Abstract
Objective
Less is known about longitudinal changes in quality of life between treatment completion and early survivorship among multimorbid cancer survivors. The current study describes longitudinal changes in quality of life among a multimorbid cohort of US Veterans diagnosed and treated for colorectal cancer.
Materials and methods
A sample of 68 multimorbid adults with colon and/or rectal cancer who received one or more treatment options (surgery, chemo or radiation therapy) was recruited. Participants were not excluded by cancer stage unless they reported being in hospice or similar status. Comprehensive assessments of quality of life and treatment side-effects were conducted 6, 12, and 18 months after diagnosis. Descriptive statistics characterized treatment side-effects and changes in quality-of-life domains longitudinally. Multivariate Analysis of Variance identified sociodemographic and clinical variables associated with quality of life changes.
Results
Many physical symptoms improved from 6 to 18 months following diagnosis, while some remained stable. Sexual symptoms worsened, attributable to increasing rates of dysfunction in older patients. Low education attainment was predictive of worse physical symptoms (F = 5.59, p = .023) and associated with body concerns (F = 5.7; p = .005) over time. Advanced cancer stage (F = 4.94; p < .04) and receipt of chemotherapy (F = 4.21; p < .05) independently predicted body concerns in multivariate analyses.
Conclusion
Endorsement of physical and sexual symptoms and body concerns occurs in different patterns over time among multimorbid colorectal cancer survivors. Low education attainment is consistently associated with physical symptoms and body concerns. Cancer stage and chemotherapy are predictive of body concerns, but not physical or sexual symptoms.
Keywords: Cancer survivors, Colorectal cancer, Multimorbidity, Quality of life, Symptoms
1. Introduction
In the United States, nearly two thirds of colorectal cancer (CRC) patients are survivors at 5 years following diagnosis because of early detection from screening, improved treatment options, and active surveillance following treatment.1 With enhanced survival, quality of life is an increasingly important outcome, particularly for older adults.2 Validated CRC-specific quality-of-life surveys include side-effects (treatment and cancer related) presenting as distinct symptoms that impact daily function and activities.3,4 They also assess emotional well-being in terms of body image, functional status, and future perspective.3,4
Prior studies using cancer-specific quality-of-life measures have characterized quality of life in CRC survivors in the early survivorship period (i.e., from diagnosis, surgical resection and adjunct therapy, and into the first year posttreatment). In these settings, quality of life is typically lower among younger patients (i.e., 60 years or less)4–6 and those with perioperative complications.5 Evidence for differences in quality of life among patients by surgery type is mixed.5,7 Few of these studies measured longitudinal changes in quality of life between treatment completion and early survivorship, and they typically did not include highly comorbid populations, such as U.S. Veterans.8 These studies have demonstrated improvements in global quality of life over time4,9; however, they have not typically looked at prevalence over time of CRC-specific symptoms, longitudinal differences among domains of CRC-specific quality of life, or the sociodemographic and clinical predictors of these differences.
The current study addresses this gap by accomplishing the following study aims. First, we characterize longitudinal changes in CRC-specific quality-of-life domains among CRC patients enrolled in the VetCares study of U.S. Veteran cancer survivors from 6 to 18 month postdiagnosis, a key transition period after surgery when most patients begin to enter posttreatment survivorship. Second, we analyze differences in the patterns of change among physical symptoms, sexual function, and body image found in the CRC-specific quality-of-life domains. Finally, we conducted multivariate analyses to identify sociodemographic and clinical predictors of declines in CRC-specific quality-of-life domains.
2. Methods
2.1. Study Design and Setting
Participants were recruited from tumor registries and patient databases from VA medical centers in Boston and Houston for a longitudinal cohort study from November 2009 through April 2013. Sample size was determined on the basis of power analyses; complete protocol methods, including nonresponder information, are described elsewhere.2 In brief, participants completed comprehensive biopsychosocial assessments at 6, 12, and 18 months after diagnosis for head and neck, esophageal, gastric, and colorectal cancer, confirmed by pathology.
