Abstract
Background
We aimed to assess trends in hospitalization, outcomes, and resource utilization among patients admitted with adult congenital heart disease (ACHD).
Methods and Results
We used the 2003–2012 US Nationwide Inpatient Sample for this study. All admissions with an ACHD were identified using standard ICD codes. Resource utilization was assessed using length of stay, invasive procedure utilization, and cost of hospitalization. There was a significant increase in the number of both simple (101%) as well as complex congenital heart disease (53%)–related admissions across 2003–2012. In addition, there was a considerable increase in the prevalence of traditional cardiovascular risk factors including older age, along with a higher prevalence of hypertension, diabetes, smoking, obesity, chronic kidney disease, and peripheral arterial disease. Besides miscellaneous causes, congestive heart failure (11.8%), valve disease (15.5%), and cerebrovascular accident (26.1%) were the top causes of admission to the hospital among patients with complex ACHD, simple ACHD without atrial septal defects/patent foramen ovale and simple atrial septal defects/patent foramen ovale patients, respectively. In‐hospital mortality has been relatively constant among patients with complex ACHD as well as simple ACHD without atrial septal defects/patent foramen ovale. However, there has been considerable increase in the average length of stay and cost of hospitalization among the ACHD patients during 2003–2012.
Conclusions
There has been a progressive increase in ACHD admissions across 2003–2012 in the United States, with increasing healthcare resource utilization among these patients. Moreover, there has been a change in the cardiovascular comorbidities of these patients, adding a layer of complexity in management of ACHD patients.
Keywords: adult congenital heart disease, cost of illness, hospital admission, length of stay, mortality, trends
Subject Categories: Congenital Heart Disease, Quality and Outcomes, Mortality/Survival
Introduction
There has been a dramatic increase in the proportion of babies with congenital heart disease (CHD) surviving to adulthood. Advances in pediatric surgery and pediatric cardiology are directly responsible for this favorable outcome.1 Over 90% of children born with CHD in the modern era are now expected to survive to adulthood, and a large majority can expect near normal life expectancy. Consequently, the number of adults with CHD now exceeds the number of babies born with CHD annually and contributes to a significant increase in the healthcare burden worldwide.2 The spectrum of CHD is extensive and may range from simple disorders such as patent foramen ovale (PFO) or atrial septal defects (ASD) with few hemodynamic consequences to complex disorders such as tetralogy of Fallot or transposition of great arteries that usually require surgical correction in early years of life.
Despite surgical correction, patients with CHD retain a lifelong risk for cardiovascular complications arising from residual defects and the clinical sequelae of the disease. Normal physiologic conditions such as pregnancy and stressors such as noncardiac surgery often require special integrated care in this population. In the United States, a large increase in both simple and complex CHD‐related hospitalizations, along with a considerable increase in the cardiac procedures performed among this cohort has been previously reported.3 Although care for this population is ideally provided at centers of excellence, fragmentation of care remains an issue and patients with CHD often present to small hospitals without expertise in CHD care. In this study, we evaluated the trends in hospitalizations for adult congenital heart disease (ACHD) between the years 2003–2012, stratified into simple and complex disorders. In addition, we evaluated the trend in healthcare resource utilization, reasons for admission, and outcomes among these patients.
Methods
Data Source
Data were obtained from the Nationwide Inpatient Sample (NIS) database from 2003 to 2012. The NIS is sponsored by the Agency for Healthcare Research and Quality as a part of the Healthcare Cost and Utilization Project. The NIS data set across 2003–2011 contains discharge level data from ≈8 million hospitalizations annually from about 1000 hospitals across the United States. In 2012, the sampling strategy was altered to include a larger sample of hospitals contributing a randomly selected subsample of hospitalizations to the database. This database is designed to represent a 20% stratified sample of all hospitals in the country. Criteria used for stratified sampling of hospitals into the NIS include location (urban or rural), teaching status, geographic region, patient volume, and hospital ownership. Every hospital has been classified into small, medium, and large size based on the number of beds available for in‐hospital admissions. The cut points for classification differed according to geographic location of the hospital and the teaching status.4 All data available from the Healthcare Cost and Utilization Project have been de‐identified and hence the analysis is exempt from the federal regulations for the protection of human research participants. The data set was obtained from the Agency for Healthcare Research and Quality after completing the data use agreement with Healthcare Cost and Utilization Project.
Study Population
The NIS database provides up to 15 diagnoses and 15 procedures for each hospitalization record for the years 2003–2009. The number of diagnoses coded in the database was expanded to 25 for the years 2010–2012. All these have been coded using the standard International Classification of Diseases, 9th edition, Clinical Modification (ICD‐9 CM) codes. All adult hospitalizations (>18 years) with a diagnosis code corresponding to known ACHD were included in our study. The list of simple, complex, and unclassified ACHD, according to the Bethesda classification, along with their ICD‐9 codes is shown in Table 1.1 It must be noted that patients with simple ACHD with coexisting complex lesions or pulmonary hypertension were classified as complex ACHD, according to the recommendations of the Bethesda classification.1 For the purpose of the study, the simple ACHD cohort was further classified into 2 subgroups. The first group consisted of simple lesions excluding secundum ASD/PFO (referred to as simple ACHD without ASD/PFO) and the second group consisted of patients with secundum ASD/PFO, in the absence of pulmonary hypertension (referred to as simple ASD/PFO).
Table 1.
ICD‐9 Codes for Congenital Heart Defects Included in Our Analysis
| Type of Lesion | ICD‐9 Code |
|---|---|
| Simple CHD | |
| Ventricular septal defect | 745.4 |
| Ostium secundum type atrial septal defect | 745.5 |
| Other bulbus cordis anomaly or septal defect | 745.8 |
| Unspecified defect of septal closure | 745.9 |
| Congenital stenosis of aortic valve | 746.3 |
| Congenital insufficiency of aortic valve | 746.4 |
| Congenital mitral stenosis | 746.5 |
| Congenital mitral insufficiency | 746.6 |
| Coronary artery anomaly | 746.85 |
| Complex CHD | |
| Common truncus | 745.0 |
| Transposition of great vessels | 745.1 |
| Complete transposition of great vessels | 745.10 |
| Double outlet of right ventricle | 745.11 |
| Corrected transposition of great vessels | 745.12 |
| Other transposition of the great vessels | 745.19 |
| Tetralogy of Fallot | 745.2 |
| Common ventricle | 745.3 |
| Endocardial cushion defect | 745.6 |
| Endocardial cushion defect, unspecified type | 745.60 |
| Ostium primum defect | 745.61 |
| Other endocardial cushion defect | 745.69 |
| Cor biloculare | 745.7 |
| Anomalies of pulmonary valve | 746.0 |
| Pulmonary valve anomaly, unspecified | 746.00 |
| Pulmonary atresia | 746.01 |
| Pulmonary stenosis | 746.02 |
| Pulmonary valve anomaly, other | 746.09 |
| Tricuspid atresia and stenosis | 746.1 |
| Ebstein's anomaly | 746.2 |
| Hypoplastic left heart syndrome | 746.7 |
| Other unspecified anomalies of the heart | 746.8 |
| Subaortic stenosis | 746.81 |
| Cor triatriatum | 746.82 |
| Infundibular pulmonic stenosis | 746.83 |
| Obstructive anomalies of heart, NEC | 746.84 |
| Congenital heart block | 746.86 |
| Patent ductus arteriosus | 747.0 |
| Coarctation of aorta | 747.1 |
| Coarctation of aorta | 747.10 |
| Interruption of aortic arch | 747.11 |
| Anomalies of aorta | 747.2 |
| Anomaly of aorta, unspecified | 747.20 |
| Anomalies of aortic arch | 747.21 |
| Atresia and stenosis of aorta | 747.22 |
| Other congenital anomalies of the aorta | 747.29 |
| Anomalies of pulmonary artery | 747.3 |
| Anomaly of great veins | 747.4 |
| Anomaly of great veins, unspecified | 747.40 |
| Total anomalous pulmonary venous connection | 747.41 |
| Partial anomalous pulmonary venous connection | 747.42 |
| Other anomalies of great veins | 747.49 |
| Unclassified CHD | |
| Malposition of heart and cardiac apex | 746.87 |
| Other congenital anomalies of heart | 746.89 |
| Other congenital anomalies of heart | 746.9 |
The stratification into simple, complex, and unclassified categories was based on the 32nd Bethesda Conference document and other published reports. Simple diagnoses with coexisting complex diagnoses or pulmonary hypertension were classified as complex. CHD indicates congenital heart disease; ICD, International Classification of Diseases; NEC, Not Elsewhere Classified.
