Abstract
Background
Few studies have examined the associations of weight changes from young adulthood to middle age with blood pressure (BP) and hypertension among Hong Kong Chinese women.
Methods and Results
Weight at age 18 (W18), current weight (Wcurrent), height, BP, demographics, and lifestyle factors were obtained from 1253 female nurses (35–65 years) by a self‐administered questionnaire through mail survey in Hong Kong. The conditional relative weight (CRW; a residual of Wcurrent regressed on W18) was used to express the relative weight change from age 18 to current age. The study results show that from young adulthood to middle age, 76.9%, 15.1%, and 8.0% of women had weight gain, weight loss, and stable weight, respectively. Women in the weight loss group had heavier W18 than those in the weight gain group (P<0.05). Higher weight gain was associated with higher BP (P for trend <0.01). Women who belonged to the heaviest 10% both at age 18 and at present had highest BP than women in other weight categories. By giving W18, a 1‐kg increase in weight change predicted 0.63 and 0.42 mm Hg increases in systolic and diastolic BP, respectively (both P<0.001) and 12% greater odds of being hypertension (95% confidence interval, 1.08, 1.17). The CRW was positively associated with BP and hypertension; no interaction was found between CRW and Wcurrent on BP/hypertension.
Conclusions
A majority of Chinese women tended to become heavier throughout adult life. More weight gain led to the higher BP. Weight change is an independent predictor for later‐life BP and hypertension.
Keywords: blood pressure, epidemiology, hypertension, obesity, women
Subject Categories: Women, Epidemiology, Hypertension, Obesity
Introduction
Body size at any stage of the life course is recognized as having an important influence on blood pressure (BP).1 Although the origins of high BP are not well understood, excessive weight gain is a strong risk factor for hypertension.2 Many previous studies have showed that excessive weight gain is a strong predictor of both increased BP and increased incidence of hypertension.3, 4, 5, 6 In contrast, short‐term weight loss seems to be a promising strategy for reducing BP and treatment of hypertension.7, 8, 9, 10, 11 Scientific evidences strongly support the concept that lifestyle modification can have powerful effects on BP.12 One method to achieve this is maintaining optimal body weight.1 However, it is not easy to keep a stable weight during adult life, because persons are more likely to increase their body weight by age.13 Therefore, understanding of trends in long‐term weight change and their association with later life BP is crucial for effective means of preventing hypertension and such means would have great clinical and public health importance.
Some previous studies investigating the association between long‐term weight change and BP14, 15, 16, 17 have indicated that weight change has a persistent influence on BP. However, in the patterns of weight change, the magnitude of independent effect of weight change on BP are not well understood. What are the trends of weight change from young adulthood to middle age among different weight category populations? And how much weight change contributes to BP that is independent of current weight? These questions need to be further addressed. Moreover, no studies of this type have been conducted in the Hong Kong population. The magnitude of weight change during adult life in Hong Kong Chinese might be different from Western populations because of dissimilarity in lifestyles and dietary patterns. Although the Hong Kong Chinese have lower BMI than most Western populations,18 the prevalence of hypertension is increasing in recent years.19 It implicates that Hong Kong Chinese may be susceptible to elevated BP even with a relatively small weight gain. Thus, an accurate estimation of the strength of association between weight change and BP in this relatively lean population is important from a public health perspective.
The current study aimed to understand the pattern of weight change from young adulthood to middle age in a sample of Hong Kong Chinese women and examine the associations between weight change and later‐life BP/hypertension, to identify the group of people who were more likely to have elevated BP and hypertension in later life. Furthermore, the independent contribution of weight change to BP/hypertension was also studied.
Methods
Data Sources
Data for this study were drawn from a life‐course epidemiology study entitled “Hong Kong Women's Health Study (HKWHS),” which was conducted from 2009 to 2011. The HKWHS investigated the impacts of early‐life exposures (eg, birth weight, shift work in young adulthood) on later‐life outcomes (eg, hypertension, diabetes). The target population was Chinese female nurses aged 35 to 65 years and resident in Hong Kong. Data collection was through self‐administered questionnaire sent by post. Together with the questionnaire, an informed consent form, a prepaid return envelope, and a paper tape measure were attached.20 The Association of Hong Kong Nursing Staff (AHKNS) helped in conducting systematic sampling of eligible nurses from their membership database. The sample size calculation, sampling method, as well as the procedure of mailing survey have been described in detail before.20, 21 The study protocol was approved by the research ethics committee of the Chinese University of Hong Kong. In total, 3 rounds of mailing were conducted, and 1253 valid questionnaires were received. All participants returned the signed statement of informed consents.
Measurements
Weight at age 18, current weight, and body mass index
Participants were asked to write down the exact value of their weight at age 18 (kilograms or pounds), current weight (kilograms or pounds), and height (centimeters) on the questionnaire. These values were converted to metric units (kilograms or meters) for analysis. Body mass index (BMI) was calculated as the ratio of weight (in kg)/square of height (in m2). Overweight was defined as 23.0 kg/m2 ≤ BMI <25.0 kg/m2 and obesity as BMI ≥25 kg/m2 according to the World Health Organization standard for Asian adults,22 respectively. An acceptable validity of self‐reporting for present anthropometric variables has been identified and reported.23 A subsample of 144 nurses (144 of 1253; 11.5%) was invited to have their body measurements at our research center. We then compared the self‐reported values with the measured data to assess the validity. The self‐reported and measured anthropometries were highly correlated (correlation coefficients ranged from 0.72 to 0. 96). An overall agreement of 84.7% was observed in the classification of BMI between self‐reported and measured values. Sensitivity was 84.6% and specificity 95.7% for overweight/obesity detection, respectively.23
For the recalled weight at age 18, learned from some previous studies,6, 24 we considered that the participants might enter the university or nursing school at around 18 years old; at that time physical examination for entrance might be conducted, and accordingly, the body weight information should be recorded. Hence, in the questionnaire, participants were asked to try to recall their body weight value from the school physical examination at that time, so as to help them to report the accurate information of weight at age 18.
Weight change from age 18 to current age, and conditional relative weight
For participants whose BP was normal,25 weight change was defined as:
For participants who had hypertension, weight change was defined as change in weight from age 18 to the time point at which they were diagnosed with hypertension:
This “time point weight” was obtained as follows: each participants was asked whether her body weight has changed after being diagnosed with hypertension; if no, current weight was used; if yes, then asked how much her weight has changed (in kg) since she has been diagnosed with hypertension.