2.2. Participants and Data Sources
In this article we describe disease-specific findings focusing on 68 men with a new diagnosis of CRC who had complete data for all interview periods. Subjects were eligible if they received CRC treatment beyond watchful waiting were considered “cancer survivors” by virtue of not being in hospice care. Twelve additional subjects of the parent study met eligibility criteria and enrolled in the parent study at 6 month postdiagnosis but did not complete the 18 month longitudinal assessment. There were no significant differences in sociodemographic or clinical characteristics including cancer stage and treatment except for the presence of a spouse/partner between these 12 and the current study cohort of 68 subjects. Participants completed face-to-face interviews at each assessment. To reduce measurement bias, trained interviewers read each question to the participant and recorded his responses. For items with a Likert scale response format, participants were given a copy of the scale to reference. Most interviews occurred in person at a location convenient for the participant, usually at the local VA medical center or the patient’s home. This study was approved by the Institutional Review Boards and Research and Development Committees of the VA Boston Healthcare System and the Michael E. DeBakey VA Medical Center and Baylor College of Medicine in Houston, and informed consent was provided by participants.
2.3. Variables, Data Sources, and Measurement
2.3.1. Demographic Variables
Participants reported their age, gender, racial/ethnic identity, and level of education, and whether they had a partner or spouse. For the purpose of making clinically-meaningful distinctions, we divided age into younger (<65 years) and older (65+); race/ethnicity into African American or Hispanic or other versus Caucasian; and education into high school or less versus any college.
2.3.2. Clinical Variables
Information about the cancer diagnosis, including cancer organ site and stage, was obtained from the participants’ medical record. Participants reported whether they had received surgery, chemotherapy, and/or radiation, confirmed in the medical record. Comorbidity ratings used electronic-medical-record extraction of diagnoses to create a Deyo adjustment of the Charlson Comorbidity Index,10 which employs outpatient ICD-9 data to create an index that predicts 10-year mortality for a patient who may have a range of comorbid conditions, such as heart disease, heart failure, or cancer (a total of 22 conditions). Each condition was assigned a score of 1, 2, 3, or 6, depending on the risk of dying associated with each. Scores were summed to provide a total score.
2.3.3. Disease-specific Symptoms
The European Organization for Research and Treatment of Cancer (EORTC) Colorectal (CRC) module was used to measure long-term treatment side effects.3 The EORTC quality-of-life questionnaire is part of an integrated system for assessing the health-related quality of life in cancer patients participating in international clinical trials. For this study, items were rated 1–4, with higher scores indicating more symptom experience in the past week, to correspond with other scales used in the study, with 1 = not at all, 2 = a little, 3 = quite a bit, 4 = very much. The EORTC CRC module has a branching tree, depending on whether the patient, post-operatively, had a surgical ostomy requiring self-management.3 Study participants completed only those items relevant to their clinical course.
To enhance the ease of clinical interpretation, we developed three symptom subscales using the established CRC module items guided by a systematic process that ensured content and face validity as well as reliability of items within the symptom subscales. First, we grouped the symptoms using a consensus process guided by the expertise of three interdisciplinary clinicians (JM, AN, DA) into broad symptom subscales: physical, sexual, and body concern. The sexual subscale was drawn from items 32–33 on the EORTC CRC module. The body-concern subscale was drawn from items 13–17 and 24 or 31 in the EORTC CRC module, depending on whether the patient endorsed having an ostomy. The physical subscale was further divided into four symptoms’ groups: urinary (items 1–3), abdominal (items 7–9 and [19, 20, 22, 23] or [26, 27, 29, 30]) pain (items 4–6 and 21 or 28), and oral (items 10, 12). Symptom subscales were standardized by dividing the total number of items within each scale, to permit comparisons across subscales. We then conducted a test of reliability using Cronbach’s alpha, a coefficient of internal consistency for the physical and body concerns subscales at T1 and T3. We could not obtain alpha’s for the sexual subscale since it only contains two items. Alpha’s were appropriate at each time point, and considered good at T3 for both physical (α = .78) and body concerns (α = .79) subscales.
2.4. Statistical Methods
We first examined symptom prevalence through descriptive statistics. Each individual EORTC CRC item was dichotomized as the percent of participants endorsing a given symptom. To capture clinically meaningful endorsement, we rated symptoms as present if participants reported “quite a bit” or more during the past week. Symptom presence at 6 and 18 months was then compared with the McNemar nonparametic test for two related dichotomous variables based on the chi-square statistic to identify significant differences between study endpoints. We then compared relationships among physical, sexual, and body-concern symptoms by producing Pearson correlations between symptom subscales across the three study time points.
