Abstract
Different patterns of gray matter volume (GMV) abnormalities have been reported between chronic patients with deficit schizophrenia (DS), relative to nondeficit schizophrenia (NDS) patients. However, it is not clear whether these differences are characteristic to the pathophysiology of DS or due to the effects of medications or illness durations. To address this issue, GMV in 88 first-episode, drug-naive patients with schizophrenia (44 DS and 44 NDS), 67 of their first-degree relatives and 84 healthy controls were assessed using voxel- based morphometry (VBM) and compared between groups. Correlations between GMV and clinical symptoms in patients were also assessed. Compared to controls, DS patients displayed more severe GMV reduction in the cerebellar culmen than NDS patients. GMV reduction in culmen was also observed in the first-degree relatives of DS (but not NDS) patients, suggesting possible different genetic risk in DS and NDS. The left insula was significantly smaller in DS patients than both NDS patients and controls, and smaller GMV of this region was associated with more severe negative symptoms in patients. Our results collectively indicate that DS might represent a distinct subtype of schizophrenia from NDS and the GMV change in left insula may be a morphological signature of DS.
Keywords: Deficit Schizophrenia, Gray matter volume, Voxel-based morphometry
1. Introduction
Deficit schizophrenia (DS) is a syndrome with enduring, primary negative symptoms in patients with schizophrenia (Kirkpatrick and Galderisi, 2008). Patients with DS comprises 15~20% of schizophrenia cases in epidemiological samples(Messias et al., 2004). Relative to non-deficit schizophrenia (NDS) patients, DS patients have more severe negative symptoms, worse long-term prognosis, poorer premorbid adjustment, greater cognitive impairment, and a high frequency of family history with schizophrenia (Kirkpatrick et al., 2000; Kirkpatrick et al., 2001; Tek et al., 2001; Galderisi et al., 2002; Cohen et al., 2007; Réthelyi et al., 2011). Consequently, it has been suggested that DS could be a distinct disease entity from nondeficit forms of schizophrenia (Galderisi and Maj, 2009). It was also hypothesized that DS and NDS might involve different pathophysiological changes in the brain (Kirkpatrick et al., 2001; Kirkpatrick and Galderisi, 2008). Indeed, several previous studies reported different patterns of grey matter volume (GMV) abnormalities in DS and NDS patients, although the results remain inconclusive (Sigmundsson et al., 2001; Galderisi et al., 2008; Cascella et al., 2010; Fischer et al., 2012; Volpe et al., 2012). For instance, using the voxel-based morphometry (VBM), Cascella et al. reported that GMV abnormalities in the left insula, bilateral superior temporal gyrus, and left precuneus were characteristic of DS (Cascella et al., 2010). In addition, Fischer et al. found that the reduction in GMV of bilateral superior prefrontal and superior and middle temporal gyrus was only associated with DS (Fischer et al., 2012). However, some other studies suggested that the difference in GMV abnormality between DS and NDS was largely a matter of severity rather than a characteristic feature. Using the volumetric approach, Galderisi et al. showed no selective regional change in GMV that was specific to patients with DS, with only a greater GMV reduction in the right temporal lobe in patients with DS than patients with NDS (Galderisi et al., 2008). Meanwhile, another volumetric study performed by Volpe et al. found that both DS and NDS patients showed reduction in GMV in the dorsolateral prefrontal cortex as compared with healthy controls (HCs), though with a greater reduction in GMV found in patients with NDS (Volpe et al., 2012). Similarly, ÖZDEMİR et al. reported less GMV in several brain regions in NDS patients than DS patients (ÖZDEMİR et al., 2012). Nevertheless, Voineskos et al. found that DS and NDS did not differ significantly on either cortical thickness reduction or surface areas and subcortical volumes (Voineskos et al., 2013).
A number of confounding factors, such as small sample sizes, heterogeneous treatment conditions, durations of illness and/or different imaging techniques may count for inconsistent results of previous studies (Galderisi and Maj, 2009). The majority of previous imaging studies on DS had relatively small sample sizes (from 8 to 34). In addition, previous brain structural analysis used the data from chronic patients with DS or NDS, with the duration of illness usually longer than 10 years (Galderisi et al., 2008; Cascella et al., 2010; Fischer et al., 2012; Volpe et al., 2012). Particularly, since antipsychotics was likely to be less effective with negative symptoms (Leucht et al., 2003), possible different treatment effects of antipsychotics on patients with DS or NDS may also compound the comparison between the two subpopulations (Kirkpatrick and Galderisi, 2008). Considering the progressive loss of cortical GMV in brain regions involving frontal and temporal lobes in patients with schizophrenia (Nakamura et al., 2007; Yoshida et al., 2009; Vita et al., 2012), chronicity and longer duration of the illness might also significantly affect the results of aforementioned studies.
It has also been shown that family members of DS patients have more severe subclinical negative symptoms such as social withdrawal, and an increased risk of schizophrenia (Kirkpatrick et al., 2001) compared with relatives of NDS probands (Hong et al., 2003). However, the manifestation of potentially different genetic risk in GMV alterations between the DS and NDS remains unknown.
For the purpose of investigating the characteristic pattern of gray matter abnormalities occurring at the onset of DS and NDS and the potential genetic risk, the present study has gathered a relatively large sample of structural magnetic resonance imaging (MRI) dataset in first-episode, DS and NDS patients (most of them were treatment-naïve), their first-degree relatives and matched healthy controls. GMV in these subjects has been analyzed by utilizing the VBM method. The relationship between regional GMV and the symptomology has also been examined.
