Abstract
Background
Tissue Doppler index E/è is used clinically and in multidisciplinary research for estimation of left ventricular filling pressure (LVFP) and diastolic dysfunction (DD)/heart failure with preserved ejection fraction (HFpEF). Its diagnostic accuracy is not well studied.
Methods and Results
From the PubMed, Scopus, Embase, and Cochrane databases, we identified 24 studies reporting E/è and invasive LVFP in preserved EF (≥50%). In random‐effects models, E/è had poor to mediocre linear correlation with LVFP. Summary sensitivity and specificity (with 95% CIs) for the American Society of Echocardiography–recommended E/è cutoffs (lateral, mean, and septal, respectively) to identify elevated LVFP was estimated by using hierarchical summary receiver operating characteristic analysis. Summary sensitivity was 30% (9–48%), 37% (13–61%), and 24% (6–46%), and summary specificity was 92% (82–100%), 91% (80–99%), and 98% (92–100%). Positive likelihood ratio (LR+) was <5 for lateral and mean E/è. LR+ was slightly >10 for septal E/è obtained from 4 studies (cumulative sample size <220). For excluding elevated LVFP, summary sensitivity for E/è (lateral, mean, and septal, respectively) was 64% (38–86%), 36% (3–74%), and 50% (14–81%), while summary specificity was 73% (54–89%), 83% (49–100%), and 89% (66–100%). Because of data set limitations, meaningful inference for identifying HFpEF by using E/è could not be drawn. With the use of quality assessment tool for diagnostic accuracy studies (Quality Assessment of Diagnostic Accuracy Studies questionnaire), we found substantial risks of bias and/or applicability.
Conclusions
There is insufficient evidence to support that E/è can reliably estimate LVFP in preserved EF. The diagnostic accuracy of E/è to identify/exclude elevated LVFP and DD/HFpEF is limited and requires further validation in a well‐designed prospective clinical trial.
Keywords: diagnostic accuracy, diastolic dysfunction, E/è, heart failure with preserved ejection fraction, left ventricular filling pressure, tissue Doppler imaging
Subject Categories: Echocardiography, Heart Failure, Diagnostic Testing, Meta Analysis
Introduction
Diastolic dysfunction (DD) is an important cause of heart failure (HF) with preserved ejection fraction (pEF) and a major public health issue.1, 2, 3 Epidemiological studies indicate that varying severities of DD are present in the community.1 DD is predictive of developing overt HF and all‐cause mortality.1, 3
Left ventricular (LV) end‐diastolic pressure (LVEDP) or pulmonary capillary wedge pressure (PCWP) is frequently used to assess LV diastolic function. Echocardiography is the mainstay for the noninvasive evaluation and quantitation of diastolic function.4, 5 Myocardial stiffness and relaxation abnormalities in DD result in elevated LVFP that is indirectly evaluated with echocardiography.4, 5 Early mitral annular velocity (è) obtained with the use of tissue Doppler imaging provides assessment of LV myocardial relaxation; è <8 to 10 cm/s (based on the location lateral or septal annulus or mean of lateral and septal) is indicative of impaired myocardial relaxation.4, 5 In conjunction with mitral peak early filling velocity E, the ratio of E/è is used to estimate LVFP and its use is recommended by the American Society of Echocardiography (ASE) and European Society of Cardiology (ESC) for evaluating DD/HFpEF.4, 5 E/èlateral >12, E/èmean >13, or E/èseptal >15 indicates elevated LVFP, whereas E/è <8 (any location) indicates normal LVFP.4 When E/è falls into the intermediate zone (8< E/è <12–15), additional echocardiographic indices are used to estimate LVFP.4, 5
E/è is extensively used both clinically and in multidisciplinary clinical research as a noninvasive surrogate for LVFP and diastolic function.6, 7, 8 However, its diagnostic accuracy is not well studied. We, therefore, decided to evaluate the diagnostic accuracy of E/è in evaluating LVFP and DD/HFpEF.
Methods
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses statement and the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The protocol for this review is described in Appendix S1.
Data Sources and Searches
Original clinical studies that evaluated LVFP and DD/HFpEF by using echocardiography and invasive techniques were screened from PubMed, Scopus, Embase, and Cochrane Library databases to February 2015 through the use of a number of search strategies with assistance from the librarian (Appendix S2).
Study Selection
We included studies that provided data on the diagnostic accuracy of pulsed‐wave tissue Doppler imaging index E/è to estimate LVFP and to identify DD/HFpEF.
For primary analysis, the studies were included if the participants had pEF cohort defined as ≥50% and corresponding echocardiographic E/è and invasive LVFP measurements at rest. The study had to evaluate the E/è–LVFP relationship and/or provide such data that a 2×2 table of true‐positive, false‐positive, false‐negative, and true‐negative values could be created for statistical analysis as described later. Table 1 describes the characteristics of the preserved LVEF patient cohort and data for primary analysis from the selected studies.
Table 1.
Data for Primary Analysisa
| Study (Reference) | LVEF, % | N | Age, y (mean±SD) | Indication for Catheterization | Echo and Catheterization Timing | Correlation to LVFP | 2×2 to Predict LVFP | 2×2 to Predict DD/HFpEF (Composite Reference Test)b | Patient Comorbidities, % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF | CAD | HTN | DM | |||||||||
| Ommen et al (2000)15 | >50 | 64 | — | Clinically indicated | Simultaneous | L, S, M (LVMDP) | S (LVMDP, from graph) | — | — | — | — | — |
| Gonzalez‐Vilchez et al (2002)16 | >50 | 32 | 66±13 | Clinically indicated | Sequential | L (PCWP) | — | — | — | — | — | — |
| Rivas‐Gotz et al (2003)18 | ≥55 | 55 | 64±2 | ICU or cath lab | Simultaneous | L, S, M (PCWP) | L, S (PCWP, from graph) | — | — | — | — | — |
| Dokanish et al (2004)19 | ≥50 | 19 | 57±13 | ICU or CCU | Simultaneous | — | M (PCWP, from graph) | — | — | — | 58 | 21 |
| Mansencal et al (2004)20 | >50 | 20 | 66±10 | Chest pain/CAD | ≤1 h | L (Pre‐A) | L (Pre‐A, from graph) | — | 5 | 100 | 5 | — |
| Hadano et al (2005)23 | >50 | 65 | 66±9 | Clinically indicated | ≤3 h | L (LVEDP, PCWP) | L (PCWP, from graph) | — | — | 28 | — | — |
| Kidawa et al (2005)24 | >50 | 50 | — | Coronary angiography | Simultaneous | L, S (LVEDP) | L (LVEDP, from graph) | — | — | — | — | — |
| Kasner et al (2007)26 | >50 | 55 | — | 43 exercise dyspnea/12 chest pain | 3 to 5 h | L (LVEDP) | — | — | 78 | 0 | 62 | 9 |
| Wang et al (2007)29 | >50 | 20 | — | ICU or cath lab | Simultaneous | M (PCWP) | — | — | — | — | — | — |
| Dokanish et al (2008)30 | >50 | 32 | — | Dyspnea | Sequential | M (Pre‐A) | — | — | — | — | — | — |
| Rudko et al (2008)32 | >50 | 39 | 64±5 | Clinically indicated | Simultaneous | S (LVMDP) | S (LVMDP, from graph) | — | 23 | 77 | 51 | — |
| Dini et al (2010)33 | >50 | 55 | 71±12 | LV dysfunction | ≤1 h | — | M (Pre‐A, from text) | — | 100c | — | — | — |
| Dokanish et al (2010)34 | ≥50 | 122 | 55±9 | Coronary angiography | Sequential | M (Pre‐A) | — | — | — | 65 | 88 | 55 |
| Dokanish et al (2010)35 | ≥50 | 122 | 55±9 | Coronary angiography | Sequential | M (LVEDP) | M (LVEDP, from graph) | — | — | 65 | 88 | 43 |
| Kasner et al (2010)37 | >50 | 33 | — | 21 exercise dyspnea/12 chest pain | Simultaneous | L (LVEDP) | — | — | 64 | 0 | 61 | 9 |
| Penicka et al (2010)38 | >50 | 30 | 67±9 | Chronic NYHA II/III dyspnea | Simultaneous | — | — | L, S, M (LVEDP, from text) | 67 | 0 | 70 | 27 |
| Bhella et al (2011)39 | >50 | 11 | 73±7 | Clinical research, HFpEF | Simultaneous | M (PCWP) | M (PCWP, from graph) | — | 100 | 0 | 100 | 55 |
| Hsiao et al (2011)40 | >50 | 100 | 69±13 | Coronary angiography | Sequential | L, S, M (Pre‐A) | — | — | — | 100 | 72 | 47 |
| Maeder et al (2011)42 | >50 | 36 | 56±17 | 11 PAH/15 HF/10 healthy volunteers and atypical patients | Sequential | L, S, M (PCWP) | — | — | 42 | — | — | — |
| Özer et al (2011)43 | >50 | 45 | 62±10 | Coronary angiography | ≤24 h | L, S, M (LVEDP) | L, S, M (LVEDP, from text) | — | — | 100 | 64 | 42 |
| Previtali et al (2012)46 | ≥55 | 57 | — | Clinically indicated | ≤1 h | L, S, M (LVEDP, Pre‐A) | L (LVEDP, from graph) | — | 0 | — | — | — |
| Manouras et al (2013)48 | ≥55 | 38 | — | Coronary angiography | Simultaneous | L, S, M (LVEDP, Pre‐A) | M (Pre‐A, from graph) | — | — | 0 | — | — |
| Hajahmadi Poorrafsanjani et al (2014)50 | ≥50 | 76 | — | Coronary angiography/mild valve disease | Next day | L (LVEDP) | — | — | — | — | — | — |
| Tatsumi et al (2014)51 | ≥50 | 22 | 65±18 | Clinically indicated | Not reported | S (PCWP) | — | — | — | — | — | — |
Empty cells are the result of no available data. 2×2 indicates set of true‐positive, false‐positive, false‐negative, and true‐negative values for recommended by American Society of Echocardiography E/è cutoffs; CAD, coronary artery disease; cath lab, catheterization laboratory; CCU, critical care unit; DD, diastolic dysfunction; DM, diabetes mellitus; HF, heart failure (clinical diagnosis); HTN, hypertension; ICU, intensive care unit; L, S, and M, lateral, septal, and mean E/è; LVEDP, left ventricular end‐diastolic pressure; LVEF, left ventricular ejection fraction; LVFP, left ventricular filling pressure; LVMDP, left ventricular mean diastolic pressure; N, number of patients; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; pEF, preserved ejection fraction; Pre‐A, left ventricular pre–A wave diastolic pressure.
