Abstract
The World Health Organization reports that 47.5 million people are affected by dementia worldwide. With aging populations and 7.7 million new cases each year, the burden of illness due to dementia approaches crisis proportions. Despite significant advances in our understanding of the biology of Alzheimer’s disease (AD), the leading dementia diagnosis, the actual causes of dementia in affected individuals are unknown except for rare fully penetrant genetic forms. Evidence from epidemiology and pathology studies indicates that damage to the vascular system is associated with an increased risk of many types of dementia. Both Alzheimer’s pathology and cerebrovascular disease increase with age. How AD affects small blood vessel function and how vascular dysfunction contributes to the molecular pathology of Alzheimer’s are areas of intense research. The science of vascular contributions to cognitive impairment and dementia (VCID) integrates diverse aspects of biology and incorporates the roles of multiple cell types that support the function of neural tissue. Because of the proven ability to prevent and treat cardiovascular disease and hypertension with population benefits for heart and stroke outcomes, it is proposed that understanding and targeting the biological mechanisms of VCID can have a similarly positive impact on public health.
Keywords: Vascular contributions to cognitive impairment and dementia, VCID; Vascular dementia; Vascular cognitive impairment, VCI; Alzheimer’s disease; Cardiovascular; Cerebrovascular
Dementia Diagnosis, Disease Burden
Dementia appeared in medical texts during antiquity to describe clinical syndromes of diminished cognitive function associated with a variety of neurological conditions (Boller and Forbes 1998). Over time dementia evolved from a broad concept that included any type of mental incapacitation, reversible or irreversible, into a modern diagnosis with highly specialized clinical meaning that itself continued to change, including very recently. Under the now out–of–date DSM IV-TR, dementia is characterized by greater memory impairment than expected with normal aging, may include other cognitive impairment (e.g., executive function, attention, visuospatial abilities, judgment, reasoning, and emotional control), and interferes with a person’s ability to function at work or in other everyday activities (DSM IV Task Force 2004; Dementia: a public health priority 2012). While impairment in social and/or occupational activities remains central, in DSM 5 the diagnosis of dementia has been subsumed under major neurocognitive disorder and is characterized by a progressive decline in one or more cognitive domains that may or may not include memory (DSM 5 Task Force 2013).
The World Health Organization reports that 47.5 million people are affected worldwide in 2015, and has projected 75.6 million people with dementia in 2030. The social and economic implications are enormous, with an estimated annual global societal cost of $604 billion, corresponding to 1.0 % of the worldwide gross domestic product (Dementia: Fact Sheet No. 362 2015). Challenges to progress in dementia research and treatment include limited drug targets and significant diagnostic uncertainty, with most diagnoses relying on clinical correlates and postmortem pathological assessment rather than on bona fide causes or response to therapeutics (DSM 5 Task Force 2013).
