Abstract
Stem cell replacement is providing hope for many degenerative diseases that lack effective therapeutic methods including multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system. Transplantation of neural stem cells or mesenchymal stem cells is a potential therapy for MS thanks to their capacity for cell repopulation as well as for their immunomodulatory and neurotrophic properties. Induced pluripotent stem cell (iPSC), an emerging cell source in regenerative medicine, is also being tested for the treatment of MS. Remarkable improvement in mobility and robust remyelination have been observed after transplantation of iPSC-derived neural cells into demyelinated models. Direct reprogramming of somatic cells into induced neural cells, such as induced neural stem cells (iNSCs) and induced oligodendrocyte progenitor cells (iOPCs), without passing through the pluripotency stage, is an alternative for transplantation that has been proved effective in the congenital hypomyelination model. iPSC technology is rapidly progressing as efforts are being made to increase the efficiency of iPSC therapy and reduce its potential side effects. In this review, we discuss the recent advances in application of stem cells, with particular focus on induced stem/progenitor cells (iPSCs, iNSC, iOPCs), which are promising in the treatment of MS.
Keywords: Multiple sclerosis, experimental autoimmune encephalomyelitis, neural stem cells, mesenchymal stem cells, induced pluripotent stem cells, direct transdifferentiation, remyelination
1. Introduction
Multiple sclerosis (MS) is a common neurological disorder that targets white matter in the central nervous system (CNS), i.e., brain and spinal cord [1, 2]. MS remains one of the major causes of disability in young adults. Globally, the median estimated prevalence of MS is 30 per 100,000 and the median estimated incidence of MS is 2.5 per 100,000, with rates varying widely in different regions [3]. While the exact etiology is unknown, an aberrant immune response against CNS antigens, especially the activation of T helper cells, is thought to play a critical role in this disease [4]. MS is characterized by a cascade of pathological events ranging from inflammatory cell infiltration, demyelination in multiple areas of the CNS, axonal degeneration, and neuron and oligodendrocyte loss [5]. The diagnosis of MS is based on clinical dysfunction and radiological abnormalities distributed throughout the CNS. Current therapeutic methods include relapse treatment, disease-modifying treatment and treatment of symptoms [6–8]. Drugs such as interferon-β, fingolimod and natalizumab can slow disease progression and reduce relapse risk, but they cannot rectify the imbalanced immune response or repair demyelinated axons and impaired neural cells [9–15].
Because of their self-renewal and differentiation ability, stem cells have been recently proposed as a promising therapy for various degenerative disorders, including MS. Neural stem cells (NSCs) and mesenchymal stem cells (MSCs) are the most common stem cell types used in the treatment of MS. Studies have demonstrated that injection of NSCs or MSCs can alleviate neurological dysfunction and promote remyelination in experimental autoimmune encephalomyelitis (EAE), a widely used animal model of MS [16, 17]. It has been shown that the therapeutic effects of NSCs and MSCs are due to their neural cell repopulation and immunomodulatory properties, which make stem cell therapy more effective than current drugs. Not only can they halt the inflammatory demyelinating process in CNS, but they also differentiate into neurons and oligodendrocytes, the myelinating cells in CNS, to repair damaged tissues.
Eight years ago, a method of preparing another kind of stem cells, induced pluripotent stem cells (iPSCs), was developed by Yamanaka et al. [18]. Mouse somatic cells were reprogrammed into iPSCs by the introduction of four reprogramming factors. Soon afterward, iPSCs were also successfully generated from cells of MS patients [19, 20]. Amelioration of disability and myelin regeneration were observed after transplantation of iPSCs–derived NSCs and oligodendrocyte progenitor cell (OPCs) into EAE mice and other demyelinated animal models [21, 22]. Compared with other stem cells, iPSCs have the great advantage of being obtained from the patient’s somatic cells, thus avoiding both the risk of transplant rejection and ethical concerns. It is expected that iPSCs derived from MS patients will differentiate into neural lineages and tissues in vitro for disease modeling, pathomechanism exploration, drug testing and will then be transplanted into the patient to repair demyelinated lesions. A significant effort is being made to achieve that goal. Here we discuss the recent advances in stem cell therapy for MS, focusing on iPSC-based stem cell therapy.
2. Application of NSCs and MSCs in EAE
Before the development of iPSCs, other types of stem cells, NSCs and MSCs in particular, were studied in EAE mice [23, 24]. Convincing results have been observed and mechanisms underlying their effects have been studied, providing a strong basis for the potential use of iPSCs in demyelinating diseases. We will therefore first discuss recent progress in the application of NSCs/MSCs in EAE.