A conditional relative weight (CRW) was calculated to express the relative weight change from age 18 to current age, which was uncorrelated with weight at age 18, to estimate the independent contribution of weight change on BP. CRW was defined as the amount by which the weight at the adulthood exceeds that which would have been predicted at age 18; it was calculated as standardized residuals by regressing current weight on weight at age 18.25 A positive CRW indicates gaining more in weight than would be expected from a given weight at age 18.
BP and hypertension
Participants were asked to report the BP value from the most recent body check; and if a sphygmomanometer was available, they were requested to measure the BP for at least twice by themselves or by other health care professionals, and then calculate the mean BP. Those taking antihypertension medicine at the time of the questionnaire were required to report the BP value before medication started. The validity of self‐reported BP has been identified elsewhere23 the overall agreement between self‐reported and measured data was 87% for systolic BP (SBP) and 72% for diastolic BP (DBP), respectively. Three instances would be considered as preliminary hypertension cases: (1) reported first time physician‐diagnosed primary hypertension; (2) reported high BP: SBP ≥140 mm Hg and/or DBP ≥90 mm Hg,26 even without physician‐diagnosed hypertension; and (3) currently on antihypertension medication. Once a hypertension case was reported, we contacted the person by telephone again and verified the hypertension status. For the first instance, the participant was asked to recall when and where she was diagnosed with hypertension. For the second instance, she was required to recheck her BP value. And for the third instance, the reason for taking antihypertension medicine was recorded. Those who gave definite answers and conformed to hypertension criteria were considered as true cases.
Covariates
The covariates included waist circumference (WC), age, menopause status, marital status, education level, smoking, drinking, physical activity, salt intake during recent 5 years, and family history of hypertension.
Statistical Analysis
One‐way ANOVA or Pearson chi‐square test or Fisher's exact test were conducted to estimate the preliminary associations between weight change and BP and other variables, where appropriate. Stratified analysis was used to examine the BP levels among different BMI groups (overweight/obesity vs no) at age 18 and at current. Furthermore, weight at age 18 and current weight were divided into 4 categories according to the percentile distribution: bottom 10%; 10% to 50%; 50% to 90%; and top 10%. The mean of BP was calculated by cross‐tabulating each of the 2 variables' percentile distributions. Thus, there would be 16 (4×4) means of BP according to 16 types of changes in body weight centile between age 18 and current age. One‐way ANOVA was used to test the overall difference of BP among these categories. ANCOVA was performed to examine the associations of current weight percentile and weight at age 18 percentile with BP. Finally, multiple linear regression and logistic regression models were applied to investigate the associations of body weight and weight change with BP and hypertension, respectively. The unexplained residual regression models25 were used to further estimate the association of CRW with BP and hypertension. The first step was to examine the respective contributions of body weight and CRW on BP/hypertension using separate simple regression models without adjustment, followed by adjusted models. We also included CRW and weight at age 18 into one model to see their total contributions on BP/hypertension and tested the interaction as well. SPSS software (version 20.0; SPSS, Inc., Chicago, IL) was used for data analysis. Statistical significance was defined as P value <0.05.
Results
Of the 1253 participant, around three fourths were married (73.3%) and received tertiary or above education (72.1%). Mean age was 45.6±7.6 years. Table 1 shows the characteristics of the participants and their differences according to 3 status of change in weight from age 18 to present: weight loss (reduced weight >1 kg); weight gain (increased weight >1 kg); and stable (weight change within 1 kg). The proportion of weight gain, weight loss, and stable weight in the total sample was 76.9%, 15.1%, and 8.0%, respectively. The changes in weight ranged from a minimum of −16 kg to a maximum of 36.29 kg, with a mean change of 5.8±6.8 kg. Most of the changes were within −2.0 kg to +11.0 kg (accounted for 70.3%). The mean of BMI increased from 19.7 kg/m2 at age 18 to 22.0 kg/m2 at current age. On average, participants in the weight gain group were older, taller, with higher proportion of married people, had higher waist circumference, and higher current weight and BMI than the weight loss and stable groups. In contrast, participants in the weight loss group were heavier than the weight gain group at age 18 and had a higher physical activity level currently. The mean of BP and prevalence of hypertension were significantly higher among the weight gain group (Table 1). No significant difference was found among the 3 groups in terms of antihypertension medication use, lifestyle factors (smoking and drinking), salt intake, menopause status, family history of hypertension, and education level (all P>0.05). Figure 1 shows the trend of BP according to the magnitude of weight change. The results indicated that more weight gain was associated with higher BP, whereas more weight loss led to lower BP (P for trend <0.001).
Table 1.
Participants' Characteristics According to Weight Change Status*
| Na | Totalb | Weight Change Status From Age 18 to the Current Agec | P Valued | |||
|---|---|---|---|---|---|---|
| Weight Loss | Stable | Weight Gain | ||||
| n (%) | 1243 | 188 (15.1) | 100 (8.0) | 955 (76.9) | ||
| Age, y | 1240 | 45.6 (7.6) | 44.4 (8.0) | 45.2 (7.7) | 46.0 (7.2) | 0.026 |
| Current height, cm | 1244 | 158.4 (5.5) | 157.1 (5.6) | 157.7 (5.7) | 158.6 (5.5) | 0.001 |
| Current weight, kg | 1243 | 55.1 (8.1) | 50.4 (6.3) | 50.3 (6.3) | 56.5 (8.0) | <0.001 |
| Current BMI, kg/m2 | 1238 | 22.0 (3.0) | 20.4 (2.2) | 20.2 (2.4) | 22.5 (3.0) | <0.001 |
| Current waist circumference, cm | 1230 | 75.3 (8.4) | 70.7 (6.8) | 69.6 (6.6) | 76.8 (8.1) | <0.001 |
| Weight at age 18, kg | 1247 | 49.4 (6.3) | 54.5 (7.3) | 50.2 (6.2) | 48.3 (5.5) | <0.001 |
| BMI at age 18, kg/m2 | 1242 | 19.7 (2.5) | 22.1 (2.7) | 20.2 (2.4) | 19.2 (2.1) | <0.001 |
| Weight change from age 18 to current, kg | 1243 | 5.8 (6.8) | −4.1 (2.9) | 0.1 (0.8) | 8.3 (5.5) | <0.001 |
| Systolic blood pressure, mm Hg | 1217 | 113.5 (13.4) | 109.5 (11.5) | 111.8 (13.6) | 114.5 (13.6) | <0.001 |
| Diastolic blood pressure, mm Hg | 1217 | 70.4 (9.9) | 67.2 (9.0) | 69.1 (10.1) | 71.1 (10.0) | <0.001 |
| Hypertensione | 1214 | 127 (10.6) | 6 (3.3) | 9 (9.2) | 112 (12.1) | 0.002 |
| Anti‐hypertension medication usee | 1214 | 45 (3.7) | 2 (1.1) | 4 (4.1) | 39 (4.2) | 0.129 |
| Physical activity, higher than averagee | 1241 | 495 (39.9) | 89 (47.3) | 45 (45.0) | 360 (38.1) | 0.038 |
| Smokere | 1246 | 18 (1.5) | 2 (1.1) | 2 (2.0) | 14 (1.5) | 0.814 |
| Drink alcohole | 1239 | 81 (6.5) | 11 (5.9) | 8 (8.0) | 61 (6.5) | 0.778 |
| Menopausee | 1234 | 363 (29.4) | 42 (23.0) | 27 (27.0) | 293 (31.1) | 0.073 |
| Salt intake: ≥5 g/daye | 1229 | 412 (33.5) | 55 (29.6.3) | 33 (34.0) | 321 (34.3) | 0.457 |
| Family history of hypertensione | 1245 | 798 (64.1) | 113 (60.8) | 70 (70.0) | 611 (64.4) | 0.296 |
| Marital status: marriede | 1250 | 916 (73.3) | 123 (65.4) | 61 (61.0) | 725 (75.9) | <0.001 |
| Education level: Tertiary education and abovee | 1245 | 898 (72.1) | 133 (71.7) | 74 (74.0) | 686 (72.1) | 0.874 |
BMI indicates body mass index.