Hypotheses regarding the cause of dementia have also changed over time. As recently as the 1960s, a vascular etiology was the prevailing view (Kling et al. 2013), and today published estimates of the prevalence of vascular dementia vary from extremely rare to common (Fitzpatrick et al. 2004; Fitzpatrick et al. 2005; Gorelick et al. 2011; Rizzi et al. 2014). This variability is due to numerous factors including the lack of widely applicable definitive diagnostic tools, the heterogeneity of vascular contributions to dementia, different methods utilized in study cohorts, age of affected individuals, and comorbidities. Beta-amyloid and abnormal forms of tau, which were discovered by the mid-1980s (Weingarten et al. 1975; Glenner and Wong 1984; Neve et al. 1986), are core features of Alzheimer’s disease (AD) pathology; however, beta-amyloid and tau co-occur with other pathologic changes in persons with AD and other dementias, as well as in persons without manifest dementia (Jellinger and Attems 2015). While standardized criteria have been developed for AD (McKhann et al. 2011), differential diagnoses with incomplete knowledge of cause remain challenging across the dementia spectrum (Montine et al. 2014). Moreover, disease onset and progression can transpire over many years, and correlates can be subtle and are frequently shared among different types of dementias. Examples of such correlates include an incremental decline in the ability to form new memories, gradual loss of hippocampal volume, and pathologic changes evaluated during life with neuroimaging or biomarkers, or and postmortem by neuropathologic evaluation (Montine et al. 2012; Hyman et al. 2012). It is increasingly reported that mixed pathology dementias account for half or more of all dementia cases, with beta-amyloid and vascular disease constituting the most frequent combination of pathologies (Langa et al. 2004; Jellinger and Attems 2007; Schneider et al. 2007, 2009; Battistin and Cagnin 2010; Gardner et al. 2013; Attems and Jellinger 2014). Atherosclerosis, arteriosclerosis, microinfarcts, silent stroke, and diffuse white matter disease are all associated with increased risk of dementia (Gorelick et al. 2011; Bangen et al. 2015; Gorelick 2015; Hachinski and World Stroke 2015). Recent evidence suggests an association between mid-life hypertension (Gottesman et al. 2014), a major risk factor for stroke and diffuse white matter disease, and mid-life obesity (Chuang et al. 2015; Bischof and Park 2015) with future risk of dementia. Stroke rates have declined continuously over the past 5 decades in developed countries (Feigin et al. 2009, 2014) due to prevention strategies such as blood pressure control (Lackland et al. 2014). Consistent with the link between stroke and dementia, epidemiologic data suggest declining age-specific population risk in high-income countries, although the total number of people affected by dementia continues to increase (Matthews et al. 2013; Langa 2015). While the overall burden of cerebrovascular disease in persons affected by cognitive impairment and dementia is not yet well documented, stroke followed by dementia and the prevalence of vascular pathology in AD together indicate vascular contributions to dementia in millions of people in the United States alone.
The Science of VCID
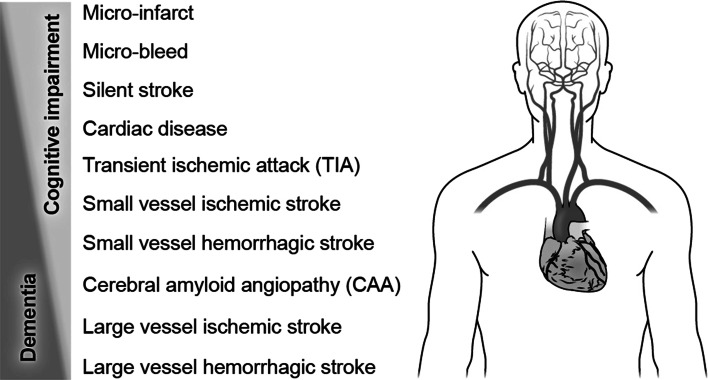
Vascular contributions to cognitive impairment and dementia was first coined as a phrase (Gorelick et al. 2011), and later as the acronym VCID (Snyder et al. 2015). VCID is proposed here as a field of research investigating the hypothesis that significant disease burden due to cognitive decline results from damage to brain function by vascular insults including clinical stroke, silent infarcts and microinfarcts, leukoaraiosis, cerebral amyloid angiopathy (CAA), transient ischemic attack (TIA), and micro-bleeds (Fig. 1) (Breteler 2000a, b; Gorelick et al. 2011; Iadecola 2013; Attems and Jellinger 2014). Although beyond the main scope of this discussion, examples of monogenetic disorders that can result in younger onset VCID include CADASIL and CARASIL (Gorelick et al. 2011; Iadecola 2013). The clinical scope of sporadic VCID science is illustrated in Fig. 2 by its overlapping relationship with cognitive decline including in clinical AD, and with cardiovascular and cerebrovascular disease including stroke. At the level of cellular and molecular mechanisms, the concept of the neurovascular unit has advanced integrated studies of the vessel and the tissue that it supplies (Lo and Rosenberg 2009; Iadecola 2010). Accordingly, the scope of mechanism-oriented VCID research is best represented as the aging neurovascular unit integrating, and failing to cope with, biological insults due to vascular disease, Alzheimer’s biology, metabolic disease, and immune affront (Fig. 3) (Neuwelt et al. 2011; Dirnagl 2012; Sa-Pereira et al. 2012; Langer and Chavakis 2013; Zlokovic 2013; Courties et al. 2014; ElAli et al. 2014; Hill et al. 2014; Winkler et al. 2014; Lourenco et al. 2015; Mezger et al. 2015; McCarthy and Kosman 2015).