For primary analysis, the studies were included if the participants had preserved LVEF cohort defined as ≥50% and provided corresponding echocardiographic E/è and invasive LVFP measurements at rest. Further, data were available such that a 2×2 table of true‐positive, false‐positive, false‐negative, and true‐negative values could be created for statistical analysis.
Clinical DD/HFpEF was described in the study based on composite of clinical signs and symptoms of HF with invasive parameters of DD with preserved LVEF.
Patient group included HF stages B, C, and D.
For secondary analysis, we also included supplemental studies that either used a lower LVEF threshold to identify preserved LV systolic function (ie, LVEF ≥40% or 45%) or had no criteria for normal LVEF but the mean and SD for LVEF of the study satisfied the condition that mean minus 2 SDs ≥40%. For a normal distribution, the latter condition assumes that ≈98% of participants have LVEF ≥40%. This allowed for the inclusion of all clinically relevant studies for secondary analysis because LVEF between 40% and 50% is sometimes used to indicate pEF. Table 2 describes the characteristics of the patient cohort and data for secondary analysis from the selected studies.
Table 2.
Data for Supplemental Analysisa
| Study (Reference) | LVEF, % | N | Age, y (mean±SD) | Indication for Catheterization | Echo and Catheterization Timing | Correlation to LVFP | 2×2 to Predict LVFP | 2×2 to Predict DD/HFpEF (Composite Reference Test)b | Specific Reason for Excluding From Primary Analysis | Patient Comorbidities, % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF | CAD | HTN | DM | ||||||||||
| Nagueh et al (1997)13 | >50 | 26 | — | Clinically indicated | Simultaneous | — | L (PCWP, from graph) | — | E/A <1 | 0 | — | — | — |
| Nagueh et al (1998)14 | >45 | 49 | — | ICU or cath lab | Simultaneous | L (PCWP) | L (PCWP, from graph) | — | LVEF not ≥50%, sinus tachycardia >100 bpm | — | — | — | — |
| Poerner et al (2003)17 | ≥55 | 85 | 60±10 | Angina/positive exercise test | 3±2.5 h | L, S, M (LVEDP, Pre‐A) | — | — | E/A >0.9 | — | — | — | — |
| Arques et al (2005)21 | >50 | 38 | 76±8 | Clinically indicated | Not reported | — | L (HF, limited data) | ASE guidelines cutoff data not available | 47 | 0 | 100 | 39 | |
| Bruch et al (2005)22 | >45 | 28 | 68±10 | Congestive HF; NYHA 2.4±0.4 | ≤4 h | M (LVEDP, PCWP) | M (LVEDP, from text) | — | LVEF not ≥50% | 100 | 50 | 75 | — |
| Hadano et al (2005)23 | >50 | 65 | 66±9 | Clinically indicated | ≤3 h | — | L (LVEDP, from graph) | — | Repetitive analysis of same study | — | 28 | — | — |
| Weber et al (2006)25 | >50 | 126 | 59±9 | Coronary angiography | Not reported | — | — | S (DD/HF, limited data) | ASE guidelines cutoff data not available | 35 | 49 | 58 | 17 |
| Kasner et al (2007)26 | >50 | 55 | — | 43 exercise dyspnea/12 chest pain | 3 to 5 h | — | — | L (HF, limited data) | ASE guidelines cutoff data not available | 78 | 0 | 62 | 9 |
| Min et al (2007)27 | ≥50 | 55 | 59±10 | Clinically indicated | Simultaneous | S (LVEDP) | S (LVEDP, from graph) | — | 8< E/è <15 | — | 56 | 46 | 31 |
| Poerner et al (2007)28 | 67±10 | 176 | 65±10 | Coronary angiography | 1±2.5 h | — | L (LVEDP, from text/graphs) | — | Assumption: LVEF >40% | — | 70 | 63 | 25 |
| Dokanish et al (2008)30 | >50 | 32 | — | Dyspnea | Sequential | — | M (Pre‐A, limited data) | — | ASE guidelines cutoff data not available | — | — | — | — |
| Ng et al (2008)31 | 61±5.6 | 20 | — | Clinically indicated | Sequential | — | M (LVEDP, limited data) | — | AUC ROC only | — | — | — | — |
| Dokanish et al (2010)34 | ≥50 | 122 | 55±9 | Coronary angiography | Sequential | — | M (Pre‐A, from graph) | — | Repetitive analysis of another study22 | — | 65 | 88 | 55 |
| Jaubert et al (2010)36 | >45 | 59 | 64±12 | Clinically indicated | Same morning | — | L (LVEDP, from text) | — | LVEF not ≥50% | — | 49 | 58 | 36 |
| Kasner et al (2010)37 | >60 | 33 | — | 21 exercise dyspnea/12 chest pain | Simultaneous | — | — | L (HF, limited data) | AUC ROC only | 64 | 0 | 61 | 9 |
| Penicka et al (2010)38 | >50 | 30 | 67±9 | Chronic NYHA II/III dyspnea | Simultaneous | — | L, S, M (LVEDP, from text) | — | Uncertainty with10% patientsc | 67 | 0 | 70 | 27 |
| Hsiao et al (2011)40 | >50 | 376 | 69±13 | Coronary angiography, HF survey | Sequential | — | L, S, M (Pre‐A, limited data) | — | ASE guidelines cutoff data not available | — | 100 | 72 | 47 |
| Kasner et al (2011)41 | >50 | 180 | — | 107 exercise dyspnea/73 chest pain | Simultaneous | — | — | L (HF, limited data) | AUC ROC only | 59 | 0 | 43 | 8 |
| Maeder et al (2011)42 | >50 | 36 | 56±17 | 11 PAH/15 HF/10 healthy volunteers and atypical patients | Sequential | — | L, S, M (PCWP, limited data) | — | AUC ROC only | 42 | — | — | — |
| Yesildag et al (2011)44 | 62±7 | 29 | 53±10 | Clinically indicated | Same day | L, S (LVEDP) | — | — | Assumption: LVEF >40% | — | — | — | — |
| Hsiao et al (2012)45 | >50 | 376 | — | Clinically indicated | Sequential | — | M (Pre‐A, limited data) | — | ASE guidelines cutoff data not available | — | — | — | — |
| Arques, 201347 | ≥50 | 36 | 66±10 | Clinically indicated | Same morning | — | L (LVEDP, from text) | — | ASE guidelines cutoff data not available | — | 53 | 67 | 42 |
| Manouras et al (2013)48 | ≥40 | 65 | 66±9 | Coronary angiography | Simultaneous | L, S, M (LVEDP, Pre‐A) | L, M (LVEDP, Pre‐A, from text) | — | LVEF not ≥50% | — | 0 | 45 | 42 |
| Weber et al (2013)49 | >50 | 359 | 64±9 | Coronary angiography | Not reported | — | — | S, M (HF, limited data) | AUC ROC only | 20 | 49 | 83 | 24 |
Empty cells are the result of no available data. 2×2 indicates set of true‐positive, false‐positive, false‐negative, and true‐negative values for recommended by American Society of Echocardiography E/è cutoffs; CAD, coronary artery disease; cath lab, catheterization laboratory; CCU, critical care unit; DD, diastolic dysfunction; DM, diabetes mellitus; E/A, the ratio of the early (E) to late (A) ventricular filling velocities; HF, heart failure (clinical diagnosis); HTN, hypertension; ICU, intensive care unit; L, S, and M, lateral, septal, and mean E/è; LVEDP, left ventricular end‐diastolic pressure; LVEF, left ventricular ejection fraction; LVFP, left ventricular filling pressure; LVMDP, left ventricular mean diastolic pressure; N, number of patients; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; pEF, preserved ejection fraction; Pre‐A, left ventricular pre–A wave diastolic pressure; ROC AUC, area under receiver operating characteristic curve.
For supplemental analysis, we included studies that either used a lower LVEF threshold to identify preserved LV systolic function (ie, LVEF ≥40% or 45%) or had no criteria for normal LVEF but the mean and standard deviation for LVEF of the study satisfied the condition that mean minus 2 SDs ≥40%. For a normal distribution, the latter condition assumes that about 98% of participants have LVEF ≥40%. This allowed for inclusion of all clinically relevant studies for secondary analysis since LVEF between 40% to 50% is sometimes used to indicate preserved LVEF.
