Abstract
The machinery at the eukaryotic replication fork has seen many new structural advances using electron microscopy and crystallography. Recent structures of eukaryotic replisome components include the Mcm2-7 complex, the CMG helicase, DNA polymerases, a Ctf4 trimer hub and the first look at a core replisome of 20 different proteins containing the helicase, primase, leading polymerase and a lagging strand polymerase. The eukaryotic core replisome shows an unanticipated architecture, with one polymerase sitting above the helicase and the other below. Additionally, structures of Mcm2 bound to an H3/H4 tetramer suggest a direct role of the replisome in handling nucleosomes, which are important to DNA organization and gene regulation. This review provides a summary of some of the many recent advances in the structure of the eukaryotic replisome.
Introduction
The structure of DNA is elegant in its simplicity, requiring only four nucleotide bases to encode the blueprints for virtually every living organism. The DNA structure immediately suggested the need for an unwinding enzyme, and perhaps a polymerase enzyme for its replication [1]. But despite its elegance, replication of DNA is quite complicated and numerous unanticipated factors were required, and are still being discovered today [2,3]. Many of the proteins that replicate DNA act together in a complex with moving parts referred to as a ‘replisome’, loosely analogous to a sewing machine. The core replication proteins, present in all cell types from bacteria to archaea and eukarya, include a helicase that separates the duplex, a priming enzyme that synthesizes a short RNA primer, DNA polymerases that extend primed sites to synthesize two new daughter strands, ring-shaped DNA sliding clamps that bind and tether the polymerases to DNA, a clamp loader that assembles the clamps on DNA, and a single-strand (ss) DNA binding protein [4,5]. DNA polymerases can only extend DNA in the 3′–5′ direction because the dNTP substrates are 5′ activated. Therefore, only one strand of the antiparallel duplex is synthesized in the same direction as fork unwinding (i.e. the leading strand), while the other strand is extended in the opposite direction, which requires repeated reinitiation events resulting in the creation of Okazaki fragments (i.e. the lagging strand). Each Okazaki fragment is initiated by an RNA primase, and the RNA is later removed and replaced with DNA, enabling ligase to seal Okazaki fragments together. The decision to replicate DNA is made at origin sequences in a highly regulated process that precedes replisome assembly [6,7]. However, eukaryotic replisomes are also regulated in a wide variety of ways. For example, checkpoint and DNA damage control pathways result in post-translational modifications that regulate the activity and stability of eukaryotic replisomes [8,9]. Eukaryotic replisomes must also function with nucleosomes carrying post-translational modifications that are important to gene expression.
Structural information is highly informative about function. Indeed, there are numerous examples of functional insights derived from protein structures that were unanticipated by genetics or biochemistry alone. Thus, new methods in electron microscopy (EM) analysis for high resolution single particle 3D reconstruction is rapidly advancing the structural biology field, and DNA replication is no exception. Here we review recent structures determined by EM and also crystallography that relate specifically to the eukaryotic replisome. We start with the helicase, which acts as the central organizer of the eukaryotic replisome.
The CMG Helicase
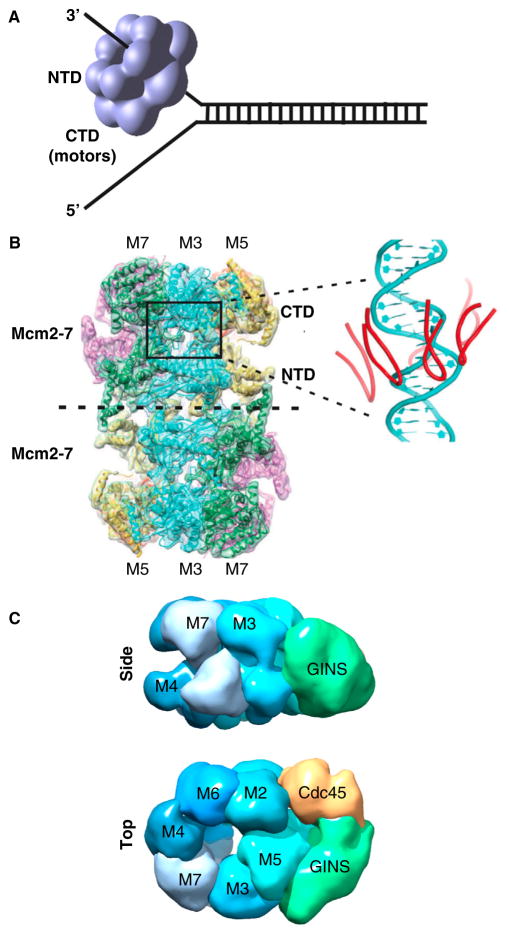
Cells from all three domains of life — bacteria, archaea and eukaryotes — utilize hexameric helicases that encircle DNA to unwind the replication fork (reviewed in [10–13]). Hexameric helicases appear as two rings stacked on top of one another because each subunit is dumbbell shaped, having an amino-terminal domain (NTD) and carboxy-terminal domain (CTD). Thus, viewed from the side, the hexamer has two tiers that appear as two stacked rings (Figure 1A). The ATPase motor regions are contained within the CTD tier, while the NTD tier forms tight interactions that hold the hexamer together. The eukaryotic CMG helicase contains a hexameric core, but functions as an 11-subunit complex that can be pulled out intact from budding yeast [14] and was isolated and characterized in the Drosophila system [15,16]. The hexameric core of CMG is the Mcm2-7 complex of motor subunits, along with five additional accessory factors that do not have ATP sites but are required for significant activity [17,18]. The Mcm2-7 of Drosophila and human display no activity without the five tightly associated accessory factors, Cdc45 and the four-subunit GINS complex (Psf1,2,3 and Sld5) [15,19]. The Cdc45/GINS accessory factors are proposed to act by bringing the Mcm2-7 motor subunits into the proper conformation for helicase activity [15,16]. Archaea contain only one Mcm subunit that forms a homohexamer and has unwinding activity without other proteins [20,21]. The CTD motor domains of the Mcm subunits are constructed from the AAA+ fold (ATPases associated with diverse cellular activities), as are the eukaryotic viral helicases, bovine papilloma virus E1 (BPV E1) and simian virus 40 large T-antigen (T-Ag) (reviewed in [12]). In contrast, the CTD motor domains of bacterial DnaB and bacteriophage T4 and T7 homohexameric helicases are constructed from the RecA fold [12].
Figure 1. The eukaryotic CMG helicase.
(A) Steric exclusion model of helicase unwinding. All replicative helicases contain hexameric motor rings that can encircle and translocate along one strand of ssDNA, excluding the other strand. Individual subunits of hexameric helicases are composed of amino- and carboxy-terminal domains (NTD and CTD, respectively); the ATP site is in the CTD. (B) CryoEM atomic structure of the Mcm2-7 double hexamer of S. cerevisiae. The inset, right, shows the six h2i loops in the interior channel that closely approach DNA modeled into the structure. Reprinted by permission from Macmillan Publishers Ltd: Nature [40], copyright 2015. (C) EM 3D reconstruction of S. cerevisiae CMG in side and carboxy-terminal top views. Representations were of EMD 6463 from [43] using Chimera.
