Abstract
Objective
The subjective feeling of loss of control (LOC) over eating is common among eating disordered individuals and has predicted weight gain in past research. Restrained eating and negative affect are risk factors for binge eating (which involves LOC), but intense feelings of pleasure derived from palatable foods might also predict the emergence or intensification of LOC eating. The Power of Food Scale (PFS; Lowe et al., 2009) assesses preoccupation with the pleasure derived from palatable food.
Method
The current sample (n = 294) comprised female college freshmen at risk for weight gain. LOC was assessed using an abbreviated version of the Eating Disorders Examination interview. LOC was assessed at baseline, 6 weeks and 6, 12 and 24 months follow-ups.
Results
Among those exhibiting LOC eating at baseline, (and controlling for baseline depression, restrained eating and body image dissatisfaction), those scoring higher on the PFS at baseline showed a smaller reduction in LOC frequency over time relative to those scoring lower. Using the same covariates, the PFS predicted the first emergence of LOC over two years among those showing no LOC at baseline.
Conclusions
These results suggest that powerful hedonic attraction to palatable foods may represent a risk factor for the maintenance of LOC in those initially experiencing it and the emergence of LOC eating in those who are not. An enhanced ability to identify individuals at increased risk of developing or maintaining LOC eating could be useful in prevention programs.
Keywords: loss of control, binge eating, Power of Food Scale, hedonic hunger
Binge eating is defined as the consumption of an amount of food that is definitely larger than what most individuals would eat under similar circumstances, while experiencing a sense of lack of control (LOC) over eating (i.e., a feeling that one cannot stop eating or control what or how much one is eating; American Psychiatric Association 2013). Binge eating occurs across the entire weight spectrum – that is, from emaciated individuals with anorexia nervosa to those with extreme obesity (Sarwer, Dilks, & West-Smith, 2011). It is associated with depression and other mental health problems (Grilo, 2006).
Binge eating is usually defined in an all-or-none manner. For example, the Eating Disorders Examination, which is often used to diagnose binge eating, defines both the “large amount of food” and the “feelings of loss of control” criteria as either present or absent (Cooper & Fairburn, 1993). However, the processes that contribute to the development of binge eating presumably emerge gradually over time. Therefore, because it is generally more effective to prevent the emergence of a behavioral disorder than to treat it once it exists, it would be desirable to identify variables that predict the emergence of binge eating or its two components.
The subjective sense of loss of control over eating is a defining feature of binge eating. Furthermore, the loss of control over eating predicts future weight gain (Sonneville et al., 2013; Tanofsky-Kraff et al., 2009) and the development of sub-clinical and clinical levels of binge eating (Hilbert, Hartmann, Czaja, & Schoebi, 2013; Tanofsky-Kraff et al., 2011). LOC is also pathognomonic independently of how much food is eaten during an episode (Tanofsky-Kraff et al., 2011). Evidence indicates there is little or no difference in psychopathology or other clinical characteristics between those who experience objective binge episodes (OBEs; involving consumption of a clearly excessive amount of food) and those who experience only subjective binge episodes (SBEs; Mond, Latner et al. 2010). Gaining insight into the development of feelings of loss of control, irrespective of the amount of food eaten, is therefore clinically important.
In the research literature, two sources of loss of control eating have been extensively investigated. One is dietary restraint (Polivy & Herman, 1985; Wilson, Fairburn, & Agras, 1997) and the other is negative affect (Fairburn, 2008; Heatherton & Baumeister, 1991). However there is a third possible cause of LOC eating that has not received much attention. This is the rewarding value of food. Many observers have pointed out similarities between over-consumption of palatable foods and consumption of addictive drugs (e.g., Stice et al., 2013). It has been established that the pleasure derived from eating palatable food continues to motivate food intake even in the absence of physiological hunger (Birch, Fisher, & Davison, 2003; Lowe & Levine, 2005). Most individuals will eat some palatable food when not hungry simply because it is available or offered (e.g., a mid-afternoon snack; having dessert after dinner). However, highly palatable food is usually available almost everywhere in modern cultures. As a result, some individuals find that palatable food captures their attention and interest even in the absence of typical eating cues (e.g., meal time, seeing food on TV).
