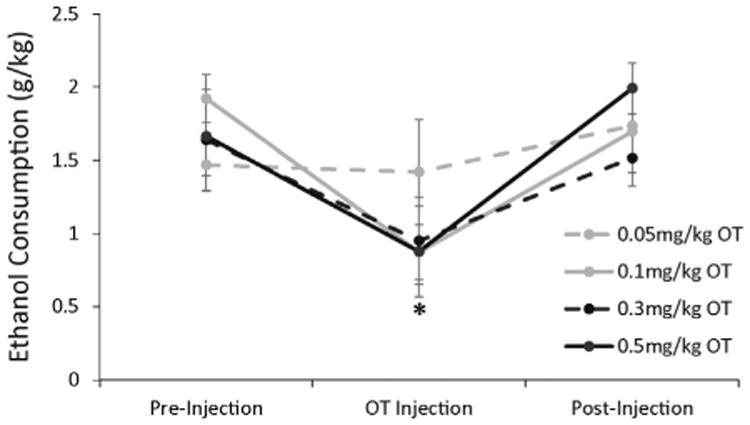
Oxytocin inhibits total ethanol consumption during three-bottle choice limited access. Rats had access to ethanol and saccharine solutions for 2-h daily, preceded by either saline or oxytocin I.P. injections 30 min earlier. Pre-injection and post-injection treatment periods represent average ethanol consumption for all rats after daily saline injections during the 4-day periods before and after oxytocin injections, respectively. Oxytocin injection data represent average ethanol consumption across all rats for each dose of oxytocin. All rats received each dose of oxytocin once in a randomized, non-confounded order via a Latin Squares design. Each line represents a different dose that was administered. Data are represented as mean (total ethanol consumption [g/kg]) ± SEM. * indicates p < 0.05 compared to pre- and post-treatment days.
The effects of 0.05, 0.1, 0.3 and 0.5 mg/kg oxytocin on the consumption of saccharin solution (with 0% ethanol) were also quantified (Fig. 2). Although there was nosignificant effect of any oxytocin dose on saccharine consumption compared to saline injections (pre- and post-oxytocin treatment) as measured by ANOVA (F(2,6) = 1.5; p > 0.1), the large degree of variability in daily saccharine consumption across animals and across days limits any conclusions. Additionally, due to the unequal variances of the data on different treatment days, the assumption of homoscedasticity prior to application of ANOVA was violated. Therefore, we also tallied the number of animals showing a decrease in saccharine consumption on the oxytocin treatment day compared to consumption after a saline injection the day before. After 0.05 mg/kg oxytocin, 50% of animals decreased ethanol consumption compared to the previous day while 25% of animals decreased saccharine consumption after this dose of oxytocin. After 0.1 mg/kg, 100% of animals decreased ethanol consumption and 25% decreased saccharine consumption; after 0.3 mg/kg, 100% of animals decreased ethanol consumption and 50% decreased saccharine consumption; and after 0.5 mg/kg, 100% of animals decreased ethanol consumption and 75% decreased saccharine consumption. Thus, on oxytocin treatment days, we observed less predictable instances of decreased saccharine consumption compared to the 100% occurrence of decreased ethanol intake after 0.1, 0.3 and 0.5 mg/kg oxytocin doses.
Fig. 2.