2.1. NSCs and EAE
NSCs are multipotent cells that can be obtained from multiple tissues, including embryo, bone marrow, fetal and adult nervous systems [25]. When NSCs were injected intraventricularly into Lewis EAE rats to test their potential effects on the acute phase of MS, these cells attenuated the clinical severity of EAE and CNS inflammation [26, 27]. Further, NSCs were also injected both intravenously (i.v.) and intracerebroventricularly (i.c.v.) into myelin oligodendrocyte glycoprotein (MOG) 35–55 peptide-induced EAE mice, and these cells were located exclusively in the lesioned areas of the CNS, where they effectively promoted remyelination and functional recovery [28].
In attempting to improve NSC transplantation, its therapeutic mechanism has been extensively investigated. NSC transplantation was originally viewed as a means of cell replacement based on their capacity to differentiate into myelin-forming cells and neurons [28]. In addition, a paracrine mechanism might also be involved in the beneficial effects of NSCs, as, for example, injected NSCs induced an upregulation of growth factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and promoted proliferation and differentiation of endogenous OPCs [28]. Moreover, transplanted NSCs might promote blood-brain barrier (BBB) integrity and attenuate CNS inflammatory responses, e.g., decreased expression of intercellular adhesion molecule and leukocyte-activating factor, inhibited lymphocyte proliferation and CNS infiltration [29, 30]. NSCs also induced apoptosis of infiltrating CD4+ T cells and increased the percentage of regulatory T cells, thus protecting neural tissues from inflammatory damage and reducing neurological disability [29].
Various modified methods have been tested to enhance the efficacy of NSC transplantation. For example, a great improvement in functional recovery was achieved by pre-treating MOG-induced EAE mice with osthole, a natural coumarin with a broad spectrum of pharmacological activities, including anti-inflammation, immunomodulation, and neuroprotection [31]. Similar results were also obtained by transfecting NSCs with IL-10, a potent immunomodulatory cytokine [32], and Neurotrophin 3, a neurotrophic factor that promotes neuron and oligodendrocyte development and survival [33]. Using in vivo MRI tracking analysis, studies have demonstrated that inflammatory signals in the CNS, such as increased concentrations of chemokines, attracted transplanted cells into the demyelinated areas [34, 35]. Thus, transfection of NSCs with a chemokine receptor, CCR-5, significantly enhanced the number of transplanted NSCs and their speed in reaching demyelinated foci, resulting in improvement of neurological function [36]. Moreover, after transplanting NSCs with Olig2, a greater number of NSCs differentiated into OPCs, making the therapy more effective [37].
NSC transplantation has proved to be a promising approach for different EAE models. With the development of iPSC technology, generation of induced NSCs (iNSCs) from human and mouse somatic cells by directly reprogramming with a single transcription factor (TF), or a set of them, is providing new avenues for MS treatment, which will be discussed in 4.1.
2.2. MSCs and EAE
MSCs, another type of multipotent cells, have been found in virtually all tissues, including bone marrow (BM) and adipose tissue [23]. Two early studies demonstrated that transplanted BM-MSCs alleviated pathological features and improved functional recovery in both myelin proteolipid protein (PLP)-induced and MOG-induced EAE mice [38, 39]; more recently, other studies showed that MSCs from BM and adipose tissue reduced infiltration of inflammatory cells into the CNS [40–42]. Moreover, MSCs from MOG-induced EAE mice had similar biological properties as MSCs from healthy donors, making possible the use of autologous MSCs from MS patients [43]. To improve the effects of MSCs therapy, different modified methods were tested. For instance, inhibition of autophagy in MSCs by knockdown of Becn1 significantly improved their therapeutic effects [44].
The therapeutic mechanism of MSCs in alleviating the clinical course of EAE is still controversial. It was found that the majority of transplanted MSCs migrated to lymphoid organs and induced a T-cell unresponsive state in MOG-induced EAE mice [38]. By using enhanced green fluorescent protein (eGFP) to track MSCs, Gerdoni et al. did not find any GFP+ neural cells within the brain parenchyma [17], indicating that MSCs might exert their beneficial effects by modulating the immune system, but not by neural cell repopulation [45, 46]. Nevertheless, MSCs also promoted endogenous neural generation though BDNF secretion [39]. Thus, MSCs exerted therapeutic effects on EAE not only through their immunomodulation function, but also their neurotrophic capacity.