The first diagnosis in the database is referred to as the “principal diagnosis” and is considered the primary reason for admission to the hospital. In cases where the principal diagnosis was designated ACHD, the second diagnosis was utilized to determine the primary reason for admission to the hospital. In addition to the ICD‐9 codes, we used the Healthcare Cost and Utilization Project Clinical Classification Software to identify patient comorbidities and procedures.5 Clinical Classification Software has been developed by the Agency for Healthcare Research and Quality for clustering patient diagnoses and procedures into a manageable number of clinically meaningful categories.5 The ICD‐9/Clinical Classification Software codes for principal diagnoses and procedures performed during the hospitalization are shown in Tables 2 and 3, respectively.
Table 2.
ICD‐9‐Based and CCS‐Based Codes for Reason for Hospital Admission
| Diagnosis/Procedure | CCS Codes | ICD 9 Codes |
|---|---|---|
| Pregnancy/pregnancy‐related complications | 177 to 196 | |
| Acute MI | 100 | |
| Coronary artery disease | 101 | |
| Congestive heart failure/cardiomyopathy | 108 | 425.xx |
| Endocarditis | 391.1; 421.0; 421.9; 424.91; 421.1; 036.42; 074.22; 093.2; 098.84 | |
| Cerebrovascular disease/transient cerebral ischemia | 109 to 113 | |
| Cardiac dysrhythmias/syncope/conduction disorders | 105; 106; 245 | |
| Valvular heart disease | 96 | |
| Nonspecific chest pain | 102 | |
| Pulmonary hypertension | 416.x | |
| Venous thromboembolism | 453.4; 453.41; 453.42; 453.2; 453.3; 453.8; 453.9; 451.11; 451.19; 451.2; 451.81; 451.83; 451.84; 451.89; 415.19; 415.11; 453.82; 453.82; 453.83; 453.85; 453.86; 453.87; 453.89; 415.1x; 415.0 | |
| Aortic aneurysm/peripheral/visceral aneurysm | 115 | |
| Pneumonia/COPD/respiratory failure/respiratory disorders | 122 to 134 |
COPD indicates chronic obstructive pulmonary disease; CCS, Clinical Classification Software; ICD, International Classification of Diseases; MI, myocardial infarction.
Table 3.
ICD‐9‐Based and CCS‐Based Codes for Diagnostic and Therapeutic Procedures
| Procedure | CCS Code | ICD 9 Code |
|---|---|---|
| ASD/PFO repair (open) | 35.71; 35.51; 35.61 | |
| ASD/PFO repair (closed) | 35.52 | |
| PCI | 45 | 36.06; 36.07; 00.41; 00.42; 00.43 |
| CABG | 44 | |
| Heart valve procedures (valvotomy/valvuloplasty/repair/replacement) | 43 | |
| Cardiopulmonary bypass | 39.61 | |
| Infusion of vasopressor | 00.17 | |
| Pacemaker | 377.x; 378.x | |
| ICD | 379.4 to 379.7 | |
| Mechanical circulatory support | 37.61 (IABP); 37.68 (Impella, tandem heart) | |
| Mechanical ventilation | 216 | |
| Transfusion | 222 | |
| Swan‐Ganz catheterization | 89.64; 37.21; 37.23 | |
| Diagnostic angiography | 47 | 88.50 |
ASD indicates atrial septal defects; CABG, coronary artery bypass graft; CCS, Clinical Classification Software; IABP, intra aortic balloon pump; ICD, International Classification of Diseases; PCI, percutaneous coronary intervention; PFO, patent foramen ovale.
Study Outcomes
The primary aim of the study was to evaluate the trend in the hospitalization of adults across 2003–2012, stratified by the presence of simple, complex, and unclassified ACHD. In addition, we evaluated the changes in the distribution of demographic and clinical characteristics among these patients. Furthermore, we assessed the trends in in‐hospital mortality and resource utilization among the study population across 2003–2012. Resource utilization was assessed by evaluating the trends in various procedures (Table 3), length of stay (LOS), and total cost of hospitalization across the study period. The NIS database provides the total charges associated with each hospital stay that were claimed by the respective hospital. The total charges of each hospital stay were converted to cost estimates using the group average all‐payer in‐hospital cost and charge information from the detailed reports by hospitals to the Centers of Medicare and Medicaid Services. All costs and charges were then converted to projected estimates for the year 2012, after accounting for annual inflation rates based on consumer price index data available from the Bureau of Labor Statistics.6
Statistical Analysis
Continuous variables are presented as mean± SD and categorical variables are presented as proportions. The Student t test was utilized for comparing means of continuous variables between 2 categories. For comparing the means of continuous variables between 3 or more categories, we utilized 1‐way ANOVA. The χ2 test was utilized for comparison of categorical variables.
Survey statistics traditionally used to analyze complex semirandom survey designs were employed to analyze these data. Since the data from NIS represent a collection of scattered hospital clusters, analysis was structured to account for a complex, multistage, probability sampling. NIS recommends the use of “strata” for constructing analysis clusters, which include geographic census region, hospital ownership, teaching status, urban/rural location, and bed size. Furthermore, the analysis is further stratified into individual hospitals, which serve as primary sampling units for the analysis. In the NIS database, each hospital admission is linked to a “discharge weight” and a “trend weight” that can be utilized to calculate projected national estimates for all hospital‐related outcomes, after accounting for the hierarchical structure of the data set. The nonparametric test for trend across ordered groups by Cuzick was utilized to determine the significance of differences in ACHD prevalence across the study period.7 All statistical analyses were performed using the statistical software Stata v 13.1 (Stata Corp, College Station, TX). All statistical tests were 2‐tailed; a P‐value <0.05 was considered significant.
