Abstract
Background
Epidemiological evidence suggests a cardioprotective role of α‐linolenic acid (ALA), a plant‐derived ω‐3 fatty acid. It is unclear whether ALA is beneficial in a background of high marine ω‐3 fatty acids (long‐chain n‐3 polyunsaturated fatty acids) intake. In persons at high cardiovascular risk from Spain, a country in which fish consumption is customarily high, we investigated whether meeting the International Society for the Study of Fatty Acids and Lipids recommendation for dietary ALA (0.7% of total energy) at baseline was related to all‐cause and cardiovascular disease mortality. We also examined the effect of meeting the society's recommendation for long‐chain n‐3 polyunsaturated fatty acids (≥500 mg/day).
Methods and Results
We longitudinally evaluated 7202 participants in the PREvención con DIeta MEDiterránea (PREDIMED) trial. Multivariable‐adjusted Cox regression models were fitted to estimate hazard ratios. ALA intake correlated to walnut consumption (r=0.94). During a 5.9‐y follow‐up, 431 deaths occurred (104 cardiovascular disease, 55 coronary heart disease, 32 sudden cardiac death, 25 stroke). The hazard ratios for meeting ALA recommendation (n=1615, 22.4%) were 0.72 (95% CI 0.56–0.92) for all‐cause mortality and 0.95 (95% CI 0.58–1.57) for fatal cardiovascular disease. The hazard ratios for meeting the recommendation for long‐chain n‐3 polyunsaturated fatty acids (n=5452, 75.7%) were 0.84 (95% CI 0.67–1.05) for all‐cause mortality, 0.61 (95% CI 0.39–0.96) for fatal cardiovascular disease, 0.54 (95% CI 0.29–0.99) for fatal coronary heart disease, and 0.49 (95% CI 0.22–1.01) for sudden cardiac death. The highest reduction in all‐cause mortality occurred in participants meeting both recommendations (hazard ratio 0.63 [95% CI 0.45–0.87]).
Conclusions
In participants without prior cardiovascular disease and high fish consumption, dietary ALA, supplied mainly by walnuts and olive oil, relates inversely to all‐cause mortality, whereas protection from cardiac mortality is limited to fish‐derived long‐chain n‐3 polyunsaturated fatty acids.
Clinical Trial Registration
URL: http://www.Controlled-trials.com/. Unique identifier: ISRCTN35739639.
Keywords: fatty acid, nutrition, sudden cardiac death
Subject Categories: Diet and Nutrition
Introduction
Consistent evidence suggests that fatal coronary heart disease (CHD), the leading cause of death worldwide, can be prevented by various dietary components.1 Consumption of fish and long‐chain n‐3 (ω‐3) polyunsaturated fatty acids (LCn‐3PUFA), mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), has been associated with a lower risk of CHD, particularly sudden cardiac death (SCD) in persons without prior cardiovascular disease (CVD).2, 3 α‐Linolenic acid (ALA) is a shorter chain n‐3 fatty acid readily available from plant sources. Although the conversion of ALA into longer chain derivatives is limited (<5%), increased consumption of ALA translates into modest increases in the concentrations of EPA, but not DHA, in plasma and cell pools in a process controlled by both endocrine and dietary factors.4 By enhancing EPA synthesis, ALA might be cardioprotective in persons who do not eat fish; however, the issue of whether ALA is protective on its own, particularly if the diet provides sufficient amounts of EPA and DHA, remains unsettled.5 As reported in a recent meta‐analysis of observational studies, higher ALA intake is associated with a moderately lower risk of CVD, particularly fatal CHD.6 The unexplained high heterogeneity in this meta‐analysis highlights the need for additional well‐designed observational studies and large randomized clinical trials to evaluate the effects of ALA intake on the primary prevention of CVD.
We hypothesized that ALA intake contributes to the primary prevention of fatal CVD and all‐cause mortality, even in a background of high intake of fish‐derived LCn‐3PUFA, such as that reported in the Spanish population.7, 8, 9 To test this hypothesis, we longitudinally investigated the association of baseline dietary ALA intake with all‐cause and cardiovascular mortality in a cohort of older persons at high cardiovascular risk enrolled into the PREvención con DIeta MEDiterránea (PREDIMED) study, a randomized nutrition intervention trial for the primary prevention of CVD conducted in Spain.10 In addition, we examined the effects on mortality of exposure to LCn‐3PUFA.