Clinical DD/HFpEF was described in studies based on composite of clinical signs and symptoms of HF with invasive parameters of DD with preserved LVEF. Some of these studies also included BNP (brain natriuretic peptide) or NT‐proBNP (N‐terminal of the prohormone brain natriuretic peptide) biochemical levels in composite reference definition. No uniform definition was used for clinical diagnosis of DD/HFpEF across these studies.
Elevated LVFP group included 3 patients who had LVEDP >16 mm Hg only after hemodynamic interventions.
We excluded studies where the cohort of pEF patients could include ≥10% of patients with moderate to severe valvular heart disease, hypertrophic or restrictive cardiomyopathy, age <18 years, congenital heart disease, acute coronary syndromes, septic shock, cardiac transplant, atrial fibrillation, or <10 participants. We did not exclude studies that did not explicitly describe the abovementioned conditions in either inclusion/exclusion criteria or baseline patient characteristics. We excluded studies if study reference tests were based only on noninvasive criteria of DD/HFpEF.
Quality Assessment and Data Extraction
The selected studies were analyzed for the risk of bias and applicability concerns by consensus of 2 investigators (O.F.S. and H.G.). We used the Quality Assessment of Diagnostic Accuracy Studies questionnaire9 optimized to our study questions (Appendix S3). Risk of bias was tested for 4 domains: patient selection, index test, reference test, and flow and timing. Applicability was tested for patient selection, index, and reference test domains (Table 3). The questionnaire was expanded to incorporate risks of differential verification on index test accuracy in clinical study (Appendix S3). Index tests were E/èlateral, E/èseptal, or E/èmean. Reference tests were invasive LVFP measurements of LVEDP, LV mean diastolic pressure (LVMDP), LV pre‐A diastolic pressure (Pre‐A), PCWP, or invasively proven DD/HFpEF (Tables 1 and 2).
Table 3.
Summary of QUADAS‐2 Assessment of Selected Studies
| Study (Reference) | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Nagueh et al (1997)13 | Unclear | Low | Low | Low | Low | Low | Low |
| Nagueh et al (1998)14 | Low | Low | Low | Low | High | Low | Low |
| Ommen et al (2000)15 | Low | Low | Low | Low | Low | Low | Low |
| Gonzalez‐Vilchez et al (2002)16 | High | Low | Low | Low | High | Low | Low |
| Poerner et al (2003)17 | Low | Low | Low | Low | Low | High | Low |
| Rivas‐Gotz et al (2003)18 | Low | Low | Low | Low | Unclear | Unclear | Low |
| Dokanish et al (2004)19 | Unclear | Low | Low | Low | High | Unclear | Low |
| Mansencal et al (2004)20 | Low | Low | Low | Low | High | Unclear | Unclear |
| Arques et al (2005)21 | High | Low | High | High | High | Low | Low |
| Bruch et al (2005)22 | Unclear | Unclear | Unclear | Low | High | Low | Low |
| Hadano et al (2005)23 | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Kidawa et al (2005)24 | Low | Low | Low | Low | Low | Unclear | Low |
| Weber et al (2006)25 | Low | Low | Unclear | Unclear | High | Unclear | Low |
| Kasner et al (2007)26 | High | Low | Low | Low | High | Low | Low |
| Min et al (2007)27 | Low | Low | Low | Low | Low | Unclear | Low |
| Poerner et al (2007)28 | Low | Low | Low | Low | Low | High | Low |
| Wang et al (2007)29 | Unclear | Low | Low | Low | Unclear | Low | Low |
| Dokanish et al (2008)30 | Low | Low | Low | Low | Unclear | Unclear | Low |
| Ng et al (2008)31 | Unclear | Low | High | Low | Unclear | Low | Low |
| Rudko et al (2008)32 | Low | Low | Low | Low | High | Unclear | Low |
| Dini et al (2010)33 | Unclear | Low | Unclear | Low | Low | Unclear | Low |
| Dokanish et al (2010)34 | Low | Low | Unclear | Low | Unclear | Unclear | Low |
| Dokanish et al (2010)35 | Low | Low | Unclear | Low | Unclear | Unclear | Low |
| Jaubert et al (2010)36 | Low | Low | Low | Low | Low | Low | Low |
| Kasner et al (2010)37 | High | Low | Low | Low | High | Unclear | Low |
| Penicka et al (2010)38 | High | Unclear | Unclear | Low | Unclear | Unclear | Low |
| Bhella et al (2011)39 | High | Low | Low | Low | High | Unclear | Low |
| Hsiao et al (2011)40 | Unclear | Low | Low | Low | High | Low | Low |
| Kasner et al (2011)41 | Unclear | Low | Low | Low | Unclear | Low | Low |
| Maeder et al (2011)42 | Low | Low | Low | Low | Low | Unclear | Low |
| Özer et al (2011)43 | Low | Low | Low | Low | High | Low | Low |
| Yesildag et al (2011)44 | Unclear | Low | Low | Low | Low | Unclear | Low |
| Hsiao et al (2012)45 | Unclear | Low | Low | Low | Unclear | Low | Low |
| Previtali et al (2012)46 | Unclear | Low | Low | Low | Low | Unclear | Low |
| Arques, 201347 | Unclear | Low | Low | Low | Unclear | Unclear | Low |
| Manouras et al (2013)48 | Low | Low | Low | Low | Unclear | Unclear | Low |
| Weber et al (2013)49 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Low |
| Hajahmadi Poorrafsanjani et al (2014)50 | Low | Low | Low | Low | Unclear | Unclear | Low |
| Tatsumi et al (2014)51 | Unclear | Low | Low | Unclear | Unclear | Unclear | Low |
QUADAS‐2 questionnaire optimized to our study questions is presented below. QUADAS‐2 indicates Quality Assessment of Diagnostic Accuracy Studies 2.
Numerical data were extracted by O.F.S. and confirmed by C.G.S. or H.G. A set was obtained of true‐positive, false‐positive, false‐negative, and true‐negative (2×2) values for E/è cutoffs to identify or exclude elevated LVFP or DD/HFpEF. In some studies (specified in Results), 2×2 values were manually extracted from the scatter plots (LVFP versus E/è). Linear regression coefficient for E/è–LVFP relationship was obtained if provided in the study text. In addition, data for LV relaxation time constant (τ) and LV stiffness parameters invasive reference standards were obtained if provided.
Data Synthesis and Analysis
E/è–LVFP linear regression
To summarize the relationship between E/èlateral, E/èseptal, or E/èmean and invasive LVFP (LVEDP, PCWP, LVMDP, or Pre‐A, separately), we calculated the weighted summary linear regression coefficient (r) by using the continuous random‐effects model.10 We chose a random‐effects model because we anticipate variation in the effect size among our studies because of substantial heterogeneity in the design and patient population among the individual studies. The model can be presented as Yi=μ+εi+ξi, where εi≈N (0, νi 2) and ξi≈N (0, τ2). Yi is effect size (linear regression coefficient), μ is the mean of the underlying effect size distribution, νi is the sampling variance for the effect size (within study variance), and τ2 is the variance of overall effect size distribution (study‐to‐study variance). We used the DerSimonian–Laird method for calculating τ2. In some cases, when τ2=0, the summary effect size was estimated in a fixed‐effect model by using the inverse‐variance method. In the latter method, the summary effect Y is computed as a weighted sum of individual study effects, where the weights are proportional to the inverse of the within‐study variance. Initially, only primary data (Table 1) were analyzed. Then, the secondary analysis was performed with and without supplemental data (Table 2) to evaluate the impact of heterogeneous study designs on the effect size. Possible confounding factors such as the timing of echocardiographic and invasive measurements (simultaneous versus not simultaneous) and the prevalence of specific patient population/disease were independently considered in subgroup analysis. Heterogeneity among the studies was estimated with use of the I 2 statistic.
Diagnostic accuracy of ASE‐recommended E/è cutoffs
For evaluation of diagnostic accuracy of E/è to identify either elevated or normal LVFP, we relied on the ASE‐recommended E/è cutoff values.4 Specifically, E/èlateral >12, E/èmean >13, or E/èseptal >15 indicates elevated LVFP, whereas E/è <8 (any location) indicates normal LVFP. We also used ASE‐recommended threshold values for quantifying elevated LVFP that are LVEDP >16 mm Hg and PCWP >12 mm Hg.4 For LVMDP and Pre‐A, values >12 and >15 mm Hg, respectively, were used because these values were used in individual studies. Sensitivity and specificity with 95% CI for individual studies were computed based on a diagnostic random‐effects model.10 To obtain summary points that take into account within‐study variability and between‐study variability (heterogeneity), we performed hierarchical summary receiver operating characteristic analysis by using the Rutter and Gatsonis model.11 Estimation was carried out via a Bayesian approach, implemented via a Gibbs sampler. Summary point from the hierarchical summary receiver operating characteristic analysis was then used to calculate positive likelihood ratio (LR+). The summary sensitivity and specificity values were also used to calculate the relationship of positive predictive value (PPV) with prevalence for elevated or normal LVFP ranging from 5% to 95%, which was compiled and graphed by using MATLAB R2013b. Separate analyses were performed for E/èlateral, E/èmean, and E/èseptal.
For the primary analysis, we pooled all invasive reference methods for evaluating LVFP (ie, LVEDP, PCWP, Pre‐A, LVMDP) because the number of “primary data” studies (Table 1) was limited. This allowed a pragmatic statistical analysis with direct clinical applicability. Then, we performed the secondary hierarchical summary receiver operating characteristic analysis with and without supplemental data (Table 2) to assess diagnostic accuracy for LVEDP, PCWP, and Pre‐A separately (if ≥3 studies were available) and to assess the effects of the confounding factors mentioned above.