Such preoccupation with palatable food may also manifest itself when individuals begin to eat such foods and find it difficult to limit consumption to an appropriate amount (e.g., having one serving of potato chips for a snack or one slice of pie for a dessert). Experiencing powerful and recurrent motivations to consume palatable foods when not food deprived has been called “hedonic hunger” by Lowe and Butryn (2007; Lowe & Levine, 2005). Unlike the experience of strong and consistent urges to eat when calorically deprived – which is adaptive – the experience of strong and consistent urges to eat for pleasure in the absence of a need for energy is likely to be maladaptive. In particular, if such eating urges are strong enough they may result in feelings of LOC over eating when eating does occur, in the consumption of excessive calories, or both. This pattern is reminiscent of the development of drug addiction. In fact, there are striking similarities in the pattern of brain activation seen during the consumption of addictive drugs and highly palatable foods (Volkow, Wang, Fowler, & Telang, 2008). Thus, just as individuals experimenting with drugs may find over time that it becomes harder and harder to control their desire to consume drugs, those who derive the greatest pleasure from the consumption of highly palatable foods may also begin to develop feelings of loss of control when they start eating such foods.
Prior research on the developmental precursors of loss of control eating and binge eating has mostly involved individuals who were already overweight or obese. One study of binge eating in children and adolescents (Tanofsky-Kraff et al., 2007) found that consumption of a “forbidden” food tended to precede binge episodes. In another, children and adolescents with certain variants of the FTO gene were more likely to report LOC eating and to consume a greater percentage of fat in a self-selected buffet meal (Tanofsky-Kraff et al., 2009). These studies support the hypothesis that an irresistible drive to consume highly palatable foods may contribute to the development of LOC eating. However in the current study our interest was in examining the initial development of LOC episodes among individuals who were not obese and were not experiencing LOC. A novel aspect of the current study is that it examines the development of LOC feelings among individuals without an existing weight or eating problem.
Finding certain foods intensely pleasurable could, over time, culminate in the development of LOC feelings when consumption of such foods is imminent or underway. The Power of Food Scale (PFS; Lowe et al. 2009) was designed to measure the intense attraction to palatable foods and is therefore a suitable means for testing this hypothesis. That is, individuals who score high on the PFS but have never experienced LOC eating may have a heightened susceptibility to develop such feelings in the future. The PFS consists of 15 items that describe preoccupation with palatable foods, but it purposefully excludes items describing amount of palatable foods respondents typically consume. Thus the measure taps the anticipatory, rather than the consummatory, phase of eating.
In one study (Lowe et al., 2009) the PFS was correlated with the Disinhibition (r = 0.61) and Hunger (r = 0.63) factors of the Eating Inventory (Stunkard & Messick, 1985) and the Emotional Eating (r = 0.54) and External Eating (r = 0.66) subscales from the Dutch Eating Behavior Questionnaire (Lowe et al., 2009;van Strien, Frijters, van Staveren, Defares, & Deurenberg, 1986). However, in contrast to these other measures, the PFS has little or no relation with body mass index (BMI; Cappelleri et al., 2009, Lowe et al., 2009, Rejeski et al. 2012). In a study where participants carried chocolates with them for two days but were instructed not to eat them, the PFS predicted the frequency and intensity of chocolate cravings – and the degree of distress associated with them (Forman et al., 2007). In the same study, the PFS also predicted who ate the chocolates despite instructions not to. Appelhans et al. (2011) found that recently fed obese individuals who scored high on the PFS ate more palatable (but not bland) food, but only if they also scored low on a measure of inhibitory control. Finally, Witt and Lowe (2014) showed that PFS scores correlated with binge eating frequency in those with either bulimia nervosa or anorexia nervosa. Despite this pattern of findings, the PFS items merely assess the degree to which respondents have frequent thoughts about and experience intense enjoyment from eating palatable foods. Although there is nothing inherently maladaptive about dwelling on the pleasure experienced from eating good-tasting food, it is possible that those who exhibit these characteristics most frequently start to experience adverse consequences of having “too much of a good thing.” One adverse consequence could be that such individuals start to ruminate about delicious foods and start having difficulty controlling their consumption of such foods.