Results
Trends in ACHD Admission
We included a total of 195 306 admissions with ACHD across 2003–2012, of which 128 697 (65.9%) involved subjects with simple lesions, 48 096 (24.6%) were individuals with complex lesions, and the remaining 18 513 (9.5%) were unclassified lesions. This projects to an estimated population of 962 668 admissions nationally across the 10‐year period. Figure 1A demonstrates the trend in annual admissions (SD) of patients with ACHD across the United States during 2003–2012. There has been a considerable increase in the ACHD admissions from 63 950 (3427) in 2003 to 116 085 (2943) in 2012, corresponding to an 81.5% increase in admission numbers during the 10‐year period (P‐trend<0.001). Figure 1B demonstrates the annual ACHD admissions after stratifying by the ACHD category. The number of admissions with simple ACHD lesions increased from 40 061 (2461) in 2003 to 80 710 (2202) in 2012, corresponding to a 101.4% increase in the annual admission rates across 2003–2012 (P‐trend<0.001). In addition, there has been a 52.8% increase in the admission rates for complex ACHD (P‐trend<0.001) and 35.2% increase in the admission rates for unclassified ACHD (P‐trend<0.001). It was not possible to separate patients with secundum ASD from those with PFO, and this group accounted for the majority of admissions with simple ACHD. As a result, we performed the analysis for trends after eliminating this clinical entity. Figure 2 demonstrate a trend towards an increasing number of hospital admissions of patients with ACHD, even after eliminating those with secundum ASD/PFO.
Figure 1.
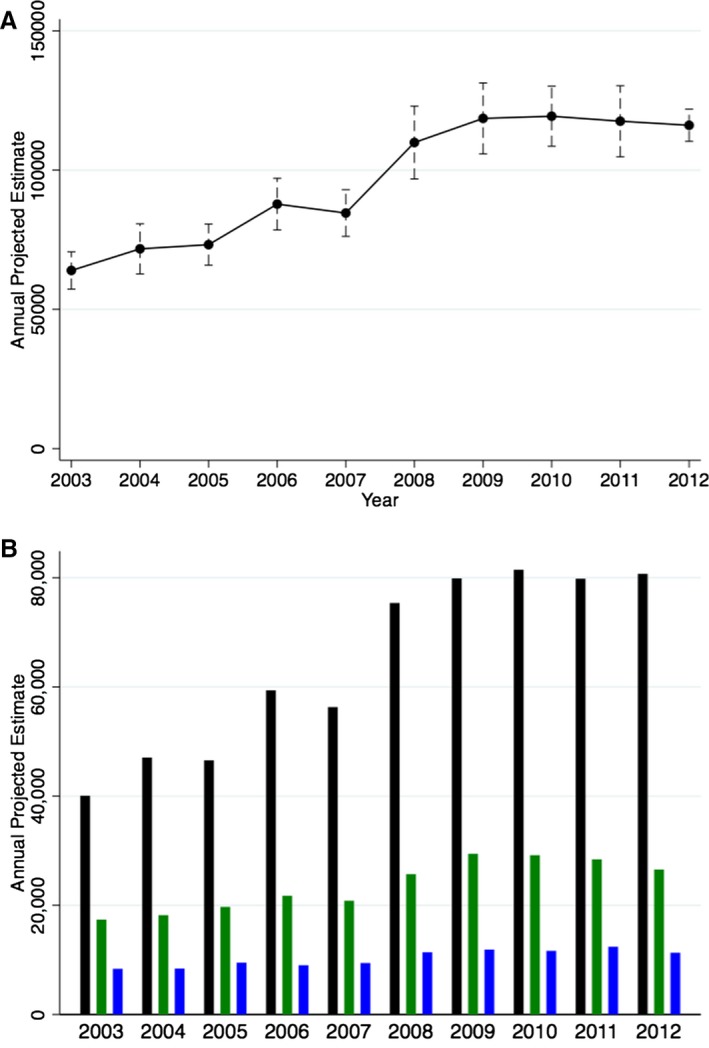
Projected annual national estimates of adult congenital heart disease admissions. A, Demonstrates the overall annual national estimates with 95% CIs. B, Demonstrates the national annual estimates, stratified into simple (black bars), complex (green bars), and unclassified defects (blue bars).
Figure 2.
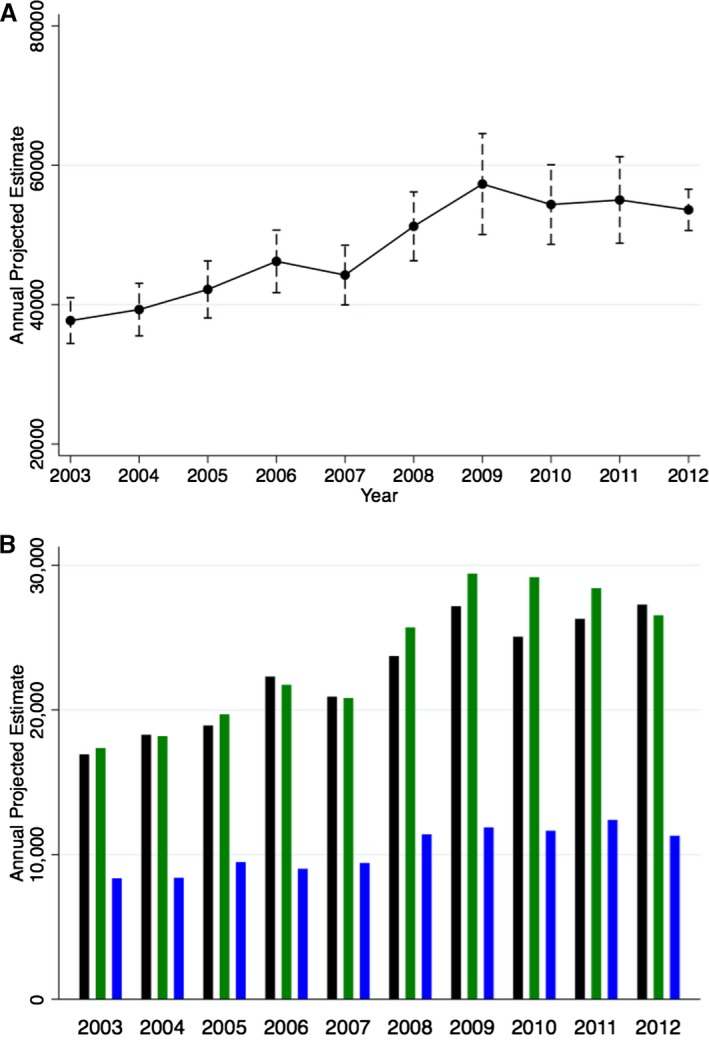
Projected annual national estimates of ACHD admissions after excluding patients with simple secundum ASD/PFO. A, Demonstrates the overall annual national estimates with 95% CIs. B, Demonstrates the projected annual national estimates, stratified into simple without ASD/PFO (black bars), complex (green bars), and unclassified defects (blue bars). ACHD indicates adult congenital heart disease; ASD, atrial septal defects; PFO, patent foramen ovale.
Trends for the annual number of admissions for specific simple and complex defects are shown in Tables 4 and 5, respectively. The number of admissions with simple ASD/PFO increased steeply from 26 246 (2050) in 2003 to 62 485 (1677) in 2012 (138.1% increase, P‐trend<0.001). Moreover, the number of admissions with congenital aortic stenosis/aortic insufficiency also increased from 8692 (594) in 2003 to 18 865 (805) in 2012 (117.0% increase, P‐trend<0.001). Among the complex disorders, aortic anomalies and tetralogy of Fallot were the 2 most common diseases encountered. The aortic anomalies increased from 4562 (412) in 2003 to 6611 (376) (44.9% increase), followed by a slight decline to 5190 (182) admissions in 2012. The prevalence of coexisting pulmonary hypertension with various ACHD increased from 6289 (384) in 2003 to 14 545 (497) in 2012, corresponding to a 131.3% increase during the 10‐year period (P‐trend<0.001).
Table 4.