Diagnostic accuracy of optimal E/è cutoffs
Descriptive summary for optimal E/è cutoff values from the receiver operating characteristic (ROC; which are different across the studies) to identify elevated/normal LVFP is also provided as part of secondary analysis. In studies that did not provide the optimal cutoff, we created the ROC curve and identified the optimal cutoff as the point on the ROC curve closest to (0,1 on x‐y coordinate).
Statistical software
OpenMetaAnalyst software12 for Windows (64‐bit version; Microsoft) was used for statistical analysis including graphic presentations of forest plots of sensitivity and specificity and hierarchical summary ROC curves.
Results
Summary of the study selection is presented in Figure 1. Amongst 21 013 unique citations, we reviewed full text of 314 studies; 275 studies (listed in Appendix S4) were excluded with reasons, and 39 studies13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 met our inclusion criteria. Data for the primary analysis were available in 24 studies (Table 1).
Figure 1.

Summary of the literature search. Primary analysis studies include data for patients with LVEF ≥50%. Supplemental analysis studies include data either for patients with LVEF ≥40% and/or with preselected echocardiographic indices (eg, the ratio of the early (E) to late (A) ventricular filling velocities (E/A) <0.9). DD/HFpEF represents invasively proven DD/HFpEF (i.e. clinical diagnosis of DD/HFpEF based on clinical sign and symptoms with evidence of elevated LVFP or impaired LV relaxation/chamber stiffness with or without additional biochemical markers and/or other ancillary tests). DD indicates diastolic dysfunction; HF, heart failure; LVEF, left ventricular ejection fraction; LVFP, left ventricular filling pressure; pEF, preserved ejection fraction.
Quality Assessment
On Quality Assessment of Diagnostic Accuracy Studies analysis for assessment of bias, we found that patient selection domain had high or unclear risk in a substantial number of studies (50% among primary studies, 51% for all selected studies) (Table 3 and Figure 2). The major reasons for such risk were convenience sampling, lack of comprehensive exclusion/inclusion criteria, and/or recruiting patients with specific symptoms or disease. Similarly for evaluating applicability, patient selection domain had a high or unclear risk in a majority of studies (83% among primary studies, 82% for all selected studies). Important reasons for this were lack of patient characteristics (diagnosis, comorbidities), selection of inappropriate control groups, and high (up to 100%) prevalence of certain disease phenotypes (eg, all coronary artery disease or HFpEF). For reference and index test, interobserver and intraobserver variabilities were not described in a substantial number of studies (67% among primary studies, 51% for all selected studies). Overall, only 2 studies (one for primary analysis) had low risk in all aspects of Quality Assessment of Diagnostic Accuracy Studies questionnaire.
Figure 2.
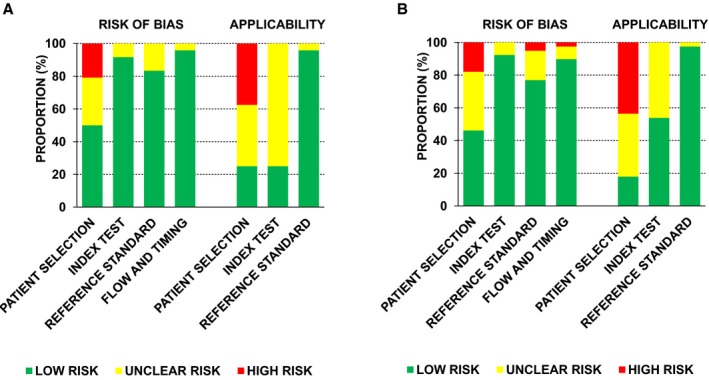
Summary of quality assessment analysis (Quality Assessment of Diagnostic Accuracy Studies [QUADAS 2]). A, QUADAS 2 bar charts for primary analysis studies (n=24). B, QUADAS 2 bar charts for all selected studies (n=39).
Correlation Effect Size for Estimating LVFP
Primary data analysis
Summary estimates of linear regression coefficient (r) with 95% CIs for E/è and LVFP for primary studies are shown in Table 4. All the summary point estimates are <0.5, revealing poor to mediocre correlation.
Table 4.
Correlation (r) Between Invasive Measurements of LVFP and E/è
| E/è | LVFP, mm Hg | ||||
|---|---|---|---|---|---|
| Location | Measure | LVEDP | PCWP | Pre‐A | LVMDP |
| All primary studies | |||||
| Lateral | r (95% CI) Studies Heterogeneity Total patients | 0.44 (0.30–0.57) (N=823, 24, 26, 37, 43, 46, 48, 50); (I 2=61, P=0.013) (n=419) | 0.46 (0.19– 0.73) (N=416, 18, 23, 42); (I 2=79, P=0.003 (n=188) | 0.23 (0.10–0.36) (N=420, 40, 46, 48); (I 2=0, P=0.56) (n=215) | 0.4 (N=115); N/A (n=64) |
| Septal | r (95% CI) Studies Heterogeneity Total patients | 0.28 (0.08–0.49) (N=424, 43, 46, 48); (I 2=54, P=0.09) (n=190) | 0.48 (0.26–0.70) (N=318, 42, 51) (I 2=42, P=0.18) (n=113) | 0.24 (0.09–0.40) (N=340, 46, 48); (I 2=15, P=0.31) (n=195) | 0.47 (0.30–0.64) (N=215, 32); (I 2=0, P=1.00) (n=103) |
| Mean | r (95% CI) Studies Heterogeneity Total patients | 0.38 (0.11–0.65) (N=435, 43, 46, 48); (I 2=81, P=0.001) (n=262) | 0.49 (0.25–0.73) (N=418, 29, 39, 42); (I 2=51, P=0.10) (n=122) | 0.31 (0.07–0.55) (N=530, 34, 40, 46, 48); (I 2=82, P<0.001) (n=349) | 0.45 (N=115); N/A (n=64) |
| Primary studies with simultaneous measurements of echocardiographic and invasive parameters | |||||
| Lateral | r (95% CI) Studies Heterogeneity Total patients | 0.51 (0.36–0.67) (N=324, 26, 48); (I 2=0, P=0.40) (n=143) | 0.7 (0.51–0.89) (N=118); N/A (n=55) | 0.4 (0.19–0.61) (N=148); N/A (n=38) | 0.4 (N=115); N/A (n=64) |
| Septal | r (95% CI) Studies Heterogeneity Total patients | 0.18 (−0.08 to 0.43) (N=224, 48); (I 2=31, P=0.23) (n=88) | 0.55 (0.32–0.78) (N=118) N/A (n=55) | 0.02 (−0.30 to 0.34) (N=148); N/A (n=38) | 0.47 (0.30–0.64) (N=215, 32); (I 2=0, P=1.00) (n=103) |
| Mean | r (95% CI) Studies Heterogeneity Total patients | 0.18 (−0.14 to 0.50) (N=148); N/A (n=38) | 0.6 (0.42–0.78) (N=318, 29, 39); (I 2=0, P=0.91) (n=86) | 0.21 (−0.11 to 0.53) (N=148); N/A (n=38) | 0.45 (N=115); N/A (n=64) |
Heterogeneity among the studies was estimated by using the I 2 statistic, and the corresponding P values are provided. N indicates number of studies; n, total patients N/A, not applicable.
Secondary data analysis
In Table 4, we also present summary estimates of linear regression coefficients in studies where invasive and echocardiographic measurements were performed simultaneously. As apparent, in only a few studies were simultaneous measurements performed for each individual LVFP parameter. The results remain consistent with the primary analysis with no obvious trends. While in some studies the r estimates were somewhat higher, in others they were lower compared with the entire primary combined data set (Table 4). Increased r values were mainly observed in studies that measured PCWP. However, these data cannot be applied to the general patient cohort because 2 of the 3 studies were conducted in the intensive care unit setting.18, 29 These 2 studies did not provide detailed patient characteristics. The third study had an extremely small sample size.39 On secondary analysis with an additional 6 studies from Table 2, the summary estimates remained similar (Appendix S5). Because of the limited number of studies that specified patient characteristics for the pEF cohort, no conclusive effect of prevalence of HFpEF, coronary artery disease, hypertension, and diabetes mellitus on r estimate was found (Appendix S5).
Diagnostic Accuracy of E/è for Identification of Elevated LVFP
Primary data analysis
The summary data for the diagnostic accuracy of E/è to identify elevated LVFP demonstrated wide CIs for sensitivity and specificity (Figure 3A, 3D, and 3G). There was significant heterogeneity for E/èlateral specificity and E/èmean sensitivity measurements. The summary sensitivity and specificity for E/èlateral (n=6), E/èmean (n=6), and E/èseptal (n=4) were 30% and 92%, 37% and 91%, and 24% and 98%, respectively (Figure 3B, 3E, and 3H). Only E/èseptal had sufficiently high LR+ (slightly above 10) in identifying elevated LVFP, whereas LR+ for E/èlateral and E/èmean was low (3.8 and 4.1, respectively).
Figure 3.