The purpose of the present study was to test the predictions that PFS scores would be cross-sectionally and prospectively related to LOC eating. Relations were examined in a sample of college women who were at elevated risk for future weight gain, either because they had elevated body dissatisfaction (Haines, Neumark-Sztainer, Wall, & Story, 2007; Stice, 2002; van den Berg & Neumark-Sztainer, 2007) or were above average in weight suppression (defined as the difference their highest weight ever and current weight; (Lowe et al., 2006) or had dieted to lose weight in the past (Lowe, Doshi, Katterman, & Feig, 2013). To our knowledge, risk and maintenance factors for LOC eating (as opposed to binge eating) have not been identified. Factors that previously have been associated with onset of binge eating include starting BMI, depressive symptoms, body dissatisfaction, and dietary restraint; BMI, depressive symptoms, and body dissatisfaction have shown relations with change in binge eating over time (Bearman, Stice, & Chase, 2003; Stice, Rohde, Shaw, & Marti, 2013). Although these factors primarily have been examined with respect to bulimic symptoms, binge eating disorder diagnosis, or objective binge eating only (vs. subjective LOC eating), all were included as covariates to determine if the PFS was independently related to LOC eating above and beyond these variables.
Methods
Participants
Participants were 294 female first-year students (Mage = 18.24, SD = 0.44; MBMI = 23.65 kg/m2, SD = 2.88) recruited from two universities in Philadelphia in 2008 and 2009. They were volunteers in a randomized, controlled weight gain prevention trial. Participants with a history of or current eating disorder were excluded. All participants were required to have one or more of the following characteristics, which were expected to confer an elevated risk for weight gain: self-reported history of dieting, above-average body dissatisfaction, or elevated weight suppression (defined as being at least 1.8 kg below one's previous highest weight, based on the mean weight suppression of a college sample in which weight suppression was a prospective predictor of weight gain – see Lowe et al., 2006). The sample was 17.7% Asian/Pacific Islander, 11.4% African American, 5.5% Hispanic, 57.8% European American, and 7.6% other/mixed racial heritage. The percentage of participants providing follow up data was was 76% at 6 months, 69% at 12 months, and 63% at 24 months.
Procedures
Participants were recruited for a study that had two aims: 1) to examine the effectiveness of a weight gain prevention intervention, and 2) to examine predictors of change in eating disorder symptoms over two years. The weight gain prevention intervention did not produce differences in weight change at any follow-up period but to be conservative treatment condition was used as a covariate in all analyses.
After a baseline assessment, participants were randomly assigned to an experimental intervention or an assessment-only control condition. The intervention was conducted in six weekly, 1-hour, closed group sessions, with six to eight participants per group. The intervention comprised education and techniques for instigating behavior change (e.g., principles of energy balance, food intake self-monitoring; Bearman, Stice et al. 2003), and components from Lowe's nutrition-focused weight control program (Lowe, 2003; Lowe et al., 2008).
Treatment aimed to modify eating behaviors and increase physical activity using guidelines from behavior therapy for obesity (Butryn, Webb, & Wadden, 2011). There was also a focus on qualitative aspects of food itself, including learning how to reduce the energy density of foods and increase the purchase and use of pre-portioned foods (Rolls, Drewnowski, & Ledikwe, 2005). This component included advice about incorporating such foods into participants' “personal food environments” (Lowe, 2003). Finally the intervention drew on the social and clinical psychology literature on persuasion principles (strategic self-presentation, foot-in-the-door techniques, motivational interviewing) in an effort to maximize the likelihood that participants would make lasting changes to their health behaviors (Stice, Shaw, Becker, & Rohde, 2008). The current study examined data from assessments conducted at baseline, 6 months, 12 months, and 24 months. Assessments were conducted by an assessor who was blind to treatment condition.
The study procedures were approved by the Institutional Review Board at Drexel University and the University of Pennsylvania; it has also been registered at ClinicialTrials.com (number NCT00456131).
Measures
Objective height and weight
Participants were weighed in light clothing using an electronic scale that measured to the nearest 0.01 kg. Height was measured to the nearest 0.01 cm with a stadiometer. BMI (kg/m2) was calculated for each participant. BMI has been found to be a sensitive measure of adiposity that is adjusted for variation in height.
Loss of control eating
Eating behavior was assessed using the abbreviated version of the Eating Disorder Examination (EDE; Cooper & Fairburn 1993), a structured interview that includes items related to episodes of LOC eating. LOC was operationalized as any reported LOC eating in the past three months including objective binge episodes and subjective binge episodes. Because objective binges comprised a minority of all LOC episodes at each assessment point, LOC was measured irrespective of the amount of food eaten at each episode.