3. Application of iPSC-based cell therapy
The breakthrough innovation of iPSCs has sparked an enormous global effort to carry out disease modeling, drug screening and regenerative therapy. The reprogrammed cells have similar morphologies to embryonic stem cells (ESCs) and can differentiate into cell types representing all three embryonic germ layers [18]. Subsequent successful attempts to generate human iPSCs from both healthy and diseased individuals have brought us several steps closer to clinical application [47–49]. This technology makes it possible for pluripotent cells reprogrammed from the MS patient’s somatic cells to differentiate in vitro into the desired cell lineages or tissues for both pathophysiological research and in vivo cell-based treatment. The therapeutic effects of these cells have been tested in EAE and other demyelinated model systems, as summarized in Table 1.
Table 1.
Summary of effective iPSC-derived cell therapies for demyelinated models
| Transplanted cells | Intermediate cells | Original cells | Demyelinated models | Conclusions | Refs |
|---|---|---|---|---|---|
| OPCs | iPSC | Mouse embryonic fibroblast | Cuprizone-induced model | Developed into mature OLs and contributed to remyelination | [53] |
| OPCs | iPSC | Human dermal fibroblast | Lysolecithin-induced model | Improved remyelination of the optic chiasm, as well as nerve function | [54] |
| OPCs | iPSC | Human fibroblast, Human keratinocyte | Congenital hypomyelination model | Formed myelin in the brains of shiverer mice and increased their survival | [22] |
| OPCs | iPSC | Fibroblast of PPMS patient | Congenital hypomyelination model | Differentiated to OLs in vitro and successfully ensheathed axons in vivo | [20] |
| NSCs | iPSC | Mouse embryonic fibroblast | MOG-induced EAE | Restrained demyelination, promoted remyelination; Trophic effect limited CNS-confined inflammation | [21] |
| iNSCs | iNSCs | Mouse embryonic fibroblast | Congenital hypomyelination model | Generated MBP+ myelin sheets in white-matter tracts of the cerebellum | [65] |
| iOPCs | iOPC | Mouse embryonic fibroblast | Congenital hypomyelination model | Generated compact myelin in vitro and in vivo | [66] |
| iOPCs | iOPC | Rat fibroblast | Congenital hypomyelination model | Differentiated into myelinating OLs and formed myelin in vivo | [67] |
3.1. IPSCs can differentiate into oligodendrocyte lineage in vitro
Successful differentiation of iPSCs into oligodendrocyte lineage is fundamental for the treatment of MS. In 2010, Tokumoto compared the differentiating ability of mouse iPSCs with ESCs. After induction, OPC marker A2B5 positive cells and oligodendrocyte-specific cell surface marker O4 positive cells were observed in both iPSC- and ESC-derived cells [50]. Human iPSCs were first induced to differentiate to oligodendrocytes in 2011 [51]. Fibroblast-derived iPSCs from healthy adults were treated with epidermal growth factor-dependent differentiation protocol and eventually differentiated to O4+ oligodendrocytes. However, the efficiency was low (less than 0.01%). Fibroblasts from relapsing-remitting multiple sclerosis (RRMS) and primary progressive multiple sclerosis (PPMS) patients were also reprogrammed into iPSCs, and these MS-iPSCs successfully differentiated into mature neurons, astrocytes and oligodendrocytes with normal karyotypes. The MS-iPSC derived neurons were electrophysiologically functional, and the oligodendrocytes displayed positive staining for myelin basic protein (MBP) and O4. Generation of neural cells from MS-iPSCs in vitro was an important step toward achieving individualized treatment [19].
3.2. Effects of iPSC-derived NSCs on EAE
Given the therapeutic effects of subventricular zone- and BM-derived NSCs on EAE [28, 52], it has been proposed that iPSC-derived NSCs have similar effects. To test this, Laterza et al. generated NSCs from mouse iPSCs and transplanted them into MOG-induced EAE mice intrathecally. The NSC-treated EAE mice showed clinical amelioration, with reduced demyelinated areas and axonal damage in the spinal cord. However, the majority of transplanted NSCs did not differentiate into oligodendrocyte lineages in vivo, indicating that they did not participate in remyelination directly. Nevertheless, iPSC-derived NSCs promoted the survival and differentiation of endogenous myelin-forming cells by secreting a specific neurotrophin: leukaemia inhibitory factor. The trophic effect mediated by NSCs also improved BBB integrity, thus limiting the CNS-confined inflammation [21]. Given that myelin damage is often caused by CNS inflammation, exerting neuroprotective and neurotrophic effect within an inflammatory environment makes iPSC-NSCs a promising cell source for treating MS.