2. Materials and methods
2.1. Participants
A total of 239 subjects including 88 patients with first-episode schizophrenia (FES), 67 of their first-degree relatives and 84 HCs participated in this study. All patients were recruited from in-patient and out-patient psychiatric units at the Mental Health Centre of the West China Hospital, during 2006-2012. The first-episode schizophrenia sample comprised individuals presenting to the Mental Health Center who had been diagnosed as schizophrenia. To be included, an individual was required to fulfill the inclusion criteria of the present study: age between 16 and 45 years; Han Chinese; right-handed; Intelligence Quotient (IQ) ≥ 70; at the first episode of schizophrenia; treatment-naïve or had no more than 3 days of antipsychotic treatment before MRI scan.
The medical history of each patient was reviewed using the Structured Clinical Interview for the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders) Patient Edition (SCID-P)(First and Gibbon, 1997). Patients with a history of any major psychiatric disorders (such as affective and schizo-affective disorders), mental retardation, head trauma, substance abuse (including cocaine and heroin), alcoholism, any major nervous system diseases, serious endocrine or metabolic diseases were excluded. Information about previous treatment and duration of untreated psychosis (DUP) was collected at diagnosis. Diagnoses were assigned based on the diagnostic criteria for schizophrenia and schizophreniform psychosis as specified in DSM-IV. Patients initially diagnosed with schizophreniform psychosis (n = 24, 10 DS and 14 NDS) were included as they were confirmed for the diagnosis of schizophrenia after being followed up for at least 6 months. The diagnoses of DS and NDS were reached using the Schedule for the Deficit Syndrome (SDS)(Kirkpatrick et al., 1989), 12 months after admission. Eight patients (9%; 5 DS and 3 NDS) were taking low dose antipsychotics (risperidone or olanzapine; 25 to 75 mg of chlorpromazine daily dose equivalent) for less than 3 days prior to MRI. All others (91%, 39 DS and 41 NDS) were treatment-naïve before scanning. Patients also underwent further evaluation on clinical symptoms using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987).
The data of HCs were selected from our database of 158 adult HCs. These HCs were recruited between 2006 and 2012 via posting advertisements. Where available, the first-degree relatives were recruited along with patients. The inclusion criteria for HCs and relatives are as follows: Han Chinese; right-handed; IQ≥70. All controls and relatives were screened for a lifetime absence of Axis I illness of DSM-IV psychiatric illnesses with the SCID non-patient version (SCID-NP) (First and Gibbon, 1997). In addition, HCs were interviewed to ascertain that there was no psychiatric illness in their first-degree relatives. Relatives or controls with any major psychiatric disorder, serious physical illness, substance abuse (including cocaine and heroin), alcoholism, pregnancy, head trauma, or mental retardation were excluded from the study.
Relatives were assigned into 2 subgroups, relatives of DS (DS_R) or with NDS (NDS_R). Two groups of ‘healthy controls’ were age- and sex-matched with patients (HC1) and relatives (HC2) respectively. For the patient group, HCs with the same gender and similar ages were selected in a case-match manner (HC1). For the relative group, the health controls in the same age range as the relative group (16-60 years) were selected which were no significant difference in mean age and sex distribution between groups. We arranged two HC groups because the relatives were markedly older than patients (Please see 3.1. Demographic and clinical characteristics), and this could compound the brain structural difference (Nenadić et al., 2012).
Current IQ was estimated for all subjects using the seven-subtest version (including Information, Similarities, Arithmetic, Digit Span, Picture Completion, Block Design, and Digit Symbol subtests) (Schopp et al., 1998) of the Wechsler Adult Intelligence Scale -revised in China (WAIS-RC) (Gong et al., 1992).
All subjects were Han Chinese, and right-handed as assessed by the Annett Handedness Scale (Annett, 1970). After a complete description of the study, written informed consent was obtained from patients and patients’ guardians and all healthy subjects. The study was approved by the Institutional Review Board of West China Hospital, Sichuan University.
2.2. MRI data acquisition
High-resolution T1-weighted images were acquired after the admission (within 3 days) of patients using a 3-Tesla MRI system (EXCITE, General Electric, WI, USA) with a volumetric 3-dimensional Spoiled Gradient Recall sequence (repetition time, 8.5 ms; echo time, 3.4 ms; flip angle, 12°; slice thickness, 1 mm with no gap. field of view, 240×240 mm2; matrix, 256×128, 156 contiguous axial slices). The raw MRI data were inspected by two experienced neuroradiologists. No gross abnormalities were observed for any subject.
2.3. Image preprocessing
Structural data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running on Matlab7.6 (MathWorks, Inc., Natick, MA, USA). The data preprocessing included the following steps: (1) T1-weighted images were realigned and then manually reoriented so that the anterior commissure was close to coordinate [0, 0, 0]; (2) The non-parametric, non-uniformity intensity normalization (N3) technique in the MINC software package (http://wiki.bic.mni.mcgill.ca/index.php/MINC) was used to rectify the nonuniformity of high-magnetic field signals in reoriented images; (3) Images were then segmented into gray matter, white matter, and cerebrospinal fluid probability maps using the unified segmentation method (Ashburner and Friston, 2005); (4) Gray matter images were spatially normalized to the MNI space and Jacobian modulated into volume images using the Diffeomorphic Anatomical Registration using the Exponentiated Lie algebra (Dartel) toolbox(Ashburner, 2007) implemented in SPM8; and finally (5) images were smoothed with a 6 mm FWHM Gaussian kernel. A relatively small Gaussian kernel of 6 mm was chosen to improve the signal-to-noise ratio while still maintain the sensitivity to detect small brain structural changes in patients (Tognin et al., 2012; Filbey et al., 2014; García-Casares et al., 2014).