Annual Number of Admissions for Adults With Simple Congenital Heart Disease Across 2003–2012
| Lesion | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|
| Simple ASD/PFO | 23 135 (1882) | 28 776 (2697) | 27 635 (1898) | 37 072 (2504) | 35 389 (2067) | 51 647 (4142) | 52 713 (2821) | 56 401 (2895) | 53 518 (3068) | 53 430 (1464) |
| VSD | 4309 (169) | 4328 (194) | 4531 (188) | 4991 (210) | 4251 (191) | 4527 (180) | 4520 (196) | 4561 (245) | 4466 (258) | 4310 (110) |
| Congenital AI/AS | 8692 (594) | 10 115 (846) | 10 699 (911) | 12 855 (982) | 12 625 (990) | 14 547 (1130) | 17 838 (2234) | 16 137 (1383) | 18 261 (1503) | 18 865 (805) |
| Congenital MS/MR | 570 (60) | 524 (49) | 741 (160) | 692 (66) | 525 (81) | 548 (50) | 529 (35) | 707 (107) | 581 (57) | 630 (41) |
| Coronary anomalies | 4252 (341) | 4187 (258) | 4146 (291) | 5089 (370) | 4866 (302) | 5932 (410) | 6853 (455) | 5899 (381) | 5709 (353) | 6100 (332) |
The numbers in parentheses represent the standard error of the estimate. According to the Bethesda classification of congenital heart disease, simple lesions with coexisting pulmonary hypertension were classified as complex lesions (1). Hence, the estimates presented in this table do not account for patients with so‐called simple lesions in conjunction with pulmonary hypertension. AI indicates aortic insufficiency; AS, aortic stenosis; ASD, atrial septal defect; MR, mitral regurgitation; MS, mitral stenosis; PFO, patent foramen ovale; VSD, ventricular septal defect.
Table 5.
Annual Number of Admissions for Adults With Complex Congenital Heart Disease Across 2003–2012
| Lesion | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|
| TOF | 1263 (104) | 1404 (99) | 1272 (117) | 1530 (200) | 1255 (98) | 1404 (141) | 1520 (131) | 1534 (125) | 1352 (121) | 1320 (56) |
| Transposition complex | 837 (103) | 807 (103) | 1244 (237) | 1053 (217) | 820 (120) | 1122 (209) | 1094 (179) | 1221 (220) | 1063 (162) | 1060 (65) |
| Endocardial cushion defect | 514 (48) | 554 (45) | 555 (70) | 520 (63) | 419 (40) | 415 (41) | 396 (46) | 606 (50) | 489 (54) | 475 (28) |
| Pulmonary valve anomalies | 1120 (81) | 1251 (104) | 1393 (136) | 1471 (123) | 1386 (108) | 1625 (161) | 1540 (121) | 1656 (152) | 1591 (200) | 1630 (117) |
| Ebstein's anomaly | 555 (54) | 614 (48) | 682 (51) | 686 (57) | 665 (41) | 666 (71) | 877 (74) | 690 (51) | 674 (57) | 720 (38) |
| Tricuspid atresia & stenosis | 417 (65) | 420 (63) | 527 (94) | 478 (127) | 319 (40) | 533 (75) | 472 (59) | 585 (73) | 525 (65) | 425 (34) |
| Great vein anomaly | 713 (47) | 817 (64) | 854 (77) | 888 (79) | 831 (67) | 1130 (85) | 1277 (99) | 1428 (109) | 1531 (111) | 1510 (73) |
| Aortic anomalies including coarctation | 4562 (412) | 4481 (235) | 4842 (317) | 5408 (303) | 5318 (340) | 5603 (381) | 6611 (376) | 6227 (538) | 5724 (331) | 5190 (182) |
| PDA | 975 (78) | 1053 (71) | 957 (68) | 1132 (63) | 1103 (71) | 1379 (90) | 1376 (76) | 1507 (108) | 1424 (108) | 1440 (45) |
| Coexisting PH in CHD | 6289 (384) | 6667 (535) | 6534 (395) | 7953 (543) | 8578 (504) | 11 941 (788) | 14 622 (978) | 14 139 (773) | 15 074 (1051) | 14 545 (497) |
The numbers in parentheses represent the standard error of the estimate. According to the Bethesda classification of congenital heart disease, simple lesions with coexisting pulmonary hypertension were classified as complex lesions. CHD indicates congenital heart disease; PDA, patent ductus arteriosus; PH, pulmonary hypertension; TOF, tetralogy of Fallot.
Baseline Characteristics
Figure 3 demonstrates the changes in mean age (Figure 3A) and sex distribution (Figure 3B) among patients with ACHD admitted during 2003–2012. As clearly evident, there has been an increase in the mean age of patients admitted with ACHD from 53.5 years in 2003 to 57.5 years in 2012 (P‐trend<0.001) (Figure 3A). The proportion of males with ACHD admitted to the hospitals increased from 47.0% in 2003 to 50.6% in 2012 (P‐trend<0.001) (Figure 3B). Table 6 lists the trend of important cardiovascular comorbidities among patients admitted with ACHD. There was an increase in the prevalence of all cardiovascular risk factors including hypertension, diabetes, obesity, smoking, peripheral arterial disease, and chronic kidney disease from 2003 to 2012 (P‐trend<0.001 for all comparisons). Figure 4 demonstrates the primary reason for admission to the hospital among the included ACHD patients, stratified into complex ACHD (Figure 4A), simple ACHD without ASD/PFO (Figure 4B), and simple ASD/PFO lesions (Figure 4C). Besides the miscellaneous causes for admission, congestive heart failure, respiratory disorders, and arrhythmias were the top 3 reasons for admission among patients with complex ACHD. Among patients with simple ACHD without ASD/PFO, valve disease, coronary artery disease, and arrhythmias were the top 3 reasons for admission to the hospital. Among patients with simple ASD/PFO, cerebrovascular accident was a major reason for admission, contributing to 26.1% of in‐hospital admissions among these patients. Figure 5 shows the proportion of all hospitalizations that were admitted on an emergent basis, stratified into complex ACHD, simple ACHD without ASD/PFO, and simple ASD/PFO categories. The proportion of emergency admits among patients with complex ACHD increased from 43.4% in 2003 to 52.5% in 2008, followed by a gradual decline to 48.3% in 2011. The proportion of emergency admits among patients with simple ACHD without ASD/PFO remained constant across the study period (P‐trend=0.99). Among patients with simple ASD/PFO, the proportion of emergency admits increased from 42.3% in 2003 to 56.3% in 2011 (P‐trend<0.001).
Figure 3.