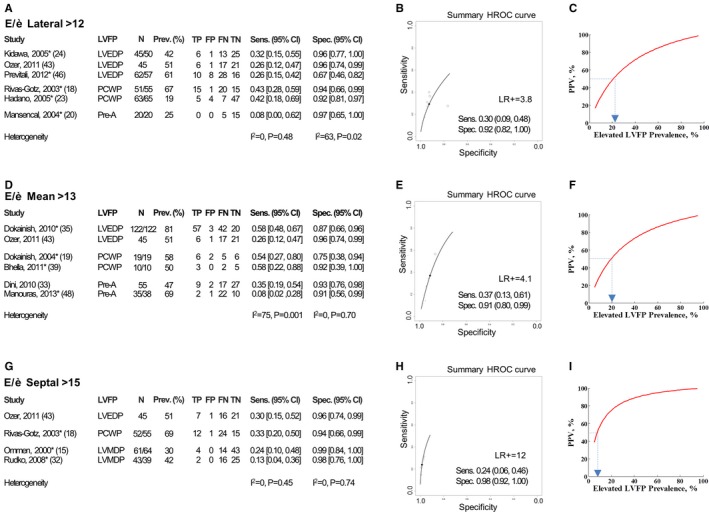
Diagnostic accuracy of E/è recommended by the American Society of Echocardiography (ASE) to identify elevated left ventricular filling pressure (LVFP). A through C, Analysis for E/èlateral (>12). A, Individual studies (reference number as listed in the main text is indicated in brackets) with corresponding LVFP measurements, sample size, elevated LVFP prevalence, diagnostic 2×2 data (true positive [TP], false positive [FP], false negative [FN], true negative [TN]), and corresponding values of sensitivity (Sens.) and specificity (Spec.) with 95% CI are described. Summary heterogeneity is described by I 2 statistic. B, The Rutter and Gatsonis11 hierarchical summary receiver operating characteristic (HSROC) analysis for recommended E/è cutoff to identify elevated LVFP is depicted. Summary sensitivity, summary specificity with 95% CI, and corresponding positive likelihood ratio (LR+) are depicted. C, Positive predictive value (PPV)–prevalence relationship for E/è to identify elevated LVFP using summary sensitivity and specificity HSROC points. Prevalence of elevated LVFP corresponding to 50% PPV for E/è is highlighted. *TP, FP, FN, TN values were extracted from the graphical data representation of LVFP vs E/è in study results; for such study, column presenting patient number (N) include 2 numbers: first number is actual counted patients in the plot, and second number is total patients in the study group. D through F, Analysis for E/èmean (>13). G through I, Analysis for E/èseptal (>15). Other description is same as for A through C.
Secondary data analysis
Because of a limited number of studies and limited data on patient characteristics, we could not identify the reasons for heterogeneity found for sensitivity, specificity, and LR+ estimates in individual studies (Appendix S6). Among studies for E/èseptal assessment, the highest individual LR+ was in Ommen et al's (2000) study15 (LR+=24), whereas LR+ values from all other E/èseptal studies were <8. For E/èseptal, 3 of the 4 studies15, 18, 32 performed simultaneous echocardiography and invasive measurements. Analysis of these 3 studies did not alter our results. For E/èlateral, only 2 of 6 studies,18, 24 and for E/èmean, only 1 of 6 studies19 reported simultaneous measurements of echocardiographic and invasive parameters. Secondary analysis with additional studies (identified in the Table 2) did not alter summary sensitivity, specificity, or LR+ (Appendix S6). Additional secondary subgroup analysis restricted to LVEDP, PCWP, or Pre‐A reference tests individually did not alter the LR+ of E/è (Appendix S6). The optimal E/è cutoffs from ROC to identify elevated LVFP in individual studies (Appendix S7) demonstrated a wide range (E/èlateral 6.6–10, E/èmean 8–15, and E/èseptal 9–13).
Diagnostic Accuracy of E/è for Identification of Normal LVFP
Primary data analysis
For the diagnostic accuracy of E/è to identify normal LVFP, we found significant heterogeneity in either the sensitivity or the specificity estimates among the studies for E/èlateral and E/èmean and not for E/èseptal (Figure 4A, 4D, and 4G).The summary sensitivity and specificity for E/èlateral (n=5) were 64% and 73%, respectively; for E/èmean (n=4), they were 36% and 83%; and for E/èseptal (n=3), they were 50% and 89%. The corresponding LR+ was 2.4, 2.1, and 4.5, respectively (Figure 4B, 4E, and 4H).
Figure 4.

Diagnostic accuracy of E/è recommended by the American Society of Echocardiography to identify normal left ventricular filling pressure (LVFP). A through C, Analysis for E/èlateral (<8). A, Individual studies (reference number as listed in the main text is indicated in brackets) with corresponding LVFP measurements, sample size, normal LVFP prevalence, diagnostic 2×2 data, and corresponding values of sensitivity and specificity with 95% CIs are described. Summary heterogeneity is described by I 2 statistic. B, Hierarchical summary receiver operating characteristic (HSROC) analysis for recommended E/è cutoff to identify normal LVFP is depicted. Summary sensitivity, summary specificity with 95% CI, and corresponding positive likelihood ratio (LR+) are depicted. C, Positive predictive value (PPV)–prevalence relationship for E/è to identify normal LVFP using summary sensitivity and specificity HSROC points. Prevalence of normal LVFP corresponding to 50% PPV for E/è is highlighted. *Same as in Figure 3. D through F, Analysis for E/èmean (<8). G through I, Analysis for E/èseptal (<8). Other description is same as for A through C.
Secondary data analysis
None of the secondary analyses improved the summary sensitivity and specificity (Appendix S8).
Clinical Context of the Findings Described Earlier
We evaluated the relationship of elevated LVFP prevalence (5–95%) and PPV of E/è (Figure 3C, 3F, and 3I). We find that for disease prevalence of ≈≤20%, the PPV to identify elevated LVFP using E/èlateral and E/èmean was <50% indicating that false positives are greater than the true positives. For E/èseptal this occurs for disease prevalence of 10% and below. Similarly we performed prevalence‐PPV relationship analysis to identify normal LVFP (Figure 4C, 4F, and 4I). We find that for normal LVFP prevalence ≤30%, the PPV to identify normal LVFP is <50% for E/èlateral and E/èmean. For E/èseptal, PPV falls to <50% for normal LVFP prevalence ≤20%.
Diagnostic Accuracy of E/è for Identification of DD/HFpEF
Only 1 study38 provided data for creating 2×2 tables for the ASE‐recommended E/è cutoff values. Therefore, we provide descriptive summary (Table 5). All studies, except 1,49 used case‐control design. In Weber et al's (2013) study,49 we find that the area under the ROC curve for the diagnostic accuracy of E/è was lower with mixed patient groups (definite HFpEF, possible HFpEF, and no HFpEF) compared with when patient group with possible HFpEF was removed from the analysis (Table 5). Different clinical criteria were used across the studies for diagnosing HFpEF, which limits the use of these data for the purpose of identifying DD/HFpEF by using E/è.
Table 5.
Identification of HFpEF/LVDD Based on E/è
| Study | Reference Test to Define HFpEF/DD | Control Patients | ROC AUC (95% CI) | E/è Cutoff | Sensitivity (95% CI)/Specificity (95% CI) | Comparison |
|---|---|---|---|---|---|---|
| Patients with HF symptoms (dyspnea) | ||||||
| Mean E/è | ||||||
| Penicka et al (2010)38 (N=30) | NYHA functional class II/III dyspnea and LVEDP >16 mm Hg at baseline or after hemodynamic interventions (N=20) | NYHA functional class II/III dyspnea and LVEDP <16 mm Hg at baseline or after hemodynamic interventions (N=10) | — | >13a | 30 (14–53)/90 (54–99) | HFpEF vs controls |
| Weber et al (2013)49 (N=359) | Definite HFpEF: dyspnea on exertion and LVEDP >16 mm Hg and NT‐proBNP >220 pg/mL (N=71) | Possible HFpEF: dyspnea on exertion not meeting criteria for definite HFpEF (N=223); no HFpEF: (dyspnea on exertion but LVEDP ≤12 mm Hg and NT‐proBNP ≤120 pg/mL (N=65) | 0.62 (0.57–0.67) | — | — | Definite HFpEF vs controls (combined possible HFpEF and no HFpEF) |
| Weber et al (2013)49 (N=136, of 359) | Definite HFpEF: see above (N=71) | No HFpEF: see above (N=65) | 0.8 (0.72–0.87) | — | — | Definite HFpEF vs no HFpEF (possible HFpEF N=223, were not included in this analysis) |
| Lateral E/è | ||||||
| Penicka et al (2010)38 (N=30) | NYHA functional class II/III dyspnea and LVEDP >16 mm Hg at baseline or after hemodynamic interventions (N=20) | NYHA functional class II/III dyspnea and LVEDP <16 mm Hg at baseline or after hemodynamic interventions (N=10) | — | >12a | 40 (21–62)/80 (46–95) | HFpEF vs controls |
| Arques et al (2005)21 (N=38) | Clinical criteria for CHF and LVEDP ≥15 mm Hg (N=18) | Acute dyspnea the result of noncardiac cause, BNP <150 pg/mL, not meeting Framingham criteria for CHF (no invasive confirmation performed) (N=20) | 0.92 (0.84–1.0) | >11b | 78 (53–90)/100 (71–100) | HFpEF vs controls |
| Septal E/è | ||||||
| Penicka et al (2010)38 (N=30) | NYHA class II/III dyspnea and LVEDP >16 mm Hg at baseline or after hemodynamic interventions (N=20) | NYHA class II/III dyspnea and LVEDP <16 mm Hg at baseline or after hemodynamic interventions (N=10) | — | >15a | 21 (9–44)/96 (55–100) | HFpEF vs controls |
| Weber et al (2006)25 (N=58, of 235) | Definite HFpEF: dyspnea and LVEDP >16 mm Hg and NT‐proBNP >125 pg/mL (N=29) | Dyspnea and LVEDP ≤16 mm Hg and NT‐proBNP ≤125 pg/mL (N=29) | 0.75 (0.62–0.86) | — | — | HFpEF vs controls |