Values reported as mean (SD) or n (%) for total sample and for each weight change status group, where appropriate.
N was the exact number of cases who had present value among 1253 participants.
Total was the exact mean (SD) or n (%) for each variable based on the exact number of cases (N).
Weight loss: participants who lost weight >1 kg; Stable: participants with weight change ≤1 kg; Weight gain: participants who gained weight >1 kg.
P values generated from 1‐way ANOVA or Pearson chi‐square test or Fisher's exact test, where appropriate.
Variables were showed the number and percentage that counted “Yes” for each weight change group.
Figure 1.
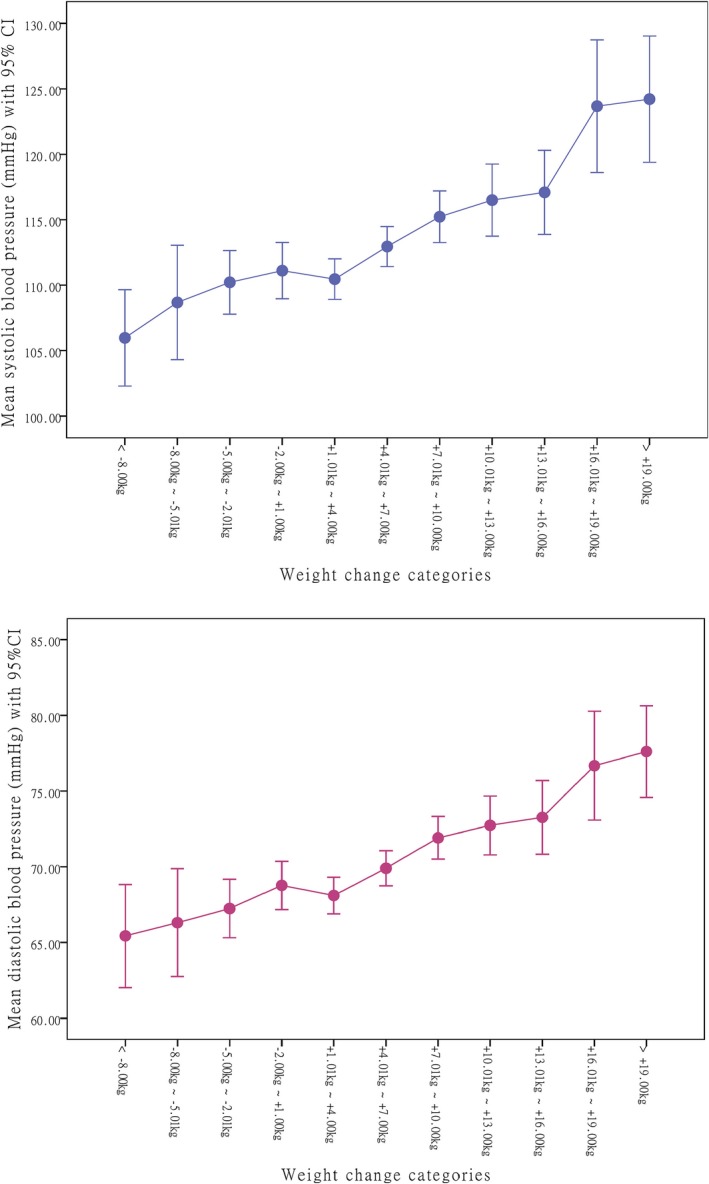
Mean of blood pressure according to the magnitude of weight change from age 18 to current age.
Figure 2 shows the cross‐tabulation analysis results by dividing the participants into groups according to their overweight/obese status (yes or no) at age 18 and at current age. Only 9.1% of participants were overweight/obese at age 18, but the proportion increased to 30.6% at current age. Among participants who were not overweight/obese at age 18, 26.8% of them became overweight/obese at current age. For those who were overweight/obese at age 18, 68.1% of them maintained the obese status through adult life. On average, the highest BP existed in those who were overweight/obese both at age 18 and currently (P<0.001). Those changed from underweight/normal weight at age 18 to overweight/obese at current age also had higher BP (P<0.001). When we compared the BP values between these 2 weight change statuses, no statistically significant difference was found (P>0.05; Figure 2).
Figure 2.