Hedonic hunger
Anticipated pleasure derived from palatable foods (one way of operationalizing hedonic hunger) was measured with the Power of Food Scale (PFS; (Lowe et al., 2009). The PFS measures appetite for palatable foods at three levels of food proximity (items describe situations where food is available but not present, present but not tasted, and tasted). Total scores represent the average response to each item, rather than a summed score. Sample items include “When I know a delicious food is available, I can't help myself from thinking about having some” and “Just before I taste a favorite food, I feel intense anticipation.” The PFS has shown high internal consistency (Cronbach's α = 0.91) and test–retest reliability (r = 0.77; Lowe, Butryn et al. 2009). The PFS was administered at baseline and at subsequent assessments.
Depressive symptoms
Depression was measured with the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff 1977). Participants completed the 20-item questionnaire to assess the frequency and duration of the symptoms associated with depression. The scale has shown high internal consistency (Cronbach's α = .90) and test-rest reliability (r = 0.91).
Restrained eating
Restrained eating was measured with the Dutch Restrained Eating Scale (van Strien et al., 1986). Participants rated the frequency with which they engage in dieting behaviors using 5-point scales ranging from never to always. This scale has high internal consistency (Cronbach's α = 0.95) and test-retest reliability (r = 0.82; (van Strien, Frijters et al. 1986), although it is not related to level of naturalistic caloric intake (Stice, Cooper, Schoeller, Tappe, & Lowe, 2007).
Body Image Dissatisfaction
Body satisfaction was measured with the Multidimensional Body-Self Relations, Body Area Satisfaction Subscale (MBSRQ-BAS; Cash and Grasso 2005). The measure asks participants to indicate how satisfied/dissatisfied they are with areas of the body on a 5-point scale from very dissatisfied to very satisfied. The MBSRQ-BAS has good internal consistency (Cronbach's α = 0.77) and test-retest reliability (r = 0.86; Cash & Grasso 2005).
Statistical Analyses
Descriptive analyses were conducted to describe the presence of loss of control eating at baseline, and the extent of LOC eating onset over the 2-year follow-up. PFS scores were compared between participants with and without LOC eating at baseline (independent samples t-test), and were tested for concurrent bivariate relationships with LOC eating frequency among those who endorsed loss of control. All available data were used in each analysis; baseline correlations included all participants who began the study, whereas correlations between baseline information and subsequent assessments included participants with available data at follow-up. We compared those who completed the study and those who did not on all of the constructs assessed at baseline; no differences were found for any of the aforementioned constructs (ps > 0.30).
Among those who reported LOC eating at baseline, a generalized linear mixed model (SAS PROC GLIMMIX, Version 9.4) was used to test longitudinal relations between baseline PFS score and change in LOC eating frequency over two years.1 Time points included assessments at baseline, 6 months, 12 months, and 24 months. GLIMMIX models used a negative binomial distribution with a log link and an adaptive quadstructure estimation method to address loss of control eating as a count variable and missing data (due to attrition). This analysis controlled for established maintenance factors for binge eating (i.e., BMI, depressive symptoms, and body dissatisfaction). A Cox proportional hazard model (SAS PROC PHREG) was used to examine the utility of PFS score for predicting LOC eating onset among participants with no LOC at baseline. Covariates included risk factors for binge eating (i.e., baseline BMI, depressive symptoms, body dissatisfaction, and dietary restraint).2 All analyses controlled for treatment condition.
Results
Baseline PFS Scores in the Current Sample Relative to Other Samples
Participants obtained a mean PFS score of 2.47 (SD = 0.81, Range = 1.0 - 5.0). A previous large sample of college females from the same university obtained mean PFS scores of 2.2 (SD = .70); the somewhat higher PFS scores of the current sample might reflect that fact that they were selected to be prone to weight gain.
PFS Scores and Initial Presence of LOC Eating
Of all participants assessed at baseline (n = 294), 55 participants (19%) reported LOC eating in the past three months. Women who reported LOC eating at baseline showed significantly higher PFS scores than those who did not (t[64] = 4.84, p < 0.001, d = 0.87). Mean PFS scores at baseline were 2.22 (SD = 0.72, 95% CI = 2.09-2.34) for those who did not develop LOC eating, and 2.82 (SD = 0.65, 95% CI = 2.60-3.04) for those who did.
PFS Scores and LOC Frequency
Across all participants, and controlling for baseline LOC eating frequency, baseline PFS scores were positively associated with the frequency of LOC episodes at 12 months and 24 months (ps < 0.05). Among the covariates, BMI and restraint predicted LOC frequency at the 12-month follow-up only. Correlation coefficients and p-values are shown in Table 1. Among those who endorsed LOC eating at baseline, PFS scores also were tested for bivariate relationships with LOC eating frequency at baseline and follow-up points. All available data were used in each analysis; correlations between baseline experiences and experiences at subsequent assessments included only participants with available data at follow-up.