Methods
Setting
This study was conducted within the frame of the PREDIMED trial, the design of which has been described in detail.11 Briefly, the PREDIMED study is a multicenter, parallel‐group, randomized clinical trial to assess the effects of the Mediterranean diet (MedDiet) on the primary prevention of CVD in a high‐risk cohort (PREDIMED website, http://www.predimed.es; ISRCTN registration, http://www.Controlled-trials.com/ISRCTN35739639). From October 2003 to June 2009, a total of 8713 candidates were screened for eligibility, and 7447 were randomly assigned to 1 of the 3 interventions. Participants were men aged 55 to 80 years and women aged 60 to 80 years at high cardiovascular risk but with no CVD at enrollment. Criteria for eligibility were the presence of either type 2 diabetes or at least 3 cardiovascular risk factors: current smoking, hypertension, dyslipidemia, overweight or obesity, and family history of early onset CHD. Main exclusion criteria were a prior history of CVD, any severe chronic illness, substance abuse, and history of allergy or intolerance to olive oil or nuts (supplemental foods given in 2 arms of the study). The study protocol was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the institutional review boards of all the recruiting centers. Written informed consent was obtained from all study participants.
Assessment of Risk Factors
Participants were considered to have diabetes, hyperlipidemia, or hypertension if they had a previous diagnosis of these conditions and/or they were treated with antidiabetic, cholesterol‐lowering, or antihypertensive agents, respectively. Smoking status was categorized into never, current, or past smoking, according to self‐reports. Physical activity was determined with the Minnesota Leisure‐Time Physical Activity questionnaire and expressed in minutes at a given metabolic equivalent per day.12 Height, weight, and waist circumference were measured with standard methods.
Dietary Intake
Dietary intake was assessed with a validated 137‐item semiquantitative food‐frequency questionnaire13 administered at baseline and yearly during follow‐up. In face‐to‐face interviews, participants were asked about the frequency of consumption of each food item during the past year, specifying usual portion sizes. Nine possibilities of frequency were offered, ranging from never to >6 times/day. Information on seafood products was collected in 8 items of the food‐frequency questionnaire (uncanned fatty fish; lean fish; smoked/salted fish; mollusks; shrimp, prawn, and crayfish; octopus, baby squid, and squid; fatty fish canned in oil; fatty fish canned in salted water) and 6 items regarding major sources of ALA (soybean oil, walnuts, margarine, corn oil, sunflower oil, olive oil). Consumption of flaxseed and canola oils was not considered because these oils are not consumed in Spain. Nutrient intakes were computed using Spanish food composition tables. The validation of the food‐frequency questionnaire against four 3‐day food records showed energy‐adjusted intraclass correlation coefficients of 0.506 and 0.728 for LCn‐3PUFA and ALA, respectively (P<0.001, both).
After the screening visit, suitable candidates were randomly assigned to 1 of 3 interventions: MedDiet with extra virgin olive oil, MedDiet with nuts, or control diet. Quarterly individual and group sessions were scheduled for the 2 MedDiet groups; in them, participants were educated on how to follow the MedDiet and received supplemental foods at no cost. Extra virgin olive oil (1 L/week) was provided to 1 group, and 30 g/day of mixed nuts (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds) were provided to the other group. During the first 3 years of the trial, participants in the control group were scheduled for yearly visits in which they received a leaflet explaining the low‐fat diet. The realization that the more infrequent visits in this group might be a limitation of the trial prompted a protocol amendment in October 2006; thereafter, participants in the control group received personalized advice and group sessions with the same intensity and frequency as those in the MedDiet groups. In the quarterly sessions, participants were educated on how to follow a low‐fat diet and received small nonfood gifts, such as kitchenware, tableware, aprons, or shopping bags. In each session, a dietary screener of adherence to the MedDiet was used to track diet changes. The score was determined by 12 questions on food consumption frequency and 2 questions on food consumption habits considered characteristic of the MedDiet (each question scored 0 or 1).14
End Point Ascertainment
The end points of interest in the present analysis were all‐cause and total CVD mortality; fatal CHD (acute myocardial infarction, unstable angina pectoris, and other forms of chronic ischemic heart disease); SCD, as ascertained by the criteria defined by Buxton and collaborators15; and fatal stroke. Information on mortality was updated yearly until completion of the trial and every 2 years thereafter by the end point adjudication committee, the members of which were blinded to treatment allocation and to the dietary habits of participants. Different sources of information were used: yearly questionnaires and examinations for all participants, contact with primary care physicians, yearly review of medical records, and linkage to the National Death Index. Medical records of deceased participants were requested. Only end points that were confirmed by the adjudication committee and that occurred between October 1, 2003, and June 30, 2012 (date of the last update in the extended follow‐up of the PREDIMED cohort, 2 years after the end of the trial), were included in the analyses.