Symptom change over all three time points was analyzed, using Repeated Measures MANOVA with adjustment for demographic (dichotomous for age, race, and education attainment) and clinical (cancer stage [split 1–3 versus 4], use of chemotherapy, use of radiation therapy) factors, and overall comorbidity score [continuous]. We conducted analyses in SPSS 21.0 (IBM, Armonk, NY).
3. Results
3.1. Participants and Descriptive Data
Table 1 describes the baseline characteristics of the study population overall and stratified by dichotomous age categories. The study cohort includes 68 men with a mean age of 65.76 (SD = 8.89, range 51–88). There were no significant differences in socio-demographic or clinical traits by age categories. At initial tumor board, American Joint Committee on Cancer Stage was I (29.4), II (29.4%), III (27.9%) or IV (13.2%). Younger compared to older patients were more likely to be stage IV (22.8% versus 3%, p = .02). For treatment, all but three (95.6%) received surgery, 37 (54.4%) received chemotherapy, and 10 (14.7%) received radiation therapy. All who received radiation also received chemotherapy, and no subjects received radiation alone. Overall comorbidity scores ranged from 2 to 18, with a median of 4 and mean of 6.18 (3.64).
Table 1.
Descriptive characteristics of the study population stratified by age.
| Variable | Category | Total (N = 68)
|
Younger (<65 years) (N = 35)
|
Older (≥65 years) (N = 33)
|
Statistic
|
|---|---|---|---|---|---|
| % | % | % | C (p)a | ||
| Education | High School (some/graduate) | 38.2 | 42.9 | 33.3 | .10 (.42) |
| Any college | 61.8 | 57.1 | 66.7 | ||
| Race and ethnicity | Hispanic (any race) | 8.8 | 14.3 | 3.0 | .21 (.20) |
| African-American | 17.6 | 20.0 | 15.2 | ||
| Non-Hispanic White | 73.5 | 65.7 | 81.8 | ||
| Cancer stage | Stage 1 | 25.7 | 33.3 | .21 (.20) | |
| Stage 2 | 29.4 | 28.6 | 30.3 | ||
| Stage 3 | 27.9 | 22.9 | 33.3 | ||
| Stage 4 | 13.2 | 22.9 | 3.0 | ||
| Treatments | Surgery | 95.6 | 97.1 | 93.9 | .08 (.52) |
| Chemotherapy | 54.4 | 57.1 | 51.5 | .06 (.64) | |
| Radiation | 14.7 | 17.1 | 12.1 | .07 (.56)b | |
| Comorbidities — continuous | Deyo mean (standard deviation) | 6.18 (3.64) [range 2–18] | 6.57 (4.15) | 5.76 (3.00) | .92 (.36)c |
Pearson contingency coefficient comparing nominal × nominal categories.
No one received radiation alone; of those 37 who got chemotherapy 10 also got radiation.
t-Test statistic (p).
3.2. Symptom Endorsement
Six months after diagnosis, patients report a range of symptoms (Table 2), especially urinary frequency, bowel frequency, abdominal distress (gas, bloating), oral symptoms (dry mouth, loss of taste), sexual problems (both interest and function), and negative body concerns (such as feeling worried about health, less attractive, or less masculine). Physical and negative body concerns were highly correlated with each other at all three time points (6 months: r = 0.61, < .01; 12 months: r = .59, p < .01; 18 months: r = .55, p < .01). Physical and sexual symptoms were significantly correlated at 6 months after diagnosis (r = .36, p < .01), but not so at 12 (r = .10) and 18 months (r = .20). Sexual and body-concern symptoms were only modestly related at all three time periods (r = .20; r = .25; r = .22, respectively), reaching statistical significance only at 12 months (p < .01). At the initial assessment (6 months after diagnosis), there were no significant associations between symptom subscales (physical, sexual, or body-concern) and receipts of chemotherapy or radiation.
Table 2.