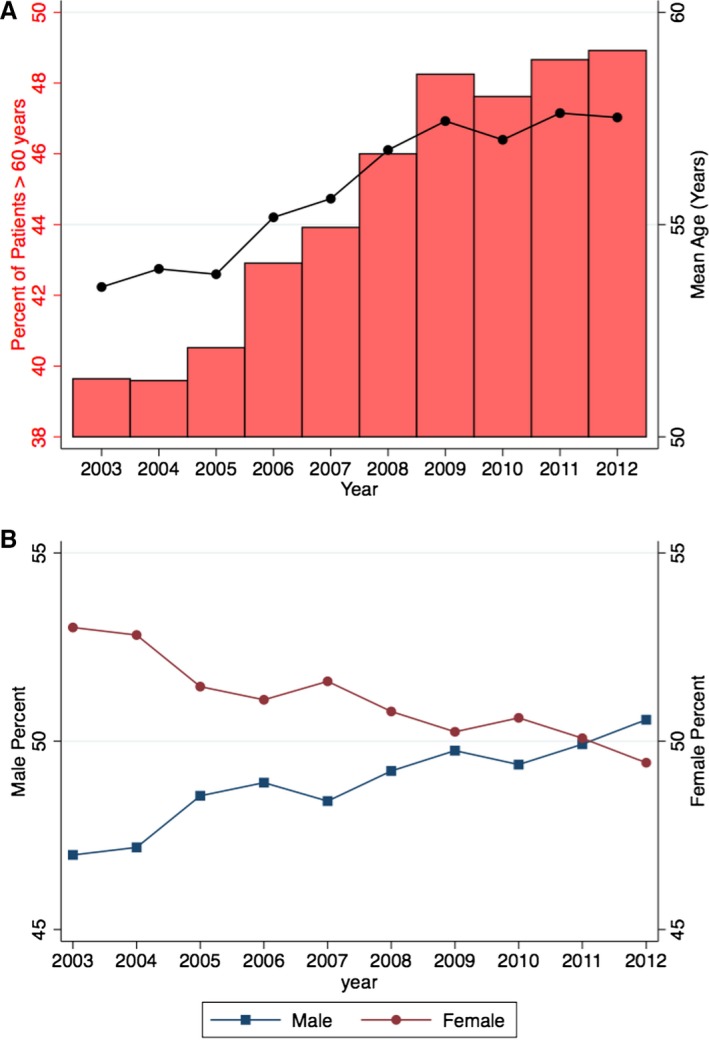
The figure demonstrates the changes in age (A) and sex distribution (B) among patients with congenital heart disease admitted during 2003–2012. In (A), the line demonstrates the trend in the mean age of patients over the study duration and the bars reflect the percentage of patients aged >60 years of age. In (B), blue line with squares demonstrates males and red line with circles demonstrates females.
Table 6.
Trends in the Prevalence (%) of Cardiovascular Comorbidities in the Study Population Across 2003–2012
| Year | Hypertension | Diabetes | Obesity | Smoking | PAD | CKD |
|---|---|---|---|---|---|---|
| 2003 | 37.3 | 13.1 | 5.3 | 14.8 | 5.7 | 2.8 |
| 2004 | 39.5 | 14.3 | 5.8 | 15.2 | 6.2 | 2.8 |
| 2005 | 39.2 | 13.9 | 6.1 | 17.0 | 6.6 | 3.5 |
| 2006 | 43.9 | 15.4 | 6.8 | 18.1 | 7.3 | 6.1 |
| 2007 | 45.2 | 17.0 | 7.9 | 19.1 | 8.3 | 7.5 |
| 2008 | 48.1 | 18.3 | 8.9 | 19.3 | 9.0 | 9.0 |
| 2009 | 50.2 | 19.6 | 10.5 | 22.1 | 10.5 | 10.3 |
| 2010 | 51.3 | 20.2 | 10.7 | 23.2 | 9.8 | 10.8 |
| 2011 | 53.2 | 21.0 | 12.3 | 25.7 | 10.8 | 12.3 |
| 2012 | 54.4 | 21.7 | 13.0 | 28.3 | 10.9 | 12.1 |
CKD indicates chronic kidney disease; PAD, peripheral arterial disease.
Figure 4.
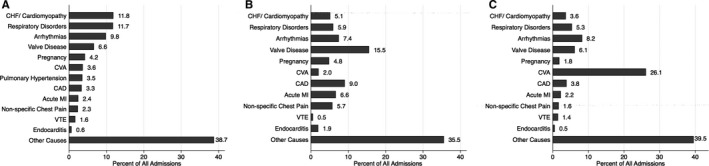
This figure demonstrates the primary reasons for admission to the hospital among the included ACHD patients, stratified into complex ACHD (A), simple ACHD without ASD/PFO (B), and simple ASD/PFO lesions (C). The numbers next to the bars represent percentage of all patients in that particular stratum. ACHD indicates adult congenital heart disease; ASD, atrial septal defect; CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; MI, myocardial infarction; PFO, patent foramen ovale; VTE, venous thromboembolism.
Figure 5.
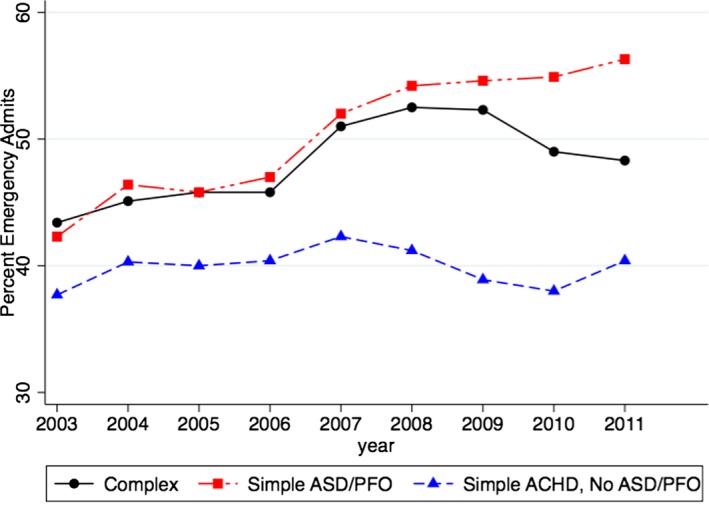
This figure demonstrates the proportion of emergency admissions across the study period, stratified into complex ACHD, simple ACHD without ASD/PFO, and simple ASD/PFO lesions. These estimates were only available from 2003 to 2011. ACHD indicates adult congenital heart disease; ASD, atrial septal defects; PFO, patent foramen ovale.
Resource Utilization and Outcomes
Figure 6 demonstrates the in‐hospital mortality among patients admitted with complex ACHD, simple ACHD without ASD/PFO, and simple ASD/PFO. As evident in this figure, there was no significant difference in mortality in both complex (P‐trend=0.52) and simple ACHD without ASD/PFO (P‐trend=0.06) categories across the study period. However, among patients with simple ASD/PFO, there was a small but significant increase in in‐hospital mortality from 2003 to 2012. This was accompanied by a gradual increase in the proportion of patients admitted with cerebrovascular accidents from 24.5% in 2003 to 28.3% in 2012. Figure 7 demonstrates the trend in LOS and cost of hospitalization among patients with simple (Figure 7A) and complex ACHD (Figure 7B). There was a significant increase in LOS from 5.5 (0.1) days in 2003 to 6.0 (0.1) days in 2012 among patients admitted with simple ACHD (P‐trend<0.001). Similarly, there was a significant increase in LOS from 6.1 (0.2) days in 2003 to 6.9 (0.2) days in 2012 among patients admitted with complex ACHD (P‐trend<0.001). As seen in Figure 7, there has been a considerable increase in the cost of hospitalization among patients with simple as well complex ACHD (P‐trend<0.001 for both comparisons).
Figure 6.