Statistical Analyses
Follow‐up time was calculated as the interval between the date of randomization and the date of death, the date of the last visit, or the last recorded clinical event of participants still alive, whichever came first. After excluding participants with reported total energy intake outside predefined limits (>4000 or <800 kcal/day in men and >3500 or <500 kcal/day in women, n=153), those with incomplete baseline dietary data (n=78), and those with implausible intakes of either ALA (>10 g/day) or LCn‐3PUFA (>4 g/day) (n=14), 7202 study participants (n=2376 control diet, n=2469 MedDiet plus extra virgin olive oil, and n=2357 MedDiet plus nuts) remained for inclusion in the analyses. The 2 exposures of interest were (1) meeting the International Society for the Study of Fatty Acids and Lipids (ISSFAL) recommendation to consume at least 500 mg/day of EPA plus DHA for primary prevention (yes or no) and (2) meeting the ISSFAL recommendation to have an intake of ALA of 0.7% of total energy (yes or no). Both recommendations were released in June 2004.16
Multivariate Cox proportional hazards models were used to assess the associations between both exposures (all models included reciprocal adjustment for the 2 types of n‐3 examined) and the risk of the 5 prespecified end points. We initially performed sex‐specific analyses, but results by sex were similar, and no interactions existed between sex and any of the exposures for any of the dependent variables (data not shown); therefore, we report pooled data for men and women. No significant interactions were found between the 2 exposures of interest and the intervention group. In all cases, analyses were stratified by recruitment center and were adjusted for age, sex, intervention group, body mass index, smoking status (never, former, or current smoker), physical activity (minutes at a given metabolic equivalent per day), total energy intake (kcal/day), history of diabetes (yes or no), history of hyperlipidemia (yes or no), history of hypertension (yes or no), alcohol intake (g/day), and dietary factors (fiber, vegetables, fruits, and red meat in g/day). In addition, we used Cox regression models to assess the risk of the prespecified end points according to the joint categories of meeting target intake recommendations for ALA and LCn‐3PUFA intake (yes or no) and the intervention group (3 groups, 2 dummy variables). To verify the proportional hazards assumption in Cox regression models, we created time‐by‐covariate interactions for each variable included into the model. When introducing products between the variables and a linear function of time in each model, no significance was found for any interaction of interest.
Baseline differences in demographic, clinical, and selected dietary variables were assessed by ANOVA or chi‐square tests, as appropriate. The level of significance for all statistical tests was P<0.05 for bilateral contrasts. Analyses were done using SPSS statistical software, version 19 (IBM Corp).
Results
The mean age of participants at inclusion was 67 years, and 57.5% of them were women. Of the whole cohort (n=7202), 5452 participants (75.7%) met the ISSFAL target recommendation for LCn‐3PUFA intake of at least 500 mg/day, whereas 1615 (22.4%) met the recommendation of ALA intake of at least 0.7% of daily energy. Table 1 summarizes the baseline clinical characteristics and treatment regimens of the whole cohort and by sex. Clinical information by meeting the recommendations can be found in Table 2. Participants meeting the ALA recommendations were slightly older, leaner, more physically active, and smoked less than participants meeting the LCn‐3PUFA recommendation.
Table 1.