Fig. 1.
The science of vascular contributions to cognitive impairment and dementia (VCID) includes many and diverse vascular diagnoses and conditions, a number of which are represented here. Clinical outcomes range from cognitive impairment (top left) to dementia (bottom left), and cognitive outcomes associated with vascular disease vary not only with the amount and type of injury, but also due to other contributing factors such anatomical location, comorbidities, and many other factors. Cognitive impairment may be associated with small vessel insults and limited brain comorbidities, and can be recognized as vascular cognitive impairment (VCI), mild cognitive impairment (MCI), or may go undetected. Dementia may be associated with larger vascular insults (small vessel disease is also typically present), especially in the presence of significant brain comorbidities such as beta-amyloid, tauopathy, TDP-43-opathy, and Lewy bodies/alpha-synuclein
Fig. 2.
VCID science overlays relevant diagnoses and conditions including cognitive decline and dementia, AD, cardio- and cerebrovascular disorders (CVD), and stroke. This schematic represents relationships among VCID and the indicated clinical conditions (CVD, stroke, cognitive decline and dementia, AD) in living persons, and does not represent pathologic outcomes
Fig. 3.
VCID is interdisciplinary in nature, with the neurovascular unit impacted by the biology of Alzheimer’s disease, stroke, metabolism, and immune function. An integrated multidisciplinary approach is required to gain an understanding of the mechanistic relationships between vascular biology and cognitive outcomes
The unorthodox scope of VCID has resulted in relevant research being separated by and largely embedded in traditional fields of science and clinical practice. This separation is reinforced by disciplinary boundaries at multiple levels including academic departments, professional societies, and funding agencies. Further fragmenting VCID science, and obscuring scientific opportunities to many who are otherwise poised to move the field forward, is that relevant studies appear in the literature under multiple and often interchangeable designations such as VCI, vascular dementia, vascular brain injury, and multi-infarct dementia, among others. Adding further confusion is that even though such designations are clinically oriented, for example VCI and vascular dementia, they are often ambiguously tied to specific diagnoses and their definitions and application vary by region, by practice, and over time.
Despite such impediments, epidemiology and neuropathology literatures emerged over the past 25 years that support a significant role for cerebrovascular biology in cognitive decline and dementia (Breteler 2000a, b; Chui 2006; Knopman and Roberts 2010; Montine and Montine 2013; Gardener et al. 2015). Vascular pathology is now widely known to be a prominent feature of AD, particularly among the oldest old (Snowdon et al. 1997; Schneider et al. 2007; James et al. 2012), and studies designed to identify and target mechanisms that underlie VCID are underway (Zlokovic 2011; Iadecola 2013). The enormous potential public health impact of VCID science remains largely untapped, however, in part because the science is challenging and in early stages, and in part because of the currently fragmented state and relatively modest scale of VCID research relative to disease burden. Many scientists with the knowledge, interest, and skills needed to move the field forward are either not engaged in VCID research, or work in relative isolation because of VCID’s overlapping position relative to traditional diagnostic and disciplinary boundaries. VCID science is a mechanistically oriented field that cuts across traditional boundaries to solve vascular mechanisms that contribute to numerous diagnoses of cognitive decline, and to facilitate synergy among researchers with diverse expertise, some of whom may have not previously recognized this field, or their ability to contribute.