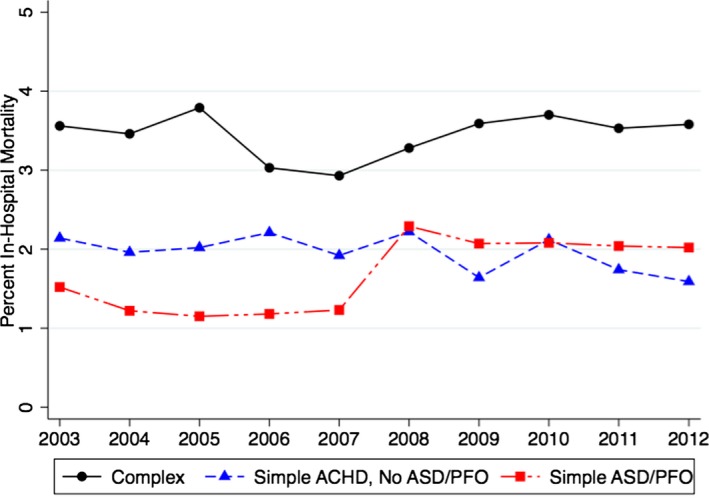
This figure demonstrates the annual in‐hospital mortality among patients admitted with complex ACHD, simple ACHD without ASD/PFO, and simple ASD/PFO. As evident in this figure, there was no significant difference in mortality in both complex ACHD (P‐trend=0.52) and simple ACHD without ASD/PFO (P‐trend=0.06) across the study period. ACHD indicates adult congenital heart disease; ASD, atrial septal defects; PFO, patent foramen ovale.
Figure 7.
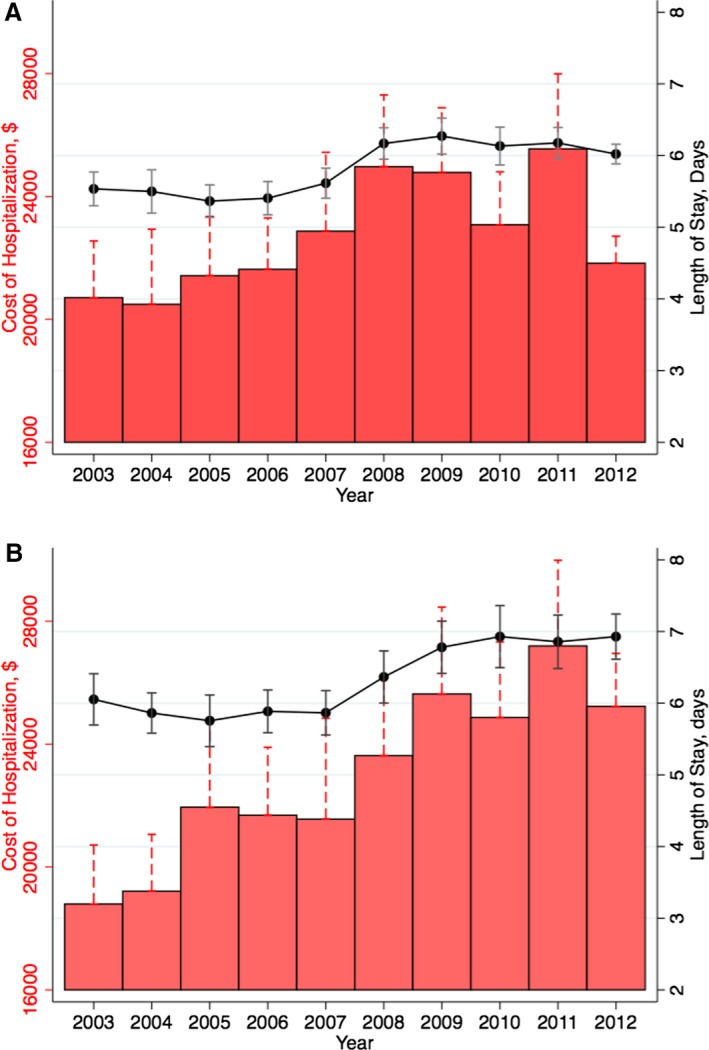
Resource utilization in simple (A) and complex adult congenital heart disease (B), evaluated using length of stay (days) and cost of hospitalization (US dollars, adjusted for inflation to 2012 estimates). In both panels, the line represents mean length of stay flanked by 95% CIs for respective years and the bars represent the annual mean cost of hospitalization.
Figure 8 demonstrates the proportion of patients presenting to small‐ and medium‐sized hospitals across the study duration. With an increase in the number of ACHD admissions, we noted a significant increase in the number of admissions to small‐ and medium‐sized hospitals from 2003 to 2012. Although relatively constant across the study duration, the proportion of all ACHD admissions presenting to small/medium‐sized hospitals was sizeable (≈30% of all admissions). Interestingly, there were small but significant differences in the case mix presenting at the small‐, medium‐, and large‐sized hospitals. We noted that 27.1% of ACHD admissions at small‐sized hospitals represented complex ACHD, compared to 25.4% at medium‐sized hospitals and 24.1% at large‐sized hospitals.
Figure 8.
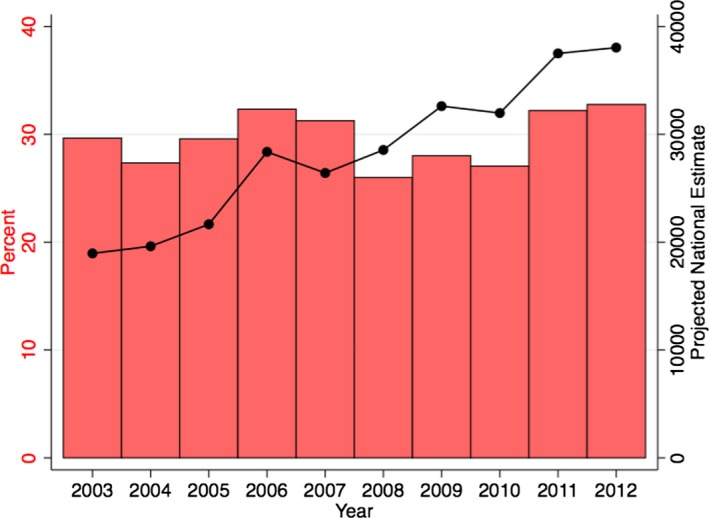
The figure quantifies hospital admissions of patients with adult congenital heart disease (ACHD) to small‐ or medium‐sized hospitals. The red bars represent the proportion of all ACHD admissions that present to the small/medium‐sized hospitals across the study duration. The black line demonstrates the projected annual number of admissions to small or medium‐sized hospitals.
Table 7 demonstrates the trend in utilization of invasive procedures among ACHD patients across 2003–2012. We observed a 49.7% increase in the number of open ASD/PFO repairs across 2003–2009 followed by a gradual slow decline in the procedural volume during 2010–2012. Similarly, there was a 52.6% increase in percutaneous ASD/PFO closures from 2003 to 2008, followed by a rather steep decline in procedural volume from 2010 to 2012 (60.6% decrease). In addition, there was a substantial increase in cardiac surgeries utilizing cardiopulmonary bypass from 10 934 (992) procedures in 2003 to 19 755 (1066) procedures in 2012 (80.7% increase, P‐trend<0.001). There were significant increases in volumes noted for heart valve procedures, percutaneous coronary intervention, bypass surgery, as well as pacemaker and implantable cardioverter defibrillator implantations. With respect to diagnostic coronary angiography, there was a significant increase in volume during 2003–2009, followed by a slow progressive decline during 2010–2012. The utilization of blood product transfusion has increased from 5159 (457) in 2003 to 14 380 (552) in 2012, corresponding to an increase of 178.7% across the study period (P‐trend<0.001).
Table 7.