2.4. Statistical analysis
2.4.1. Demographic data
Statistical analysis of demographic data was conducted using SPSS16.0 (SPSS Inc., Chicago, IL, USA). Where appropriate parametric (analysis of variance, ANOVA or Student's t-test) and non-parametric (Mann-Whitney's U test) procedures were used to assess the group difference. For categorical data, χ2 tests were applied. Student's t-test or one-way ANOVA were used to compare the distribution and differences of categorical and continuous data between groups as appropriate. As mentioned earlier, due to the age differences between groups of patient and relatives, comparison among the patient groups (DS, NDS and HC1) and the relative groups (DS_R, NDS_R and HC2) were performed respectively.
2.4.2. GMV analysis
Statistical analysis for preprocessed sMRI data was performed with the following steps:
-
(1)
In patient groups, voxel-wise comparisons of GMV were performed using a series of two sample t-tests in SPM8, including: DS vs. HC1, NDS vs. HC1, and DS vs. NDS, with age and gender as nuisance variable. Correction for multiple comparisons was conducted at combined voxel-level P < 0.0001 and cluster-level P < 0.05(FWE corrected).
The comparisons of relatives were carried out: HC2 vs. DS_R, HC2 vs. NDS_R, and DS_R vs. NDS_R. Considering that the gray matter volume changes might be smaller in relatives than probands according to recent meta-analyses (Allen et al., 2009; Chan et al., 2011), and less statistical power due to a relatively smaller sample size of relative groups, a less conservative threshold of uncorrected P <0.001 and cluster extent of 100 voxels was used in these comparisons.
For all voxel-wise comparisons mentioned above, an explicit gray matter mask constructed from all participants were applied to ensure that only voxels within the gray matter were analyzed. To further evaluate the impact of head size, we also specified a model with WBV (the sum of total gray matter volume and total white matter volume, an index of head size), age and gender as nuisance variables. The results remained the same after adjusting for the effect of WBV, age and gender (Please see Figure S1 and Table S1).
-
(2)
Each individual cluster that showed significant difference between groups identified in step (1) was defined as a region of interest (ROI). For each ROI, the GMV were extracted from each subject and compared between groups using ANOVA in SPSS.
-
(3)
To assess the functional significance of GMV alterations in patients, values of GMV of each ROI were correlated with PANSS scores, DUP and IQ scores. The correlation analysis was carried out using Pearson's correlation and Spearman's correlation (for variables with nonnormal distribution) in SPSS 16.0. Correlations were calculated in all FES patients (combining DS and NDS), and also in DS and NDS group separately.
2.5. Evaluating potential confounding effects of DUP and symptomatic severity
Considering some clinical variables, such as the DUP and symptomatic severity, were different between DS and NDS group (see Table 1) and may affect the GMV of patients (Ziermans et al., 2010; Vita et al., 2012), a series of regression model with DUP (or one of PANSS total and PANSS positive subscale scores) as a covariate was specified in SPM8 to assess the effects of these clinical profiles on regional GMV. The gray matter mask used in voxel-wise comparisons was also applied in these regression analyses. An exploratory threshold of uncorrected P < 0.001 and a minimum cluster extent of 100 voxels were adopted. This threshold was used to minimal false negative effect in contrasts, so that even small effects would not missed. These regression analyses were carried out in all FES patients (combining DS and NDS), to provide information about the effects of DUP and symptomatic severity on the GMV in patients.
Table 1.
Demographic summary of the patients their first-degree relatives and the health control.
| NDS | DS | HC1 | Statistic, P value | NDS_R | DS_R | HC2 | Statistic, P value | |
|---|---|---|---|---|---|---|---|---|
| N | 44 | 44 | 44 | 42 | 25 | 40 | ||
| Age | 23.16±6.99 | 22.91±6.89 | 22.55±6.25 | F2, 129=0.017, 0.983 | 43.00±7.86 | 44.00±8.06 | 42.53±9.19 | F2, 90=0.186, 0.831 |
| Female/Male | 18/26 | 18/26 | 18/26 | χ2 = 0, 1 | 23/19 | 12/13 | 22/18 | χ2 = 0.365, 0.833 |
| tGMV | 0.74±0.07 | 0.73±0.09 | 0.74±0.06 | F2, 129=0.008, 0.923 | 0.68±0.06 | 0.69±0.07 | 0.70±0.07 | F2, 90=0.854, 0.429 |
| WBV | 1.17±0.12 | 1.16±0.13 | 1.18±0.09 | F2, 129=0.398, 0.673 | 1.11±0.09 | 1.12±0.11 | 1.14±0.11 | F2, 90=0.871, 0.422 |
| Education | 12.52±2.77 | 11.45±2.66 | 12.72±2.41 | F2, 129=2.575, 0.080 | 10.00±3.88 | 9.00±3.78 | 10.23±4.71 | F2, 90=0.944, 0.392 |
| IQ | 97.85±13.85 | 90.87±20.22 | 118.50±14.93 | F2, 129=30.607, 0.000* | 94.44±15.78 | 90.62±20.02 | 103.50±16.89 | F2, 90=4.571, 0.013* |
| DUP | 8.83±13.33 | 19.89±29.88 | U88=604.5, 0.002* | |||||
| Age of Onset | 22.85±7.65 | 21.79±7.56 | T86=0.902, 0.370 | |||||
| PANSS-T | 88.90±17.61 | 95.95±16.82 | T86=−2.144, 0.0350* | |||||
| PANSS-P | 24.67±6.39 | 21.95±6.88 | T86=2.497, 0.014* | |||||
| PANSS-N | 16.70±5.77 | 27.57±7.53 | T86=−7.548, 0.000* | |||||
| PANSS-G | 46.27±9.43 | 46.43±9.68 | T86=−0.011, 0.991 |
Note: Demographic data are shown as mean±standard deviation
significant different between groups at p<0.05;
Aberrations: DS, deficit schizophrenia; NDS, nondeficit schizophrenia; HC1, healthy controls who age-and sex-matched with patients; DS_R, first degree relatives of DS patients; NDS_R first degree relatives of NDS patients; HC2, healthy controls who age-and sex-matched with relatives; tGMV, total gray matter volume; WBV, whole brain volume= total gray matter volume + total white matter volume; DUP, duration of untreated psychosis (in months); PANSS-T, PANSS total scores; PANSS-P, PANSS positive symptoms subscale scores; PANSS-N, PANSS negative symptoms subscale scores; PANSS-G, PANSS general psychopathological symptoms subscale scores.