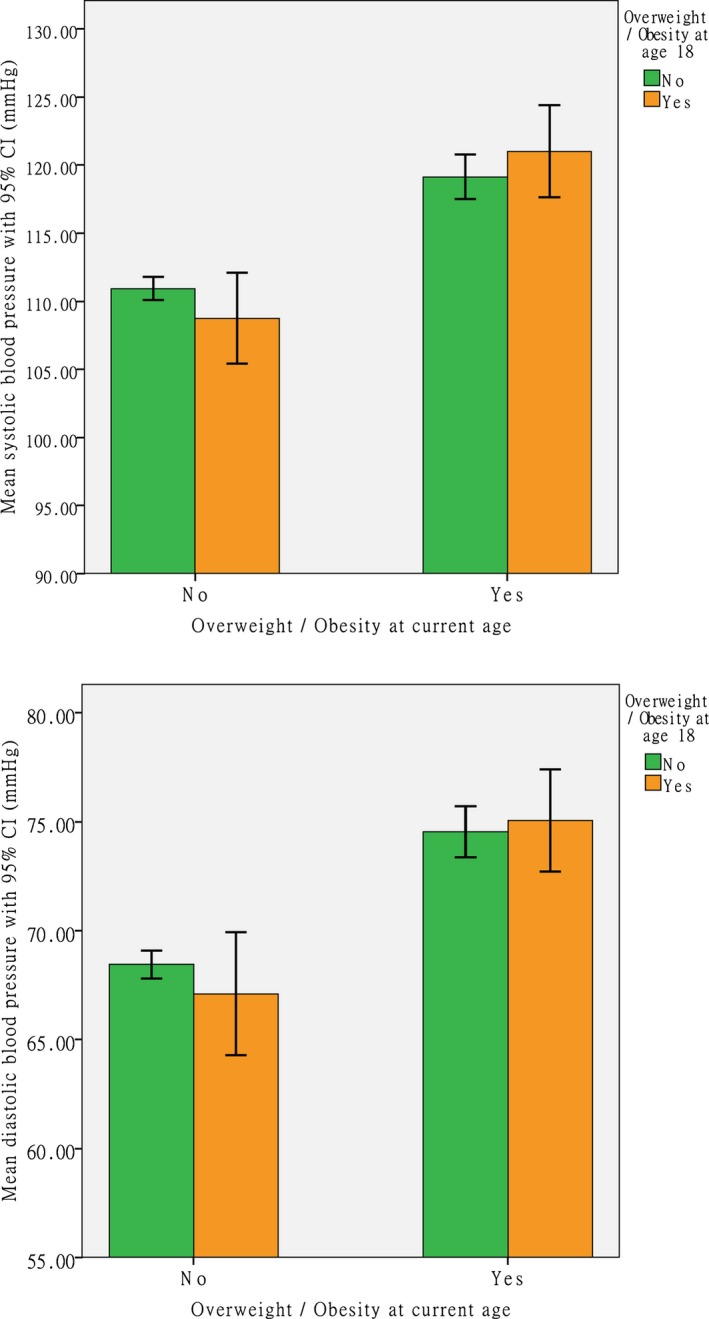
Mean of blood pressure from the cross‐tabulation analysis by dividing the participants into groups according to their overweight/obese status (yes or no) at age 18 and at current age.
The variation of BP according to percentile strata of current weight and weight at age 18 is shown in Table 2 and Figure 3. The latter further demonstrates the trends of change in mean BP according to percentile strata. No participants in the lightest and heaviest 10% strata at age 18 went across to the heaviest and lightest 10% strata at current age, respectively. The participants belonging to the current heaviest 10% strata had higher BP than those in the other 3 percentile strata (SBP: 122.9 vs 114.5, 111.1, and 109.4). The highest SBP and DBP were observed in the heaviest 10% participants both at age 18 and at present (SBP: 124.6 mm Hg; DBP: 77.8 mm Hg). ANCOVA analysis showed a significant association between current weight percentile and BP. No significant interaction was found between weight at age 18 and current weight percentile on BP (Table 2).
Table 2.
Cross‐Tabulation of Mean Blood Pressure According to the Percentile Distributions of Weight at Age 18 and Current Weight
| Current Weight Percentile | Weight at Age 18 Percentile | N | Mean (SD) | |
|---|---|---|---|---|
| Systolic Blood Pressure | Diastolic Blood Pressure | |||
| Bottom 10% | Bottom 10% | 49 | 111.0 (13.8) | 69.6 (9.5) |
| 10% to 50% | 63 | 108.8 (12.8) | 66.0 (10.0) | |
| 50% to 90% | 9 | 104.1 (14.4) | 64.2 (9.4) | |
| Top 10% | 0 | — | — | |
| Total | 121 | 109.4 (13.4) | 67.3 (9.9) | |
| 10% to 50% | Bottom 10% | 55 | 115.8 (14.8) | 71.8 (10.6) |
| 10% to 50% | 266 | 111.3 (12.5) | 68.7 (9.7) | |
| 50% to 90% | 160 | 109.6 (10.6) | 67.8 (8.7) | |
| Top 10% | 12 | 105.9 (7.2) | 64.7 (8.3) | |
| Total | 493 | 111.1 (12.3) | 68.6 (9.5) | |
| 50% to 90% | Bottom 10% | 16 | 118.6 (13.5) | 73.0 (9.4) |
| 10% to 50% | 162 | 114.9 (14.1) | 71.7 (10.0) | |
| 50% to 90% | 222 | 114.2 (11.9) | 71.3 (8.7) | |
| Top 10% | 58 | 113.4 (11.5) | 69.1 (8.7) | |
| Total | 458 | 114.5 (12.7) | 71.2 (9.2) | |
| Top 10% | Bottom 10% | 0 | — | — |
| 10% to 50% | 13 | 119.7 (15.2) | 74.6 (9.9) | |
| 50% to 90% | 68 | 122.0 (15.3) | 76.0 (10.8) | |
| Top 10% | 55 | 124.6 (15.4) | 77.8 (11.0) | |
| Total | 136 | 122.9 (15.3) | 76.6 (10.8) | |
| P value* | Current weight percentile | <0.001 | <0.001 | |
| Weight at age 18 percentile | 0.243 | 0.411 | ||
| Interaction | 0.568 | 0.413 | ||
P values generated from ANCOVA analysis. Covariates in models were age, height, waist circumference, physical activity (higher than average or not), and marital status (married or not).
Figure 3.