Participant Clinical Characteristics and Treatment Regimes at Baseline by Sex
| All Participants (n=7202) | Men (n=3063) | Women (n=4139) | |
|---|---|---|---|
| Age, y | 67.0 (66.9–67.2) | 66.0 (65.8–66.3) | 67.8 (67.6–67.9) |
| Weight, kg | 76.7 (76.5–77.0) | 82.2 (81.8–82.6) | 72.7 (72.4–73.0) |
| Body mass index, kg/m2 | 30.0 (29.9–30.1) | 29.3 (29.2–29.4) | 30.5 (30.3–30.6) |
| Waist circumference, cma | 100 (100–101) | 103 (103–104) | 98 (98–99) |
| Family history of early onset CHD, n (%)b | 1607 (23.0) | 523 (17.6) | 1084 (27.0) |
| Energy expenditure in physical activity, MET‐min/day | 231 (226–237) | 310 (300–321) | 173 (168–178) |
| Smoking status, n (%) | |||
| Current | 1003 (13.9) | 776 (25.3) | 227 (5.5) |
| Former | 1170 (24.6) | 1477 (48.2) | 293 (7.1) |
| Never | 4429 (61.5) | 810 (26.4) | 3619 (87.4) |
| Hypertension, n (%) | 5959 (82.7) | 2386 (77.9) | 3573 (86.3) |
| Use of antihypertensive drugs, n (%) | 5006 (69.5) | 2019 (65.9) | 2987 (72.2) |
| Dyslipidemia, n (%) | 5202 (72.2) | 2031 (66.3) | 3171 (76.6) |
| Use of statins, n (%) | 2897 (40.2) | 1103 (36.0) | 1794 (43.3) |
| Type 2 diabetes, n (%) | 3516 (48.8) | 1674 (54.6) | 1845 (44.6) |
| Use of oral hypoglycemic agents, n (%) | 2146 (29.8) | 1022 (33.4) | 1124 (27.2) |
| Use of insulin, n (%) | 374 (5.2) | 153 (5.0) | 221 (5.3) |
Values are n (%), except for age, weight, body mass index, and physical activity, expressed as means (95% CI). CHD indicates coronary heart disease; MET‐min, minutes at a given metabolic equivalent level (units of energy expenditure in physical activity, 1 MET‐min is roughly equivalent to 1 kcal).
Data from n=6997 participants (2979 men and 4018 women).
Data from n=6983 participants (2968 men and 4015 women).
Table 2.
Baseline Demographic and Medical History by Meeting the ISSFAL Recommendations
| Meeting the ISSFAL Recommendation for ALAa | Meeting the ISSFAL Recommendation for LCn‐3PUFAb | |||
|---|---|---|---|---|
| Yes (n=1615) | No (n=5587) | Yes (n=5452) | No (n=1750) | |
| Age, y | 67.3 (67.0–67.6) | 67.0 (66.8–67.1)c | 66.7 (66.6–66.9)d | 68.0 (67.7–68.3)c |
| Sex, men n (%) | 682 (42.2) | 2381 (42.6) | 24.1 (44.6) | 632 (36.1)c |
| Weight, kg | 74.9 (74.4–75.5) | 77.3 (77.0–77.6)c | 76.9 (76.6–77.3)d | 76.1 (75.6–76.7)c |
| Body mass index, kg/m2 | 29.3 (29.1–29.4) | 30.2 (30.1–30.3)c | 29.9 (29.8–30.0)d | 30.1 (30.0–30.3)c |
| Waist circumference, cme | 99 (98–99) | 101 (101–101)c | 101 (100–101)d | 100 (100–101) |
| Family history of early‐onset CHD, n (%)f | 375 (23.2) | 1232 (22.1) | 1227 (22.5) | 380 (21.7) |
| Energy expenditure in physical activity, MET‐min/day | 263 (251–276) | 222 (216–228)c | 236 (229–242)d | 217 (206–227)c |
| Smoking status, n (%) | c | d | c | |
| Current | 178 (11.0) | 825 (14.8) | 789 (14.5) | 214 (12.2) |
| Former | 407 (25.2) | 1363 (24.4) | 1410 (25.9) | 360 (20.6) |