Clinical symptoms reported at 6, 12, and 18 months after diagnosis (N = 68).
| 6-Month % | 12-Month % | 18-Month % | pa | |
|---|---|---|---|---|
| Physical symptoms’ subscale | ||||
| Urinary symptoms’ items | ||||
| 1. Urinate frequently during day (#1) | 50.0 | 38.2 | 33.8 | .043 |
| 2. Urinate frequently at night (#2) | 33.8 | 32.8 | 25.0 | .238 |
| 3. Urinary leakage (#3) | 4.4 | 7.5 | 5.9 | 1.00 |
| Abdominal symptoms’ items | ||||
| 4. Bloated feeling in abdomen (#7) | 8.8 | 4.5 | 8.8 | 1.00 |
| 5. Blood in stools (#8) | 1.5 | 1.5 | 2.0 | 1.00 |
| 6. Mucus in stools (#9) | 4.4 | 1.5 | 2.9 | 1.00 |
| 7. Unintentional gas (#19 or 26) | 17.6 | 20.0 | 16.9 | .508 |
| 8. Stool leakage (#20 or 27) | 2.9 | 3.6 | 1.5 | 1.00 |
| 9. Frequent bag changes or BM during day (#22 or 29) | 16.2 | 19.6 | 13.8 | .727 |
| 10. Frequent bag changes or BM at night (#23 or 30) | 13.2 | 7.4 | 3.1 | .039 |
| Pain symptoms’ items | ||||
| 11. Pain with urination (#4) | 0.0 | 1.5 | 1.5 | + |
| 12. Pain in abdomen (#5) | 2.9 | 3.0 | 0.0 | 1.00 |
| 13. Pain in buttocks/anal/rectal area (#6) | 1.4 | 4.5 | 1.5 | 1.00 |
| 14. Sore skin around stoma/anal area (#21 or 28) | 2.9 | 7.1 | 1.5 | + |
| Oral symptoms’ items | ||||
| 15. Dry mouth (#10) | 19.1 | 13.4 | 14.7 | .581 |
| 16. Problems with sense of taste (#12) | 25.0 | 13.4 | 2.9 | .000 |
| Sexual symptoms’ subscale | ||||
| 17. Loss of interest (#32) | 35.0 | 37.7 | 38.3 | 1.00 |
| 18. Dysfunction (erectile, pain, discomfort) (#33) | 17.6 | 45.3 | 50.0 | .022 |
| Body concern symptoms’ subscale | ||||
| 19. Worry about health (#13) | 20.6 | 19.4 | 20.6 | 1.00 |
| 20. Worry about weight (#14) | 16.2 | 23.9 | 20.6 | .629 |
| 21. Feel less attractive (#15) | 9.0 | 10.4 | 10.4 | 1.00 |
| 22. Feel less masculine (#16) | 11.8 | 16.4 | 11.8 | 1.00 |
| 23. Dissatisfied with body (#17) | 17.6 | 10.4 | 14.9 | 1.00 |
| 24. Embarrassed because of stoma or BM (#24 or 31) | 6.0 | 8.9 | 12.5 | .237 |
Note: Percent refers to individuals endorsing the symptom as “quite a bit” or more during the past week.
+ = value cannot be determined.
BM = bowel movement.
Nonparametric testing comparing distributions at 6 and 18 months.