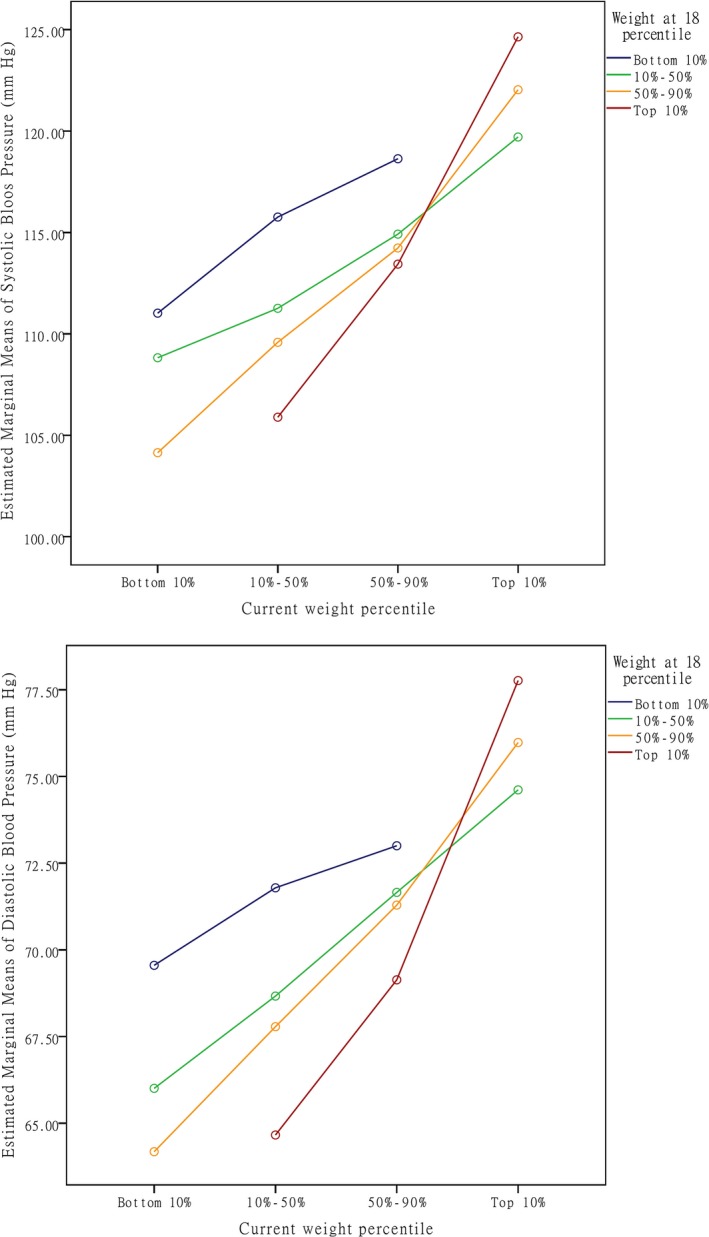
Mean blood pressure according to weight at age 18 percentiles and current weight percentiles.
The correlations between weight at age 18, current weight, weight change from age 18 to current age, and CRW are shown in Table 3. Both weight at age 18 and weight change were positively correlated with current weight (both P<0.01). CRW had no significant correlation with weight at age 18 (P>0.05). Table 4 shows the multiple linear regression and logistic regression results of weight at age 18, current weight, weight change, and CRW on BP/hypertension. Age, height, and WC all had significant associations with both BP and body weight; they were treated as confounders and thereby adjusted in the regression models. Weight at age 18 and current weight were both positively associated with BP (all P<0.01). Weight change also had significant association with BP (coefficient: 0.52 for SBP and 0.38 for DBP; both P<0.001); this association was attenuated, but still statistically significant, after adjustment for age, height, and waist circumference. By giving weight at age 18, a 1‐kg increase in weight change during adulthood predicted a 0.63 and 0.42 mm Hg increase in SBP and DBP, respectively. Similar results were found when CRW was used as the predictor. Higher CRW predicted higher BP (Table 4). In the adjusted models, a 1‐kg increase in CRW was associated with an increase in SBP and DBP of 0.44 and 0.30 mm Hg, respectively. No significant interaction between weight change and current weight, as well as CRW and current weight on BP, was found (all P>0.05). In the logistic regression analysis, significant associations of hypertension with weight at age 18, current weight, weight change, and CRW were found (all P<0.01). The adjusted odds of having hypertension was 12% greater with a 1‐kg increase in weight change by controlling weight at age 18 (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.08, 1.17; P<0.001). The result from CRW showed a very similar OR in the combined model (Table 4). We also conducted subsample analysis for BP by excluding the subjects with antihypertension medication use. Similar relationships were found (Table 5).
Table 3.
Correlations Between Weight at Age 18, Current Weight, Weight Change, and Conditional Relative Weight
| Current Weight | Weight at 18 | Weight Change From 18 Years to Current | Conditional Relative Weight | |
|---|---|---|---|---|
| Current weight | ||||
| Correlation coefficient | 1 | 0.574a | 0.652a | 0.818a |
| P value | 0.000 | 0.000 | 0.000 | |
| Weight at 18 | ||||
| Correlation coefficient | 0.574a | 1 | −0.247a | 0.000 |
| P value | 0.000 | 0.000 | 0.987 | |
| Weight change from 18 years to current | ||||
| Correlation coefficient | 0.652a | −0.247a | 1 | 0.969a |
| P value | 0.000 | 0.000 | 0.000 | |
| Conditional relative weight | ||||
| Correlation coefficient | 0.818a | 0.000 | 0.969a | 1 |
| P value | 0.000 | 0.987 | 0.000 | |
Correlation is significant at the 0.01 level (2‐tailed).
Table 4.