| Weber et al (2013)49 (N=359) | Definite HFpEF: dyspnea on exertion, LVEDP >16 mm Hg, NT‐proBNP >220 pg/mL (N=71) | Possible HFpEF: dyspnea on exertion not meeting criteria for definite HFpEF (N=223): no HFpEF: dyspnea on exertion but LVEDP ≤12 mm Hg and NT‐proBNP ≤120 pg/mL (N=65) | 0.75 (0.70–0.79) | — | — | Definite HFpEF vs controls (combined possible HFpEF and no HFpEF) |
| Weber et al (2013)49 (N=136, of 359) | Definite HFpEF (N=71): see above | No HFpEF (N=65): see above | 0.82 (0.75–0.88) | — | — | Definite HFpEF vs no HFpEF (possible HFpEF N=223 were not included in this analysis) |
| HFpEF patients vs no HF symptoms patients | ||||||
| Lateral E/è | ||||||
| Kasner et al (2007)26 (N=55) | Dyspnea, exercise intolerance, τ >48 ms, and LVEDP ≥12 mm Hg, and/or β >0.015 mL−1 and/or b >0.19 mm Hg/mL (N=43) | Chest discomfort, no HF symptoms, no significant heart disease (N=12) | 0.91 (–) | >8b | 83 (70–92)/92 (59–99) | HFpEF vs controls |
| Kasner et al (2010)37 (N=33) | Dyspnea, exercise intolerance, and LVEDP ≥16 mm Hg τ >48 ms and, and/or β >0.015 mL−1 and/or b >0.19 mm Hg/mL (N=21) | Chest discomfort, no HF symptoms, no significant heart disease (N=12) | 0.83 (–) | — | — | HFpEF vs controls |
| Kasner et al (2011)41 (N=180) | Dyspnea, exercise intolerance, and LVEDP ≥12 mm Hg or τ ≥48 ms or dP/dtmin ≥−1100 mm Hg/s or PCWP >12 mm Hg or PCWP‐at‐stress >20 mm Hg (N=107) | Chest discomfort, no HF symptoms, no significant heart disease (N=73) | 0.86 (–) | — | — | HFpEF vs controls |
| DD patients vs no DD patients | ||||||
| Septal E/è | ||||||
| Weber et al (2006)25 (N=126, of 235) | Definite DD: LVEDP>16 mm Hg and NT‐proBNP > 125 pg/mL (N=44) | LVEDP ≤16 mm Hg and NT‐proBNP ≤125 pg/mL (N=82) | 0.78 (0.69–0.87) | >11.2b | 73 (58–84)/73 (63–82) | Definite DD vs controls (possible DD N=109 (LVEDP >16 mm Hg or NT‐proBNP >125 pg/mL) were not included) |
Empty cells are the result of no available data. CHF indicates congestive heart failure; dP/dtmin, the minimum rate of pressure change in the left ventricle; HF, heart failure; DD, diastolic dysfunction; LVEDP, left ventricular end‐diastolic pressure; N, number of patients; NT‐proBNP, the N‐terminal of the prohormone brain natriuretic peptide; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; pEF, preserved ejection fraction; ROC AUC, area under receiver operating characteristic curve.
American Society of Echocardiography–recommended cutoff values.
Optimal cutoff values.
LV Relaxation Time Constant (τ) and LV Chamber Stiffness
Only 1 study26 reported a relationship between E/èlateral and τ. Low correlation of E/èlateral with τ (r±SE 0.34±0.13) was found.26 Two studies26, 37 from the same group of investigators assessed the relationship of E/èlateral with LV chamber stiffness. There was a moderate correlation of E/èlateral with LV stiffness parameters in both studies26, 37 (average r±SD 0.59±0.08 for β [mL−1] and 0.54±0.11 for b [mm Hg/mL]). These 2 studies had a high risk of bias and applicability for patient selection domain on the Quality Assessment of Diagnostic Accuracy Studies analysis (Table 3).
Discussion
To best of our knowledge, this is the first comprehensive meta‐analysis of the diagnostic accuracy of tissue Doppler echocardiographic index, E/è, to estimate LVFP in patient cohorts with pEF and for the identification of DD/HFpEF. We find that the evidence for the use of E/è for routine clinical practice in estimating LVFP in pEF is limited. Further, because of multiplicity of study designs, no conclusive inference can be drawn for the use of E/è in diagnosing DD/HFpEF in routine clinical applications.
Based on ASE and ESC guidelines,4, 5 E/è is an important noninvasive parameter with which to evaluate diastolic function. From a clinical perspective, this application of E/è can be grouped into 2 important concepts: (1) estimation of LVFP—this includes either semiquantitative estimate of LVFP (ie, normal versus elevated) and/or direct quantitative estimate of LVFP, and (2) clinical diagnosis of DD/HFpEF. Extensive use of E/è as a noninvasive surrogate for LVFP and diastolic function has been observed in recent clinical research in cardiac and multidisciplinary fields.6, 7, 8, 52, 53, 54 The poor‐to‐mediocre correlation of E/è to LVFP indicates that E/è alone may not be reliable to estimate LVFP. Therefore, its use for directly estimating LVFP is not recommended until well validated in robust clinical studies.
For the semiquantitative estimate of LVFP, we find that (1) the summary specificity of the ASE‐recommended E/è for identifying elevated LVFP is high but the summary sensitivity is very low. Except for E/èseptal, the resulting LR+ is also low. (2) For identifying normal LVFP, the LR+ of E/è is low for all locations. To provide a practical framework for interpreting/use of these findings in the clinical practice, we describe 3 representative scenarios based on E/èseptal that demonstrates the best LR+ for identifying elevated LVFP. These scenarios are based on PPV–patient prevalence relationship obtained in our study results (Figures 3I and 4I).
Scenario 1: Low Prevalence of Elevated LVFP
Here, E/èseptal <8 generally suggests normal LVFP, while E/è >15 may be misleading (Figure 5A). However, the E/èseptal <8 value is found only in a small subset with a large number of patients in indeterminate zone. This is consistent with previous reported observations of E/è that has a broad range of values in healthy people with evidence of increasing E/è with age.55, 56 In our analyzed cohorts, the majority of whom underwent clinically recommended cardiac catheterization, only 36% to 64% with normal LVFP were noted to have E/è <8 (Figure 4). In the community setting, disease prevalence of confirmed HFpEF is low (≈1%) in adults >45 years old, whereas moderate to severe DD with pEF is estimated at <6%.1 Therefore, routine use of estimating E/è to evaluate elevated LVFP or DD in ambulatory clinical situations requires further testing and validation.
Figure 5.
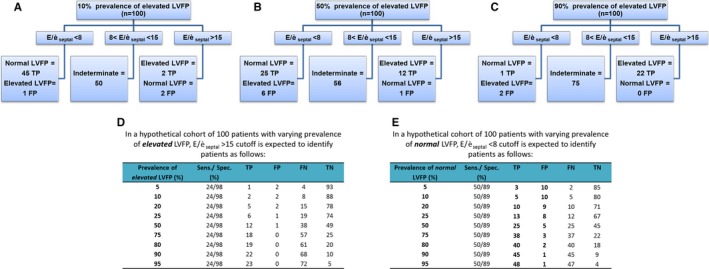
Estimates for use of American Society of Echocardiography–recommended E/èseptal cutoffs in patient group with varying prevalences of elevated left ventricular filling pressure (LVFP). A through C, Summary outline of application of E/èseptal for evaluating elevated and normal LVFP in representative examples with disease prevalence of elevated LVFP set at 10%, 50%, and 90% for n=100. More than half of the patients are in the indeterminate zone regardless of the disease prevalence. In the low‐prevalence (A, 10%) scenario, abnormal E/è is noted in a few patients and can be misleading in a substantial number of these patients. Normal E/è value is suggestive of normal LVFP in the majority of patients. For intermediate prevalence (B, 50%), abnormal E/è is suggestive of elevated LVFP, while normal E/è value is suggestive of normal LVFP in the majority of patients. In the high‐prevalence (C, 90%) scenario, even more patients are found in the indeterminate zone. Abnormal E/è is suggestive of elevated LVFP in most instances. Normal E/è is misleading in most cases here and cannot be used to rule out elevated LVFP. D and E, Estimated 2×2 distributions of patients after application of E/èseptal cutoffs with hierarchical summary receiver operating characteristic–calculated summary sensitivity and specificity for evaluating elevated (D) or normal (E) LVFP (Figures 3 and 4).
Scenario 2: High Prevalence of Elevated LVFP
Here E/èseptal >15 generally suggests elevated LVFP, while E/èseptal <8 may be misleading (Figure 5C). Further, this abnormal E/è finding will be present only in a small subset of patients with elevated LVFP. Based on our analysis, we find that only 24% to 44% with elevated LVFP have increased E/è (Figure 3) and, importantly, 10% to 26% patients with elevated LVFP have normal E/è <8 (Figure 4). Similar observations have been made in the clinical trials of proven HFpEF where ≈30% have no echocardiographic evidence of DD.57
Scenario 3: Intermediate Prevalence of Elevated LVFP
Here E/èseptal values >15 or <8 may correctly identify about one‐third of total patients with either elevated or normal LVFP. More than half would remain in the indeterminate zone, and some would be falsely classified (Figure 5B).