3.3. Change Over Time
3.3.1. Physical Symptoms
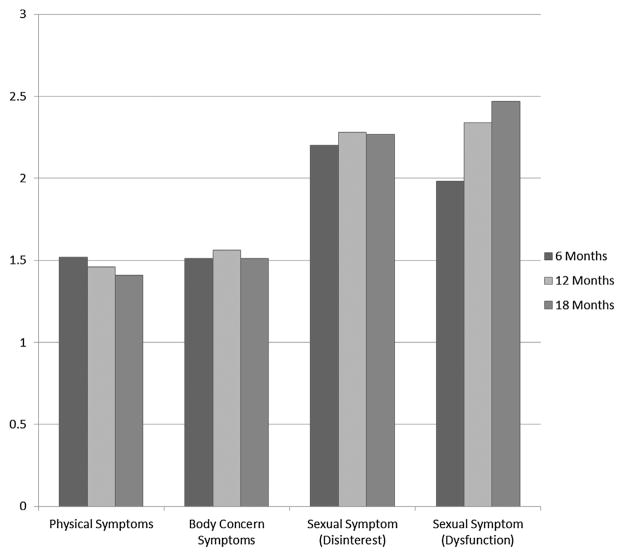
Fig. 1 illustrates patterns in symptom severity and change over time when stratified into the three symptom scales (i.e., physical, sexual and body concern), with additional breakdown for sexual symptoms by disinterest and dysfunction. Physical symptoms improved at each time point from 6 (M = 1.52, SD = .28), to 12 (M = 1.46, SD = .31) to 18 months (M = 1.41, SD = .30) (F = 3.06; p < .05). Specific physical symptoms were generally stable in their prevalence across all three time points (see Table 2), but some of the most highly endorsed physical complaints at 6 months were less commonly reported by 18 months. For example, urinary frequency during the day was highly prevalent at 6 months (50%) but less so by 12 and 18 months (38% and 34%, respectively), as was bowel frequency at night. Dramatic changes in problems with taste also were endorsed over time, by from one quarter of respondents at 6 months down to 3% at 18 months.
Fig. 1.
Physical, body concern and sexual symptoms endorsed over time. This figure illustrates the endorsement of physical symptoms, body concerns, sexual disinterest and dysfunction at 6, 12, and 18 month postdiagnosis for colorectal cancer. Participants endorsed the amount of symptoms experienced over the prior week from 1 = “not at all”, to 2 = “a little”, to 3 = “quite a bit”. The improvement in physical symptoms was significant over time (p < .05). Changes in body concerns and sexual disinterest did not significantly change. The worsening of sexual-dysfunction symptoms was also significant over time (p < .01).
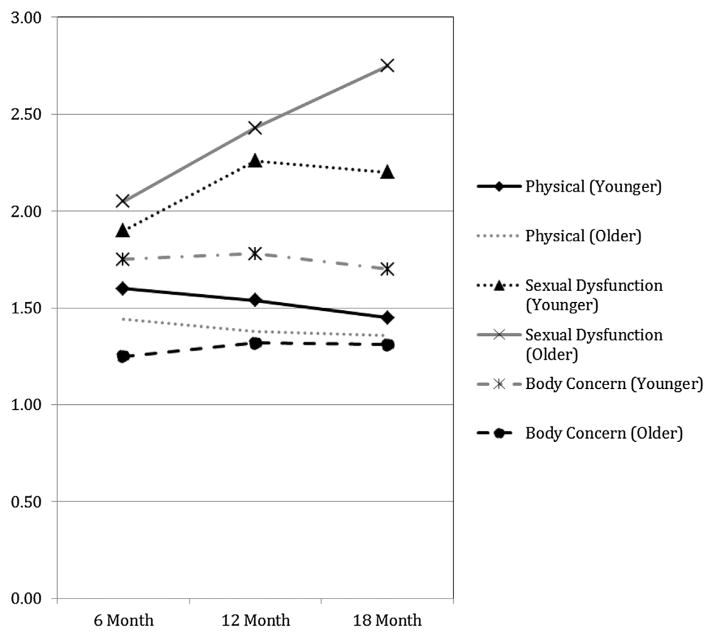
There was slightly more endorsement of physical symptoms among younger adults at 6 months, but this difference narrowed at 18 months and was not statistically significant over time (p > .2) (see Fig. 2). In repeated-measures MANOVA, only those with a lower education level displayed significantly worse symptom complaints over time (F = 5.59, p = .02). There were no statistically significant differences between subject effects for age, race, cancer stage, receipt of chemotherapy or radiation treatment, or comorbidity burden over time.
Fig. 2.
Differences in symptom complaints stratified by age over time. This figure illustrates the differences in endorsement of physical symptoms, body concerns, sexual disinterest and dysfunction stratified by age at 6, 12, and 18 month postdiagnosis for colorectal cancer. Younger is defined as all participants less than 65 years of age. Conversely, older is defined as all participants aged 65 years and older. Participants endorsed the amount of symptoms experienced over the prior week from 1 = “not at all”, to 2 = “a little”, to 3 = “quite a bit”. Differences in physical symptoms by age were not statistically significant at any time point. Differences in sexual dysfunction were similar at 6 months but became significantly different at 18 months (p < .01), with older participants reporting much more dysfunction. Younger participants consistently reported more body concerns than younger participants over time (p < .04).