Multiple Linear Regression Coefficients for Adult Blood Pressure (mm Hg) Per 1 Unit Increase in Current Weight, Weight at Age 18, Weight Change From 18 Years to Current, and Conditional Relative Weight (CRW); Odds Ratio by Binary Logistic Regression Analysis for Being Hypertension Per 1 Unit Increase in Current Weight, Weight at Age 18, Weight Change From 18 Years to Current, and CRW (N=1243)
| Simple Modelsa | Adjusted Modelsb | Combined Models 1c | Combined Models 2d | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Systolic blood pressure | ||||||||
| Current weight | 0.52 (0.43, 0.61) | <0.001 | 0.62 (0.48, 0.75) | <0.001 | ||||
| Weight at age 18 | 0.24 (0.12, 0.36) | <0.001 | 0.23 (0.10, 0.35) | 0.001 | 0.60 (0.45, 0.76) | <0.001 | 0.43 (0.31, 0.57) | <0.001 |
| Weight change from 18 years to current | 0.52 (0.42, 0.63) | <0.001 | 0.28 (0.15, 0.41) | <0.001 | 0.63 (0.48, 0.78) | <0.001 | ||
| Conditional relative weighte | 0.62 (0.51, 0.73) | <0.001 | 0.44 (0.30, 0.59) | <0.001 | 0.63 (0.50, 0.72) | <0.001 | ||
| Diastolic blood pressure | ||||||||
| Current weight | 0.35 (0.29, 0.42) | <0.001 | 0.40 (0.30, 0.50) | <0.001 | ||||
| Weight at age 18 | 0.14 (0.05, 0.23) | 0.002 | 0.13 (0.03, 0.23) | 0.008 | 0.38 (0.26, 0.50) | <0.001 | 0.27 (0.17, 0.37) | <0.001 |
| Weight change from 18 years to current | 0.38 (0.30, 0.46) | <0.001 | 0.20 (0.10, 0.29) | <0.001 | 0.42 (0.30, 0.53) | <0.001 | ||
| Conditional relative weighte | 0.44 (0.35, 0.52) | <0.001 | 0.30 (0.20, 0.41) | <0.001 | 0.42 (0.30, 0.53) | <0.001 | ||
| Hypertension | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Current weight | 1.09 (1.07, 1.12) | <0.001 | 1.12 (1.08, 1.17) | <0.001 | ||||
| Weight at age 18 | 1.04 (1.01, 1.07) | 0.003 | 1.05 (1.02, 1.09) | 0.002 | 1.13 (1.08, 1.18) | <0.001 | 1.09 (1.05, 1.14) | <0.001 |
| Weight change from 18 years to current | 1.11 (1.08, 1.14) | <0.001 | 1.07 (1.02, 1.09) | 0.001 | 1.12 (1.08, 1.17) | <0.001 | ||
| Conditional relative weighte | 1.12 (1.09, 1.15) | <0.001 | 1.09 (1.05, 1.13) | <0.001 | 1.12 (1.08, 1.17) | <0.001 |
Simple models: blood pressure was regressed on current weight, weight at age 18, weight change from 18 years to current, and conditional relative weight, respectively; without adjustment for covariates.
Adjusted models: blood pressure was regressed on current weight, weight at age 18, weight change from 18 years to current, and conditional relative weight, respectively; adjusted for age, height, and waist circumference.
Combined models 1: included weight at age 18 and weight change from 18 years to current into 1 regression model, adjusted for age, height, and waist circumference.
Combined models 2: included weight at age 18 and conditional relative weight into 1 regression model, adjusted for age, height, and waist circumference.
Conditional relative weight (CRW): calculated as a residual of current weight regressed on weight at age 18. CRW=Exact current weight−Expected current weight; Expected current weight=a+b×weight at age 18.
Table 5.
Multiple Linear Regression Coefficients for Adult Blood Pressure (mm Hg) Per 1 Unit Increase in Current Weight, Weight at Age 18, Weight Change From 18 Years to Current, and Conditional Relative Weight (CRW)
| Simple Models* | Adjusted Models† | Combined Models 1‡ | Combined Models 2§ | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Systolic blood pressure | ||||||||
| Current weight | 0.46 (0.37, 0.55) | <0.001 | 0.56 (0.42, 0.70) | <0.001 | ||||
| Weight at age 18 | 0.17 (0.05, 0.28) | 0.005 | 0.15 (0.03, 0.28) | 0.018 | 0.52 (0.36, 0.67) | <0.001 | 0.36 (0.22, 0.49) | <0.001 |
| Weight change from 18 years to current | 0.49 (0.39, 0.60) | <0.001 | 0.30 (0.17, 0.42) | <0.001 | 0.60 (0.45, 0.75) | <0.001 | ||
| Conditional relative weight|| | 0.57 (0.46, 0.68) | <0.001 | 0.44 (0.30, 0.59) | <0.001 | 0.60 (0.45, 0.75) | <0.001 | ||
| Diastolic blood pressure | ||||||||
| Current weight | 0.32 (0.25, 0.38) | <0.001 | 0.35 (0.25, 0.45) | <0.001 | ||||
| Weight at age 18 | 0.09 (0.01, 0.18) | 0.041 | 0.08 (−0.02, 0.18) | 0.106 | 0.31 (0.19, 0.43) | <0.001 | 0.21 (0.11, 0.31) | <0.001 |
| Weight change from 18 years to current | 0.36 (0.28, 0.44) | <0.001 | 0.20 (0.11, 0.30) | <0.001 | 0.39 (0.27, 0.50) | <0.001 | ||
| Conditional relative weight|| | 0.41 (0.33, 0.49) | <0.001 | 0.30 (0.19, 0.40) | <0.001 | 0.39 (0.27, 0.50) | <0.001 | ||
Only included subjects without antihypertension medication use (n=1169).
Simple models: blood pressure was regressed on current weight, weight at age 18, weight change from 18 years to current, and conditional relative weight, respectively; without adjustment for covariates.
Adjusted models: blood pressure was regressed on current weight, weight at age 18, weight change from 18 years to current, and conditional relative weight, respectively; adjusted for age, height, and waist circumference.
Combined models 1: included weight at age 18 and weight change from 18 years to current into 1 regression model, adjusted for age, height, and waist circumference.
Combined models 2: included weight at age 18 and conditional relative weight into 1 regression model, adjusted for age, height, and waist circumference.
Conditional relative weight (CRW): calculated as a residual of current weight regressed on weight at age 18. CRW=Exact current weight−Expected current weight; Expected current weight=a+b×weight at age 18.
Discussion
In this study, we found that: (1) weight at age 18 and at current were both positively associated with later‐life BP and hypertension, with a stronger association found for current weight; (2) most women tended to become heavier throughout adult life; (3) in a long‐term period, more weight gain led to higher BP and higher risk of hypertension; and (4) weight change from young adulthood to middle age was an independent predictor for later‐life BP and hypertension. Our findings are consistent with some previous population‐based studies6, 14, 17, 27, 28, 29 that sustained weight loss during adult life had a stronger apparent protective effect on adult BP, whereas excessive weight gain (relative to the population) was associated with higher BP at midlife.