3.3. Effects of iPSC-derived OPCs on chemically-induced demyelinated and congenital hypomyelination models
In addition to iPSC-derived NSCs, another common cell source for transplantation was iPSC-derived OPCs. The myelin-forming capacity of iPSC-derived OPCs was examined in vivo by injecting them into the demyelinated corpus callosum of cuprizone-fed mice; the implanted OPCs developed into mature MBP+ oligodendrocytes that contributed to the remyelination of the corpus callosum axons [53]. OPCs derived from human iPSCs were also transplanted into the lysolecithin-induced demyelinated rat optic chiasm [54]. Obvious remyelination was revealed by luxol fast blue myelin-specific staining after transplantation. The visual evoked potential recording also reflected functional improvement. The transplanted cells differentiated into mature oligodendrocytes and integrated within the chiasm, contributing to remyelination and functional recovery.
Human iPSC-derived OPCs were also tested in shiverer mouse, a genetic model of congenital hypomyelination [22]. Mice transplanted with iPSC-OPCs exhibited markedly improved survival, with reduced mortality over a 9-month period of observation. Robust donor-derived myelination, with a higher proportion of ensheathed axons in recipient brains, was revealed by confocal images [22]. MS-iPSC-derived OPCs were also injected into shiverer mice to evaluate their myelinogenic ability. After 16 weeks, human MBP+ oligodendrocytes were found diffusely throughout the engrafted corpus callosum, and about 30% of host mouse axons were ensheathed [20]. Together, these results provided direct evidence for the myelinogenic effect of iPSC-derived OPCs in vivo.
3.4. Modifications to improve the generation and migration of iPSC-derived OPCs
Although iPSC-derived OPCs can differentiate into mature oligodendrocytes and form myelin in vivo, it is difficult to acquire large numbers of autologous OPCs [55]. Modifying the protocols to generate sufficient iPSC-OPCs in a relatively short time is crucial. In an attempt to do so, Douvaras et al. induced OPCs from iPSCs by dual inhibition of SMAD signaling in adherent cultures and addition of retinoic acid and/or sonic hedgehog; 44%-70% OPCs were obtained after 75 days of differentiation, compared with the minimum of 120 days previously required [20]. Furthermore, it was found that the differentiation rate of iPSCs-derived OPCs into O4+ oligodendrocytes was increased by overexpression of p27, and the differentiation efficiency was enhanced over eightfold compared with the control experiments [56].
While iPSC-derived OPCs have the ability to generate and restore the myelin sheath, they have limited capacity to migrate along the axons [53]. To increase the migratory capacity of these cells, overexpression of sialyltransferase X (STX) was induced in iPSC-derived OPCs via lentiviral transduction, after which transfected cells were injected into the cuprizone-fed demyelinated mice to examine their migratory and myelinogenic behavior. At 3 weeks after implantation, STX-transfected OPCs survived and repopulated the corpus callosum along its entire width on the unilateral side of the injection. A significant increase in migration along the axons was revealed compared with control OPCs [57].
4. Direct transdifferentiation of somatic cells to NSCs and OPCs
After the successful reprogramming of somatic cells to iPSCs, a more direct lineage conversion of fibroblasts to functional neurons by defined factors was achieved in 2010 [58]. This novel approach made it possible for a fully differentiated cell to be transformed into another specialized cell type without passing through the pluripotency stage. Subsequently, strategies were developed for the generation of other induced neural lineage cells using specific sets of TFs in optimal culture conditions [59, 60].
4.1. Induced neural stem cells (iNSCs)
In 2011, Kim et al. first showed that four Yamanaka reprogramming factors in combination with NSC-permissive culture condition directly transformed fibroblasts to iNSCs [61]; these iNSCs differentiated to neurons and astrocytes, but self-renewed for only 3–5 passages. Thier et al. also generated iNSCs that could be expanded for more than 50 passages by constitutively inducing Sox2, Klf4 and c-Myc while strictly limiting Oct4 activity [62]. The iNSCs not only had a similar genome-wide transcriptional profile compared to brain-derived NSCs, but they could also differentiate into neurons, astrocytes, and oligodendrocytes. Tripotent, self-renewing iNSCs were also induced using a combination of TFs: Brn4/Pou3f4, Sox2, Klf4, c-Myc, plus E47/Tcf3 [63]. Furthermore, Ring et al. reported the generation of iNSCs by direct reprogramming with a single TF: Sox2. The implanted Sox2-induced iNSCs survived and was integrated into mouse brains and did not generate tumors [64].