3.3.2. Sexual Symptoms
As a whole, sexual symptoms significantly worsened over time (F = 3.31, p < .05) from 6 (M = 2.07, SD = .57), to 12 (M = 2.31, SD = .62) to 18 months (M = 2.37, SD = .72), and no demographic or clinical variables demonstrated significant associations with sexual symptoms over time. As noted in Fig. 1, the worsening sexual symptoms over time were entirely because of increasing sexual dysfunction over time from 6 months (18%) to 18 months (50%). At 18 months, sexual dysfunction was considerably higher in older men (63%) than in younger men (39%) (χ2 = 3.30, p < .05), although they had started as equivalent at 6 months (Fig. 2). In contrast, there were considerable (one third) but stable reports of disinterest over time. Complaints of sexual disinterest were higher in younger men. At 18 months 55% of younger versus 19% of older men (19%) reported disinterest (χ2 = 8.15, p < .01). In repeated-measures MANOVA, there were no statistically significant between-subject effects for age, race, or cancer stage, receipt of chemotherapy, or comorbidity burden over time. Receipt of radiation did approach statistical significance (F = 3.61; p = .064).
3.3.3. Body Concern
Body-concern symptoms were reported by 10–20% of the sample and remained stable over time from 6 (M = 1.51, SD = .58), to 12 (M = 1.56, SD = .60) to 18 months (M = 1.51, SD = .58). These differences were not significant (F = 0.56), as shown in Table 2. Younger subjects reported significantly higher body concerns than older subjects at 6 months (see Fig. 2). These differences narrowed slightly by 18 months but remained significantly different over time (F = 3.43, p < .04). Education was also associated with significant differences in body concerns over time (F = 5.7, p = .005). In repeated-measures MANOVA, cancer stage (F = 4.94, p = .032) and receipt of chemotherapy (F = 4.21, p < .05) were both significant predictors of body-concern symptoms over time. No other demographic variables, receipt of radiation, or comorbidity burden were associated with body concern in multivariate analysis.
4. Discussion
CRC prevalence is increasing worldwide,11 because of prolonged survival from improved care after diagnosis, even among older adults with multiple morbidities.12 As a result, patients are increasingly interested in survivorship outcomes that include quality of life. The current study advances our understanding of quality of life among a population of older CRC patients with multiple morbidities (median comorbidity score = 4), who received a range of treatments (all but three had surgery, and half received chemotherapy). This study targeted an intermediate survivorship timeframe (6–18 months after diagnosis), compared with most prior studies that report findings on long-term quality-of-life changes (3 plus years postdiagnosis).
The findings of the current study present novel results, as well as confirm findings of prior studies.5–7 Participants in this study endorsed a range of negative physical symptoms, sexual problems, and body concerns. Physical symptoms were often highly prevalent, including urinary frequency, unintentional gas, frequent bowel movements or changes of ostomy bag, and dry mouth; while there was infrequent endorsement of pain symptoms. Many physical symptoms improved over time (from 6 to 18 months following diagnosis), while some symptoms remained stable. Sexual functioning worsened over time, attributable to increasing rates of erectile dysfunction, particularly in older patients. About 10–20% of participants endorsed one or more items related to body concerns (e.g., worries about weight and health, feeling less attractive or masculine, and dissatisfied with one’s body) at 6 months, and these rates remained stable at 18 months. Endorsement of body concerns was strongly correlated with physical symptoms, but no consistent patterns emerged among other symptom areas.
Prior studies of quality of life following CRC treatment have typically used general cancer quality-of-life measures. The current study significantly contributes to the literature because it used a CRC-specific quality-of-life measure and characterized distinct symptoms domains at three discrete time points. One prior study that used the same measure focused on the longer-term interval of 1 to 3 years postdiagnosis, reporting largely unchanged symptoms (e.g., urinary and gastrointestinal symptoms, sexual complaints, and body image), except for a significant decline in sexual activity.13 These differences in trajectories of quality-of-life symptoms may reflect differences in time frame compared with the current study and may be related to different methods for determining symptom prevalence. The 6- to 18-month window described in the current study may represent an important time for improvement, stabilization, or decline after diagnosis.