| Never | 1030 (63.8) | 3399 (60.8) | 3253 (59.7) | 1176 (67.2) |
| Hypertension, n (%) | 1237 (82.2) | 4632 (82.9) | 4484 (82.2) | 1475 (84.3)c |
| Use of antihypertensive drugs, n (%) | 1088 (67.4) | 3918 (70.1)c | 3749 (68.8) | 1257 (71.8)c |
| Dyslipidemia, n (%) | 1188 (73.6) | 4014 (71.8) | 3937 (72.2) | 1265 (72.3) |
| Use of statins, n (%) | 666 (41.2) | 2231 (39.9) | 2173 (39.9) | 724 (41.4) |
| Type 2 diabetes, n (%) | 776 (48.0) | 2740 (49.0) | 2640 (48.4) | 876 (50.1) |
| Use of oral hypoglycemic agents, n (%) | 440 (27.2) | 1706 (30.5)c | 1591 (29.2) | 555 (31.7)c |
| Use of insulin, n (%) | 79 (4.9) | 295 (5.3) | 265 (4.9) | 109 (6.2)c |
Values are n (%), except for age, weight, body mass index, and physical activity, expressed as means (95% CI). ALA indicates α‐linolenic acid; CHD, coronary heart disease; ISSFAL, International Society for the Study of Fatty Acids and Lipids; LCn‐3PUFA, long‐chain n‐3 (ω‐3) polyunsaturated fatty acids; MET‐min, minutes at a given metabolic equivalent level (units of energy expenditure in physical activity, 1 MET‐min is roughly equivalent to 1 kcal).
0.7% energy, as a healthy ALA intake.
A minimum intake of 500 mg/day of combined eicosapentaenoic and docosahexaenoic acids, for primary cardiovascular protection.
P<0.005 compared with participants meeting the recommendation (obtained by the chi‐square test or ANOVA, as appropriate).
P<0.005 compared with participants meeting the ALA recommendation (obtained by the chi‐square test or ANOVA, as appropriate).
Data from n=6997 participants (2979 men and 4018 women).
Data from n=6983 participants (2968 men and 4015 women).
Intake of energy, nutrients, and key foods are shown in Table 3 (whole cohort and by sex) and Table 4 (by meeting the ISSFAL recommendations). A total of 4292 participants (60%) reported consumption of walnuts at least once per week, whereas only 119 (<2%) reported similar consumption of soybean oil. The main sources of ALA were walnuts (mean, 27% of total ALA), olive oil (23%), eggs and meat (20%), and dairy products (12%). Vegetable fats other than olive oil supplied <3% of total ALA. The Pearson correlation coefficients between the baseline estimated dietary ALA intake and consumption of walnuts, soybean oil, margarine, olive oil, and corn oil were 0.941 (P<0.001), 0.084 (P<0.001), 0.069 (P<0.001), 0.099 (P<0.001), and 0.024 (P=0.044), respectively. Consumption of sunflower oil was unrelated to dietary ALA. By definition of the study groups, intake of ALA and LCn‐3PUFA and their main parent foods (walnuts and seafood, respectively) differed between groups. Compared with the participants meeting the LCn3‐PUFA recommendation, those meeting the ALA recommendation adhered more to the MedDiet and consumed more total fat and ω‐6 polyunsaturated fatty acids, fruit, and fiber and less carbohydrate, cereals, cholesterol, and alcoholic beverages.
Table 3.