Annual Number of Various Invasive Procedures Performed Among Adults With Congenital Heart Disease Across 2003–2012
| Procedure | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|
| ASD/PFO repair (operative) | 4704 (461) | 4771 (418) | 5181 (597) | 5620 (581) | 5323 (578) | 6145 (560) | 7042 (839) | 5874 (551) | 6465 (697) | 5740 (302) |
| ASD/PFO repair (percutaneous) | 3533 (869) | 4433 (881) | 3258 (375) | 5296 (808) | 3799 (480) | 5390 (950) | 3864 (557) | 3226 (383) | 2756 (608) | 2125 (167) |
| PCI | 1842 (141) | 1972 (190) | 2252 (183) | 3231 (284) | 2585 (199) | 3499 (272) | 3550 (246) | 3168 (220) | 3063 (205) | 3305 (139) |
| CABG | 3474 (287) | 3723 (331) | 3873 (305) | 5182 (479) | 4649 (431) | 6166 (515) | 6599 (737) | 5288 (448) | 5974 (514) | 5280 (216) |
| Heart valve procedures a | 7529 (763) | 7740 (817) | 8992 (1005) | 10 847 (1048) | 9731 (990) | 12 172 (1122) | 16 027 (2680) | 13 138 (1386) | 15 304 (1659) | 15 810 (889) |
| Cardiac surgeries with CPB | 10 934 (992) | 11 279 (1034) | 12 569 (1217) | 14 248 (1307) | 13 161 (1273) | 15 855 (1376) | 20 385 (3148) | 17 315 (1635) | 19 589 (2068) | 19 755 (1066) |
| Pacemaker | 1808 (126) | 2036 (143) | 2194 (173) | 2561 (225) | 2232 (149) | 2884 (204) | 3618 (321) | 3136 (227) | 3296 (280) | 2855 (116) |
| ICD | 668 (55) | 901 (90) | 840 (68) | 935 (107) | 921 (68) | 1118 (66) | 1168 (101) | 990 (84) | 1032 (121) | 990 (49) |
| MCS | 432 (36) | 488 (56) | 626 (57) | 761 (75) | 594 (43) | 1005 (76) | 1015 (97) | 1197 (95) | 1111 (82) | 1140 (54) |
| Transfusion | 5159 (457) | 5837 (465) | 7039 (664) | 8366 (817) | 8848 (665) | 12 232 (1027) | 14 446 (1531) | 13 608 (1015) | 15 208 (1211) | 14 380 (552) |
| Swan‐Ganz catheterization | 6325 (505) | 7069 (841) | 6678 (604) | 7911 (765) | 6862 (565) | 9919 (1191) | 9205 (796) | 8004 (6240 | 8009 (1132) | 7715 (340) |
| Diagnostic angiography | 12 603 (854) | 13 838 (1326) | 13 377 (979) | 16 521 (1319) | 14 669 (1039) | 19 226 (1673) | 19 878 (1349) | 17 375 (1035) | 17 402 (1489) | 16 930 (620) |
ASD indicates atrial septal defect; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; ICD, implantable cardioverter defibrillator; MCS, mechanical circulatory support (including intra‐aortic balloon pump); PCI, percutaneous coronary intervention; PFO, patent foramen ovale.
Numbers in parentheses indicate standard error of the estimate.
Heart valve procedures include valvotomy or valvuloplasty or valve repair or valve replacement.
Discussion
Our study has evaluated contemporary trends in the care of ACHD patients in the United States. We have observed several important findings. First, there has been a significant increase in the number of both simple (101%) as well as complex ACHD (53%)–related admissions over 2003–2012. Importantly, the prevalence of concomitant pulmonary hypertension in ACHD increased by 131% over the study period. Second, there has been a significant change in the comorbidities of patients with ACHD. Over the study duration, there was a considerable increase in the prevalence of comorbidities including hypertension, diabetes, smoking, obesity, chronic kidney disease, and peripheral arterial disease. Third, besides miscellaneous causes, congestive heart failure, valve disease, and cerebrovascular accident were the top causes of admission to the hospital among patients with complex ACHD, simple ACHD without ASD/PFO, and simple ASD/PFO patients, respectively. Fourth, a large proportion of patients were admitted on an “emergent” basis, highlighting the healthcare burden imposed by these patients upon emergency medical services. Fifth, although relatively constant across the study duration, the proportion of all ACHD admissions presenting to small/medium‐sized hospitals was sizeable (≈30% of all admissions). Last, there has been a considerable increase in the average LOS and cost of hospitalization among the ACHD patients during 2003–2012.
The overwhelming growth in the ACHD population in the United States is likely attributable to a significant improvement in the surgical and medical approach to children with CHD, which has led to marked improvement in survival to adulthood and overall life expectancy. In addition, there has been an increase in the availability and utilization of sophisticated diagnostic imaging such as cardiac magnetic resonance and computed tomography, which has likely widened the ACHD pool. Furthermore, management of a larger number of patients in ACHD centers of excellence has possibly led to improvement in outcomes and resultant survival.8 As of 2015, there are 114 self‐designated ACHD specialty centers in the United States, with 799 median patient visits per year.9 However, it is estimated that more than 500 000 patients with complex CHD require specialized ACHD care in the United States, exposing a major gap in current healthcare delivery to these patients.10, 11 Despite establishment and growth of ACHD centers across the nation, a sizeable proportion of all ACHD admissions continue to present to small/medium‐sized hospitals.
The ACHD population is aging and is progressively becoming more complex, in terms of comorbidities and repeated interventions for underlying CHD. Our results demonstrated a significant increase in the mean age of the ACHD population across the study period, with approximately half the patients aged >60 years in 2012. In addition, there was an increase in the risk‐factor profile of these patients, which adds a layer of complexity in management of ACHD patients. Increase in hypertension, diabetes, and obesity have been traditionally associated with diastolic dysfunction among adult patients. Although such data are scarce for ACHD patients, it has been suggested that these observations might apply to this cohort of patients as well.12 Changing prevalence of comorbidities among ACHD patients is likely going to impact the severity as well as nature of hospital presentation and would most certainly impact management strategies and response to treatment.
A substantial number of patients in this cohort were admitted with cerebrovascular accidents, cardiomyopathy/heart failure, acute myocardial infarction, coronary artery disease, which might emphasize the importance of interplay of traditional cardiovascular risk factors with ACHD. It must be acknowledged that a sizeable proportion of patients were admitted with miscellaneous noncardiac reasons, pregnancy and respiratory disorders, which emphasizes the importance of multispecialty collaboration in delivering care to these patients. For example, pregnancy in these patients is an especially challenging scenario and requires collaboration between high‐risk obstetrics, neonatology, anesthesiology, and ACHD specialists.
One of the important findings of our study was a progressive rise in the proportion of patients admitted on an emergent basis across 2003–2012. This finding is in contrast to the trends reported by Opotowsky et al during 1998–2005.3 This suggests a change in insurance patterns of the ACHD patients that leads them to use the emergency department as the source of care. We observed that the proportion of ACHD patients with private insurance reduced from 43.6% in 2003 to 32.0% in 2012. It has been previously reported that ACHD patients without private insurance are likely to use the emergency department as their usual source of care.13 This increase in the number of patients presenting to the emergency department emphasizes the need for familiarity with ACHD by emergency department physicians. The impact of the Affordable Care Act on these detrimental trends during the recent years remains to be seen.14 In addition, a substantial proportion of patients admitted to the hospital present with acute conditions such as myocardial infarction, cerebrovascular accident, and venous thromboembolism. Changing patterns in acute conditions among patients with ACHD over the last decade might also be responsible for the trends in emergency admits noted in our study. The increase in proportion of emergency admissions among patients with simple ASD/PFO might be attributable to an increase in the incidence of cerebrovascular accidents in this cohort. In addition, this proportion might be confounded due to an increased detection of interatrial shunting among patients presenting with cerebrovascular accidents during subsequent hospitalization, due to widespread availability of imaging and echocardiography, along with possibly an increase in image resolution and standardization of protocols to evaluate for intracardiac shunting throughout the hospital.