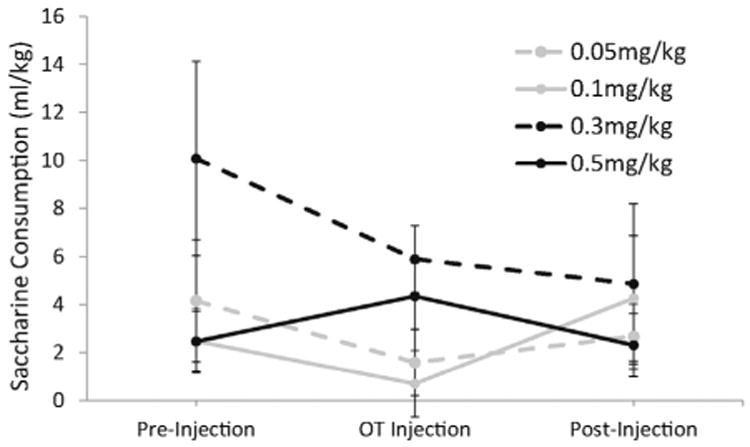
The effects of saline and oxytocin injections on saccharine consumption during three-bottle choice limited access. Rats had access to ethanol and saccharine solutions for 2-h daily, preceded by either saline or oxytocin I.P. injections 30 min earlier. Pre-injection and post-injection treatment periods represent average saccharine consumption for all rats after daily saline injections during the 4-day periods before and after oxytocin injections, respectively. Oxytocin injection data represent average saccharine consumption across all rats for each dose of oxytocin. Each line represents a different dose that was administered. Data are represented as mean (total saccharine consumption [ml/kg]) ± SEM.
2.2. Operant responding for ethanol and plain gelatin after oxytocin administration
When a second set of animals was allowed to operantly self-administer ethanol gel (N = 6) or plain gel (N = 6) in daily 30-min sessions, stable baseline consumption was attained of about 15 g plain gel/kg body wt and 12 g/kg ethanol gel (1.2 ± 1.3 g/kg body wt) as shown in Fig. 3 (Days 1–3). On day 4, animals received either a saline injection or 0.3 mg/kg oxytocin injection 30 min prior to the start of the operant session. As shown in panel A, oxytocin decreased ethanol gel consumption by about 30% (to about 0.8 g ethanol/kg body wt) but decreased plain gel consumption by less than 10%. These observations were supported by a significant treatment by gel type interaction (F(1,10) = 7.94, p < 0.05). When data was analyzed separately for ethanol and plain gel, there was a significant treatment by day interaction for ethanol gel (F(2,4) = 12.74, p < 0.05) but not for plain gel (F(2,4) = 2.38, p = 0.2). Thus, the effects of oxytocin decreased ethanol intake from about 1.2 g/kg to 0.75 g/kg ethanol during the 30-min operant session.
Fig. 3.
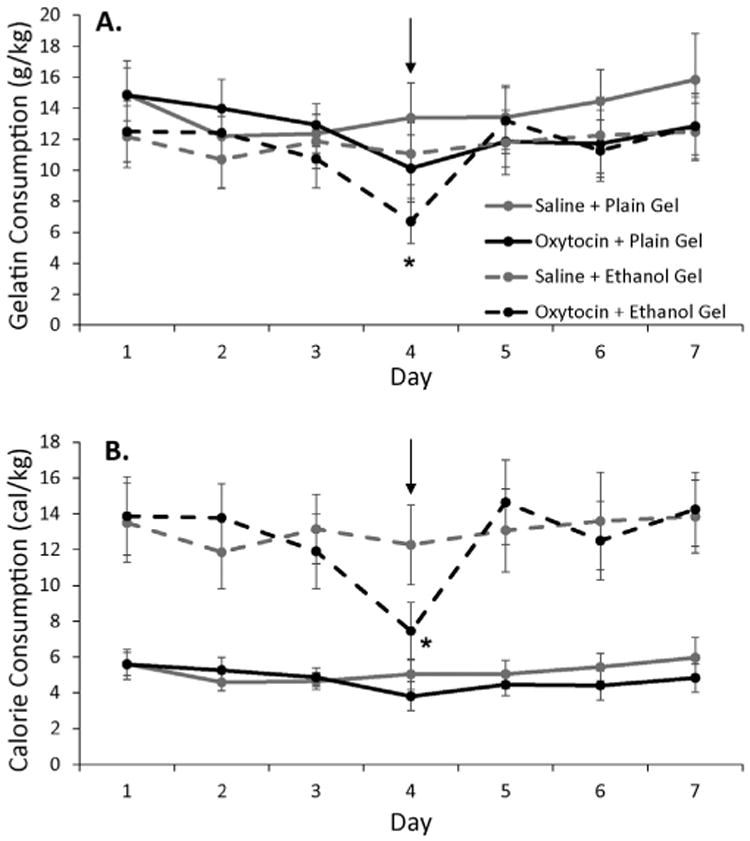
The effect of 0.3 mg/kg oxytocin on ethanol gel or plain gel operant self-administration. Rats were injected I.P. with either oxytocin (indicated by arrow) or saline (all other days) 30 min prior to the start of the operant sessions. Separate groups of rats responded for either ethanol gel (n = 6) or plain gel (n = 6) reinforcement during the 30-min sessions. Panel A shows the consumption of plain gel and ethanol gel in grams of gel per kg body weight. Since the ethanol gel contained 10% ethanol (w/w) the baseline ethanol intake on saline injection days was about 1.2 g/kg ethanol/kg body wt (12 g gel/kg body wt). Panel B shows the consumption of plain and ethanol gel in calories consumed per kg body weight. Oxytocin significantly decreased ethanol consumption to a greater extent than plain gel consumption compared to saline injections both when graphed as grams of gel or calories. Shown are means ± SEM. * indicates p < 0.05 compared to same day ethanol gel consumption after saline injection.