In our study, women who were thin at age 18 tended to gain body weight whereas those relatively heavy at young adulthood tended to reduce body weight in later life. This may be a common social‐psychological behavior. However, effective control of weight in a long‐term is not easy, and the unconsciously excessive weight gain may increase the risk of obesity and hypertension. Thus, a life‐course weight management is needed. In our study, the highest BP was found in those who were overweight/obese both at young adulthood and at middle age, whereas those who reduced their weight to normal/underweight at middle age had significantly lower BP. The implication is that a persistent overweight/obese status may have a long‐term negative impact on BP. Hence, maintaining an optimal body weight throughout adult life is important. In our study, we also observed higher BP level among those who had normal weight in young adulthood but became overweight/obese in middle age. It indicated that getting fat from young adulthood to middle age might lead to higher BP. The relatively small sample size and cross‐sectional design might be the reason that we could not detect which weight change status, that is, being overweight or obese consistently throughout young adulthood to middle age, or being normal weight in young adulthood and becoming overweight or obese in middle age, is more harmful in terms of BP control. We suggest that large cohort studies with long‐term follow‐up should be conducted in the future to address this issue. For women who are overweight/obese at young adulthood, reducing body weight to an optimal level is more beneficial to their later‐life health. In the Nurses' Health Study, the investigators found that the benefit from weight loss was largely limited to women who had a higher baseline BMI.17 Our study was consistent with this finding. Furthermore, among women who were in the lightest 10% at present, those who were also in the lightest 10% at 18 years old had the highest BP. This indicated that being too thin at young adulthood may also have a deleterious effect on later‐life BP. We suggest that an increase in body weight through changes of diet and exercise patterns at young adulthood may be beneficial to later‐life BP for those underweight young adults.
Some experts30 pointed out that weight gain stimulates sympathetic activation and that insulin and leptin are likely to be involved. However, the exact mechanism is still not clear. More studies are needed to investigate the underlying physiological mechanisms of weight‐change–induced BP elevation. One strength of our study is that we used CRW to estimate the independent contribution of weight change on BP and hypertension. It permits a more straightforward way to estimate the effect in a single model. In principle, all coefficients show their own effect without mutual influence, which makes the interpretation of the model easier.25 Our study also has some limitations. First, the self‐reported measures and the recall of weight at age 18 may lead to some misclassification of weight change status. However, it is unlikely to overturn the associations observed in our study because both change in weight and CRW demonstrated significant associations with BP by regression models as well as by stratified analyses. Moreover, it is reasonable to believe that participants could report anthropometric information with high accuracy in our study given that nurses are well educated with professional health‐related knowledge. In addition, some previous studies also collected the recalled weight information by participants themselves.6, 17, 24, 31 The second limitation is we only collected data on body weight at 2 time points. The lack of detailed weight cycling information limits a comprehensive examination of the relationships between changes in weight and BP. The third limitation is that we did not measure height at age 18. Age at menarche has been widely used to evaluate the maturation timing of females. Previous study32, 33 showed that nearly all Chinese girls (99.3%) had experienced onset of menstruation before age 18. Hence, we believe that a majority of Hong Kong women had achieved their final height before age 18. Thus, we used current height to calculate BMI at age 18 in our study. Finally, our cross‐sectional study did not allow an absolutely identical time point for calculating weight change between participants with and without hypertension. But we suggest that calculating exact values of weight change until the hypertension occurring is more important, because changes in weight after hypertension diagnosis does not contribute to hypertension development. Furthermore, for those that gained weight over time but their BP was still in the normal range (normotensive participants), though they gained more weight during the relatively longer “follow‐up time” than those with hypertension, as long as their BP values were in the normal range, their weight changes still reflected the true magnitude of weight change before hypertension development. Thus, we suggest that the different calculations used in our study could not fundamentally change the weight change/BP associations. We further conducted 2 sensitivity analyses by excluding those diagnosed with hypertension and those who had weight changes after hypertension diagnosis, respectively. The observed weight change/BP associations were similar to the whole sample analysis (data not shown).
In a public health perspective, primary prevention of hypertension provides an opportunity to contain the high costs involved in managing hypertension and its complications.34 A 10‐kg weight gain in our study is estimated to be associated with a 6.3 mm Hg increase in SBP at the population level. This effect is equivalent to an extra 5.8 g of salt intake per day35 and to an estimated increase of ≈5.4% in the mortality risk of cardiovascular disease.36 Therefore, we believe that the associations we observed between weight change and BP/hypertension are of public health significance. These estimated effects highlight the importance of public health initiatives to maintain an optimal body weight during the life course and to prevent too low/too high body weight in a population. Appropriate weight control strategy in a general population is warranted. The modification of lifestyle factors at the population level is challenging, but is important to prevent cardiovascular events and complications.37
In summary, our results support a positive association between change in body weight and later‐life BP independent of initial and attained body weight. Maintaining an optimum body weight throughout adulthood may be beneficial in the primary prevention of hypertension.