The hexameric helicases are thought to unwind DNA by encircling one DNA strand and translocating along it, excluding the other strand and thus acting as a wedge to unwind the duplex, referred to as the steric exclusion model of DNA unwinding (illustrated in Figure 1A) [22–26]. However, many hexameric helicases have also been demonstrated to translocate over double-stranded (ds)DNA [19,27–29]. Thus, it remains possible that dsDNA enters the helicase for strand separation inside the hexamer, with one (or two) strand(s) extruded out the side(s) between the NTD and CTD, referred to as the side channel extrusion or plowshare model of unwinding [30,31]. While the AAA+ helicases and RecA-based hexameric helicases operate by the steric exclusion mechanism, they have opposite directions of translocation [32]. The eukaryotic AAA+ based hexameric helicases travel 3′–5′, placing them on the leading strand of a replication fork [22,23]. In contrast, RecA based bacterial helicases travel 5′–3′, placing them on the lagging strand [25].
Unidirectional translocation along DNA requires a protein to have two or more grips to DNA, enabling it to ‘walk’ or ‘inch-worm’ along it. Inchworm processes have been determined for the non-replicative monomeric helicases and their crystal structures show that they use two RecA domains to bind ssDNA [33,34]. As ATP is hydrolyzed, the distance between the two domains changes, enabling movement along DNA with repeated cycles of hydrolysis. Most crystal structures of hexameric helicases lack DNA, but their motor domains contain conspicuous loops that protrude into the central channel and are thought to function with DNA [23,25,30,35]. The BPV E1 and bacterial DnaB helicase structures are determined in complex with a ssDNA oligonucleotide and confirm that loops within the central channel bind DNA [23,25]. In BPV E1, the motor domains are arranged in a right-hand spiral and the loops point up or down depending on the nucleotide bound state [23]. It is proposed that BPV E1, and probably other hexameric helicases, have six grips to DNA (i.e. DNA binding loops on each subunit) and that sequential ATP hydrolysis around the ring moves DNA through the central pore of the helicase [13]. Although sequential hydrolysis occurs in a rotary fashion, neither the helicase nor DNA actually need to rotate in this model.
Formation of eukaryotic CMG is segregated into two distinct stages of the cell cycle (reviewed in [6,7,36–38]. In G1 phase, two Mcm2-7 hexamers are assembled around dsDNA adjacent to an origin requiring ORC (origin recognition complex), Cdc6 and Ctd1. In proceeding to S phase, kinases and several other initiation factors (e.g. Sld2, Sld3, Dpb11, Mcm10) remodel the double hexamer to form two CMG complexes that each encircle a ssDNA for bidirectional replication. The entire process of initiation and formation of replication forks has recently been reconstituted from pure proteins [39]. The cryoEM atomic resolution structure of the Saccharomyces cerevisiae Mcm2-7 double hexamer has also recently been determined (Figure 1B) [40]. Among the many interesting details, six DNA binding loops located in the AAA+ region of the motor domains are arranged in a right hand spiral, similar to BPV E1. In addition, the NTD of each Mcm2-7 hexamer contains loops that protrude into the central channel and are associated with OB domains. Genetic studies have demonstrated that the OB domains of S. cerevisiae Mcm4, Mcm6 and Mcm7 are required for replication in vivo, and the crystal structure of the analogous loops in an archaeal Mcm NTD reveal that these OB domains bind ssDNA [41].
Using single particle EM, the 3D structure of Drosophila melanogaster CMG was determined [42], and more recently the 18 Å resolution EM structure of S. cerevisiae CMG [43]. Figure 1C shows the top and side views of CMG. The side-view demonstrates the two-tiered structure of the Mcm2-7 ring. The top view shows that the Cdc45 and GINS accessory factors are located to one side of the Mcm2-7 ring and form a secondary, smaller channel. It has been proposed that the lagging strand might occupy the secondary channel [42]. Another possible function for the accessory factors stems from the need for the Mcm2-7 ring to open at the Mcm2/5 interface during origin assembly [44]. The accessory factors span the Mcm2/5 interface, and recent studies indicate they may prevent DNA from escaping through the Mcm2/5 interface that periodically re-opens during helicase action [45]. The GINS tetramer also functions as an organizing center for the binding of other replisome proteins, as will be discussed below.
DNA Polymerases and the Ctf4 Trimer Hub
Eukaryotes require three B family DNA polymerases to propagate fork movement [46]. These include Pol epsilon, Pol delta and Pol alpha-primase. Many genetic and biochemical experiments in budding and fission yeast assign Pol epsilon to the leading strand and Pol delta to the lagging strand [47–51], although one report indicates Pol delta functions on both strands [52]. Both Pol epsilon and Pol delta function with the circular PCNA clamp for processive synthesis [46,48]. The circular processivity clamp was the first protein discovered to encircle DNA and the first of the many replisome proteins to be solved structurally [53,54].
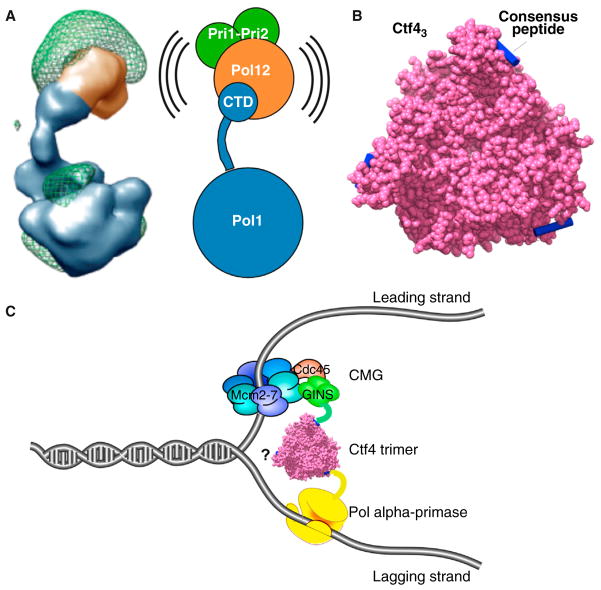
Pol alpha primase consists of 4 subunits, and unlike the other two replicative polymerases, it lacks a proofreading 3′-5′ exonuclease and contains an additional RNA primase activity [55]. Crystal structures exist for individual subunits of Pol alpha [56–58], while single particle EM 3D reconstruction studies have provided the 3D architecture of the Pol alpha holoenzyme [59] (Figure 2A). The largest subunit, Pol1, contains the DNA polymerase as well as a CTD that is connected to the polymerase region by a flexible stalk [59]. The second largest subunit, Pol12, and the two smallest subunits that function together for primase activity, bind to the CTD at the end of the flexible stalk of Pol1. Pol alpha holoenzyme generates a hybrid RNA–DNA primer of about 25–30 nucleotides [55]. After about 7 ribonucleotides, the RNA switches from the primase to the Pol1 site for DNA extension [57,60]. This switch occurs without enzyme dissociation and it is proposed that the flexible tether between the two active centers may facilitate this action [56].
Figure 2. Pol alpha–primase and the Ctf4 trimer.