The effects of iNSCs were also examined in a congenital hypomyelination model. Lujan et al. injected iNSCs that were eGFP positive into the neonatal brain of shiverer mouse [65]. Ten weeks after transplantation, eGFP+ cells and MBP+ myelin sheaths were observed in white-matter tracts of the cerebellum. This result indicates that iNSCs differentiated into myelinating oligodendrocytes and restored myelin sheaths.
4.2. Induced oligodendrocyte progenitor cells (iOPCs)
Najm et al. [66] found that forced expression of sets of 8 or 3 defined TFs could directly reprogram mouse fibroblasts into myelinogenic iOPCs, obviating the use of iPSCs. The 8 TFs (Olig1, Olig2, Nkx2.2, Nkx6.2, Sox10, ST18, Gm98 and Myt1) or 3 TFs (Nkx6.2, Sox10, Olig2)-induced mouse fibroblast globally expressed OPC genes and could differentiate into cells with a typical morphology of oligodendrocytes. The iOPCs generated characteristically MBP+ myelin sheaths after being transplanted into organotypic slice cultures, which provided a complex 3D tissue representative of the CNS. Compact myelin sheaths around dorsal column axons were also restored. Furthermore, the authors found that the efficiency of generating functional iOPCs was enhanced by increasing the viral titer of the TFs [66].
Consistent with Najm’s study, Yang et al. [67] also generated iOPCs from mouse and rat fibroblasts using 3 TFs (Sox10, Olig2 and Zfp536). These iOPCs exhibited properties of bona fide OPCs; they gave rise to MBP+ cells that showed mature oligodendrocytic morphologies and myelinated the axons of dorsal root ganglion neurons in vitro. When iOPCs were injected into shiverer mouse brains, small scattered groups of MBP+ cells forming tube-like structures that surrounded axons were detected at all injection sites of brain. The myelin formation of transplanted iOPCs was also confirmed by ultrastructural analysis. These results strongly indicated the myelinogenic capacity of iOPCs.
Although iNSCs and iOPCs have not been tested in EAE mice, they may be more advantageous than other types of stem cells. First, iNSCs and iOPCs have shown their myelinogenic ability in vitro and in vivo, which is advantageous over MSCs. Second, a relatively simpler generation protocol makes their use less effort- and cost-consuming than iPSCs. Last but not least, iNSCs and iOPCs are autologous cells, making them promising candidates for individualized cell treatment compared to those derived from ES cells or stem cells of umbilical cord blood. Certainly, the therapeutic effects of iNSCs and iOPCs on EAE/MS need to be evaluated by further studies.
5. Therapeutic mechanisms of stem cells
Although spontaneous recovery is observed in the early stage of MS, endogenous processes of repair and remyelination are typically incomplete and ultimately fail in the setting of recurrent episodes. Exogenous stem cell transplantation has therefore been advocated for regenerative medicine. Accumulated studies have shown the therapeutic effects of NSCs or MSCs on EAE. iPSC-based stem cells, including iPSCs-derived NSCs and OPCs, iNSCs, iOPCs, have also shown their potential in treatment of EAE/MS. The underlying mechanisms for the beneficial effects of stem cells may include: 1) as an exogenous source of neuron/oligodendrocyte repopulation; 2) immunomodulation, thus converting the CNS microenvironment from a hostile to a supportive one; and 3) neurotrophic effects, thus promoting endogenous neuron/oligodendrocyte differentiation and regeneration.
In the acute phase of EAE, the immunoregulatory property of stem cells protects tissue from attacks of abnormal inflammation; in the chronic phase, stem cells secrete neurotrophic factors to promote endogenous remyelination. Furthermore, NSCs also produce matrix metallo proteinases that degrade extracellular matrix and cell surface molecules that impede axonal regeneration, thus enabling axons to extend through the glial scar [68]. Meanwhile, stem cells also provide exogenous neurons and oligodendrocytes to restore myelin and axons.
6. Challenges confronting clinical application of iPSC-derived stem cells in MS
IPSCs are similar to ESCs in morphology and ability to proliferate and differentiate, which makes them a promising candidate for regenerative medicine. The fact that they are easily obtained from somatic cells makes their use free from ethical concerns. Furthermore, patient-derived iPSCs are immunologically privileged for transplantation, eliminating the need for lifelong immunosuppressive drugs. These advantages make iPSC-derived stem cells attractive for clinical application in MS treatment. However, many obstacles need to be surmounted before this can become a reality. These obstacles include the genomic instability of iPSCs, which is potentially tumorigenic. Some TFs, such as c-Myc, used to generate iPSCs are potent oncogenes [69]. In addition to these safety concerns, current methods for generating iPSCs are inefficient and time-consuming, and there remains a tremendous gap between generation of iPSC-derived stem cells for research and for therapeutic purposes [70, 71].