We next converted the gel consumption data into calories consumed per kg body wt, since the caloric content of the ethanol and plain gel differs considerably (although not to the extent as with ethanol vs saccharine solutions). As shown in panel B of Fig. 3, there was significantly higher caloric consumption when rats had ethanol gel as a reward compared to plain gel. After 0.3 mg/kg oxytocin injection, caloric consumption in ethanol gel groups was decreased to that of plain gel groups with very little effect on caloric consumption of plain gel. There was a significant treatment by gel type by day interaction (F(2,20) = 9.78, p < 0.05).
When the pattern of gel consumption during the 30 min operant session was grouped into 3-min bins and analyzed (Fig. 4, panels A and B), oxytocin significantly decreased both plain gel and ethanol gel consumption in the first 15 min of each session compared to saline injection, but there appeared to be a greater inhibition in the ethanol gel group. ANOVA revealed significant treatment × time interactions for both types of gel (plain gel: F(1,5) = 8.21, p < 0.05; ethanol gel F(1,5) = 32.71, p < 0.05) but there was not a significant gel type by treatment by time interaction. Similar findings were found when data were converted to calories instead of grams of gel (Fig. 4, panels C and D) with oxytocin decreasing caloric consumption in the ethanol gel group to that in the plain gel group.
Fig. 4.
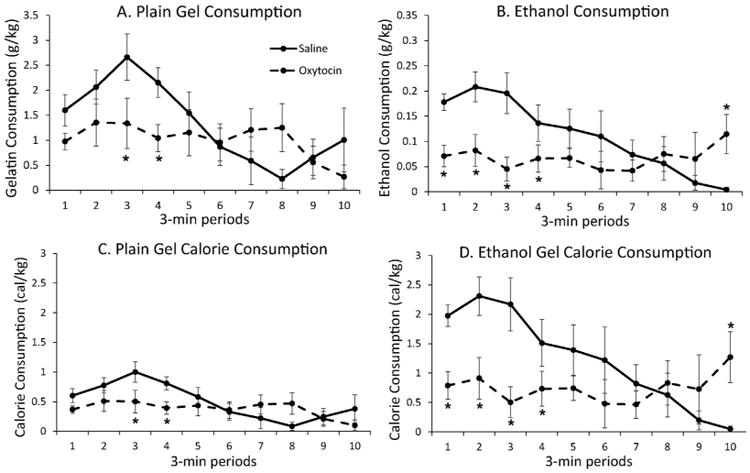
Time course of oxytocin inhibition of plain and ethanol gel consumption and calorie consumption during an operant session. Rats were given 0.3 mg/kg oxytocin or saline via i.p. injection 30 min prior to the start of the operant session in which they responded for either plain gel (n = 6) or 10%-ethanol gel (n = 6). Oxytocin significantly lowered both ethanol and plain gel consumption during the first 15 min of the session compared to saline injection. Consumption is shown for each 3 min period of the operant session. Panel A shows plain gel consumption, expressed as grams of gel consumed per kg body weight. Panel B shows ethanol consumption, expressed as grams of ethanol consumed per kg body weight. Panel C shows plain gel calorie consumption, and Panel D shows ethanol calorie consumption, both expressed as calories consumed per kg body weight. Shown are means ± SEM. * indicates p < 0.05 compared to saline treatment at that time point.