(A) EM 3D reconstruction of the four-subunit Pol alpha from S. cerevisiae. The Pol1 polymerase subunit contains a CTD linked by a flexible tether to which the other subunits attach. Adapted from [59] by permission of Oxford University Press. (B) Surface representation of the C-half of S. cerevisiae Ctf4 trimer (magenta), with bound peptides modeled into the peptide binding pockets (blue). Drawn using Chimera and PDB 4CBH from [61]. (C) Cartoon of a Ctf4 trimer binding GINS within CMG (leading strand), and Pol alpha, inferred from Ctf4–peptide interactions. One subunit of Ctf4 may interact with other binding partners yet to be identified (question mark). Both B and C are reprinted by permission from Macmillan Publishers Ltd: Nature [61], copyright 2014.
Mass spectrometry analysis of CMG pull-outs from cell lysates reveal that Pol alpha associates with CMG, along with many other proteins including Ctf4, Tof1, Mrc1, Csm3, TopoI, Mcm10, and FACT [14]. Thus, these many factors are thought to travel as a complex at the replication fork, referred to as the RPC (replisome progression complex) [14]. Recent EM studies reveal that Ctf4 is a homotrimer; each subunit is composed of amino- and carboxy-terminal halves that are connected by a highly flexible linker [61]. Ctf4 is known to bind Pol alpha [62] and Ctf4 also binds the GINS complex [63,64]. The crystal structure of the carboxy-terminal half of Ctf4 reveals a homotrimer that can bind consensus peptide sequences found in both Pol1 (of Pol alpha) and Sld5 (of GINS) (Figure 2B) [61]. Hence, the Ctf4 trimer is proposed to bridge leading and lagging strand components within the replisome, as illustrated in Figure 2C. Since Ctf4 is a homotrimer, it may bind other proteins yet to be identified (Figure 2C). The fact that Ctf4 binds a short peptide sequence suggests it might act as a trafficking center like the PCNA clamp, which interacts with numerous partners in a dynamic fashion through binding of a PIP motif (PCNA-interacting peptide) present in many replication, repair and cell cycle regulatory factors [65,66]. By analogy to PCNA, the Ctf4 trimer may recruit various factors to the fork in a dynamic fashion as they are needed.
Pol epsilon and Pol delta are composed of multiple subunits; their largest subunit is the DNA polymerase [46]. Interestingly, the Pol2 subunit of Pol epsilon consists of two polymerase sequences linked head-to-tail; the amino-terminal half encodes the active polymerase while the carboxy-half encodes an inactive polymerase [67]. Crystal structures of the active polymerase region of Pol2, and of Pol delta have been determined and, as with all other DNA polymerases, they have the shape of a right hand [68,69]. The catalytic amino-terminal half of Pol2 is unique in containing a novel ‘processivity’ domain that closes the cleft between the thumb and fingers to completely encircle DNA [68,70]. Unlike other B-family polymerases, Pols alpha, delta and epsilon contain two cysteine-rich regions in their carboxy-terminal domain that form two metal binding sites; CysA binds a zinc and CysB binds an iron-sulphur cluster [71]. In studies of Pol delta the CysA site is required for function with PCNA and the CysB site is required to bind Pol 31, the second largest subunit, also called the B subunit [71]. The B subunits of Pol alpha (i.e. Pol12) and Pol epsilon (Dpb2) share homology to the B subunit of Pol delta, although the function of B subunits have thus far remained enigmatic.
Reconstitution of a Leading/Lagging Strand Replisome
Recent studies have reconstituted a functional asymmetric replisome of Saccharomyces cerevisiae using pure proteins, and have determined the minimal set required for leading/lagging strand replication in vitro [72,73]. The studies show that Pol epsilon/RFC/PCNA functions with CMG on the leading strand, while Pol delta/RFC/PCNA is much less active and can not gain access to the leading strand in the presence of Pol epsilon. The studies also showed that priming by Pol alpha occurs on both strands, but requires interaction with CMG when the RPA single strand binding protein is present. Unexpectedly, Pol epsilon/RFC/PCNA could not extend lagging strand primers. However, as expected, Pol delta/RFC/PCNA was highly active in extension of lagging strand primers [72,73]. Hence, assembly of a three polymerase leading/lagging strand asymmetric replisome with Pol epsilon and Pol delta on the leading and lagging strands is inherent within the 31 distinct subunits comprised by CMG, Pol alpha, Pol epsilon, Pol delta, the RFC clamp loader, PCNA clamp and RPA [72,73]. Therefore, the numerous additional factors that travel with the eukaryotic replisome are not required for asymmetric polymerase function at the fork, and likely facilitate replication control.
Study of subunit interactions among the replisome proteins established several important protein–protein interactions. In fact, the eukaryotic replisome proteins adhere to one another much tighter than proteins of the E. coli and phage T4 and T7 replisomes. Reconstitution studies demonstrated that Pol epsilon binds CMG to form a 15-protein polymerase–helicase complex, referred to as CMGE [74], which likely explains the much greater activity of Pol epsilon with CMG compared to Pol delta with CMG. Ctf4 forms an isolatable complex with CMG [64]. EM structural studies of replisome complexes will be discussed below.
Structure of the Core Eukaryotic Replisome
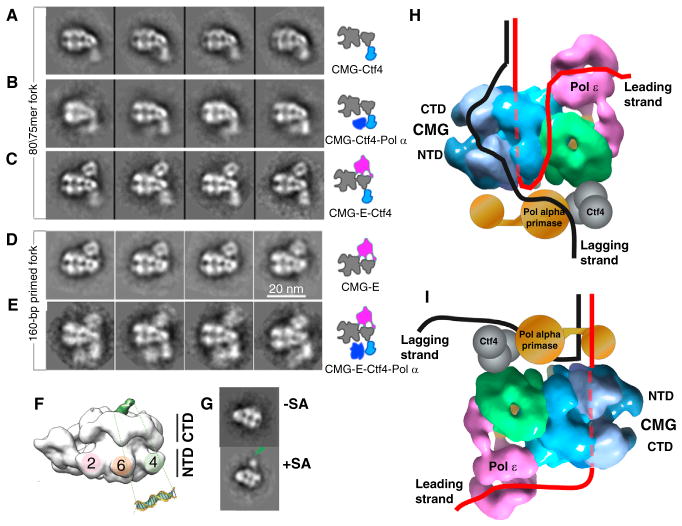
We have recently determined the 3D structure of the CMG–Pol epsilon complex (CMGE; Figure 3), and arrangement of Ctf4-Pol alpha with CMGE, by single particle electron microscopy (Figure 4) [43]. Figure 4A shows 2D class averages of CMG and CMGE. Pol epsilon can be seen as an extra appendage on CMG with multiple connections to CMG. The 16 Å resolution CMGE structure shows that Pol epsilon is positioned over the top of the GINS/Cdc45 accessory factors (Figure 3B). The Pol epsilon density extends above the plane of the CTD-tier of the Mcm2-7 ring. The subunit assignments of CMG and placement of Pol epsilon at the CTD-tier of Mcm2-7 within CMG were confirmed by application of a powerful new technique that can map subunits within EM structures [43]. This technique, cross-linking with mass spectrometry readout (CX-MS), is performed by reacting a multiprotein complex with a bifunctional cross-linker, then digestion with protease followed by mass spectrometry to identify the cross-linked peptides [75]. The CX-MS technique, applied to CMGE, provided over 550 cross-linked positions, among which over 150 were intersubunit cross-links [43]. The intersubunit cross-links provide information about the proximity of subunits within the complex, and the CX-MS data confirmed the arrangement of each of the 11 CMG subunits and that Pol epsilon is on the CTD-side of CMG.