Research Priorities
The relationship between vascular disorders and cognitive decline has been recognized in all national plans to address dementia, starting with the French prototype in 2001 (Les plans Alzheimer 2001). The U.S. National Plan to Address AD includes related dementias that are designated as frontotemporal, Lewy body, mixed, and vascular dementia (National Plan to Address Alzheimer’s Disease: 2015 Update 2015). Over the past several years, a coordinated worldwide movement has been evolving (Rosow et al. 2011; Prince et al. 2015), including a 2015 World Dementia Council statement suggesting that “Regular physical activity and management of cardiovascular risk factors (e.g., diabetes, obesity, smoking, and hypertension) are associated with a reduced risk of cognitive decline and may reduce the risk of dementia” (Steven 2015; Baumgart et al. 2015).
VCID research priorities developed under national plans and in other forums have highlighted prevention as well as addressing vascular contributions in the context of mixed etiology dementias such as typical late onset AD (Gorelick et al. 2011; Dementia: a public health priority 2012; Vickrey et al. 2013; Montine et al. 2014; WHO takes up the baton on dementia 2015; Recommendations from the NIH AD Research Summit 2015). Near-term priorities include: development and validation of imaging and biospecimen-based biomarkers; improved experimental models; and a better understanding of underlying molecular and physiological mechanisms including for diffuse white matter disease, infarction, microhemorrhage, glymphatic flow, vascular autoregulation, metabolism including lipidomics and diabetes, immune trafficking, and interactions between Alzheimer’s pathophysiology and vascular dysfunction (Jiwa et al. 2010; Sperling et al. 2011; Gorelick et al. 2011; Gardner et al. 2013; Montine et al. 2014; Roh and Lee 2014; Snyder et al. 2015). Advancing these priorities will provide answers to a number of critical questions about VCID that will help shape the future development of interventions for vascular contributions to cognitive decline, as well as for dementia overall. For example: when do vascular contributions pose a definitive burden that significantly impacts cognitive outcomes? Conversely, under what circumstances may vascular pathology be an incidental bystander? When is vascular pathology synergistic with versus additive to other pathologies when it comes to impacting cognitive outcomes, including dementia? Under what circumstances are vascular contributions the main or even sole cause of dementia? At what point in disease progression can vascular pathologies that are relevant to dementia be stopped and even reversed to stop and/or reverse cognitive decline? The science of VCID provides common ground and a framework for the interdisciplinary synergy that will be needed to answer these critical questions.
Conclusion
Numerous studies over several decades have linked cardio- and cerebrovascular risk factors to cognitive impairment and dementia, including AD. The science of VCID creates a focus on opportunities for synergy toward understanding mechanistic relationships between vascular biology and the diverse cell and tissue types that the vasculature supports and interacts with to determine cognitive outcomes. VCID is interdisciplinary by nature and defines a research domain that, while not defined in a traditional sense by clinical terminology, overlays multiple clinical diagnoses. In 2014, the NIH officially recognized VCID as a field by tracking spending on VCID research in NIH Reporter, aligning the acronym with “vascular cognitive impairment/dementia.” Because VCID cuts across diseases and specialties, several international professional organizations have expressed interest in this emerging area, including the Alzheimer’s Association, the American Stroke Association, the International Congress on Vascular Dementia, and VasCog. The concept of VCID is timely, dovetailing with the increasing recognition of the prominence of mixed dementias that include a vascular component, as well as major national and international planning efforts that highlight the importance of this area and opportunities for collaborative action (Hachinski 2013; Vogel 2014; Feldman et al. 2014; Montine et al. 2014; Snyder et al. 2015; Mason 2015; Recommendations from the NIH AD Research Summit 2015; National Alzheimer’s Project Act 2015; Prince et al. 2015). VCID as a mechanistic and research-oriented scientific framework that overlays diagnoses will help drive important hypothesis testing research that will ultimately lead to improved understanding, prevention, and treatment of dementia.