Table 1.
Correlations between baseline PFS scores, covariates, and LOC frequency over two years, among college women who endorse LOC at baseline and reported LOC at follow-up points (n = 37).
| PFS Scores at Baseline | LOC Frequency (Baseline) | LOC Frequency (6 months) | LOC Frequency (1 year) | LOC Frequency (2 years) |
| PFS Total | 0.13 | 0.23 | 0.39* | 0.56** |
| Covariates at Baseline | LOC Frequency (Baseline) | LOC Frequency (6 months) | LOC Frequency (1 year) | LOC Frequency (2 years) |
| BMI | -0.27 | 0.23 | 0.32t | 0.23 |
| MBSRQ-BAS | 0.01 | -0.17 | -0.27 | -0.31 |
| CESD | -0.22 | 0.06 | 0.07 | 0.22 |
| Dutch | -0.16 | 0.31 | 0.39t | 0.24 |
Note: MBSRQ-BAS = Multidimensional Body Self-Relations Questionnaire-Body Area Satisfaction subscale (body dissatisfaction); CESD = Center for Epidemiological Studies-Depression scale; Dutch Restrained Eating scale
p = 0.05
p < 0.05
p < 0.01
p < 0.001
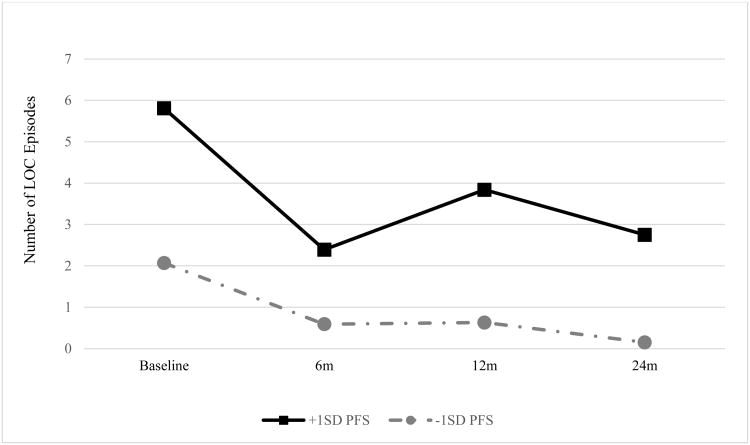
In the subset of participants who reported LOC eating at baseline (n = 55), episodes occurred approximately once per month at the start of the study (M = 3.13, SE = 0.70). LOC eating frequency showed a non-significant decrease over time (p = 0.46). A significant main effect of baseline PFS score on LOC eating frequency (t = 2.19, p = 0.03) indicated a positive correlation between PFS scores and the frequency of LOC episodes over time. This effect was qualified by an interaction with assessment point (t = 8.40, p < 0.001); time moderated the effect of PFS score on LOC frequency, indicating that individuals who scored higher on the PFS at baseline changed differently than those who scored lower. Although both groups showed decreases in LOC eating over time, the decrease shown by higher-PFS participants was smaller than the decrease shown by lower-PFS participants (see Figure 1).” For reference purposes, the high-PFS group (with scores at least one standard deviation above the mean) places their scores in the clinical range (i.e., between the mean scores previously obtained by obese and bulimic individuals, respectively – Schultes et al., 2010; Witt & Lowe, 2014).
Figure 1.
Frequency of LOC eating episodes (past three months) over two years, by baseline PFS score. LOC frequency for those who endorsed LOC eating at baseline. PFS scores represent +/-1 standard deviation from the grand mean.
PFS Score as a Risk Indicator for LOC Onset
Of participants who denied LOC eating at baseline, 37 reported LOC at later time points: 19 at 6 months, 10 at 12 months, and 8 at 24 months. PFS total scores for participants with no LOC eating at baseline who reported new onset at any follow-up time point (n = 37) were compared to those of participants with no reported LOC eating at any time point (n = 125). With respect to weight change from baseline to the three follow-up time points, women who developed LOC eating during the study interval did not differ from those who did not (ps > 0.12). Controlling for the aforementioned covariates, PFS score predicted participants who did and did not develop LOC eating over two years (Wald χ2 = 3.99, p = 0.04, hazard ratio [HR] = 1.65).3
Discussion
Findings from the present study contribute to our understanding of LOC eating by identifying the Power of Food Scale as a predictor of: (1) change in LOC frequency among those who exhibit episodes at the start of college, and (2) onset of LOC eating during the first two years of college, among those with no episodes at the start of college. With respect to change among those who initially reported LOC, the reason for the decline in LOC frequency is not known. Given that all participants were freshmen, however, it could be that they were unprepared for the newfound freedom to eat and drink whatever and whenever they pleased at the start of college, but learned improved control over such intake as the year progressed. Although, on average, all participants who endorsed LOC eating at the start of college showed a decline in frequency over time, the decline was smaller in those with higher relative to lower PFS scores (with reductions in the two groups being approximately 50% and 100%, respectively). This effect was independent of other known maintenance factors for binge eating (e.g., depressive symptoms).