Figure 3. The leading strand CMGE replisome.

(A) EM 2D averages of S. cerevisiae CMG are in the top row and CMGE (i.e. CMG + Pol epsilon) is in the bottom row. (B) 3D reconstruction of S. cerevisiae CMGE. CMGE is drawn with Chimera using EMD-6465 from [43].
Figure 4. Core replisome architecture.
(A–E) 2D class averages of complexes as depicted in the illustrations to the right of each row of images. Adapted from Figure 5 of [42]. (F) DNA path through CMG determined in the Drosophila system [79]. The DNA duplex region (green) was visible in the negative stain 3D reconstruction. (G) This was confirmed by a streptavidin-biotin tag (SA) on the 20 bp duplex. Both F and G are adapted with permission from Figure 2 of [79]. (H) Combining the DNA path from the Drosophila CMG–DNA structure (79) with the S. cerevisiae CMGE structure and the class averages of panels A–E [42] places the Pol epsilon on the top (C-face) of CMG adjacent to the parental duplex. After the unwound leading strand exits the NTD of CMG, it needs to make a U-turn to reach Pol epsilon on the top, illustrated as taking an external path, but the ssDNA may take an internal path through CMG. In the steric exclusion model, the lagging strand is on the outside of CMG to reach Pol alpha on the N-side. CMGE was drawn with Chimera using EMD-6465 from [42]. (I) If DNA were to enter the NTD of the Mcm2-7, the Pol epsilon would reside below the CMG and Pol alpha primase would ride near the DNA split point, where it could prime the lagging strand.
The crystal structure of the catalytic amino-terminal half of Pol2 can be docked reasonably well into the stalk of density that extends above CMG in the EM structure of CMGE, although given that Pol2 may contain two polymerase structures we can not be sure this density is the catalytic polymerase [43]. The Dpb3 and Dpb4 subunits are small histone fold proteins and the known dimensions of the homologous Drosophila Dsl1/Dpb4 heterodimer [76] suggests a possible location of Dpb3/4 in the CMGE structure (Figure 3B). The density of Pol epsilon in the CMGE complex accounts for about 70% of the Pol epsilon mass, suggesting that a region of Pol epsilon may have sufficient flexibility to be lost from the averaged density, although other explanations are possible (discussed in [43]). An earlier EM study of Pol epsilon demonstrated that Pol2 is a compact globular shape, without a flexible hinge between the active and inactive polymerase halves [77]. It is not known which region of Pol epsilon, if any, is missing from the observed density. But if some region of Pol epsilon, such as the catalytic amino-terminal half of Pol2, were flexibly attached and thus not present in the density, the CX-MS technique that uses a short cross-linker reveals that it must be within or adjacent to the observed density.
Despite decades of research on bacterial replisomes, the structure of a replisome has not yet been imaged. Replisome structural studies have been hampered by weak connections among core components of bacterial replisomes. For example, the E. coli DnaB helicase, DnaG primase and DNA Pol III do not form sufficiently strong connections to isolate complexes among any two of them. Unlike bacterial replisome proteins, several components of the eukaryotic replisome form complexes with sufficient stability to be isolated. We have determined the architecture of the eukaryotic core replisome by building the replisome up in stages during single particle electron microscopy [43]. Figure 4 shows 2D class averages of replisome subcomplexes, arriving at a final core replisome of 20 different proteins that contains the CMG helicase, leading strand Pol epsilon, Ctf4 scaffold trimer, and lagging strand Pol alpha polymerase–primase. The 2D averages in Figure 3A show side views of CMG and the characteristic two layer CTD–NTD structure of the Mcm2-7 subunits with the GINS/Cdc45 as a knob to the right of Mcm2-7. Figure 4A shows 2D averages of the CMG–Ctf4 complex, in which Ctf4 is located on the NTD side of CMG. The Ctf4 used for the CMG–Ctf4 complex is full-length protein and appears fuzzy, consistent with the flexible joint observed between the amino- and carboxy-terminal halves of Ctf4 in an EM study [61]. As discussed earlier, Ctf4 binds to Pol alpha [61,62], and thus Ctf4 is a proxy for the location of the lagging strand Pol alpha. Figure 4B shows 2D class averages of CMG–Ctf4–Pol alpha in which Pol alpha density is observed adjacent to Ctf4, and located on the amino-terminal side of the Mcm ring. This is consistent with interaction between Pol alpha and the Mcms reported in an earlier study [78]. Figure 4C shows 2D class averages of CMGE–Ctf4, demonstrating that Pol epsilon and Ctf4 occupy opposite sides of CMG. Figure 4D is a side view of CMGE in the presence of a primed forked DNA. Figure 4E shows 2D averages of the 20 protein CMGE–Ctf4–Pol alpha core replisome with a primed replication fork substrate. The core replisome inferred from the 2D images, along with the CMGE structure, is shown in the diagram to the right in Figure 4E.
The EM structural results reveal that the two polymerases are on opposite sides of the CMG helicase [43]. Thus, one polymerase must ride on top of the helicase, ahead of the unwinding point. This was unexpected, as it has been widely thought that DNA polymerases would follow behind the helicase to operate on the unwound strands. Thus, the question arises as to which polymerase is sitting on top of CMG, above the unwinding point, and which polymerase is below. While the EM studies of Figure 4A–E were performed in the presence of forked DNA, EM grids were prepared by the negative stain procedure which does not clearly visualize DNA. Hence, the DNA path is not determined in the replisome EM structure study.
DNA Threading through the Replisome
The path of DNA through the Drosophila CMG has previously been determined in EM studies by the Botchan and Berger labs [79], and given the similarity in CMG structures one may presume the DNA path will be the same in S. cerevisiae CMG. The Drosophila CMG–DNA EM study used a primed DNA structure and negative stain, and the 20 bp duplex portion of the DNA was observed protruding from the CTD tier (Figure 4F). To confirm the assignment of DNA one end of the duplex was labeled with biotin-streptavidin (Figure 4G) [79]. The result demonstrates that the leading strand enters into the Mcm2-7 ring of CMG at the CTD face. The CTD-to-NTD direction of leading strand DNA passage through the Mcms is also consistent with studies of archaeal MCM helicase [80].