The current study also characterized demographic and clinical predictors of quality-of-life symptoms from 6 to 18 months following diagnosis. We found that comorbidity and treatment-related variables were not associated with quality-of-life domains in multivariate analysis, except for a significant association between cancer stage and greater body concerns. This finding is counter-intuitive in some aspects. At both the symptom and subscale levels there are few significant associations with chemotherapy or radiation treatment over time. Our results may simply reflect the reality of our study sample—older, multimorbid adults who received more than one cancer (and non-cancer) treatment over time. Their symptoms likely reflect this complexity. For example, sexual symptoms may arise from the combination of diabetes, pelvic radiation for rectal cancer, and the use of antidepressant medications. In our analyses, we found that older age, after adjusting for comorbidity, was associated with risk for worsening sexual function over time; even though the age groups were equivalent in sexual function initially. Interest in sexual activity however remained stable over time.
Low education attainment was also significantly associated with worse physical symptoms and more body concerns than high educational attainment. Prior studies have reported an association between low education attainment and CRC-related psychosocial distress,14 fear of cancer recurrence,15 and post-treatment symptom burden.16 This relationship may suggest less capacity to adapt to persistent physical symptoms and body-image issues, as time since diagnosis advances, among patients with lower education attainment. Higher rates of psychological distress among long-term cancer survivors with lower education attainment may also be a moderating factor for higher physical and body concern symptoms.17 These results underscore the importance of addressing health literacy gaps at diagnosis, providing clear instructions about expectations for treatment and recovery when planning CRC treatment courses and being mindful of patients’ health literacy and education levels.
The current study has limitations, as our almost exclusively male U.S. Veteran population may limit the generalizability of study findings. However, this population represents an important and highly morbid subgroup of CRC patients that is generally understudied in cancer care. About 15% (n = 12) of the subjects with colorectal cancer in the parent study did not complete 18-month assessments and were excluded from the analyses in the current study. The current results, therefore, may be biased by a survivor effect, especially the findings demonstrating limited effect of cancer stage and treatment received on physical and sexual symptoms. We used a novel method for calculating scores from the CRC-specific quality-of-life scale, which may enhance the clinical relevance of individual symptom reports, despite having less prior validation than other measures. Furthermore, our method of describing clinical symptoms for each of the three categories (physical, sexual, body concern) does not allow us to draw conclusions related to EORTC standards for minimally significant difference.3 Despite these limitations, the current study offers a number of interesting and novel findings.
The study results have important clinical implications for the longitudinal care of multimorbid CRC patients. First, physical and sexual symptoms and body concerns change in divergent patterns during the period following treatment initiation and completion. Clinicians should explore the full range of these symptoms and expect many to be dynamic in nature. Second, age and education are important predictors of endorsement of physical, sexual, and body concerns over time. These demographic traits may help clinicians tailor resources and time for counseling of patients with symptom management and avoid subsequent health service use by older, multimorbid cancer survivors.18 With the exception of body concerns, cancer stage and specific treatments have some but, surprisingly, not dominant effects on symptom endorsement. Finally, these findings could be further enhanced by an understanding of baseline quality of life prior to treatment. Future studies should begin measurement at time of diagnosis prior to any therapy and measure longitudinal changes through surgery and adjunct therapy and into survivorship.
Acknowledgments
Funding for this project was provided by the Department of Veterans Affairs Rehabilitation Research and Development Service #5I01RX000104. This material is also the result of work supported with resources and the use of facilities at the Boston VA Medical Center and the Houston VA HSR&D Center for Innovations in Quality, Effectiveness, and Safety (CIN13-413) at the Michael E. DeBakey VA Medical Center. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research team for assistance in subject recruitment and data collection. We are indebted to the Veterans who have participated in our research studies and allow us to contribute to their health care. The funders played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and/or in the decision to submit the paper for publication.
Footnotes
Disclosures and Conflict of Interest Statements
The authors disclose no actual or potential conflicts of interest, including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could influence, or be perceived to influence, their work.