Similarly, the PFS predicted LOC eating onset when controlling for previously-identified risk factors. An increase of one point in PFS score was associated with a 65% increase in risk for LOC eating onset (though because PFS scores only range between 1-5, a one-point increase is relatively large). This risk is noticeably higher than risk associated with other predictors (Stice et al., 2013). On the other hand, it does not appear that the level of LOC eating reported by participants – roughly 1 episode per month -rose to the level of clinical concern. At the same time, however, the mean baseline PFS score among those who would later develop LOC eating – 2.82 – was similar to the mean level found in a group of obese individuals who were candidates for bariatric surgery (2.8; Schultes, Ernst, Wilms, Thurnheer, & Hallschmid, 2010). If those exhibiting LOC eventually develop more frequent episodes or episodes involving objectively large amounts of food, then such eating patterns could contribute to unhealthy weight gain or eating disorders.
It may be that those who are most preoccupied with the availability of favorite foods at the start of college tend to repeatedly consume them, leading them to feel increasingly powerless to tame their desires compared to those with lower PFS scores. Even though such LOC feelings may not result in eating objectively large amounts of food, the feeling of being out of control could be quite distressing nonetheless (Mond et al., 2010).
The finding that participants who endorsed LOC eating at baseline scored significantly higher on the PFS is not surprising. PFS items reflect a preoccupation with delicious foods, and such preoccupation is a common feature of binge eating. The two most common explanations for binge eating are strict dietary restraint and the disinhibitory effect of negative affect (Polivy & Herman, 1985; Stice, 1994), but a third possibility is that subjective and objective binge eating reflect the culmination of food reward processes that become too powerful to control. In this nonclinical sample, the amount eaten in LOC episodes rarely rose to the level of an objective binge. Future research should examine whether the development of the subjective sense of LOC eating in nonclinical samples presages the eventual emergence of objective binge eating and/or weight gain.
A further question raised by the present results is why, among those who initially experienced LOC eating, the frequency of this experience declined over time, and why this decline was smaller among those who started the study with elevated PFS scores. Because the wide availability of high calorie foods on college campuses (and the surrounding areas where some students live) presumably changes little over time, the decline in LOC may reflect the development of effective eating inhibition more than a reduction in availability of tempting foods. If accurate, this perspective would also suggest that high-scoring PFS participants experienced more difficulty than their lower-scoring peers learning to inhibit their urges to eat highly palatable foods. The finding that PFS scores predicted degree of change in LOC frequency indicates that the PFS predicted both the likelihood of initial emergence of LOC and degree of LOC change in those who initially showed this characteristic. The ability of the PFS to predict frequency of binge eating among individuals showing this eating pattern was also documented by Witt et al. (2013), who found that PFS scores were positively related to binge eating frequency in patients with anorexia or bulimia nervosa.
Strengths of the present study include its recruitment of a sample that was predisposed toward weight gain (though on average did not experience significant weight change), a relatively large sample size, a longitudinal design and corresponding prospective analyses, and its use of covariates to examine alternative explanations for the findings. Limitations include the loss of participants to follow-up and the fact that all participants were female freshmen, limiting the generalizability of the findings to other populations. Although it is important to not overgeneralize the current findings, it is possible that the PFS or similar measures could be useful for identifying individuals who are at risk for developing LOC eating (and possibly objective binges) in the future. The availability of such measures could be quite helpful in preventive efforts because binge eating is relatively rare in the general population.
Acknowledgments
This research was supported by NIH grant RO1 DK072982
Footnotes
Participants who denied LOC eating at baseline were excluded from frequency prediction models because the vast majority of reports was zero, and mean estimates were close to zero for all time points. Thus, even if the time interaction were significant, this effect would not allow for clinically meaningful differentiation of change (as averages are very close to 0).