Combining the DNA path with the organization of the core CMGE–Ctf4–Pol alpha replisome presents a surprising and unanticipated architecture in which Pol epsilon is at the prow of the fork, ahead of the helicase (illustrated in Figure 4H) [43]. Therefore, when the leading strand ssDNA exits from the amino-terminal side of Mcm2-7, it must make a U-turn to reach Pol epsilon at the carboxy-terminal side of CMG, above the forked junction. The length of ssDNA required to pass through CMG and turn back to Pol epsilon at the top of CMG predicts a length of at least 40 nucleotides. Interestingly, there is experimental support in the Xenopus system for a sizable gap on the leading strand, extending 20–40 nucleotides from the forked junction [24]. The leading strand may traverse the outside of CMG to reach Pol epsilon as illustrated in Figure 4H. There remain many uncertainties about the replisome structure that will require further study. For example, the replisome structure and the DNA threading through it is an intricate process that occurs at origins and requires many factors that do not travel with the fork (e.g. ORC, Cdc6, Cdt1, Sld2, Sld3, Dpb11; reviewed in [6,7,36–38]). Furthermore, many additional factors travel with CMG at a replication fork in vivo (e.g. Mcm10, Tof1, Csm3, Mrc1, FACT and possibly others) [14]. Hence, the architecture of the minimal replisome determined by in vitro reconstitution methods may not accurately reflect the replisome architecture within cells. Thus, it remains possible that the leading DNA strand might enter the NTD tier of the Mcm2-7 when CMG is assembled in vivo, as illustrated in Figure 4I. In this case, Pol epsilon would be positioned behind CMG and Pol alpha primase would be at the top, near the DNA split point for priming the lagging strand. The other replisome enzymes required for fork propagation include the RFC clamp loader and lagging strand Pol delta. To date, replisome complexes containing Pol delta and/or the RFC clamp loader have not been obtained, and their connection to the replisome is still uncertain. Clearly, a secure view of the replisome structure and the way it binds to DNA will require further studies.
Provided Pol epsilon rides ahead of the CMG helicase, what function may this serve? One possible advantage of this structural arrangement is that Pol epsilon has been demonstrated to bind histones, and the replisome must deal with nucleosomes affixed to the parental duplex [81,82]. A replisome with Pol epsilon at the top of CMG places it in a position to encounter nucleosomes on the parental DNA. Furthermore, the Dpb3/4 histone heterodimer of Pol epsilon may function to facilitate nucleosome mobility, similar to proposals for similar histone fold heterodimers found in the CHRAC nucleosome remodeler and in RNA Pol II [83,84]. Indeed, genetic phenotypes of Dpb3 and Dpb4 mutants in yeast show a loss in ability to maintain heterochromatin during DNA replication [83,84].
Mcm2 Interaction with Nucleosomes
The amino-terminal region of Mcm2 binds to histones, and recent crystal structures of a region of Mcm2 bound to the H3/H4 tetramer shows that Mcm2 binds the H3/H4 tetramer at the same positions that DNA binds to H3/H4 (Figure 5A; compare to the structure of the full nucleosome in panel B) [85,86]. This binding mode is common to nucleosome chaperones, suggesting Mcm2 or Mcm2-containing assemblies may serve in this capacity [85]. The proposed DNA path through CMG [79] places the histone-binding region of Mcm2 on the DNA exit face of CMG, opposite the entry point of parental DNA. Thus, if Mcm2 operates on nucleosomes within CMG, it is well positioned to do so on the daughter DNA strands. The Mcm2-7 complex exists in a large excess over CMG in the cell (i.e. the Mcm paradox), and isolated Mcm2 has been demonstrated in vitro to assemble H3/H4 tetramers onto DNA, suggesting it may function alone or within excess Mcm2-7 outside the context of the replisome [85,86]. It is important to note that the crystal structure contains two Mcm2 modules bound to one H3/H4 tetramer, while there is only one copy of Mcm2 in CMG and Mcm2-7. It is therefore not yet proven that the single Mcm2 within CMG and Mcm2-7 is sufficient to bind and assemble an H3/H4 tetramer onto DNA in vitro. Among other hypotheses are that the Mcm2 protein may function alone as a nucleosome chaperone [85,86].
Figure 5. Mcm2 binds the H3/H4 heterotetramer.
(A) Crystal structure of the amino-terminal histone binding region of Mcm2 (pink) with the H3/H4 tetramer (green and blue) drawn using Chimera and PDB 5BNV from [85]. (B) Crystal structure of the nucleosome drawn using Chimera and PDB 1A0I from [90]. Histones H3 and H4 are in green and blue. Histones H2A and H2B are in orange and yellow. The location at which Mcm2 binds is not depicted but can be inferred by comparison to panel A. (C) Crystal structure of the Mcm2 histone binding region (pink) with a H3/H4 heterodimer (green and blue) and Asf1 (black) drawn using Chimera and PDB 5BNX from [85]. Figure reprinted by permission from Macmillan Publishers Ltd: Nature Structural and Molecular Biology [85], copyright 2015.
A crystal structure of Mcm2–H3/H4–Asf1 has also been determined [85,86], and in this case only one Mcm2 is bound to a heterodimer of H3/H4 to which Asf1 is also bound (Figure 5C). Asf1 disrupts H3/H4 tetramers by binding at the H3/H4 tetramerization interface, and this interaction site does not overlap with the Mcm2 binding site on H3/H4. Asf1 did not appear to have a synergistic effect with Mcm2 in H3H4 tetramer assembly reactions onto DNA in vitro, and therefore it is not yet clear whether these two factors function together [85]. It is important to note that nucleosome assembly on daughter DNA is thought to occur via the Caf1 (chromatin assembly factor 1) heterotrimer in a PCNA-dependent reaction [87]. While Asf1 is known to participate with Caf1, it is not yet known whether Mcm2 can function with Caf1. Clearly, the relevance of the Mcm2–H3/H4 interaction during replication will be important to explore in future studies.
The replisome contains many points of connection to histones beyond the Mcm2–H3/H4 interaction. The FACT complex, which binds and helps mobilize histones, is a component of the RPC and travels with replisomes [14]. FACT is a two-subunit complex that helps RNA polymerase transcribe through nucleosome-bound DNA [88]. FACT also binds Pol alpha [88], and its association with RPC suggests it may facilitate replisome progression through nucleosomes as it does for RNA polymerase [14,81]. Other interactions between nucleosome assembly factors and replisome components include binding between the RFC clamp loader and Asf1 [89], and binding between Caf1 and PCNA, required for nucleosome assembly activity as described above. Furthermore, besides the finding that both Pol epsilon and Mcm2 bind histones, recent studies show that Pol alpha also binds histones [81]. Hence, the replisome contains many sites at which it binds histones and chromatin remodeling factors. The replisome probably makes use, directly or indirectly, of several different nucleosome remodelers and chaperones for histone management during replication, as the replisome must deal with essentially every nucleosome in the entire genome. It is possible that the replisome may actively redistribute the parental histone octamers among the daughter strands.
What Needs to Be Done?