We then expressed these data as the percent of ethanol or plain gel consumed during the corresponding 3-min period of the previous day's operant session (after a saline injection). As shown in Fig. 5, there was significantly greater oxytocin inhibition in the ethanol group (F(1, 34) = 5.29, p < 0.05). Data were analyzed during the first 9 min of the operant session when the majority of gel was consumed.
Fig. 5.
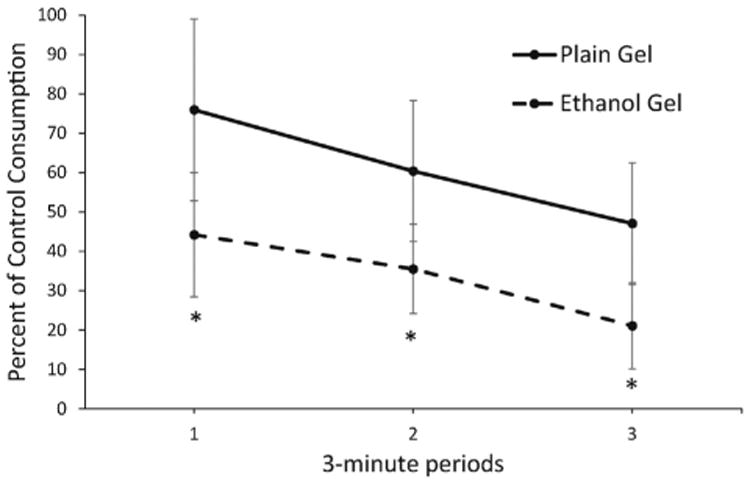
Oxytocin inhibition of ethanol gel intake is greater than inhibition of plain gel intake. Oxytocin inhibition of ethanol gel (n = 6) and plain gel (n = 6) intake is expressed as a percent of the control consumption measured during the same time period of the previous day's operant session which occurred after a saline injection. Data are presented for the first three 3-min periods of the operant session when gel consumption was highest and most consistent. Oxytocin significantly inhibited ethanol gel consumption more than plain gel. Shown are means ± SEM. * indicates p < 0.05 for an overall difference between oxytocin inhibition of plain gel and ethanol gel consumption.
3. Discussion
Using a three bottle choice experiment, peripheral administration of oxytocin significantly reduced ethanol consumption in adult male rats from an average intake of 1.6 g ethanol/kg body wt to around 1 g ethanol/kg body wt (Fig. 1). Our results are consistent with the current literature supporting the ability of peripherally administered oxytocin to decrease alcohol consumption in adolescent male rats (Bowen et al., 2011) and extends these findings to adult rats using much lower oxytocin doses with fewer sedative side effects. These outcomes suggest an inhibitory effect of oxytocin on ethanol consumption and support the observation that oxytocin inhibits ethanol reward (Bahi, 2015). However, we could not eliminate the possibility that oxytocin might have had non-selective effects on fluid consumption or caloric intake.
Based on the literature concerning the role of oxytocin in modulating food intake, we then used the “jello shot” model of operant ethanol intake (Peris et al., 2006) since it would allow for a comparison of the effect of oxytocin on the consumption of a similar caloric reward. Although there did appear to be a small inhibitory effect of oxytocin injection on plain gel responding, consumption of ethanol gel was inhibited to a greater extent, both over the whole 30-min session and during the first 9 min of the session (Figs. 3–5). Daily operant self-administration of ethanol was reduced from 1.2 g/kg body wt to less than 0.7 g/kg body wt, a significantly less intoxicating dose. It is possible that the rats decreased responding for ethanol gelatin due to the effect of oxytocin on their ability to self-titrate ethanol intoxication (Becker et al., 2013). However, it should be noted that the caloric content of the ethanol and plain gels still differed considerably due to the high caloric content of ethanol. Therefore it is possible that there is a greater inhibitory effect of oxytocin on rewards with higher caloric content regardless of the level of ethanol intoxication.