3. Results
3.1. Demographic and clinical characteristics
The demographic characteristics of participants are summarized in Table 1. All variables, except DUP, are normally distributed. HC1 have higher IQ than both DS and NDS patients. NDS patients showed a trend of higher IQ (post-hoc LSD test, P = 0.076) than DS patients. HC2 showed higher IQ than both relative groups (post-hoc LSD test, P < 0.001). There were no statistically significant differences in age, gender distribution, education years, total gray matter volume and whole brain volume in either patient analysis or relative analysis. In terms of clinical profiles, DS patients showed significantly longer DUP, higher PANSS total and negative subscale scores but lower PANSS positive subscale scores. There was no significant difference between DS and NDS in the age of illness onset and general psychopathological symptoms.
3.2. Group differences in regional GMV
3.2.1. Voxel-wise comparisons
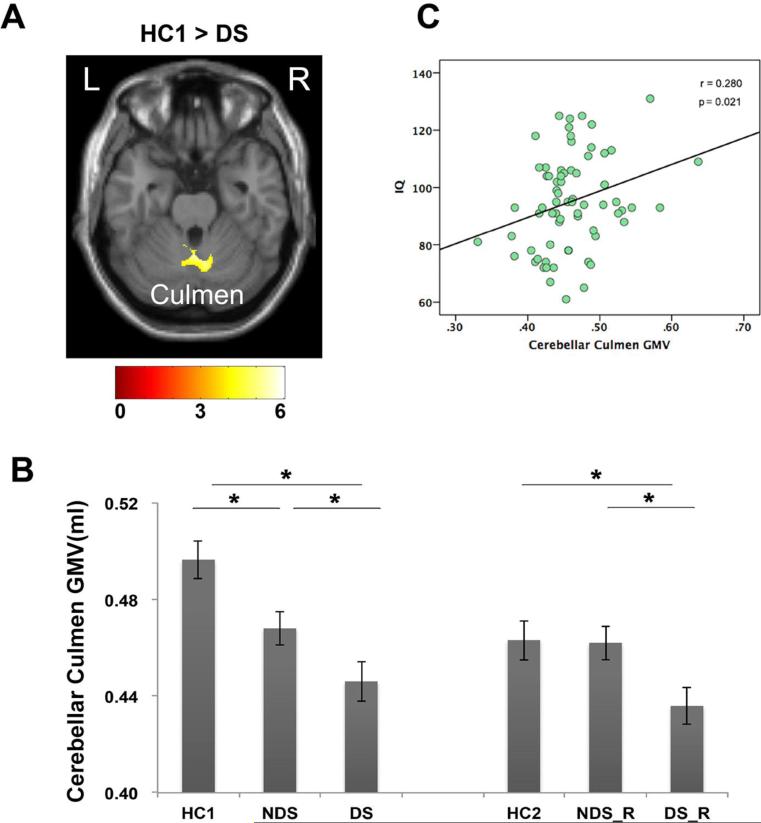
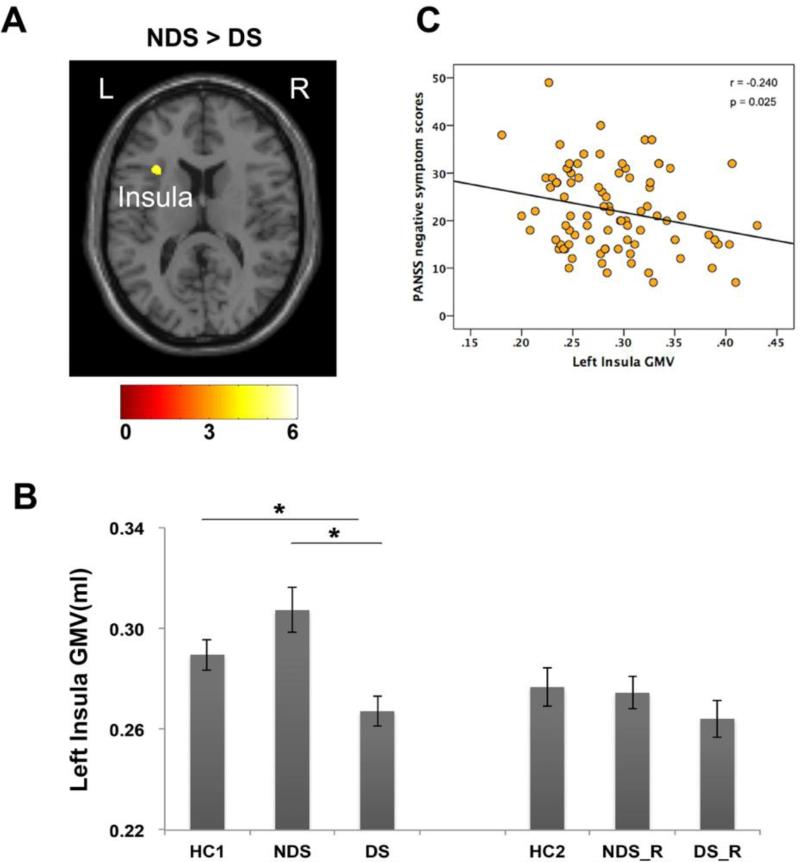
Results of voxel-wise comparisons are present in Table 2. When compared to healthy controls (HC1), FES patients (combining DS and NDS) showed significantly smaller gray matter volume in cerebellar culmen. Compared to HC1, DS patients displayed smaller GMV in cerebellar culmen as well (Figure 1A). Direct comparison between the DS and NDS patients showed that the GMV of left insula were significantly smaller in DS patients than NDS patients (Figure 2A).