Author Contributions
Concept and design: A. Naik and J. Moye
Data collection: A. Naik and J. Moye
Analysis and interpretation of data: A. Naik, N. Uy, D. Anaya, J. Moye
Manuscript writing and approval: A. Naik, N. Uy, D. Anaya, J. Moye
Contributor Information
Aanand D. Naik, Email: anaik@bcm.edu.
Natalie Uy, Email: Natalie.Uy@bcm.edu.
Daniel A. Anaya, Email: Daniel.anaya@va.gov.
Jennifer Moye, Email: Jennifer.Moye@va.gov.
References
- 1.Jansen L, Koch L, Brenner H, Arndt V. Quality of life among long-term (≥5 years) colorectal cancer survivors—systematic review. Eur J Cancer. 2010;46:2879–2888. doi: 10.1016/j.ejca.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Naik AD, Martin LA, Karel M, Wachen JS, Mulligan E, Gosian JS, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes) BMC Health Serv Res. 2013;13:93. doi: 10.1186/1472-6963-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38) Eur J Cancer. 1999;35:238–247. doi: 10.1016/s0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 4.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 5.Wilson TR, Alexander DJ, Kind P. Measurement of health related quality of life in the early follow-up of colon and rectal cancer. Dis Colon Rectum. 2006;49:1692–1702. doi: 10.1007/s10350-006-0709-9. [DOI] [PubMed] [Google Scholar]
- 6.Gall CA, Weller D, Esterman A, Pilotto L, McGorm K, Hammett Z, et al. Patient satisfaction and health-related quality of life after treatment for colon cancer. Dis Colon Rectum. 2007;50:801–809. doi: 10.1007/s10350-006-0815-8. [DOI] [PubMed] [Google Scholar]
- 7.Smith-Gagen J, Cress RD, Drake CM, Romano PS, Yost KJ, Ayanian JZ, et al. Quality-of-life and surgical treatments for rectal cancer — a longitudinal analysis using the California Cancer Registry. Psychooncology. 2010;19:870–878. doi: 10.1002/pon.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27:36–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp I, Bauhofer A, Koller M. Understanding quality of life in patients with colorectal cancer: comparison of data from a randomised controlled trial, a population based cohort study and the norm reference population. Inflamm Res. 2004;53(Suppl 2):130–135. doi: 10.1007/s00011-004-0361-6. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.Anaya DA, Becker NS, Abraham NS. Global graying, colorectal cancer and liver metastasis: new implications for surgical management. Crit Rev Oncol Hematol. 2011;77:100–108. doi: 10.1016/j.critrevonc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Cheema FN, Abraham NS, Berger DH, Albo D, Taffet GE, Naik AD. Novel approaches to perioperative assessment and intervention may improve long-term outcomes after colorectal cancer resection in older adults. Ann Surg. 2011;253:867–874. doi: 10.1097/SLA.0b013e318208faf0. [DOI] [PubMed] [Google Scholar]
- 13.Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Restrictions in quality of life in colorectal cancer patients over three years after diagnosis: a population based study. Eur J Cancer. 2006;42:1848–1857. doi: 10.1016/j.ejca.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 14.Dunn J, Ng SK, Holland J, Aitken J, Youl P, Baade PD, et al. Trajectories of psychological distress after colorectal cancer. Psychooncology. 2013;22:1759–1765. doi: 10.1002/pon.3210. [DOI] [PubMed] [Google Scholar]
- 15.Koch L, Jansen L, Brenner J, Arndt V. Fear of recurrence and disease progression in long-term cancer survivors—a systematic review of the quantitative literature. Psychooncology. 2013;22:1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 16.Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis. Cancer. 2011;117:2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman K, McCarthy EP, Recklitis CJ, Ng AK. Psychological distress in long-term survivors of adult-onset cancer. Arch Intern Med. 2009;169:1274–1281. doi: 10.1001/archinternmed.2009.179. [DOI] [PubMed] [Google Scholar]
- 18.Hermosillo-Rodriguez J, Anaya DA, Sada Y, Walder A, Amspoker AB, Berger DH, et al. The effect of age and comorbidity on patient-centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. J Geriatr Oncol. 2013;4:99–106. doi: 10.1016/j.jgo.2012.12.004. [DOI] [PubMed] [Google Scholar]