Sources of Funding
This work was supported by the Postgraduate Student Research Grant of the Jockey Club School of Public Health and Primary Care, and the Center of Research and Promotion of Women's Health, The Chinese University of Hong Kong.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002361 doi: 10.1161/JAHA.115.002361)
References
- 1. Whincup PH, Cook DG, Geleijnse JM. A life course approach to blood pressure In: Kuh D, Ben‐Shlomo Y, eds. A Life Course Approach to Chronic Disease Epidemiology. Oxford, UK: Oxford University Press; 2004:218–239. [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 3. Juhaeri, Stevens J, Chambless LE, Tyroler HA, Rosamond W, Nieto FJ, Schreiner P, Jones DW, Arnett D. Associations between weight gain and incident hypertension in a bi‐ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26:58–64. [DOI] [PubMed] [Google Scholar]
- 4. Curtis AB, Strogatz DS, James SA, Raghunathan TE. The contribution of baseline weight and weight gain to blood pressure change in African Americans: the Pitt County Study. Ann Epidemiol. 1998;8:497–503. [DOI] [PubMed] [Google Scholar]
- 5. Williams PT. Increases in weight and body size increase the odds for hypertension during 7 years of follow‐up. Obesity (Silver Spring). 2008;16:2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Field AE, Byers T, Hunter DJ, Laird NM, Manson JE, Williamson DF, Willett WC, Colditz GA. Weight cycling, weight gain, and risk of hypertension in women. Am J Epidemiol. 1999;150:573–579. [DOI] [PubMed] [Google Scholar]
- 7. Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo‐controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–172. [DOI] [PubMed] [Google Scholar]
- 8. Neaton JD, Grimm RH Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, Cutler JA, Flack JM, Schoenberger JA, McDonald R, Lewis CE, Liebson PR, Raines J, Joffrion I, Allen RE, Jones L, Parker D, De Worth JK, Anzelone E, Gunn D, George A, Montgomery J, Neri GS, Betz E, Mascitti B, Plank E, Peterson B, Remijas T, Washington W, Turner I, Stefanie L, Aye P, Madnek‐Oxman S, Jones H, Mascioli SR, Van Heel N, Bjerk C, Galle F, Laqua P, Miller M, Bell LM, Robinson ME, Thorson C, Townsend R, Caggiula A, Dianzumba S, Ciak C, Link M, Hall B, Monske M, Theobald TM, Berry M, Coyne T, Bunker CA, Kramer K, DuChene AG, Holland LA, Tze S, Sjolund S, Launer CA, Lagus J, Miller CM, Svendsen KH, Leon A, Laing B, McDonald M, Surbey D, Wiche MK, Kuiper K, Remington R, Coates TJ, Devereux R, Gifford RW Jr, Langford H, McCullough L, Tyroler HA. Treatment of mild hypertension study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 9. Jones DW. Body weight and blood pressure. Effects of weight reduction on hypertension. Am J Hypertens. 1996;9:50s–54s. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA. 1998;279:839–846. [DOI] [PubMed] [Google Scholar]
- 11. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42:878–884. [DOI] [PubMed] [Google Scholar]
- 12. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR; Writing Group of the PREMIER Collaborative Research Group . Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization . Obesity: prevention and managing the global epidemic: report of a WHO consultation. WHO Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 14. Droyvold WB, Midthjell K, Nilsen TI, Holmen J. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes (Lond). 2005;29:650–655. [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, Kawakubo K, Kashihara H, Mori K. Effect of long‐term body weight change on the incidence of hypertension in Japanese men and women. Int J Obes Relat Metab Disord. 2004;28:391–395. [DOI] [PubMed] [Google Scholar]
- 16. Lee JS, Kawakubo K, Kobayashi Y, Mori K, Kasihara H, Tamura M. Effects of ten year body weight variability on cardiovascular risk factors in Japanese middle‐aged men and women. Int J Obes Relat Metab Disord. 2001;25:1063–1067. [DOI] [PubMed] [Google Scholar]
- 17. Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–88. [DOI] [PubMed] [Google Scholar]
- 18. Ko G, Chan J. Burden of obesity–lessons learnt from Hong Kong Chinese. Obes Rev. 2008;9(suppl 1):35–40. [DOI] [PubMed] [Google Scholar]
- 19. Wong MC, Wang HH, Leung MC, Tsang CS, Lo S, Griffiths SM. The rising prevalence of self‐reported hypertension among Chinese subjects: a population‐based study from 121 895 household interviews. QJM. 2015;108:9–17. [DOI] [PubMed] [Google Scholar]
- 20. Xie YJ, Ho SC. Prenotification had no additional effect on the response rate and survey quality: a randomized trial. J Clin Epidemiol. 2013;66:1422–1426. [DOI] [PubMed] [Google Scholar]
- 21. Xie YJ, Ho SC, Liu ZM, Hui SS. Birth weight and blood pressure: ‘J’ shape or linear shape? Findings from a cross‐sectional study in Hong Kong Chinese women. BMJ Open. 2014;4:e005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organisation, International Association for the Study of Obesity, International Obesity Task Force . The Asia‐Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications; 2000. [Google Scholar]
- 23. Xie YJ, Ho SC, Liu ZM, Hui SS. Comparisons of measured and self‐reported anthropometric variables and blood pressure in a sample of Hong Kong female nurses. PLoS One. 2014;9:e107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Houston DK, Ding J, Nicklas BJ, Harris TB, Lee JS, Nevitt MC, Rubin SM, Tylavsky FA, Kritchevsky SB; Health ABC Study . Overweight and obesity over the adult life course and incident mobility limitation in older adults: the health, aging and body composition study. Am J Epidemiol. 2009;169:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keijzer‐Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–1324. [DOI] [PubMed] [Google Scholar]
- 26. Whitworth JA; World Health Organization, International Society of Hypertension Writing Group . 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 27. Li L, Law C, Power C. Body mass index throughout the life‐course and blood pressure in mid‐adult life: a birth cohort study. J Hypertens. 2007;25:1215–1223. [DOI] [PubMed] [Google Scholar]
- 28. Czernichow S, Mennen L, Bertrais S, Preziosi P, Hercberg S, Oppert JM. Relationships between changes in weight and changes in cardiovascular risk factors in middle‐aged French subjects: effect of dieting. Int J Obes Relat Metab Disord. 2002;26:1138–1143. [DOI] [PubMed] [Google Scholar]
- 29. Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences—the Tromso Study, 1986–1995. Arch Intern Med. 2000;160:2847–2853. [DOI] [PubMed] [Google Scholar]
- 30. Masuo K, Mikami H, Ogihara T, Tuck ML. Weight gain‐induced blood pressure elevation. Hypertension. 2000;35:1135–1140. [DOI] [PubMed] [Google Scholar]
- 31. Houston DK, Stevens J, Cai J, Morey MC. Role of weight history on functional limitations and disability in late adulthood: the ARIC study. Obes Res. 2005;13:1793–1802. [DOI] [PubMed] [Google Scholar]
- 32. Huen KF, Leung SS, Lau JT, Cheung AY, Leung NK, Chiu MC. Secular trend in the sexual maturation of southern Chinese girls. Acta Paediatr. 1997;86:1121–1124. [DOI] [PubMed] [Google Scholar]
- 33. Lam T, Shi H, Ho L, Stewart SM, Fan S. Timing of pubertal maturation and heterosexual behavior among Hong Kong Chinese adolescents. Arch Sex Behav. 2002;31:359–366. [DOI] [PubMed] [Google Scholar]
- 34. National High Blood Pressure Education Program working group report on primary of hypertension. Arch Intern Med. 1993;153:186–208. [PubMed] [Google Scholar]
- 35. Law M, Frost C, Wald N. By how much does dietary salt reduction lower blood pressure? I‐Analysis of observational data among populations. BMJ. 1991;302:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCarron P, Smith GD, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355:1430–1431. [DOI] [PubMed] [Google Scholar]
- 37. Cheung BM, Wat NM, Man YB, Tam S, Cheng CH, Leung GM, Woo J, Janus ED, Lau CP, Lam TH, Lam KS. Relationship between the metabolic syndrome and the development of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study‐2 (CRISPS2). Am J Hypertens. 2008;21:17–22. [DOI] [PubMed] [Google Scholar]