Table 2.
Voxel-wise comparisons of GMV between groups
| Regions | Cluster P value (FWE corrected) | Voxels | T | MNI Coordinates (x, y, z) |
|---|---|---|---|---|
| FES* < HC1 | ||||
| Cerebellar Culmen | 0.008 | 1041 | 5.20 | 10 −62 −26 |
| DS < HC1 | ||||
| Cerebellar Culmen | 0.004 | 1248 | 5.36 | 6 −57 −22 |
| DS<NDS | ||||
| Insula | 0.034 | 646 | 4.56 | −31 20 14 |
FES, n=88, the combining patient group with both DS and NDS patients.
Figure 1.
Gray matter volume reduction in DS patients and relatives. In voxel-wise comparison, DS patients exhibited smaller GMV in cerebellar culmen than matched healthy controls (A). ROI analysis showed that the first-degree relatives of DS also have less GMV than both relatives of NDS and matched healthy controls in this region (B). And GMV of cerebellar culmen was correlated with IQ in patients (C). Color bar illustrated the t-value in the voxel-wise comparisons.
Note: *, significant different between groups at p<0.05; L, Left hemisphere; R, right hemisphere.
Figure 2.
Gray matter volume difference between DS and NDS. DS patients have smaller left insula than NDS patients and matched healthy controls (A, B). And smaller GMV in this region is associate with more severe negative symptoms in patients (C). Color bar illustrated the t-value in the voxel-wise comparisons.
Note: *, significant different between groups at p<0.05; L, Left hemisphere; R, right hemisphere.
3.2.2. ROI-wise comparisons
The clusters that group difference in cerebellar culmen and left insula were defined as ROIs. The GMV values of these two ROIs were extracted for each participant and fed into ROI-wise comparisons.
In cerebellar culmen, both DS (post-hoc LSD test, P < 0.001), and NDS patients (post-hoc LSD test, P = 0.010) showed GMV reduction in this ROI when compared with HC1, with the DS group showing even less GMV than NDS (post-hoc LSD test, P = 0.046). Intriguingly, a significant GMV reduction in cerebellar culmen was also observed in DS_R (post-hoc LSD test, P = 0.023) but not in NDS_R relative to HC2 (post-hoc LSD test, P = 0.916) (Figure 1B).
In insula, ROI-wise comparisons showed that DS group had significantly less GMV than both NDS (post-hoc LSD test, P < 0.001) and HC1 (post-hoc LSD test, P = 0.027), while no significant difference was observed between NDS and HC1 (post-hoc LSD test, P = 0.087) (Figure 2B). No significant differences were found among relative groups (F2,104=0.702, P=0.498).
3.3. Correlation analysis
In the patient group combining both DS and NDS, the GMV of cerebellar culmen was positively correlated with IQ scores (r = 0.280, P = 0.021) (Figure 1C). The GMV of insula was negatively correlated with PANSS negative symptom scores (r = −0.240, P = 0.025, Figure 2C). Regardless of controlling for the effect of age, sex and WBV, all results remained the same (see Table S2 for the partial correlation results). The correlational analyses were also conducted separately in DS and NDS group. Both groups showed similar trends of correlations but no significant correlations were observed for either group, mostly likely due to smaller statistical power (see Table S3 and Table S4 for correlation results of DS and NDS groups, respectively).
3.4. The effects of DUP and symptomatic severity
In all FES patients, whole-brain voxel-wise regression analysis revealed that the GMV in left precentral gyrus was negatively associated with DUP, and the GMV in superior temporal gyrus, medial frontal gyrus and precentral gyrus were negatively associated with PANSS total scores (see Table S5). No significant associations between GMV and PANSS positive scores were observed. None of these regions were overlapped with regions identified in voxel-wise comparisons, suggesting that our results cannot be attributed to the effects of symptoms severity or DUP.
4. Discussion
The present study aimed to investigate the characteristic pattern of GMV abnormalities occurring at the onset of illness in patients with DS or NDS. We found that: (1) Compared to controls, DS patients displayed more severe GMV reduction in the cerebellar culmen than NDS patients. GMV reduction in culmen was also observed in the first-degree relatives of DS (but not NDS) patients, suggesting possible different genetic risk in DS and NDS in this region. (2) The left insula was significantly smaller in DS patients than both NDS patients and controls, and smaller GMV of this region was associated with more severe negative symptoms in patients.