The dose of oxytocin used in the operant study falls within the range of doses that have been shown to decrease food intake in obese animals (Maejima et al., 2011; Morton et al., 2012). However, these studies used repeat administrations of oxytocin and measured total food intake whereas our study used a single injection and measured consumption over a 30 min period. Although oxytocin did significantly decrease plain gel consumption in the first 15 min of the session (Fig. 4), there was a greater effect on ethanol consumption particularly when taking the different caloric values of the gel into account (Fig. 5). Thus, it is likely that oxytocin does decrease the animal's specific appetite for ethanol gel more so than overall appetite.
One of the criticisms of these data is that a fixed ratio operant schedule does not accurately characterize the motivation of the animal to obtain the reward but simply the point at which an animal is satiated. A progressive ratio operant schedule, on the other hand, uses increasing response requirements to determine the reinforcing properties of a stimulus by comparing the point at which an animal will no longer work to receive a reward. Previous studies in our lab have found that using a progressive ratio schedule, breakpoint values do not significantly differ between plain gel and ethanol gel (Li et al., 2010). Thus it will be important in future experiments to test the effects of these doses of oxytocin on motivation for ethanol and plain gel reward using progressive ratio schedules of reinforcement.
There is still no definitive mechanism describing how peripheral oxytocin administration gains access to the central nervous system. Oxytocin is a nine amino acid neuropeptide, therefore its uptake into brain extracellular fluid is vastly restricted by the blood brain barrier. Until recently, studies demonstrating oxytocin's ability to influence social development and cognition have been discussed with much controversy surrounding this blood brain barrier issue. However, work by Landgraf and Neumann (Neumann and Landgraf, 2012; Landgraf and Neumann, 2004) demonstrates the ability of exogenous neuropeptides to cross the blood brain barrier in small, but functionally significant amounts. Oxytocin administered by the I.P. route caused a 40-fold increase in plasma levels that were detectable at 10 min post-injection (Neumann and Landgraf, 2012). Similarly, microdialysate samples from the right dorsal hippocampus and left amygdala detected a rise in central oxytocin concentration within brain extracellular fluid after peripheral administration (Neumann et al., 2013). This peripheral increase in oxytocin concentration coupled with approximately 0.2% of peripheral administered oxytocin being able to cross the blood brain barrier potentially leads to direct binding of oxytocin to central oxytocin receptors (Ermisch et al., 1985; Kendrick et al., 1986; Engelmann et al., 1996). It has also been suggested that systemic effects of oxytocin may be regulated by afferent feedback from peripheral tissues that are sensitive to oxytocin (Cushing and Carter, 2000). It is possible that this feed-forward phenomenon of peripheral oxytocin leading to increases in central oxytocin levels might explain why there was not a graded dose–response curve to oxytocin in the three-bottle choice experiment. Future studies should measure central oxytocin levels using microdialysis in response to different doses of systemic oxytocin administration and correlate these levels to the degree of inhibition of ethanol intake.
The results from our experiments contribute to mounting evidence that oxytocin decreases ethanol intake, possibly via a specific action in the nucleus accumbens (Bahi, 2015) and thus could be an effective treatment for alcohol addiction. If future studies are extended to human subjects, it could pave the way for oxytocin to be introduced as a method for treating addiction to ethanol.
Acknowledgments
Dr. Anatoly Martynyuk (University of Florida College of Medicine Department of Anesthesiology) and his lab paid for rats, housing and supplies for the three-bottle choice experiment. The University of Florida University Scholars Program provided funds to support Rebecca Loveless. Erie Uy, Jeffery Uy, and Erin Delaney assisted the authors of this paper by providing feedback and suggestions on wording, layout, and figures.