There has been a rapid expansion in our knowledge of eukaryotic replisome structure in just the last two years, yet the information brings up more questions than it answers. The present model of the core replisome arrangement begs the question about how the RFC clamp loader and Pol delta fit into the replisome, if at all? Many subunits of the multiprotein polymerases are still unknown. The atomic structure of CMG helicase, the explanation of why it contains six distinct Mcm subunits, the function of Cdc45 and GINS in helicase activity, and the translocation mechanism of the complex are questions that await insight from future structural studies. There are numerous additional proteins that travel with replisomes; how are they organized in the replisome and how do they function? For example, the function of Mcm10 is unknown yet it is part of the RPC and is an essential protein in all cells examined thus far. How do the checkpoint proteins Mrc1, Tof1, and Csm3 fit into the replisome architecture and how do they perform their checkpoint function? Pol epsilon is well established to be involved in checkpoint signaling, but the mechanism and structural changes that accompany this process are completely unknown. Many replisome proteins become phosphorylated in a cell cycle fashion or upon DNA damage, yet the function of these post-translational modifications remain to be determined. Nucleosomes bind tightly to parental DNA, especially in regions of heterochromatin, yet the replisome must be capable of moving through all chromatin structures during a replication cycle. Many replisome proteins are known to bind histones or histone chaperones. How the replisome deals with nucleosomes is an important question to be addressed. The replisome must also deal with collisions with RNA polymerase and transcriptional effectors, and interdigitate with repair and recombinational processes. The replisome must also coordinate its action with huge cohesion rings that keep daughter chromosomes paired during and after replication. These are only a few of the many questions about replisomes that structural studies can inform. Crystal structure analysis as well as the new direct detector technology for EM analysis of large protein complexes promise a bright and exciting future for understanding the central and vital processes needed to duplicate chromosomes.
Acknowledgments
We appreciate help with some of the figures by Dr. Nina Yao (Rockefeller University). This work was funded by the US National Institutes of Health (GM111472 and OD12272 to H.L. and GM115809 to M.O.D.) and Howard Hughes Medical Institute (M.O.D.).
References
- 1.Watson JD, Crick FH. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953;171:964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- 2.Kornberg A, Baker TA. DNA Replication. New York: W.H. Freeman; 1992. [Google Scholar]
- 3.Barros T, Guenther J, Kelch B, Anaya J, Prabhakar A, O’Donnell M, Kuriyan J, Lamers MH. A structural role for the PHP domain in E. coli DNA polymerase III. BMC Struct Biol. 2013;13:8. doi: 10.1186/1472-6807-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benkovic SJ, Valentine AM, Salinas F. Replisome-mediated DNA replication. Annu Rev Biochem. 2001;70:181–208. doi: 10.1146/annurev.biochem.70.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Yao NY, O’Donnell ME. Comparison of bacterial and eukaryotic replisome components. In: Bradshaw RA, Stahl PD, editors. Encyclopedia of Cell Biology. Academic Press; 2015. pp. 396–417. [Google Scholar]
- 6.Costa A, Hood IV, Berger JM. Mechanisms for initiating cellular DNA replication. Annu Rev Biochem. 2013;82:25–54. doi: 10.1146/annurev-biochem-052610-094414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remus D, Diffley JF. Eukaryotic DNA replication control: lock and load, then fire. Curr Opin Cell Biol. 2009;21:771–777. doi: 10.1016/j.ceb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Rodriguez LJ, De Piccoli G, Marchesi V, Jones RC, Edmondson RD, Labib K. A conserved Pol binding module in Ctf18-RFC is required for S-phase checkpoint activation downstream of Mec1. Nucleic Acids Res. 2015;43:8830–8838. doi: 10.1093/nar/gkv799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheu YJ, Kinney JB, Lengronne A, Pasero P, Stillman B. Domain within the helicase subunit Mcm4 integrates multiple kinase signals to control DNA replication initiation and fork progression. Proc Natl Acad Sci USA. 2014;111:E1899–E1908. doi: 10.1073/pnas.1404063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell SP, Kaguni JM. Helicase loading at chromosomal origins of replication. Cold Spring Harb Perspect Biol. 2013;5:a010124. doi: 10.1101/cshperspect.a010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JM. SnapShot: nucleic acid helicases and translocases. Cell. 2008;134:888. doi: 10.1016/j.cell.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- 13.Enemark EJ, Joshua-Tor L. On helicases and other motor proteins. Curr Opin Struct Biol. 2008;18:243–257. doi: 10.1016/j.sbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol. 2006;8:358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 15.Ilves I, Petojevic T, Pesavento JJ, Botchan MR. Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Mol Cell. 2010;37:247–258. doi: 10.1016/j.molcel.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci USA. 2006;103:10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bochman ML, Bell SP, Schwacha A. Subunit organization of Mcm2-7 and the unequal role of active sites in ATP hydrolysis and viability. Mol Cell Biol. 2008;28:5865–5873. doi: 10.1128/MCB.00161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bochman ML, Schwacha A. The Mcm2-7 complex has in vitro helicase activity. Mol Cell. 2008;31:287–293. doi: 10.1016/j.molcel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Kang YH, Galal WC, Farina A, Tappin I, Hurwitz J. Properties of the human Cdc45/Mcm2-7/GINS helicase complex and its action with DNA polymerase epsilon in rolling circle DNA synthesis. Proc Natl Acad Sci USA. 2012;109:6042–6047. doi: 10.1073/pnas.1203734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong JP, Hayashi MK, Simon MN, Xu RM, Stillman B. A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 2000;97:1530–1535. doi: 10.1073/pnas.030539597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelman Z, Lee JK, Hurwitz J. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum DeltaH contains DNA helicase activity. Proc Natl Acad Sci USA. 1999;96:14783–14788. doi: 10.1073/pnas.96.26.14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell SD, Botchan MR. The minichromosome maintenance replicative helicase. Cold Spring Harb Perspect Biol. 2013;5:a012807. doi: 10.1101/cshperspect.a012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enemark EJ, Joshua-Tor L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature. 2006;442:270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- 24.Fu YV, Yardimci H, Long DT, Ho TV, Guainazzi A, Bermudez VP, Hurwitz J, van Oijen A, Scharer OD, Walter JC. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell. 2011;146:931–941. doi: 10.1016/j.cell.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itsathitphaisarn O, Wing RA, Eliason WK, Wang J, Steitz TA. The hexameric helicase DnaB adopts a nonplanar conformation during translocation. Cell. 2012;151:267–277. doi: 10.1016/j.cell.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan DL. The 3′-tail of a forked-duplex sterically determines whether one or two DNA strands pass through the central channel of a replication-fork helicase. J Mol Biol. 2000;301:285–299. doi: 10.1006/jmbi.2000.3965. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan DL, Davey MJ, O’Donnell M. Mcm4,6,7 uses a “pump in ring” mechanism to unwind DNA by steric exclusion and actively translocate along a duplex. J Biol Chem. 2003;278:49171–49182. doi: 10.1074/jbc.M308074200. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan DL, O’Donnell M. DnaB drives DNA branch migration and dislodges proteins while encircling two DNA strands. Mol Cell. 2002;10:647–657. doi: 10.1016/s1097-2765(02)00642-1. [DOI] [PubMed] [Google Scholar]