Significance of Intermediate E/è Zone
According to ASE and ESC recommendations, additional echocardiographic indices should be used to estimate LVFP when E/è falls into intermediate zone (8< E/è <12–15).4, 5 Thus, based on our analysis, more than half of the patients (eg, 50–75% for E/èseptal; Figure 5) would require additional indices. ASE guidelines state that “an Ar–A duration ≥30 ms, a change of E/A ratio with the Valsalva maneuver of ≥0.5, IVRT/TE‐è <2, PA systolic pressure ≥35 mm (in the absence of pulmonary disease), and maximal LA volume ≥34 mL/m2 are all indicative of increased LV filling pressures”.4 In the current work, we did not analyze incremental power of other echocardiographic indices that might favor the diagnostic accuracy of E/è. Because of the complexity of the topic, such analysis would require separate studies. We should, however, point out that by using broad search terminology in our search terms, we found very few studies27, 33, 34, 35, 43 that addressed this aspect in their studies. Most of them have similar study limitations as just given here (mostly with low patient cohort and heterogeneous design). Based on our study results that demonstrate the uncertainty of using the proposed E/è cutoffs, we suggest that the additional echocardiographic parameters described here (and probably others) should be evaluated across the range of E/è values and not restricted to the intermediate zone to define an optimal approach to evaluate LVFP and identify HFpEF in prospective studies. Recently, 1 such clinical trial has been proposed.58
Application of E/è in DD/HFpEF
Invasive characterization of DD relies on LV end‐diastolic pressure–volume relationship. A single measure of LVFP (eg, LVEDP) does not comprehensively characterize the diastolic properties. LV chamber stiffness is an accepted invasive method to accurately characterize LV diastolic function. Therefore, one can suggest that for accurate diagnostics of DD by E/è, there should also be a relationship between E/è and LV chamber stiffness. Based on our results, the relationship of E/è to LV chamber stiffness is not well established. We found only 2 studies26, 37 that directly compared E/è with LV chamber stiffness. These studies have significant limitations for wider applicability. Therefore, more clinical research is needed before E/è can be recommended as a surrogate for LV chamber stiffness.
The current consensus for the diagnosis of HFpEF relies on the clinical presentation and exclusion of other etiologies. In clinical practice, echocardiography is frequently used to evaluate HFpEF. There are only a small number of studies that have compared the diagnostic accuracy of E/è to evaluate clinical DD/HFpEF. These studies have significant methodological limitations such as lack of uniform reference definition and case‐control design. On area under the ROC curve analysis, although the E/è values appear to be moderate to high in identifying DD/HFpEF, the clinical applicability is again limited. Based on our analysis, no uniform/summary cutoff E/è value can be recommended.
Limitations
We performed analysis based on the published data sets and did not have access to the primary data source. Some studies did not provide the quantitative data set required for our analysis, which, in some instances, was because of a lack of statistically meaningful relationships. This suggests that our results are affected to some extent by the publication bias. Formal assessment of publication bias was not performed as the number of studies is inadequate in individual groups. Despite our best efforts, in some studies we find that the data points extracted from the graphs did not match the original sample size (Figures 3 and 4). This discordance is of small magnitude and could be the result of an inaccurate number of plotted data points and/or superimposed data points. In many studies, the timing of echocardiographic and invasive measurements was not simultaneous. It has been shown that the LVFP can rapidly change based on underlying hemodynamic status.59 Because all measurements were performed at the rest, mostly on the same day if not simultaneously, the hemodynamic conditions are expected to be similar. Overall, we find that our study results remained similar regardless of the timing of invasive and echocardiographic measurements.
We grouped studies that were not identical in study design and methods as using strict criteria would have excluded a large number of studies from the analysis and therefore made any analysis pointless. For similar reasons, including a lack of comparable and comprehensive data sets, we were unable to perform statistically meaningful subgroup analysis to evaluate possible causes for heterogeneity in correlation coefficients, sensitivity, and specificity among the studies. We have provided all the raw data that we have extracted from the selected studies that could be used in future meta‐analysis or in preparation of specific projects (eg, for assessment of a sample size).
Conclusions
There is insufficient evidence to support that E/è can reliably estimate LVFP in patients with preserved LVEF. The diagnostic accuracy of E/è to predict LVFP and evaluate DD/HFpEF is limited. Its routine clinical use requires rigorous evaluation in well‐designed prospective multicenter studies.
Sources of Funding
This work was supported by National Institutes of Health grant NHLBI R01‐HL104018 (Dr Gupta). The funding organizations did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Appendix S1. Study protocol.
Appendix S2. Data sources and search strategy.
Appendix S3. QUADAS‐2 questionnaire.
Appendix S4. Excluded full‐text studies.
Appendix S5. Secondary analysis of E/è correlation with LVFP.
Appendix S6. Secondary analysis of sensitivity/specificity of E/è cutoffs to predict elevated LVFP.
Appendix S7. Optimal cutoffs and AUC for elevated LVFP.
Appendix S8. Secondary analysis of sensitivity/specificity of E/è cutoffs to predict normal LVFP.
Acknowledgments
We thank Lee A. Vucovich, MLS, MS, AHIP, Assistant Director for Reference Services, UAB Lister Hill Library, for her valuable assistance with search strategy.
(J Am Heart Assoc. 2016;5:e002530 doi: 10.1161/JAHA.115.002530)
Accompanying Appendices S1 through S8 are available at http://jaha.ahajournals.org/content/5/1/e002530/suppl/DC1
References
- 1. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 2. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 3. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 6. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen H‐D, Tschöpe C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 7. Kim Y‐H, Kim SH, Lim SY, Cho G‐Y, Baik I‐K, Lim H‐E, Na JO, Han SW, Ko Y‐H, Shin C. Relationship between depression and subclinical left ventricular changes in the general population. Heart. 2012;98:1378–1383. [DOI] [PubMed] [Google Scholar]
- 8. Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL, Redfield MM. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 10. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 11. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–2884. [DOI] [PubMed] [Google Scholar]
- 12. Wallace B, Dahabreh I, Trikalinos T, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end‐users: R as a computational back‐end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 13. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. [DOI] [PubMed] [Google Scholar]
- 14. Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quiñones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation. 1998;98:1644–1650. [DOI] [PubMed] [Google Scholar]
- 15. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler‐catheterization study. Circulation. 2000;102:1788–1794. [DOI] [PubMed] [Google Scholar]
- 16. González‐Vilchez F, Ayuela J, Ares M, Mata NS, González AG, Durán RM. Comparison of Doppler echocardiography, color M‐mode Doppler, and Doppler tissue imaging for the estimation of pulmonary capillary wedge pressure. J Am Soc Echocardiogr. 2002;15:1245–1250. [DOI] [PubMed] [Google Scholar]
- 17. Poerner TC, Goebel B, Unglaub P, Sueselbeck T, Strotmann JM, Pfleger S, Borggrefe M, Haase KK. Detection of a pseudonormal mitral inflow pattern: an echocardiographic and tissue Doppler study. Echocardiography. 2003;20:345–356. [DOI] [PubMed] [Google Scholar]
- 18. Rivas‐Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–784. [DOI] [PubMed] [Google Scholar]
- 19. Dokainish H, Zoghbi WA, Lakkis NM, Al‐Bakshy F, Dhir M, Quinones MA, Nagueh SF. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B‐type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–2439. [DOI] [PubMed] [Google Scholar]
- 20. Mansencal N, Bouvier E, Joseph T, Farcot J‐C, Pillière R, Redheuil A, Lacombe P, Jondeau G, Dubourg O. Value of tissue Doppler imaging to predict left ventricular filling pressure in patients with coronary artery disease. Echocardiography. 2004;21:133–138. [DOI] [PubMed] [Google Scholar]
- 21. Arques S, Roux E, Sbragia P, Gelisse R, Ambrosi P, Pieri B, Luccioni R. Comparative accuracy of color M‐mode and tissue Doppler echocardiography in the emergency diagnosis of congestive heart failure in chronic hypertensive patients with normal left ventricular ejection fraction. Am J Cardiol. 2005;96:1456–1459. [DOI] [PubMed] [Google Scholar]
- 22. Bruch C, Grude M, Müller J, Breithardt G, Wichter T. Usefulness of tissue Doppler imaging for estimation of left ventricular filling pressures in patients with systolic and diastolic heart failure. Am J Cardiol. 2005;95:892–895. [DOI] [PubMed] [Google Scholar]
- 23. Hadano Y, Murata K, Liu J, Oyama R, Harada N, Okuda S, Hamada Y, Tanaka N, Matsuzaki M. Can transthoracic Doppler echocardiography predict the discrepancy between left ventricular end‐diastolic pressure and mean pulmonary capillary wedge pressure in patients with heart failure? Circ J. 2005;69:432–438. [DOI] [PubMed] [Google Scholar]