Footnotes
Author contributions: JP is the principle investigator, and designed the experiments and supervised all experimental work. RL and KM are to be considered equal first authors of this paper. KM and RL wrote the majority of the manuscript, conducted experiments, and collected data. BD, SD, and CT collected data and wrote sections of the manuscript. KW calculated all caloric data and constructed the corresponding figures. RL, CT, and BD completed statistical analyses of the data. All authors provided feedback and contributed to the final draft of the manuscript, approving it before submission.
References
- Bahi A. The oxytocin receptor impairs ethanol reward in mice. Physiol Behav. 2015;139:321–327. doi: 10.1016/j.physbeh.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Becker RO, Lazzari VM, de Azevedo MS, Morris M, Rigatto K, Almeida S, Lucion AB, Giovenardi M. Oxytocin modulates social interaction but is not essential for sexual behavior in male mice. Behav Brain Res. 2013;244:130–136. doi: 10.1016/j.bbr.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Chang WH, Lee IH, Chen KC, Chi MH, Chiu NT, Yao WJ, Lu RB, Yang YK, Chen PS. Oxytocin receptor gene Rs53576 polymorphism modulates oxytocin–dopamine interaction and neuroticism traits—a SPECT Study. Psychoneuroendocrinology. 2014;47:212–220. doi: 10.1016/j.psyneuen.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Rühle HJ, Skopková J, Hrbas P, Landgraf R. On the blood–brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Dev Cogn Neurosci. 2011;1:471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Westberg L, Sandnabba K, Jern P, Salo B, Santtila P. Associations between oxytocin receptor gene (OXTR) polymorphisms and self-reported aggressive behavior and anger: interactions with alcohol consumption. Psychoneuroendocrinology. 2012;37:1546–1556. doi: 10.1016/j.psyneuen.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Kasper J, Tikamdas R, Kim MS, MacFadyen K, Aramini R, Ladd J, Bisceglia S, Booth R, Peris J. The serotonin-2 receptor modulator, (—)-trans-PAT, decreases voluntary ethanol consumption in rats. Eur J Pharmacol. 2013;718:98–104. doi: 10.1016/j.ejphar.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. Cerebrospinal fluid levels of acetylcholinesterase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep. Neuroendocrinology. 1986;44:149–156. doi: 10.1159/000124638. [DOI] [PubMed] [Google Scholar]
- Kivimaki P, Kekkonen V, Valtonen H, Tolmunen T, Honkalampi K, Tache U, Hintikka J, Lehto S, Laukkanen E. Alcohol use among adolescents, aggressive behaviour, and internalizing problems. J Adolesc. 2014;37:945–951. doi: 10.1016/j.adolescence.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Borthaiser Z, Telegdy G. Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sci. 1985;37:17–26. doi: 10.1016/0024-3205(85)90620-4. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Zharikova A, Vaughan CH, Bastian J, Zandy S, Esperon L, Axman E, Rowland NE, Peris J. Intermittent high-dose ethanol exposures increase motivation for operant ethanol self-administration: possible neurochemical mechanism. Brain Res. 2010;1310:142–153. doi: 10.1016/j.brainres.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu BN, Nguyen TT, Newman IM. Traditional alcohol production and use in three provinces in Vietnam: an ethnographic exploration of health benefits and risks. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-731. n. pag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y1, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging. 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Gosnell BA, Schiöth HB, Grace MK, Klockars A, Olszewski PK, Levine AS. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides. 2010;31:1346–1352. doi: 10.1016/j.peptides.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012;302:E134–E144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. doi: 10.2337/db13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Zharikova A, Li Z, Lingis M, MacNeill M, Wu MT, Rowland NE. Brain ethanol levels in rats after voluntary ethanol consumption using a sweetened gelatin vehicle. Pharmacol Biochem Behav. 2006;85:562–568. doi: 10.1016/j.pbb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Flor PJ, Newmann ID, Reber SO. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addict Biol. 2013;18:66–77. doi: 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]