Baseline Intake of Energy, Nutrients, and Key Foods by Sex
| All Participants (n=7202) | Men (n=3063) | Women (n=4139) | |
|---|---|---|---|
| Energy intake, kcal/day | 2235 (2223–2248) | 2408 (2388–2427) | 2108 (2093–2123) |
| Carbohydrate, g/day | 234 (233–236) | 248 (245–251) | 224 (222–226) |
| Fiber, g/day | 25.2 (25.0–25.4) | 25.7 (25.4–26.0) | 24.9 (24.6–25.2) |
| Protein, g/day | 91 (91–92) | 94 (94–95) | 89 (88–90) |
| Fata | 97 (96–98) | 103 (102–104) | 92 (92–93) |
| Saturated fatty acids | 24.8 (24.6–25.0) | 26.5 (26.2–26.9) | 23.5 (23.3–23.8) |
| Monounsaturated fatty acids | 48.2 (47.8–48.5) | 51.3 (50.8–51.9) | 45.9 (45.4–46.3) |
| n‐6 polyunsaturated fatty acids | 13.0 (12.8–13.1) | 13.9 (13.7–14.1) | 12.2 (12.1–12.4) |
| ALA | 1.40 (1.38–1.42) | 1.49 (1.46–1.53) | 1.34 (1.31–1.36) |
| ALA, % of energy | 0.06 (0.06–0.06) | 0.06 (0.06–0.06) | 0.06 (0.06–0.06) |
| Meeting the ISSFAL recommendation for a healthy ALA intake, n (%)b | 1615 (22.4) | 682 (22.3) | 933 (22.5) |
| LCn‐3PUFA | 0.84 (0.83–0.85) | 0.88 (0.86–0.90) | 0.80 (0.79–0.82) |
| Meeting the ISSFAL recommendation for LCn‐3PUFA, n (%)c | 5452 (75.7) | 2431 (79.4) | 3021 (73.0) |
| Cholesterol, mg/day | 361 (359–364) | 382 (377–386) | 346 (343–343) |
| Cereals, g/day | 143 (141–145) | 161 (158–164) | 129 (127–132) |
| Vegetables, g/day | 334 (331–337) | 333 (327–338) | 335 (331–340) |
| Fruits, g/day | 368 (364–373) | 365 (358–372) | 371 (365–377) |
| Total nuts, g/day | 10.1 (9.8–10.4) | 11.2 (10.7–11.7) | 9.3 (8.9–9.7) |
| Walnuts, g/day | 5.9 (5.7–6.1) | 6.2 (5.9–6.6) | 5.7 (5.4–5.9) |
| Dairy products, g/day | 380 (375–385) | 342 (335–350) | 408 (401–415) |
| Red meat, g/day | 76 (75–77) | 88 (86–90) | 68 (67–69) |
| Seafood, g/dayd | 99 (98–100) | 102 (100–104) | 97 (95–98) |
| Alcohol, g/day | 9.1 (8.8–9.4) | 15.4 (14.7–16.0) | 3.1 (2.9–3.3) |
| Adherence to Mediterranean Diete | 8.67 (8.62–8.71) | 8.78 (8.71–8.84) | 8.59 (8.53–8.71) |
Values are n (%) or means (95% CI). ALA indicates α‐linolenic acid; ISSFAL, International Society for the Study of Fatty Acids and Lipids; LCn‐3PUFA, long‐chain n‐3 (ω‐3) polyunsaturated fatty acids.
Otherwise stated, values in g/day.
0.7% of total energy intake.
A minimum intake of 500 mg/day of combined eicosapentaenoic acid and docosahexaenoic acid, for primary cardiovascular protection.
Sum of uncanned fatty fish; lean fish; smoked/salted fish; molluscs; shrimp, prawn, and crayfish; octopus, baby squid, and squid; fatty fish canned in oil; and fatty fish canned in salted water.
Determined by 12 questions on food consumption frequency and 2 questions on food intake habits characteristic of the Mediterranean diet (each question scored 0 or 1).
Table 4.