The landscape of resource utilization in ACHD has undergone a major metamorphosis over the last decade. The advancement in therapeutic capabilities in ACHD is evidenced by a significant increase in the number of open‐heart surgeries as well as heart valve procedures that were performed in these patients. Contrary to the trends during 1998–2005, the largest increase in invasive procedures occurred for open‐heart surgeries and valvular procedures rather than ASD/PFO repair. The ASD/PFO repair trends were interesting in that the absolute numbers have gradually declined during the most recent years. This is likely attributable to a clear lack of efficacy in stroke prevention with PFO closure, contributing to declining use in clinical practice.15, 16, 17 Although it was not possible to separate the ASD/PFO cohort into ASD and PFO due to the common ICD code for these conditions, we surmise that the majority of these patients were PFO rather than ASD. The reduction in the utilization of angiography and right heart catheterization might testify to a greater utilization of advanced cardiovascular imaging serving to usurp the use of invasive testing. Arrhythmias contributed to a common reason for admission to the hospital, associated with a slow, progressive increase in the use of electrophysiology procedures across the study duration, similar to findings by Maxwell et al.18 The rise in the use of transfusion and mechanical circulatory support likely mirrors the rise in the use of open‐heart surgeries and valvular procedures over the study period.
Opotowsky et al demonstrated an increase in the total hospital charges for ACHD admissions from $691 million to $3.16 billion in 2005.3 Although costs of hospitalization were not reported, the trend certainly indicated a major increase in healthcare spending in the ACHD cohort. Due to further enhancement in the data set, we were able to calculate uniform cost estimates and observed a trend towards further increase in these costs across the United States during 2003–2012. Interestingly, there was also an associated increase in the average LOS, which might be attributable to an increase in the number of invasive procedures as well as to the rising complexity of these patients. Furthermore, the hospital costs for simple and complex CHD were comparable, demonstrating a considerable use of healthcare resources even among patients with so‐called “simple” defects.
Limitations
Our study has several limitations, which are inherent to large administrative databases. First, there may be errors in coding of diseases or procedures. Second, since the unit of analysis in the NIS database is “unique admission” rather than “unique patient,” it is possible that 1 patient might have been represented more than once, in case of repeat admission for recurrent admission. In addition, since several CHD diagnoses might coexist in 1 patient, we acknowledge that the trends might be overestimated. However, patients with more than 1 ACHD diagnosis represented a minority of patients (<5%), and the direction of trends in our analysis is largely accurate. Third, this is a retrospective observational study, which may be subject to traditional biases of observational studies such as selection bias. However, these limitations might be partially compensated due to the large size of the NIS database and a uniform representation of all regions of the United States. Fourth, a few diagnoses such as secundum ASD/PFO suffer from lack of diagnostic specificity, which may limit distinction of resource utilization in these discrete disease states. However, analysis of trends after excluding patients with secundum ASD/PFO (Figure 2) still demonstrated a progressive increase in the number of ACHD admissions across the study duration. In addition, we have presented most of our analysis after stratifying simple ACHD into simple ACHD without secundum ASD/PFO and those with simple ASD/PFO, in order to demonstrate heterogeneity in presentations and outcomes among these individuals. Furthermore, our data only pertain to hospitalized patients and do not include patients managed as outpatients or in the emergency departments.
Conclusions
There has been a significant increase in the number of both simple as well as complex ACHD‐related admissions over 2003–2012. Congestive heart failure, valve disease, and cerebrovascular accident were the top causes of admission to the hospital among patients with complex ACHD, simple ACHD without ASD/PFO, and simple ASD/PFO patients, respectively, highlighting the need for multispecialty collaborations in care of these patients. In addition, a substantial proportion of patients were admitted on an “emergent” basis across the study duration. Furthermore, there has been a considerable increase in the average LOS and cost of hospitalization among the ACHD patients during 2003–2012, possibly attributable to a large increase in the number of invasive procedures performed in these patients.
Disclosures
Dr Menon is a consultant for and has received a research grant from AstraZeneca, and is a consultant for Takeda Pharmaceuticals. All other authors have reported that they have no relevant relationships or disclosures relevant to the content of this article.
Acknowledgments
The authors would like to acknowledge the help of Kathryn Brock in editing the manuscript.
(J Am Heart Assoc. 2016;5:e002330 doi: 10.1161/JAHA.115.002330)
References
- 1. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H. Geriatric congenital heart disease: a new challenge in the care of adults with congenital heart disease? Eur Heart J. 2014;35:683–685. [DOI] [PubMed] [Google Scholar]
- 3. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. [DOI] [PubMed] [Google Scholar]
- 4. Healthcare utilization project. Agency for Healthcare Research & Quality. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed May 5, 2015.
- 5. Healthcare Cost and Utilization Project (HCUP) . HCUP Comorbidity Software 1‐14‐2013. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 6. Consumer Price Index—Guide to CPI data. United States Department of Labor. Bureau of Labor Statistics. Available at: http://www.bls.gov/cpi/cpifact8.htm. Accessed May 25, 2014.
- 7. Cuzick J. A Wilcoxon‐type test for trend. Stat Med. 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 8. Fernandes SM, Chamberlain LJ, Grady S Jr, Saynina O, Opotowsky AR, Sanders L, Wise PH. Trends in utilization of specialty care centers in California for adults with congenital heart disease. Am J Cardiol. 2015;115:1298–1304. [DOI] [PubMed] [Google Scholar]
- 9. Adult Congenital Heart Association . Available at: http://www.achaheart.org/home/clinic-directory.aspx. Accessed April 4, 2015.
- 10. Marelli AJ, Therrien J, Mackie AS, Ionescu‐Ittu R, Pilote L. Planning the specialized care of adult congenital heart disease patients: from numbers to guidelines; an epidemiologic approach. Am Heart J. 2009;157:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Webb G, Landzberg MJ, Daniels CJ. Specialized adult congenital heart care saves lives. Circulation. 2014;129:1795–1796. [DOI] [PubMed] [Google Scholar]
- 12. Tede NH, Child JS. Diastolic dysfunction in patients with congenital heart disease. Cardiol Clin. 2000;18:491–499. [DOI] [PubMed] [Google Scholar]
- 13. Gurvitz MZ, Inkelas M, Lee M, Stout K, Escarce J, Chang RK. Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood. J Am Coll Cardiol. 2007;49:875–882. [DOI] [PubMed] [Google Scholar]
- 14. 111th Congress. Patient Protection and Affordable Care Act. Public law 111‐148, US Government Printing Office; 2010. [Google Scholar]
- 15. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; Investigators R . Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. [DOI] [PubMed] [Google Scholar]
- 16. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; Investigators CI . Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. [DOI] [PubMed] [Google Scholar]
- 17. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick‐Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Juni P; Investigators PCT . Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. [DOI] [PubMed] [Google Scholar]
- 18. Maxwell BG, Steppan J, Cheng A. Complications of catheter‐based electrophysiology procedures in adults with congenital heart disease: a national analysis. J Cardiothorac Vasc Anesth. 2015;29:258–264. [DOI] [PubMed] [Google Scholar]