Stice (2002) identified distinct risk factors for the maintenance and onset of binge eating. Although BMI, depressive symptoms, and body dissatisfaction presented risk for both maintenance and onset, dietary restraint presented risk only for onset; as such, restraint was included as a covariate in onset prediction only. However, as these variables represent risk and maintenance factors for binge eating (not LOC eating), we tested our models both with and without these variables as covariates. Baseline PFS score predicted change in LOC eating frequency and onset in both cases; we chose to present the full model in order to address plausible competing explanations for PFS findings.
Analyses also tested for differences in PFS-LOC relationships by racial/ethnic group. Race/ethnicity was not a significant predictor of LOC and did not change the PFS-LOC relationship over time, and was left out of final models.
The subscale score for the availability of food did not differentiate LOC onset (Wald χ2 = 2.29, p = 0.13, HR = 1.35); subscale scores for presence of food (Wald χ2 = 3.26, p = 0.07 [marginal], HR = 1.41) and taste of food (Wald χ2 = 4.96, p = 0.02, HR = 1.51) were associated with LOC onset. When all factors were entered into a proportional hazard model simultaneously, however, only factor 2 (presence of food) remained significant (Wald χ2 = 6.14, p = 0.01, HR = 1.77).
Contributor Information
Michael R. Lowe, Drexel University
Danielle Arigo, Drexel University.
Meghan L. Butryn, Drexel University
Jennifer R Gilbert, Drexel University.
David Sarwer, Perelman School of Medicine at the University of Pennsylvania.
Eric Stice, Oregon Research Institute.
References
- American Psychiatric Association A. P. A. D. S. M. T. F. Diagnostic and statistical manual of mental disorders : DSM-5. 2013 from http://dsm.psychiatryonline.org/book.aspx?bookid=556.
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 2011;19(11):2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearman SK, Stice E, Chase A. Evaluation of an intervention targeting both depressive and bulimic pathology: A randomized prevention trial. Behavior Therapy. 2003;34(3):277–293. doi: 10.1016/S0005-7894(03)80001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls' eating in the absence of hunger. The American Journal of Clinical Nutrition. 2003;78(2):215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatric Clinics of North America. 2011;34(4):841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, Lowe MR. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes. 2009;33(8):913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- Cash TF, Grasso K. The norms and stability of new measures of the multidimensional body image construct. Body Image. 2005;2(2):199–203. doi: 10.1016/j.bodyim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Fairburn CG. Confusion over the core psychopathology of bulimia nervosa. Int J Eat Disord. 1993;13(4):385–389. doi: 10.1002/1098-108x(199305)13:4<385::aid-eat2260130406>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Fairburn CG. Cognitive behavior therapy and eating disorders. Guilford Press; 2008. [Google Scholar]
- Forman EM, Hoffman KL, McGrath KB, Herbert JD, Brandsma LL, Lowe MR. A comparison of acceptance-and control-based strategies for coping with food cravings: An analog study. Behaviour Research and Therapy. 2007;45(10):2372–2386. doi: 10.1016/j.brat.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Grilo CM. Cognitive behavioural therapy does not improve outcome in obese women with binge eating disorder receiving a comprehensive very low calorie diet programme. Evid Based Ment Health. 2006;9(1):12. doi: 10.1136/ebmh.9.1.12. [DOI] [PubMed] [Google Scholar]
- Haines J, Neumark-Sztainer D, Wall M, Story M. Personal, behavioral, and environmental risk and protective factors for adolescent overweight. Obesity. 2007;15(11):2748–2760. doi: 10.1038/oby.2007.327. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychological Bulletin. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Hartmann AS, Czaja J, Schoebi D. Natural course of preadolescent loss of control eating. Journal of abnormal psychology. 2013;122(3):684. doi: 10.1037/a0033330. [DOI] [PubMed] [Google Scholar]
- Lowe MR. Self-Regulation of Energy Intake in the Prevention and Treatment of Obesity: Is It Feasible? Obesity Research. 2003;11(S10):44S–59S. doi: 10.1038/oby.2003.223. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, Stice E, et al. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite. 2006;47(1):83–90. doi: 10.1016/j.appet.2006.03.160. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: A new dimension of appetite? Physiology & Behavior. 2007;91(4):432–439. doi: 10.1016/j.physbeh.2007.04.006. doi: http://dx.doi.org/10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Halford J, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–118. doi: 10.1016/j.appet.2009.05.016. doi: http://dx.doi.org/10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Doshi SD, Katterman SN, Feig EH. Dieting and restrained eating as prospective predictors of weight gain. Frontiers in psychology. 2013;4 doi: 10.3389/fpsyg.2013.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR, Levine AS. Eating Motives and the Controversy over Dieting: Eating Less Than Needed versus Less Than Wanted. Obesity Research. 2005;13(5):797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Tappe KA, Annunziato RA, Riddell LJ, Coletta MC, Crerand CE, McKinney S, et al. The Effect of Training in Reduced Energy Density Eating and Food Self-monitoring Accuracy on Weight Loss Maintenance. Obesity. 2008;16(9):2016–2023. doi: 10.1038/oby.2008.270. [DOI] [PubMed] [Google Scholar]