- 29.Shin JH, Jiang Y, Grabowski B, Hurwitz J, Kelman Z. Substrate requirements for duplex DNA translocation by the eukaryal and archaeal minichromosome maintenance helicases. J Biol Chem. 2003;278:49053–49062. doi: 10.1074/jbc.M308599200. [DOI] [PubMed] [Google Scholar]
- 30.Gai D, Zhao R, Li D, Finkielstein CV, Chen XS. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell. 2004;119:47–60. doi: 10.1016/j.cell.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi TS, Wigley DB, Walter JC. Pumps, paradoxes and ploughshares: mechanism of the MCM2-7 DNA helicase. Trends Biochem Sci. 2005;30:437–444. doi: 10.1016/j.tibs.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Thomsen ND, Berger JM. Running in reverse: the structural basis for translocation polarity in hexameric helicases. Cell. 2009;139:523–534. doi: 10.1016/j.cell.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Yang W. UvrD helicase unwinds DNA one base pair at a time by a two-part power stroke. Cell. 2006;127:1349–1360. doi: 10.1016/j.cell.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velankar SS, Soultanas P, Dillingham MS, Subramanya HS, Wigley DB. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 35.Singleton MR, Sawaya MR, Ellenberger T, Wigley DB. Crystal structure of T7 gene 4 ring helicase indicates a mechanism for sequential hydrolysis of nucleotides. Cell. 2000;101:589–600. doi: 10.1016/s0092-8674(00)80871-5. [DOI] [PubMed] [Google Scholar]
- 36.Botchan M, Berger J. DNA replication: making two forks from one prereplication complex. Mol Cell. 2010;40:860–861. doi: 10.1016/j.molcel.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evrin C, Clarke P, Zech J, Lurz R, Sun J, Uhle S, Li H, Stillman B, Speck C. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc Natl Acad Sci USA. 2009;106:20240–20245. doi: 10.1073/pnas.0911500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remus D, Beuron F, Tolun G, Griffith JD, Morris EP, Diffley JF. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–730. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeeles JT, Deegan TD, Janska A, Early A, Diffley JF. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature. 2015;519:431–435. doi: 10.1038/nature14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Zhai Y, Zhang Y, Li W, Yang M, Lei J, Tye BK, Gao N. Structure of the eukaryotic MCM complex at 3.8 A. Nature. 2015;524:186–191. doi: 10.1038/nature14685. [DOI] [PubMed] [Google Scholar]
- 41.Froelich CA, Kang S, Epling LB, Bell SP, Enemark EJ. A conserved MCM single-stranded DNA binding element is essential for replication initiation. Elife. 2014;3:e01993. doi: 10.7554/eLife.01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa A, Ilves I, Tamberg N, Petojevic T, Nogales E, Botchan MR, Berger JM. The structural basis for MCM2-7 helicase activation by GINS and Cdc45. Nat Struct Mol Biol. 2011;18:471–477. doi: 10.1038/nsmb.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Shi Y, Georgescu RE, Yuan Z, Chait BT, Li H, O’Donnell ME. The architecture of a eukaryotic replisome. Nat Struct Mol Biol. 2015;22:976–982. doi: 10.1038/nsmb.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samel SA, Fernandez-Cid A, Sun J, Riera A, Tognetti S, Herrera MC, Li H, Speck C. A unique DNA entry gate serves for regulated loading of the eukaryotic replicative helicase MCM2-7 onto DNA. Genes Dev. 2014;28:1653–1666. doi: 10.1101/gad.242404.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petojevic T, Pesavento JJ, Costa A, Liang J, Wang Z, Berger JM, Botchan MR. Cdc45 (cell division cycle protein 45) guards the gate of the Eukaryote Replisome helicase stabilizing leading strand engagement. Proc Natl Acad Sci USA. 2015;112:E249–E258. doi: 10.1073/pnas.1422003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgers PM. Polymerase dynamics at the eukaryotic DNA replication fork. J Biol Chem. 2009;284:4041–4045. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clausen AR, Lujan SA, Burkholder AB, Orebaugh CD, Williams JS, Clausen MF, Malc EP, Mieczkowski PA, Fargo DC, Smith DJ, et al. Tracking replication enzymology in vivo by genome-wide mapping of ribonucleotide incorporation. Nat Struct Mol Biol. 2015;22:185–191. doi: 10.1038/nsmb.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18:521–527. doi: 10.1016/j.tcb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyabe I, Kunkel TA, Carr AM. The major roles of DNA polymerases epsilon and delta at the eukaryotic replication fork are evolutionarily conserved. PLoS Genet. 2011;7:e1002407. doi: 10.1371/journal.pgen.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RE, Klassen R, Prakash L, Prakash S. A major role of DNA polymerase delta in replication of both the leading and lagging DNA. Strands Mol Cell. 2015;59:163–175. doi: 10.1016/j.molcel.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong XP, Onrust R, O’Donnell M, Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992;69:425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- 54.Stukenberg PT, Studwell-Vaughan PS, O’Donnell M. Mechanism of the sliding beta-clamp of DNA polymerase III holoenzyme. J Biol Chem. 1991;266:11328–11334. [PubMed] [Google Scholar]
- 55.Conaway RC, Lehman IR. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci USA. 1982;79:2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilkenny ML, Longo MA, Perera RL, Pellegrini L. Structures of human primase reveal design of nucleotide elongation site and mode of Pol alpha tethering. Proc Natl Acad Sci USA. 2013;110:15961–15966. doi: 10.1073/pnas.1311185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perera RL, Torella R, Klinge S, Kilkenny ML, Maman JD, Pellegrini L. Mechanism for priming DNA synthesis by yeast DNA Polymerase alpha. UK: University of Cambridge; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klinge S, Nunez-Ramirez R, Llorca O, Pellegrini L. 3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases. EMBO J. 2009;28:1978–1987. doi: 10.1038/emboj.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunez-Ramirez R, Klinge S, Sauguet L, Melero R, Recuero-Checa MA, Kilkenny M, Perera RL, Garcia-Alvarez B, Hall RJ, Nogales E, et al. Flexible tethering of primase and DNA Pol alpha in the eukaryotic primosome. Nucleic Acids Res. 2011;39:8187–8199. doi: 10.1093/nar/gkr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh H, Brooke RG, Pausch MH, Williams GT, Trainor C, Dumas LB. Yeast DNA primase and DNA polymerase activities. An analysis of RNA priming and its coupling to DNA synthesis. J Biol Chem. 1986;261:8564–8569. [PubMed] [Google Scholar]