References
- Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med 12:206. doi:10.1186/s12916-014-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Nation DA, Delano-Wood L, Weissberger GH, Hansen LA, Galasko DR, Salmon DP, Bondi MW (2015) Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Alzheimer’s Dement 11(4):394–403 e391. doi:10.1016/j.jalz.2013.12.025 [DOI] [PMC free article] [PubMed]
- Battistin L, Cagnin A (2010) Vascular cognitive disorder. A biological and clinical overview. Neurochem Res 35(12):1933–1938. doi:10.1007/s11064-010-0346-5 [DOI] [PubMed] [Google Scholar]
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer’s Dement 11(6):718–726. doi:10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Bischof GN, Park DC (2015) Obesity and aging: consequences for cognition, brain structure, and brain function. Psychosom Med 77(6):697–709. doi:10.1097/PSY.0000000000000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller F, Forbes MM (1998) History of dementia and dementia in history: an overview. J Neurol Sci 158(2):125–133 [DOI] [PubMed] [Google Scholar]
- Breteler MM (2000a) Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Ann N Y Acad Sci 903:457–465 [DOI] [PubMed] [Google Scholar]
- Breteler MM (2000b) Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging 21(2):153–160 [DOI] [PubMed] [Google Scholar]
- Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, Breitner JC, Ferruci L, Resnick SM, Thambisetty M (2015) Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry. doi:10.1038/mp.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC (2006) Vascular cognitive impairment: today and tomorrow. Alzheimer’s Dement 2(3):185–194. doi:10.1016/j.jalz.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Courties G, Moskowitz MA, Nahrendorf M (2014) The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol 71(2):233–236. doi:10.1001/jamaneurol.2013.5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementia: a public health priority (2012). World Health Organization
- Dementia: Fact Sheet No. 362 (2015) World Health Organization (WHO). http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed Mar 2015
- Dirnagl U (2012) Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 1268:21–25. doi:10.1111/j.1749-6632.2012.06691.x [DOI] [PubMed] [Google Scholar]
- DSM 5 Task Force (2013) Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn. American Psychiatric Association, Arlington [Google Scholar]
- DSM IV Task Force (2004) Diagnostic and statistical manual of mental disorders Fourth Edition Text Revision: DSM-IV-TR, 4th edn. American Psychiatric Association, Arlington [Google Scholar]
- ElAli A, Theriault P, Rivest S (2014) The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci 15(4):6453–6474. doi:10.3390/ijms15046453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8(4):355–369. doi:10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, Global Burden of Diseases I, Risk Factors S, the GBDSEG (2014) Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 383(9913):245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HH, Haas M, Gandy S, Schoepp DD, Cross AJ, Mayeux R, Sperling RA, Fillit H, van de Hoef DL, Dougal S, Nye JS, One Mind for R, the New York Academy of S (2014) Alzheimer’s disease research and development: a call for a new research roadmap. Ann N Y Acad Sci 1313:1–16. doi:10.1111/nyas.12424 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C (2004) Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 52(2):195–204 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W (2005) Survival following dementia onset: Alzheimer’s disease and vascular dementia. J Neurol Sci 229–230:43–49. doi:10.1016/j.jns.2004.11.022 [DOI] [PubMed] [Google Scholar]
- Gardener H, Wright CB, Rundek T, Sacco RL (2015) Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. doi:10.1038/nrneurol.2015.195 [DOI] [PubMed] [Google Scholar]
- Gardner RC, Valcour V, Yaffe K (2013) Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther 5(4):27. doi:10.1186/alzrt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120(3):885–890 [DOI] [PubMed] [Google Scholar]
- Gorelick PB (2015) World Stroke Day Proclamation 2015: call to preserve cognitive vitality. Stroke 46(11):3037–3038. doi:10.1161/STROKEAHA.115.011166 [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council CoE, Prevention CoCNCoCR Council Intervention on Cardiovascular S, Anesthesia (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(9):2672–2713. doi:10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH (2014) Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 71(10):1218–1227. doi:10.1001/jamaneurol.2014.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V (2013) Neurology in a globalizing world: World Congress of Neurology, Vienna, 2013. Neurology 80(24):2248–2249. doi:10.1212/WNL.0b013e318296ea48 [DOI] [PubMed] [Google Scholar]