- Mond JM, Latner JD, Hay PH, Owen C, Rodgers B. Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: Another nail in the coffin of a problematic distinction. Behaviour Research and Therapy. 2010;48(7):661–669. doi: 10.1016/j.brat.2010.03.020. doi: http://dx.doi.org/10.1016/j.brat.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Dieting and binging: a causal analysis. American Psychologist. 1985;40(2):193. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. Journal of the American Dietetic Association. 2005;105(5):98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Dilks RJ, West-Smith L. Dietary intake and eating behavior after bariatric surgery: threats to weight loss maintenance and strategies for success. Surgery for Obesity and Related Diseases. 2011;7(5):644–651. doi: 10.1016/j.soard.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. The American journal of clinical nutrition. 2010;92(2):277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Horton NJ, Micali N, Crosby RD, Swanson SA, Solmi F, Field AE. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA pediatrics. 2013;167(2):149–155. doi: 10.1001/2013.jamapediatrics.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E. Review of the evidence for a sociocultural model of bulimia nervosa and an exploration of the mechanisms of action. Clinical psychology review. 1994;14(7):633–661. [Google Scholar]
- Stice E. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychological Bulletin. 2002;128(5):825. doi: 10.1037/0033-2909.128.5.825. [DOI] [PubMed] [Google Scholar]
- Stice E, Cooper JA, Schoeller DA, Tappe K, Lowe MR. Are dietary restraint scales valid measures of moderate-to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychological Assessment. 2007;19(4):449. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]
- Stice E, Rohde P, Shaw H, Marti CN. Efficacy trial of a selective prevention program targeting both eating disorders and obesity among female college students: 1-and 2-year follow-up effects. J Consult Clin Psychol. 2013;81(1):183. doi: 10.1037/a0031235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Shaw H, Becker CB, Rohde P. Dissonance-based interventions for the prevention of eating disorders: Using persuasion principles to promote health. Prevention Science. 2008;9(2):114–128. doi: 10.1007/s11121-008-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. doi: http://dx.doi.org/10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, Olsen C, et al. A multisite investigation of binge eating behaviors in children and adolescents. Journal of Consulting and Clinical Psychology. 2007;75(6):901. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, Roza CA, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. The American journal of clinical nutrition. 2009;90(6):1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, Yanovski SZ, et al. A prospective study of pediatric loss of control eating and psychological outcomes. Journal of abnormal psychology. 2011;120(1):108. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. International Journal of Eating Disorders. 2009;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg P, Neumark-Sztainer D. Fat' n Happy 5 Years Later: Is It Bad for Overweight Girls to Like Their Bodies? Journal of Adolescent Health. 2007;41(4):415–417. doi: 10.1016/j.jadohealth.2007.06.001. [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters JER, van Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. International Journal of Eating Disorders. 1986;5(4):747–755. doi: 10.1002/1098-108X(198605)5:4<747∷AID-EAT2260050413>3.0.CO;2-6. [DOI] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GT, Fairburn CG, Agras WS. Cognitive-behavioral therapy for bulimia nervosa. In: Garner DM, Garfinkel PE, editors. Handbook of Treatment for Eating Disorders. 2nd. New york: Guilford; 1997. pp. 67–93. [Google Scholar]
- Witt AA, Lowe MR. Hedonic hunger and binge eating among women with eating disorders. International Journal of Eating Disorders. 2014;47(3):273–280. doi: 10.1002/eat.22171. [DOI] [PubMed] [Google Scholar]
- Witt AA, Lowe MR. Hedonic hunger and binge eating among women with eating disorders. Int J Eat Disord. 2014;47(3):273–280. doi: 10.1002/eat.22171. [DOI] [PubMed] [Google Scholar]