4.1. Cerebellar gray matter atrophy in first-episode patients
Our results demonstrated that FES patients (combining both DS and NDS group) showed less GMV in cerebellar culmen, and DS patients showed more severe GMV reduction in this than NDS patients. These results were in line with previous meta-analysis of GMV abnormalities in FES (Leung et al., 2011), which suggested that the GMV atrophy in cerebellar culmen might be a feature of drug-naïve patients with FES. Moreover, our results were also consistent with previous VBM studies involving patients with chronic DS and NDS (Cascella et al., 2010; ÖZDEMİR et al., 2012), in which GMV in cerebellar culmen in DS patients was found smaller than that in controls and in NDS patients. Together, these results suggest that severe reduction in GMV in cerebellar culmen was a characteristic feature for DS at the onset of illness, and likely to be maintained during the progress of illness.
With both anatomical and functional connections to the prefrontal cortex, subcortical limbic areas and brainstem nuclei, the cerebellum has been hypothesized to be involved in multiple cognitive processes such as attention, executive control and memory (Picard et al., 2008; Strick et al., 2009). As highlighted by Andreasen and Pierson, the cerebellar dysfunction might represent a fundamental aspect of cognitive impairments in psychosis, which could lead to the diversity of symptoms and cognitive dysfunction observed in schizophrenia (Andreasen and Pierson, 2008). Indeed, GMV in cerebellum culmen was associated with IQ in FES patients in this study. Moreover, worse impaired global cognitive in DS patients has been consistently reported in previous neurocognitive studies with DS and NDS (Galderisi et al., 2002; Cohen et al., 2007; Réthelyi et al., 2011). Collectively, our results suggested that worse GMV atrophy in cerebellar culmen might be associated with worse cognitive impairment in patients with FES (Galderisi et al., 2002; Cohen et al., 2007; Réthelyi et al., 2011). However, further studies with bigger sample size are needed to clarify if such association is specific to DS patients.
Interestingly, the gray matter reduction in cerebellar culmen was also observed in the first-degree relatives of DS patients but not in relatives of NDS patients. These results well agreed with the precious finding that family members of DS patients have an increased prevalence of subclinical negative symptoms compared with the relatives of NDS patients (Hong et al., 2003). Moreover, GMV reduction in cerebellum has also been reportedly related to neurological soft signs (i.e. functions known to be deficient not only in patients with schizophrenia, but also in relatives with an increased genetic liability (Niethammer et al., 2000) in FES patients (Bottmer et al., 2005). Together, these results suggest potentially different genetic risk in DS and NDS.
4.2. The brain morphological signature of DS
Our results showed the GMV atrophy in left insula was selectively affected in first-episode drug-naive DS patients, as first-episode NDS patients and healthy controls did not differ in this region. GMV atrophy of the insula cortex is one of most consistent findings in neuroanatomical studies of chronic schizophrenia, yet it's less conclusive in FES according to recent meta-analyses (Bora et al., 2011; Shepherd et al., 2012). In a recent systematic meta-analysis, Shepherd et al reported a compatible GMV loss in insula in both FES patients and chronic patients (Shepherd et al., 2012). While Bora et al reported insula GMV reduction only in chronic patients (Bora et al., 2011). Our results in line with previous reports that selective GMV reduction in left insula in chronic DS patients relative to both NDS patients and healthy controls (Cascella et al., 2010), and primarily in line with volumetric study which found a significantly deficient right posterior insula volume in drug naïve patients with predominantly negative symptoms than those with predominantly positive symptoms (Virupaksha et al., 2012). Together, these results suggested that GMV loss in left insula at the early period of illness might be characteristic to DS patients. However, it should be noted that, according to previous studies (Kubicki et al., 2002; Makris et al., 2006; Saze et al., 2007; Takahashi et al., 2009), the insula volume of both hemispheres have been shown to be reduced in schizophrenia. There is some evidence of more volume loss in left anterior insula (Makris et al., 2006), but the findings seem to be inconclusive (Saze et al., 2007) and require more independent studies.
The insula is part of the salience network, which is believed to control the interaction between self-related and goal-directed processes in brain (Menon and Uddin, 2010). Functional and structural alterations in insula may underlie impaired coordination of self-monitoring and task-performance in patients, and thus may represent a core feature of schizophrenia (Wylie and Tregellas, 2010; Palaniyappan and Liddle, 2012). Aberrant functional connectivity in this region was associated with the altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission (Manoliu et al., 2013; Manoliu et al., 2014). Also, GMV in left insula was associated with negative symptoms in a recent meta-analysis (Bora et al., 2011). Less GMV in insula was consistently found in patients with predominantly negative symptoms, both at first-episode (Virupaksha et al., 2012) and chronic stage (Sigmundsson et al., 2001). In a recent follow-up MRI study, the GMV reduction of bilateral insula was also associated with negative symptoms (Takahashi et al., 2009). These results in line with our correlation results, suggest that less GMV in left insula is associated with negative symptoms in schizophrenia. Altogether, our results suggest that selective GMV atrophy in left insula could represent a morphological signature of DS.