- 24. Kidawa M, Coignard L, Drobinski G, Krzeminska‐Pakula M, Thomas D, Komajda M, Isnard R. Comparative value of tissue Doppler imaging and M‐mode color Doppler mitral flow propagation velocity for the evaluation of left ventricular filling pressure. Chest. 2005;128:2544–2550. [DOI] [PubMed] [Google Scholar]
- 25. Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss H‐P, Pauschinger M, Tschöpe C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler‐conductance catheterization study. Circulation. 2007;116:637–647. [DOI] [PubMed] [Google Scholar]
- 27. Min P‐K, Ha J‐W, Jung J‐H, Choi E‐Y, Choi D, Rim S‐J, Jang Y, Shim W‐H, Cho S‐Y, Chung N. Incremental value of measuring the time difference between onset of mitral inflow and onset of early diastolic mitral annulus velocity for the evaluation of left ventricular diastolic pressures in patients with normal systolic function and an indeterminat. Am J Cardiol. 2007;100:326–330. [DOI] [PubMed] [Google Scholar]
- 28. Poerner TC, Goebel B, Kralev S, Kaden JJ, Süselbeck T, Haase KK, Borggrefe M, Haghi D. Impact of mitral E/A ratio on the accuracy of different echocardiographic indices to estimate left ventricular end‐diastolic pressure. Ultrasound Med Biol. 2007;33:699–707. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Khoury DS, Thohan V, Torre‐Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. [DOI] [PubMed] [Google Scholar]
- 30. Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–1509. [DOI] [PubMed] [Google Scholar]
- 31. Ng ACT, Tran DT, Newman M, Allman C, Vidaic J, Kadappu KK, Boyd A, Thomas L, Leung DY. Comparison of myocardial tissue velocities measured by two‐dimensional speckle tracking and tissue Doppler imaging. Am J Cardiol. 2008;102:784–789. [DOI] [PubMed] [Google Scholar]
- 32. Rudko R, Przewlocki T, Pasowicz M, Biernacka B, Kablak‐Ziembicka A, Tracz W. IVRT'/IVRT index is a useful tool for detection of elevated left ventricular filling pressure in patients with preserved ejection fraction. Echocardiography. 2008;25:473–481. [DOI] [PubMed] [Google Scholar]
- 33. Dini FL, Ballo P, Badano L, Barbier P, Chella P, Conti U, De Tommasi SM, Galderisi M, Ghio S, Magagnini E, Pieroni A, Rossi A, Rusconi C, Temporelli PL. Validation of an echo‐Doppler decision model to predict left ventricular filling pressure in patients with heart failure independently of ejection fraction. Eur J Echocardiogr. 2010;11:703–710. [DOI] [PubMed] [Google Scholar]
- 34. Dokainish H, Nguyen JS, Sengupta R, Pillai M, Alam M, Bobek J, Lakkis N. Do additional echocardiographic variables increase the accuracy of E/è for predicting left ventricular filling pressure in normal ejection fraction? An echocardiographic and invasive hemodynamic study. J Am Soc Echocardiogr. 2010;23:156–161. [DOI] [PubMed] [Google Scholar]
- 35. Dokainish H, Nguyen J, Sengupta R, Pillai M, Alam M, Bobek J, Lakkis N. New, simple echocardiographic indexes for the estimation of filling pressure in patients with cardiac disease and preserved left ventricular ejection fraction. Echocardiography. 2010;27:946–953. [DOI] [PubMed] [Google Scholar]
- 36. Jaubert M‐P, Armero S, Bonello L, Nicoud A, Sbragia P, Paganelli F, Arques S. Predictors of B‐type natriuretic peptide and left atrial volume index in patients with preserved left ventricular systolic function: an echocardiographic‐catheterization study. Arch Cardiovasc Dis. 2010;103:3–9. [DOI] [PubMed] [Google Scholar]
- 37. Kasner M, Gaub R, Sinning D, Westermann D, Steendijk P, Hoffmann W, Schultheiss H‐P, Tschöpe C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure‐volume loop analysis. Eur J Echocardiogr. 2010;11:743–751. [DOI] [PubMed] [Google Scholar]
- 38. Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure‐volume loop analysis. J Am Coll Cardiol. 2010;55:1701–1710. [DOI] [PubMed] [Google Scholar]
- 39. Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams‐Huet B, Thomas JD, Grayburn PA, Levine BD. Echocardiographic indices do not reliably track changes in left‐sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2011;4:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsiao S‐H, Chiou K‐R, Lin K‐L, Lin S‐K, Huang W‐C, Kuo F‐Y, Cheng C‐C, Liu C‐P. Left atrial distensibility and E/è for estimating left ventricular filling pressure in patients with stable angina. ‐A comparative echocardiography and catheterization study‐. Circ J. 2011;75:1942–1950. [DOI] [PubMed] [Google Scholar]
- 41. Kasner M, Gaub R, Westermann D, Kaplan H, Akpulat S, Steendijk P, Schultheiss H‐P, Tschöpe C. Simultaneous estimation of NT‐proBNP on top to mitral flow Doppler echocardiography as an accurate strategy to diagnose diastolic dysfunction in HFNEF. Int J Cardiol. 2011;149:23–29. [DOI] [PubMed] [Google Scholar]
- 42. Maeder MT, Karapanagiotidis S, Dewar EM, Gamboni SE, Htun N, Kaye DM. Accuracy of Doppler echocardiography to estimate key hemodynamic variables in subjects with normal left ventricular ejection fraction. J Card Fail. 2011;17:405–412. [DOI] [PubMed] [Google Scholar]
- 43. Ozer N, Okutucu S, Kepez A, Aksoy H, Deveci OS, Atalar E, Ovünç K, Aksöyek S. Diagnostic accuracy and clinical utility of echocardiographic indices for detecting left ventricular diastolic dysfunction in patients with coronary artery disease and normal ejection fraction. Anadolu Kardiyol Derg. 2011;11:666–673. [DOI] [PubMed] [Google Scholar]
- 44. Yesildag O, Koprulu D, Yuksel S, Soylu K, Ozben B. Noninvasive assessment of left ventricular end‐diastolic pressure with tissue Doppler imaging in patients with mitral regurgitation. Echocardiography. 2011;28:633–640. [DOI] [PubMed] [Google Scholar]
- 45. Hsiao S‐H, Lin K‐L, Chiou K‐R. Comparison of left atrial volume parameters in detecting left ventricular diastolic dysfunction versus tissue Doppler recordings. Am J Cardiol. 2012;109:748–755. [DOI] [PubMed] [Google Scholar]
- 46. Previtali M, Chieffo E, Ferrario M, Klersy C. Is mitral E/è ratio a reliable predictor of left ventricular diastolic pressures in patients without heart failure? Eur Heart J Cardiovasc Imaging. 2012;13:588–595. [DOI] [PubMed] [Google Scholar]
- 47. Arques S. Shortened mitral A wave deceleration time: an under‐recognized but promising Doppler‐derived index of left ventricular myocardial dysfunction in patients with normal left ventricular ejection fraction. Int J Cardiol. 2013;167:3076–3077. [DOI] [PubMed] [Google Scholar]
- 48. Manouras A, Nyktari E, Sahlén A, Winter R, Vardas P, Brodin L‐Å. The value of E/Em ratio in the estimation of left ventricular filling pressures: impact of acute load reduction: a comparative simultaneous echocardiographic and catheterization study. Int J Cardiol. 2013;166:589–595. [DOI] [PubMed] [Google Scholar]
- 49. Weber T, Wassertheurer S, O'Rourke MF, Haiden A, Zweiker R, Rammer M, Hametner B, Eber B. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–1883. [DOI] [PubMed] [Google Scholar]
- 50. Hajahmadi Poorrafsanjani M, Rahimi Darabad B. Evaluate the sensitivity and specificity echocardiography in trans‐Doppler and tissue Doppler method in the estimation of left ventricular end‐diastolic pressure. Glob J Health Sci. 2014;6:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tatsumi K, Tanaka H, Matsumoto K, Sawa T, Miyoshi T, Imanishi J, Motoji Y, Mochizuki Y, Fukuda Y, Shinke T, Hirata K. Global endocardial area change rate for the assessment of left ventricular relaxation and filling pressure: using 3‐dimensional speckle‐tracking study. Int J Cardiovasc Imaging. 2014;30:1473–1481. [DOI] [PubMed] [Google Scholar]
- 52. Ito H, Ishii K, Iwakura K, Nakamura F, Nagano T, Takiuchi S. Impact of azelnidipine treatment on left ventricular diastolic performance in patients with hypertension and mild diastolic dysfunction: multi‐center study with echocardiography. Hypertens Res. 2009;32:895–900. [DOI] [PubMed] [Google Scholar]
- 53. Kurioka S, Ose H, Fukuma K, Yoshimoto K. Severity of diabetic retinopathy is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;99:287–291. [DOI] [PubMed] [Google Scholar]
- 54. Liao Z‐Y, Peng M‐C, Yun C‐H, Lai Y‐H, Po HL, Hou CJ‐Y, Kuo J‐Y, Hung C‐L, Wu Y‐J, Bulwer BE, Yeh H‐I, Tsai C‐H. Relation of carotid artery diameter with cardiac geometry and mechanics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2012;1:e003053 doi: 10.1161/JAHA.112.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, de Gomez Diego JJ, Oliva MJ, Hagendorff A, Hristova K, Lopez T, Magne J, Martinez C, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt J‐U, Zamorano JL, Bernard A, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging. 2015;16:1031–1041. [DOI] [PubMed] [Google Scholar]
- 56. Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3:614–622. [DOI] [PubMed] [Google Scholar]
- 57. Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galderisi M, Lancellotti P, Donal E, Cardim N, Edvardsen T, Habib G, Magne J, Maurer G, Popescu BA. European multicentre validation study of the accuracy of E/è ratio in estimating invasive left ventricular filling pressure: EURO‐FILLING study. Eur Heart J Cardiovasc Imaging. 2014;15:810–816. [DOI] [PubMed] [Google Scholar]
- 59. Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Study protocol.
Appendix S2. Data sources and search strategy.
Appendix S3. QUADAS‐2 questionnaire.
Appendix S4. Excluded full‐text studies.
Appendix S5. Secondary analysis of E/è correlation with LVFP.
Appendix S6. Secondary analysis of sensitivity/specificity of E/è cutoffs to predict elevated LVFP.
Appendix S7. Optimal cutoffs and AUC for elevated LVFP.
Appendix S8. Secondary analysis of sensitivity/specificity of E/è cutoffs to predict normal LVFP.