- 61.Simon AC, Zhou JC, Perera RL, van Deursen F, Evrin C, Ivanova ME, Kilkenny ML, Renault L, Kjaer S, Matak-Vinkovic D, et al. A Ctf4 trimer couples the CMG helicase to DNA polymerase alpha in the eukaryotic replisome. Nature. 2014;510:293–297. doi: 10.1038/nature13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miles J, Formosa T. Protein affinity chromatography with purified yeast DNA polymerase alpha detects proteins that bind to DNA polymerase. Proc Natl Acad Sci USA. 1992;89:1276–1280. doi: 10.1073/pnas.89.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gambus A, van Deursen F, Polychronopoulos D, Foltman M, Jones RC, Edmondson RD, Calzada A, Labib K. A key role for Ctf4 in coupling the MCM2-7 helicase to DNA polymerase alpha within the eukaryotic replisome. EMBO J. 2009;28:2992–3004. doi: 10.1038/emboj.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang YH, Farina A, Bermudez VP, Tappin I, Du F, Galal WC, Hurwitz J. Interaction between human Ctf4 and the Cdc45/Mcm2-7/GINS (CMG) replicative helicase. Proc Natl Acad Sci USA. 2013;110:19760–19765. doi: 10.1073/pnas.1320202110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgescu R, Langston L, O’Donnell M. A proposal: Evolution of PCNA’s role as a marker of newly replicated DNA. DNA Repair (Amst) 2015;29:4–15. doi: 10.1016/j.dnarep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 67.Tahirov TH, Makarova KS, Rogozin IB, Pavlov YI, Koonin EV. Evolution of DNA polymerases: an inactivated polymerase-exonuclease module in Pol epsilon and a chimeric origin of eukaryotic polymerases from two classes of archaeal ancestors. Biol Direct. 2009;4:11. doi: 10.1186/1745-6150-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hogg M, Osterman P, Bylund GO, Ganai RA, Lundstrom EB, Sauer-Eriksson AE, Johansson E. Structural basis for processive DNA synthesis by yeast DNA polymerase varepsilon. Nat Struct Mol Biol. 2014;21:49–55. doi: 10.1038/nsmb.2712. [DOI] [PubMed] [Google Scholar]
- 69.Swan MK, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase delta. Nat Struct Mol Biol. 2009;16:979–986. doi: 10.1038/nsmb.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain R, Rajashankar KR, Buku A, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Crystal structure of yeast DNA polymerase epsilon catalytic domain. PLoS One. 2014;9:e94835. doi: 10.1371/journal.pone.0094835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Netz DJ, Stith CM, Stumpfig M, Kopf G, Vogel D, Genau HM, Stodola JL, Lill R, Burgers PM, Pierik AJ. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol. 2011;8:125–132. doi: 10.1038/nchembio.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Georgescu RE, Langston L, Yao NY, Yurieva O, Zhang D, Finkelstein J, Agarwal T, O’Donnell ME. Mechanism of asymmetric polymerase assembly at the eukaryotic replication fork. Nat Struct Mol Biol. 2014;21:664–670. doi: 10.1038/nsmb.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Georgescu RE, Schauer GD, Yao NY, Langston LD, Yurieva O, Zhang D, Finkelstein J, O’Donnell ME. Reconstitution of a eukaryotic replisome reveals suppression mechanisms that define leading/lagging strand operation. Elife. 2015;4:e04988. doi: 10.7554/eLife.04988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langston LD, Zhang D, Yurieva O, Georgescu RE, Finkelstein J, Yao NY, Indiani C, O’Donnell ME. CMG helicase and DNA polymerase epsilon form a functional 15-subunit holoenzyme for eukaryotic leading-strand DNA replication. Proc Natl Acad Sci USA. 2014;111:15390–15395. doi: 10.1073/pnas.1418334111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi Y, Fernandez-Martinez J, Tjioe E, Pellarin R, Kim SJ, Williams R, Schneidman-Duhovny D, Sali A, Rout MP, Chait BT. Structural characterization by cross-linking reveals the detailed architecture of a coatomer-related heptameric module from the nuclear pore complex. Mol Cell Proteomics. 2014;13:2927–2943. doi: 10.1074/mcp.M114.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartlepp KF, Fernandez-Tornero C, Eberharter A, Grune T, Muller CW, Becker PB. The histone fold subunits of Drosophila CHRAC facilitate nucleosome sliding through dynamic DNA interactions. Mol Cell Biol. 2005;25:9886–9896. doi: 10.1128/MCB.25.22.9886-9896.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asturias FJ, Cheung IK, Sabouri N, Chilkova O, Wepplo D, Johansson E. Structure of Saccharomyces cerevisiae DNA polymerase epsilon by cryo-electron microscopy. Nat Struct Mol Biol. 2006;13:35–43. doi: 10.1038/nsmb1040. [DOI] [PubMed] [Google Scholar]
- 78.You Z, De Falco M, Kamada K, Pisani FM, Masai H. The mini-chromosome maintenance (Mcm) complexes interact with DNA polymerase alpha-primase and stimulate its ability to synthesize RNA primers. PLoS One. 2013;8:e72408. doi: 10.1371/journal.pone.0072408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costa A, Renault L, Swuec P, Petojevic T, Pesavento J, Ilves I, MacLellan-Gibson K, Fleck RA, Botchan MR, Berger JM. DNA binding polarity, dimerization, and ATPase ring remodeling in the CMG helicase of the eukaryotic replisome. Elife. 2014;3:e03273. doi: 10.7554/eLife.03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rothenberg E, Trakselis MA, Bell SD, Ha T. MCM forked substrate specificity involves dynamic interaction with the 5′-tail. J Biol Chem. 2007;282:34229–34234. doi: 10.1074/jbc.M706300200. [DOI] [PubMed] [Google Scholar]
- 81.Foltman M, Evrin C, De Piccoli G, Jones RC, Edmondson RD, Katou Y, Nakato R, Shirahige K, Labib K. Eukaryotic replisome components cooperate to process histones during chromosome replication. Cell Rep. 2013;3:892–904. doi: 10.1016/j.celrep.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 82.Tackett AJ, Dilworth DJ, Davey MJ, O’Donnell M, Aitchison JD, Rout MP, Chait BT. Proteomic and genomic characterization of chromatin complexes at a boundary. J Cell Biol. 2005;169:35–47. doi: 10.1083/jcb.200502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iida T, Araki H. Noncompetitive counteractions of DNA polymerase epsilon and ISW2/yCHRAC for epigenetic inheritance of telomere position effect in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24:217–227. doi: 10.1128/MCB.24.1.217-227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsubota T, Tajima R, Ode K, Kubota H, Fukuhara N, Kawabata T, Maki S, Maki H. Double-stranded DNA binding, an unusual property of DNA polymerase epsilon, promotes epigenetic silencing in Saccharomyces cerevisiae. J Biol Chem. 2006;281:32898–32908. doi: 10.1074/jbc.M606637200. [DOI] [PubMed] [Google Scholar]
- 85.Huang H, Stromme CB, Saredi G, Hodl M, Strandsby A, Gonzalez-Aguilera C, Chen S, Groth A, Patel DJ. A unique binding mode enables MCM2 to chaperone histones H3–H4 at replication forks. Nat Struct Mol Biol. 2015;22:618–626. doi: 10.1038/nsmb.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Wang M, Yang N, Xu RM. Structure of the quaternary complex of histone H3–H4 heterodimer with chaperone ASF1 and the replicative helicase subunit MCM2. Protein Cell. 2015;6:693–697. doi: 10.1007/s13238-015-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 88.Wittmeyer J, Formosa T. The Saccharomyces cerevisiae DNA polymerase alpha catalytic subunit interacts with Cdc68/Spt16 and with Pob3, a protein similar to an HMG1-like protein. Mol Cell Biol. 1997;17:4178–4190. doi: 10.1128/mcb.17.7.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franco AA, Lam WM, Burgers PM, Kaufman PD. Histone deposition protein Asf1 maintains DNA replisome integrity and interacts with replication factor C. Genes Dev. 2005;19:1365–1375. doi: 10.1101/gad.1305005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]