- Hachinski V, World Stroke O (2015) Stroke and potentially preventable dementias proclamation: updated World Stroke Day Proclamation. Stroke 46(11):3039–3040. doi:10.1161/STROKEAHA.115.011237 [DOI] [PubMed] [Google Scholar]
- Hill J, Rom S, Ramirez SH, Persidsky Y (2014) Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 9(5):591–605. doi:10.1007/s11481-014-9557-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8(1):1–13. doi:10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2010) The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120(3):287–296. doi:10.1007/s00401-010-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80(4):844–866. doi:10.1016/j.neuron.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA (2012) Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 307(17):1798–1800. doi:10.1001/jama.2012.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257(1–2):80–87. doi:10.1016/j.jns.2007.01.045 [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J (2015) Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm 122(4):505–521. doi:10.1007/s00702-014-1288-x [DOI] [PubMed] [Google Scholar]
- Jiwa NS, Garrard P, Hainsworth AH (2010) Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem 115(4):814–828. doi:10.1111/j.1471-4159.2010.06958.x [DOI] [PubMed] [Google Scholar]
- Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE (2013) Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimer’s Dement 9(1):76–92. doi:10.1016/j.jalz.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Roberts R (2010) Vascular risk factors: imaging and neuropathologic correlates. J Alzheimer’s Dis 20(3):699–709. doi:10.3233/JAD-2010-091555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A, American Heart Association Stroke C, Council on C, Stroke N, Council on Quality of C, Outcomes R, Council on Functional G, Translational B (2014) Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 45(1):315–353. doi:10.1161/01.str.0000437068.30550.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM (2015) Is the risk of Alzheimer’s disease and dementia declining? Alzheimers Res Ther 7(1):34. doi:10.1186/s13195-015-0118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Foster NL, Larson EB (2004) Mixed dementia: emerging concepts and therapeutic implications. JAMA 292(23):2901–2908. doi:10.1001/jama.292.23.2901 [DOI] [PubMed] [Google Scholar]
- Langer HF, Chavakis T (2013) Platelets and neurovascular inflammation. Thromb Haemost 110(5):888–893. doi:10.1160/TH13-02-0096 [DOI] [PubMed] [Google Scholar]
- Les plans Alzheimer (2001) Ministère des Affaires sociales, de la Santé et des Droits des femmes. http://www.sante.gouv.fr/archives-les-plans-alzheimer-2001-2005-et-2004-2007.html. Accessed 26 Oct 2015
- Lo EH, Rosenberg GA (2009) The neurovascular unit in health and disease: introduction. Stroke 40(3 Suppl):S2–S3. doi:10.1161/STROKEAHA.108.534404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco CF, Ledo A, Dias C, Barbosa RM, Laranjinha J (2015) Neurovascular and neurometabolic derailment in aging and Alzheimer’s disease. Front Aging Neurosci 7:103. doi:10.3389/fnagi.2015.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason VL (2015) Alzheimer’s Association International Conference on Alzheimer’s Disease 2015 (AAIC 2015) (July 18-23, 2015—Washington, DC, USA). Drugs Today 51(7):447–452. doi:10.1358/dot.2015.51.7.2375989 [DOI] [PubMed] [Google Scholar]
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C, Medical Research Council Cognitive F, Ageing C (2013) A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 382(9902):1405–1412. doi:10.1016/S0140-6736(13)61570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RC, Kosman DJ (2015) Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci 72(4):709–727. doi:10.1007/s00018-014-1771-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7(3):263–269. doi:10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezger M, Gobel K, Kraft P, Meuth SG, Kleinschnitz C, Langer HF (2015) Platelets and vascular inflammation of the brain. Hamostaseologie 35(3):244–251. doi:10.5482/HAMO-14-11-0071 [DOI] [PubMed] [Google Scholar]