In addition to the common challenges with iPSCs, MS adds three more challenges to the complex, in vivo microenvironment surrounding transplanted iPSC-derived stem cells in CNS foci: 1) persistent CNS inflammation, including BBB disruption, high numbers of infiltrating immune cells, and increased levels of proinflammatory cytokines, nitric oxide and other cytotoxic molecules, etc. [72, 73]; 2) loss of trophic support for both oligodendrocytes and neurons [74]; and 3) accumulation of neuroregeneration inhibitors, including myelin-associated glycoprotein, oligodendrocyte myelin glycoprotein and Nogo A [75, 76]. The vicious cycle involving these mechanisms results in a hostile microenvironment, which not only leads to the failure of endogenous remyelination, but would also inhibit for the regenerating capacity of transplanted (exogenous) cells. Approaches to convert the hostile CNS microenvironment to a supportive one needs to be further studied for future MS therapy.
7. Conclusions
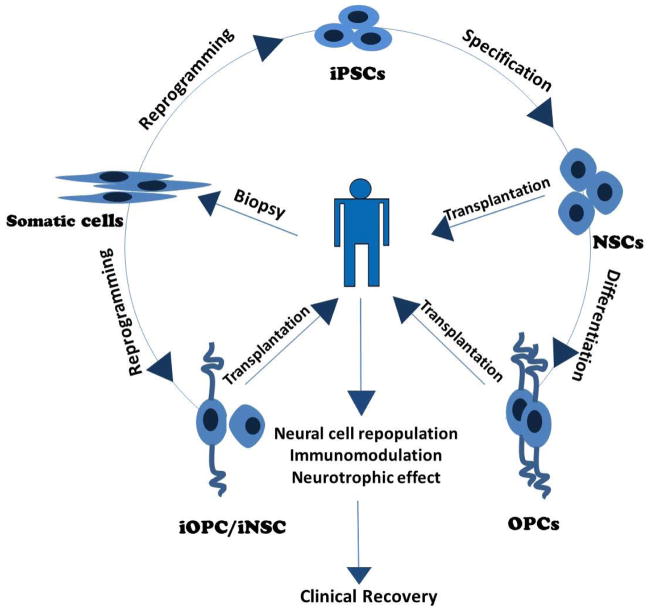
In this review we summarized the recent advances in application of iPSC-derived stem cells in treatment strategies for MS/EAE (summarized in Fig. 1). While NSCs and MSCs have long been tested as an effective MS/EAE therapy, the successful generation of iPSCs from somatic cells opens up a new era of stem cell therapy. The advantages of being easily obtained from the patient’s own tissue and being well tolerated make iPSC-derived stem cells, especially iNSCs and iOPCs, the most suitable candidates for individualized cell replacement therapy. After being examined in EAE and other demyelinated models, iPSC-derived stem cells have shown great potential in the treatment of MS. Although there are still many problems to be solved before clinical application, we anticipate that, with the dramatic progress in the iPSC field, these challenges can be met, making iPSC-derived stem cell transplantation an autologous, safe and highly effective therapy for MS.
Fig. 1. Application of induced stem cells in treatment of MS.
iPSCs: induced pluripotent stem cells; NSCs: neural stem cells; OPCs: oligodendrocyte progenitor cells; iNSC: induced neural stem cell; iOPC: induced oligodendrocyte progenitor cell.