4.3. Potential Limitations
In our sample, the DS and NDS group were different on several clinical measures such as PANSS scores and DUP, which could have affected the results. We thus assessed the effect of these clinical measures (including DUP and PANSS total and positive scores) by establish additional regression models to locate the most affected regions, and these analyses demonstrated that our results was mainly independent with the variance of these clinical measures. An alternative approach is to exclude some DS patients with long DUP (or NDS patients with short DUP). However, this alternative strategy will also bias the representation of DS patients and would reduce statistical power to demonstrate group differences, and because it would bias the representation of DUP in our sample. Another limitation of present study is that we have not controlled the effect of relatively low-dosage alcohol consumption and tobacco smoking. This is potentially important as alcohol and tobacco smoking have all been shown to have an impact on brain structure (Tregellas et al., 2007; Kühn et al., 2010). Further studies may address the issue via collect more specific information on substance use in subjects. In addition, the patients we recruited in present study were first-episode and had no more than 3 days of antipsychotic treatment, which provided an advantage in order to control potential confounders, such as the effects of medication and long-term illness course. However, this approach also raised a potential limitation, as patients who were unwilling or unable to participate in the study due to aggression or other severe psychotic symptoms were excluded. This may have led to a selective bias. In the future study, it is important to compare their clinical features of patients completed the MRI scan and those unable to tolerate or complete the MRI scan.
5. Conclusions
In conclusion, comparing to healthy controls, both DS and NDS patients demonstrated gray matter deficit in cerebellum with a more severe GMV reduction in DS patients. Interestingly, this gray matter deficit was only observed in the first-degree relatives of DS but not NDS patients. More importantly, the GMV atrophy in left insula differentially affected schizophrenia patients with and without the deficit syndrome, and the GMV of left insula was inversely correlated with primary negative symptoms. These results collectively suggest that DS might represent a distinct subtype of schizophrenia from NDS and the GMV change in left insula could be a morphological signature of DS.
Supplementary Material
Acknowledgements
This work was partly funded by National Nature Science Foundation of China (81130024, 30530300 and 30125014, TL), National Key Technology R & D Program of the Ministry of Science and Technology of China during the 12th Five-Year Plan (2012BAI01B06, TL), the Ph.D. Programs Foundation of Ministry of Education of China (20110181110014, TL), the National Basic Research Program of China (973 Program 2007CB512301, TL), and the Doctoral Fund of Ministry of Education of China (Grant No.20110181120033). The work was also supported by the National Institutes of Health Grant Numbers R01MH098003 (PI: Nanyin Zhang, PhD) from the National Institute of Mental Health and R01NS085200 (PI: Nanyin Zhang, PhD) from the National Institute of Neurological Disorders and Stroke.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Allen AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD. Endophenotypes in schizophrenia: a selective review. Schizophrenia Research. 2009;109:24–37. doi: 10.1016/j.schres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yücel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophrenia Research. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad LR, Magnotta V, Schröder J. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Research: Neuroimaging. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, Schretlen DJ. Gray-matter abnormalities in deficit schizophrenia. Schizophrenia Research. 2010;120:63–70. doi: 10.1016/j.schres.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong Q.-y. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophrenia Bulletin. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT, Jr, Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophrenia Bulletin. 2007;33:1201–1212. doi: 10.1093/schbul/sbl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M. User's guide for the structured clinical interview for DSM-IV axis I disorders: SCID-1 clinician version. American Psychiatric Publication; Washington, DC/USA: 1997. [Google Scholar]
- Fischer BA, Keller WR, Arango C, Pearlson GD, McMahon RP, Meyer WA, Francis A, Kirkpatrick B, Carpenter WT, Buchanan RW. Cortical structural abnormalities in deficit versus nondeficit schizophrenia. Schizophrenia Research. 2012;136:51–54. doi: 10.1016/j.schres.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S, Maj M. Deficit schizophrenia: An overview of clinical, biological and treatment aspects. European Psychiatry. 2009;24:493–500. doi: 10.1016/j.eurpsy.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Maj M, Mucci A, Cassano GB, Invernizzi G, Rossi A, Vita A, Dell'Osso L, Daneluzzo E, Pini S. Historical, Psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. The American Journal of Psychiatry. 2002;159:983–990. doi: 10.1176/appi.ajp.159.6.983. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Quarantelli M, Volpe U, Mucci A, Cassano GB, Invernizzi G, Rossi A, Vita A, Pini S, Cassano P. Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophrenia Bulletin. 2008;34:393–401. doi: 10.1093/schbul/sbm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Casares N, Jorge RE, García-Arnés JA, Acion L, Berthier ML, Gonzalez-Alegre P, Nabrozidis A, Gutiérrez A, Ariza MJ, Rioja J. Cognitive Dysfunctions in Middle-Aged Type 2 Diabetic Patients and Neuroimaging Correlations: A Cross-Sectional Study. Journal of Alzheimer's Disease. 2014;42:1337–1346. doi: 10.3233/JAD-140702. [DOI] [PubMed] [Google Scholar]
- Gong Y. Wechsler adult intelligence scale-revised in China Version. Hunan Medical College; Changsha, Hunan/China: 1992. [Google Scholar]
- Hong LE, Avila MT, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophrenia Research. 2003;63:39–48. doi: 10.1016/s0920-9964(02)00388-2. [DOI] [PubMed] [Google Scholar]
- Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–267. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenny PD, Alphs LD, Carpenter WT. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Research. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Archives of General Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Castle D, Murray RM, Carpenter WT., Jr Risk factors for the deficit syndrome of schizophrenia. Schizophrenia Bulletin. 2000;26:233–242. doi: 10.1093/oxfordjournals.schbul.a033443. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. 2008;7:143–147. doi: 10.1002/j.2051-5545.2008.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Shenton M, Salisbury D, Hirayasu Y, Kasai K, Kikinis R, Jolesz F, McCarley R. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. NeuroImage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biological Psychiatry. 2010;68:1061–1065. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Leucht S, Wahlbeck K, Hamann J, Kissling W. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet. 2003;361:1581–1588. doi: 10.1016/S0140-6736(03)13306-5. [DOI] [PubMed] [Google Scholar]
- Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, Chua S, McAlonan G. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophrenia Bulletin. 2011;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia research. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Doll A, Bäuml JG, Mühlau M, Schwerthöffer D, Scherr M, Zimmer C, Förstl H, Bäuml J. Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission. Frontiers in Human Neuroscience. 2013;7:1–16. doi: 10.3389/fnhum.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, Peters H, Zimmer C, Forstl H, Bauml J, Wohlschlager AM, Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia Bulletin. 2014;40:428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messias E, Kirkpatrick B, Bromet E, Ross D, Buchanan RW, Carpenter WT, Jr, Tek C, Kendler KS, Walsh D, Dollfus S. Summer birth and deficit schizophrenia: a pooled analysis from 6 countries. Archives of General Psychiatry. 2004;61:985–989. doi: 10.1001/archpsyc.61.10.985. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biological Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadić I, Sauer H, Smesny S, Gaser C. Aging effects on regional brain structural changes in schizophrenia. Schizophrenia Bulletin. 2012;38:838–844. doi: 10.1093/schbul/sbq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer R, Weisbrod M, Schiesser S, Grothe J, Maier S, Peter U, Kaufmann C, Schröder J, Sauer H. Genetic influence on laterality in schizophrenia? A twin study of neurological soft signs. American Journal of Psychiatry. 2000;157:272–274. doi: 10.1176/appi.ajp.157.2.272. [DOI] [PubMed] [Google Scholar]
- Özdemir Hİ, Eker MÇ, Zengin B, Yilmaz DA, Haznedaroglu Dİ, ÇINAR C, Kitis Ö, Akay A, Gonul AS. Gray Matter Changes in Patients with Deficit Schizophrenia and Non-Deficit Schizophrenia. Turkish Journal of Psychiatry. 2012;23:237–247. [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry & Neuroscience. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophrenia Bulletin. 2008;34:155–172. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réthelyi JM, Czobor P, Polgár P, Mersich B, Bálint S, Jekkel É, Magyar K, Mészáros Á, Fábián Á, Bitter I. General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2011:1–9. doi: 10.1007/s00406-011-0224-4. [DOI] [PubMed] [Google Scholar]
- Saze T, Hirao K, Namiki C, Fukuyama H, Hayashi T, Murai T. Insular volume reduction in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:473–479. doi: 10.1007/s00406-007-0750-2. [DOI] [PubMed] [Google Scholar]
- Schopp LH, Callahan CD, Johnstone B, Schwake CJ. Utility of a seven-subtest version of the WAIS-R among an Alzheimer's disease sample. Archives of clinical neuropsychology. 1998;13:637–643. [PubMed] [Google Scholar]
- Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biological Psychiatry. 2012;72:775–784. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams SC, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry. 2001a;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry. 2001b;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and Nonmotor Function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Soulsby B, McGorry PD, Tanino R, Suzuki M, Velakoulis D, Pantelis C. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophrenia Research. 2009;108:49–56. doi: 10.1016/j.schres.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Tek C, Kirkpatrick B, Buchanan R. A five-year followup study of deficit and nondeficit schizophrenia. Schizophrenia Research. 2001;49:253–260. doi: 10.1016/s0920-9964(00)00146-8. [DOI] [PubMed] [Google Scholar]
- Tognin S, Rambaldelli G, Perlini C, Bellani M, Marinelli V, Zoccatelli G, Alessandrini F, Pizzini FB, Beltramello A, Terlevic R, Tansella M, Balestrieri M, Brambilla P. Enlarged hypothalamic volumes in schizophrenia. Psychiatry Research. 2012;204:75–81. doi: 10.1016/j.pscychresns.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Shatti S, Tanabe JL, Martin LF, Gibson L, Wylie K, Rojas DC. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophrenia Research. 2007;97:242–249. doi: 10.1016/j.schres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Virupaksha HS, Kalmady SV, Shivakumar V, Arasappa R, Venkatasubramanian G, Gangadhar BN. Volume and asymmetry abnormalities of insula in antipsychotic-naive schizophrenia: a 3-tesla magnetic resonance imaging study. Indian Journal of Psychological Medicine. 2012;34:133–139. doi: 10.4103/0253-7176.101778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Translational Psychiatry. 2012;2:e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging Evidence for the Deficit Subtype of Schizophrenia. JAMA Psychiatry. 2013;113:472–480. doi: 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- Volpe U, Mucci A, Quarantelli M, Galderisi S, Maj M. Dorsolateral prefrontal cortex volume in patients with deficit or nondeficit schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;37:264–269. doi: 10.1016/j.pnpbp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophrenia Research. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, McCarley RW, Nakamura M, Lee KU, Koo MS, Bouix S, Salisbury DF, Morra L, Shenton ME, Niznikiewicz MA. A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala–hippocampal complex in chronic schizophrenia. Schizophrenia Research. 2009;113:84–94. doi: 10.1016/j.schres.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PCMP, Kahn RS, van Engeland H, Durston S. Progressive structural brain changes during development of psychosis. Schizophrenia Bulletin. 2010;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.