- Montine KS, Montine TJ (2013) Anatomic and clinical pathology of cognitive impairment and dementia. J Alzheimer’s Dis 33(Suppl 1):S181–S184. doi:10.3233/JAD-2012-129032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on A, Alzheimer’s A (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123(1):1–11. doi:10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, Greenberg SM, Hutton ML, Knopman DS, Kuzmichev AN, Manly JJ, Marder KS, Miller BL, Phelps CH, Seeley WW, Sieber BA, Silverberg NB, Sutherland M, Torborg CL, Waddy SP, Zlokovic BV, Corriveau RA, Committee ACO (2014) Recommendations of the Alzheimer’s disease-related dementias conference. Neurology 83(9):851–860. doi:10.1212/WNL.0000000000000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Alzheimer’s Project Act (2015) Office of The Assistant Secretary for Planning and Evaluation, U.S. Department of Health & Human Services. http://aspe.hhs.gov/national-alzheimers-project-act. Accessed 29 Oct 2015
- National Plan to Address Alzheimer’s Disease: 2015 Update (2015). Secretary of the U.S. Department of Health and Human Services, U.S. Department of Health and Human Services (HHS)
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O’Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR (2011) Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12(3):169–182. doi:10.1038/nrn2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA (1986) Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 387(3):271–280 [DOI] [PubMed] [Google Scholar]
- Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M (2015) World Alzheimer Report 2015 The global impact of dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International (ADI), London [Google Scholar]
- Recommendations from the NIH AD Research Summit (2015) National Institute on Aging. https://www.nia.nih.gov/research/recommendations-nih-ad-research-summit-2015. Accessed 21 Oct 2015
- Rizzi L, Rosset I, Roriz-Cruz M (2014) Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Res Int 2014:908915. doi:10.1155/2014/908915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Lee JH (2014) Recent updates on subcortical ischemic vascular dementia. J Stroke 16(1):18–26. doi:10.5853/jos.2014.16.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosow K, Holzapfel A, Karlawish JH, Baumgart M, Bain LJ, Khachaturian AS (2011) Countrywide strategic plans on Alzheimer’s disease: developing the framework for the international battle against Alzheimer’s disease. Alzheimer’s Dement 7(6):615–621. doi:10.1016/j.jalz.2011.09.226 [DOI] [PubMed] [Google Scholar]
- Sa-Pereira I, Brites D, Brito MA (2012) Neurovascular unit: a focus on pericytes. Mol Neurobiol 45(2):327–347. doi:10.1007/s12035-012-8244-2 [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69(24):2197–2204. doi:10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66(2):200–208. doi:10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR (1997) Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277(10):813–817 [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC (2015) Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s Dement 11(6):710–717. doi:10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7(3):280–292. doi:10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven C (2015) World Dementia Council issues risk reduction statement. Global action against dementia
- Vickrey BG, Brott TG, Koroshetz WJ, Stroke Research Priorities Meeting Steering C, the National Advisory Neurological D, Stroke C, National Institute of Neurological D, Stroke (2013) Research priority setting: a summary of the 2012 NINDS Stroke Planning Meeting Report. Stroke 44(8):2338–2342. doi:10.1161/STROKEAHA.113.001196 [DOI] [PubMed] [Google Scholar]
- Vogel L (2014) Call for collaboration in dementia research. CMAJ Canadian Medical Association journal = journal de l’Association medicale canadienne 186(15):1132. doi:10.1503/cmaj.109-4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72(5):1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO takes up the baton on dementia (2015) Lancet Neurol 14(5):455. doi:10.1016/S1474-4422(15)00022-8 [DOI] [PubMed]
- Winkler EA, Sagare AP, Zlokovic BV (2014) The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol 24(4):371–386. doi:10.1111/bpa.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723–738. doi:10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV (2013) Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 70(4):440–444. doi:10.1001/jamaneurol.2013.2152 [DOI] [PMC free article] [PubMed] [Google Scholar]