Abbreviations
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- BM
bone marrow
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- eGFP
enhanced green fluorescent protein
- ESC
embryonic stem cell
- iNSC
induced neural stem cell
- iOPC
induced oligodendrocyte progenitor cell
- iPSC
induced pluripotent stem cell
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MSC
mesenchymal stem cell
- NGF
nerve growth factor
- NSC
neural stem cell
- OPC
oligodendrocyte progenitor cell
- PLP
proteolipid protein
- PPMS
primary progressive multiple sclerosis
- RRMS
relapsing remitting multiple sclerosis
- STX
sialyltransferase X
- TF
transcription factor
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. Atlas: Multiple Sclerosis Resources in the World 2008. Geneva: World Health Organization; 2008. pp. 14–15. [Google Scholar]
- 4.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26(9):485–95. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poser CM, Brinar VV. Diagnostic criteria for multiple sclerosis. Clin Neurol Neurosurg. 2001;103(1):1–11. doi: 10.1016/s0303-8467(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 9.Khan OA, Tselis AC, Kamholz JA, Garbern JY, Lewis RA, Lisak RP. A prospective, open-label treatment trial to compare the effect of IFN beta-1a (Avonex), IFNbeta-1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing-remitting multiple sclerosis. Eur J Neurol. 2001;8(2):141–8. doi: 10.1046/j.1468-1331.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Sandberg-Wollheim M, Bever C, Carter J, et al. Comparative tolerance of IFN beta-1a regimens in patients with relapsing multiple sclerosis. The EVIDENCE study. J Neurol. 2005;252(1):8–13. doi: 10.1007/s00415-005-0589-2. [DOI] [PubMed] [Google Scholar]
- 11.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 12.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–56. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 13.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 15.Chahin S, Balcer LJ, Miller DM, Zhang A, Galetta SL. Vision in a Phase 3 Trial of Natalizumab for Multiple Sclerosis: Relation to Disability and Quality of Life. J Neuroophthalmol. 2014 doi: 10.1097/WNO.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reekmans KP, Praet J, De Vocht N, et al. Clinical potential of intravenous neural stem cell delivery for treatment of neuroinflammatory disease in mice? Cell Transplant. 2011;20(6):851–69. doi: 10.3727/096368910X543411. [DOI] [PubMed] [Google Scholar]
- 17.Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–27. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Song B, Sun G, Herszfeld D, et al. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. 2012;8(2):259–73. doi: 10.1016/j.scr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Douvaras P, Wang J, Zimmer M, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014;3(2):250–9. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laterza C, Merlini A, De Feo D, et al. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun. 2013;4:2597. doi: 10.1038/ncomms3597. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Bates J, Li X, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12(2):252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells as treatment for MS - progress to date. Mult Scler. 2013;19(5):515–9. doi: 10.1177/1352458512464686. [DOI] [PubMed] [Google Scholar]
- 24.Lutz SE, Lengfeld J, Agalliu D. Stem cell-based therapies for multiple sclerosis: recent advances in animal models and human clinical trials. Regen Med. 2014;9(2):129–32. doi: 10.2217/rme.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Hur T, Einstein O, Mizrachi-Kol R, et al. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41(1):73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- 27.Einstein O, Karussis D, Grigoriadis N, et al. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24(4):1074–82. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Pluchino S, Quattrini A, Brambilla E, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 29.Pluchino S, Zanotti L, Rossi B, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436(7048):266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 30.Einstein O, Fainstein N, Vaknin I, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007;61(3):209–18. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 31.Gao Z, Wen Q, Xia Y, et al. Osthole augments therapeutic efficiency of neural stem cells-based therapy in experimental autoimmune encephalomyelitis. J Pharmacol Sci. 2014;124(1):54–65. doi: 10.1254/jphs.13144fp. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Jiang Z, Fitzgerald DC, et al. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009;119(12):3678–91. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Yan Y, Xia Y, et al. Neurotrophin 3 transduction augments remyelinating and immunomodulatory capacity of neural stem cells. Mol Ther. 2014;22(2):440–50. doi: 10.1038/mt.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Politi LS, Bacigaluppi M, Brambilla E, et al. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells. 2007;25(10):2583–92. doi: 10.1634/stemcells.2007-0037. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Hur T, van Heeswijk RB, Einstein O, et al. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn Reson Med. 2007;57(1):164–71. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Yan Y, Ma CG, et al. Accelerated and enhanced effect of CCR5-transduced bone marrow neural stem cells on autoimmune encephalomyelitis. Acta Neuropathol. 2012;124(4):491–503. doi: 10.1007/s00401-012-0989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sher F, Amor S, Gerritsen W, et al. Intraventricularly injected Olig2-NSCs attenuate established relapsing-remitting EAE in mice. Cell Transplant. 2012;21(9):1883–97. doi: 10.3727/096368911X637443. [DOI] [PubMed] [Google Scholar]
- 38.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195(1):16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27(10):2624–35. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 41.Yousefi F, Ebtekar M, Soleimani M, Soudi S, Hashemi SM. Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE) Int Immunopharmacol. 2013;17(3):608–16. doi: 10.1016/j.intimp.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Payne NL, Sun G, McDonald C, et al. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant. 2013;22(8):1409–25. doi: 10.3727/096368912X657620. [DOI] [PubMed] [Google Scholar]
- 43.Kassis I, Petrou P, Halimi M, Karussis D. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunol Lett. 2013;154(1–2):70–6. doi: 10.1016/j.imlet.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Dang S, Xu H, Xu C, et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy. 2014;10(7):1301–15. doi: 10.4161/auto.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glenn JD, Smith MD, Calabresi PA, Whartenby KA. Mesenchymal stem cells differentially modulate effector CD8+ T cell subsets and exacerbate experimental autoimmune encephalomyelitis. Stem Cells. 2014;32(10):2744–55. doi: 10.1002/stem.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon D, Pavlovska G, Glover CP, Uney JB, Wraith D, Scolding NJ. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neurosci Lett. 2008;448(1):71–3. doi: 10.1016/j.neulet.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 48.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 50.Tokumoto Y, Ogawa S, Nagamune T, Miyake J. Comparison of efficiency of terminal differentiation of oligodendrocytes from induced pluripotent stem cells versus embryonic stem cells in vitro. J Biosci Bioeng. 2010;109(6):622–8. doi: 10.1016/j.jbiosc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa S, Tokumoto Y, Miyake J, Nagamune T. Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells. In Vitro Cell Dev Biol Anim. 2011;47(7):464–9. doi: 10.1007/s11626-011-9435-2. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Yan Y, Ciric B, et al. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010;177(4):1989–2001. doi: 10.2353/ajpath.2010.091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czepiel M, Balasubramaniyan V, Schaafsma W, et al. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia. 2011;59(6):882–92. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- 54.Pouya A, Satarian L, Kiani S, Javan M, Baharvand H. Human induced pluripotent stem cells differentiation into oligodendrocyte progenitors and transplantation in a rat model of optic chiasm demyelination. PLoS One. 2011;6(11):e27925. doi: 10.1371/journal.pone.0027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diecke S, Jung SM, Lee J, Ju JH. Recent technological updates and clinical applications of induced pluripotent stem cells. Korean J Intern Med. 2014;29(5):547–57. doi: 10.3904/kjim.2014.29.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamaki S, Tokumoto Y. Overexpression of cyclin dependent kinase inhibitor P27/Kip1 increases oligodendrocyte differentiation from induced pluripotent stem cells. In Vitro Cell Dev Biol Anim. 2014;50(8):778–85. doi: 10.1007/s11626-014-9753-2. [DOI] [PubMed] [Google Scholar]
- 57.Czepiel M, Leicher L, Becker K, Boddeke E, Copray S. Overexpression of polysialylated neural cell adhesion molecule improves the migration capacity of induced pluripotent stem cell-derived oligodendrocyte precursors. Stem Cells Transl Med. 2014;3(9):1100–9. doi: 10.5966/sctm.2014-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdullah AI, Pollock A, Sun T. The path from skin to brain: generation of functional neurons from fibroblasts. Mol Neurobiol. 2012;45(3):586–95. doi: 10.1007/s12035-012-8277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lujan E, Wernig M. The many roads to Rome: induction of neural precursor cells from fibroblasts. Curr Opin Genet Dev. 2012;22(5):517–22. doi: 10.1016/j.gde.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Efe JA, Zhu S, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108(19):7838–43. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thier M, Worsdorfer P, Lakes YB, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10(4):473–9. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Han DW, Tapia N, Hermann A, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10(4):465–72. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Ring KL, Tong LM, Balestra ME, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11(1):100–9. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109(7):2527–32. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Najm FJ, Lager AM, Zaremba A, et al. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31(5):426–33. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang N, Zuchero JB, Ahlenius H, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31(5):434–9. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci. 2007;27(17):4499–506. doi: 10.1523/JNEUROSCI.0200-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasi CE, Dereli-Oz A, Negrini S, et al. Genomic instability in induced stem cells. Cell Death Differ. 2011;18(5):745–53. doi: 10.1038/cdd.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–23. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Gao Y, Gordon A, Wang ZZ, Qian Z, Wu WS. Efficient generation of fully reprogrammed human iPS cells via polycistronic retroviral vector and a new cocktail of chemical compounds. PLoS One. 2011;6(10):e26592. doi: 10.1371/journal.pone.0026592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sobottka B, Harrer MD, Ziegler U, et al. Collateral Bystander Damage by Myelin-Directed CD8+ T Cells Causes Axonal Loss. The American Journal of Pathology. 2009;175(3):1160–1166. doi: 10.2353/ajpath.2009.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ortiz GG, Pacheco-Moises FP, Bitzer-Quintero OK, et al. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659. doi: 10.1155/2013/708659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 75.Mi S, Hu B, Hahm K, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13(10):1228–33. doi: 10.1038/nm1664. [DOI] [PubMed] [Google Scholar]
- 76.McDonald CL, Bandtlow C, Reindl M. Targeting the Nogo receptor complex in diseases of the central nervous system. Curr Med Chem. 2011;18(2):234–44. doi: 10.2174/092986711794088326. [DOI] [PubMed] [Google Scholar]