Baseline Intake of Energy, Nutrients, and Key Foods by Meeting the ISSFAL Recommendations
| Meeting the ISSFAL Recommendation for ALAa | Meeting the ISSFAL Recommendation for LCn‐3PUFAb | |||
|---|---|---|---|---|
| Yes (n=1615) | No (n=5587) | Yes (n=5452) | No (n=1750) | |
| Energy intake, kcal/day | 2320 (2294–2347) | 2210 (2196–2225)c | 2300 (2286–2314) | 2033 (2008–2058)c |
| Carbohydrate, g/day | 220 (218–222) | 239 (237–240)c | 230 (229–231)d | 249 (247–251)c |
| Fiber, g/day | 26.8 (26.5–27.2) | 24.8 (24.6–25.0)c | 25.2 (25.0–25.4)d | 25.4 (25.0–25.7) |
| Protein, g/day | 93 (92–93) | 91 (90–91)c | 93 (93–94) | 85 (84–85)c |
| Fate | 104 (103–104) | 95 (95–96)c | 98 (98–99)d | 94 (93–95)c |
| Saturated fatty acids | 25.1 (24.9–25.4) | 24.7 (24.6–24.9)c | 25.0 (24.8–25.2) | 24.2 (23.9–24.5)c |
| Monounsaturated fatty acids | 49.0 (48.4–49.5) | 48.0 (47.7–48.3)c | 48.8 (48.5–49.1) | 46.3 (45.8–46.8)c |
| n‐6 polyunsaturated fatty acids | 17.1 (16.9–17.3) | 11.8 (11.7–11.9)c | 12.9 (12.8–13.0)d | 13.2 (12.9–13.4) |
| ALA | 2.63 (2.61–2.65) | 1.05 (1.04–1.06)c | 1.43 (1.41–1.45)d | 1.32 (1.28–1.36)c |
| ALA, % of energy | 0.12 (0.12–0.12) | 0.05 (0.05–0.05)c | 0.059 (0.057–0.060)d | 0.064 (0.063–0.065)c |
| Meeting the ISSFAL recommendation for a healthy ALA intake, n (%) | — | — | 4156 (76.2) | 1296 (23.8)c |
| LCn‐3PUFA | 0.89 (0.87–0.92) | 0.82 (0.81–0.83)c | 0.99 (0.98–1.00)d | 0.37 (0.35–0.39)c |
| Meeting the ISSFAL recommendation for LCn‐3PUFA, n (%) | 1296 (80.2) | 319 (19.8)c | — | — |
| Cholesterol, mg/day | 357 (352–362) | 363 (360–366)c | 376 (373–379)d | 316 (311–321)c |
| Cereals, g/day | 132 (128–135) | 146 (144–148)c | 145 (143–147)d | 137 (133–141)c |
| Vegetables, g/day | 353 (346–360) | 329 (325–332)c | 346 (342–350) | 297 (291–304)c |
| Fruits, g/day | 399 (389–409) | 359 (354–365)c | 373 (367–378)d | 355 (345–365)c |
| Total nuts, g/day | 27 (27–28) | 5 (5–5)c | 11 (10–11)d | 8 (8–9)c |
| Walnuts, g/day | 19.4 (18.9–19.9) | 2.0 (2.0–2.1)c | 6.3 (6.0–6.5)d | 4.8 (4.4–5.2)c |
| Dairy products, g/day | 372 (361–382) | 383 (377–388) | 376 (371–382) | 391 (381–402)c |
| Red meat, g/day | 80 (77–82) | 75 (74–77)c | 81 (80–82) | 61 (60–63)c |
| Seafood, g/dayd | 105 (103–108) | 97 (96–98)c | 113 (112–114)d | 55 (54–57)c |
| Alcohol, g/day | 7.4 (6.8–8.1) | 8.6 (8.2–8.9)c | 7.7 (7.1–8.4)d | 8.5 (8.2–8.9)c |
| Adherence to Mediterranean Dietf | 9.43 (9.34–9.51) | 8.45 (8.40–8.50)c | 8.88 (8.83–8.93)d | 8.02 (7.93–8.10)c |
Values are n (%) or means (95% CI). ALA indicates α‐linolenic acid; ISSFAL, International Society for the Study of Fatty Acids and Lipids; LCn‐3PUFA, long‐chain n‐3 (ω‐3) polyunsaturated fatty acids.
0.7% of total energy intake.
A minimum intake of 500 mg/day of combined eicosapentaenoic acid and docosahexaenoic acid, for primary cardiovascular protection.
P<0.005 compared with participants meeting the recommendation (obtained by the chi‐square test or ANOVA, as appropriate).
P<0.005 compared with participants meeting the ALA recommendation (obtained by the chi‐square test or ANOVA, as appropriate).
Otherwise stated, values in g/day.
Sum of uncanned fatty fish; lean fish; smoked/salted fish; molluscs; shrimp, prawn, and crayfish; octopus, baby squid, and squid; fatty fish canned in oil; and fatty fish canned in salted water.
Determined by 12 questions on food consumption frequency and 2 questions on food intake habits characteristic of the Mediterranean diet (each question scored 0 or 1).
During a mean follow‐up of 5.9 years, we documented 431 all‐cause deaths, including 104 cases of fatal CVD (55 cases of fatal CHD, 32 cases of SCD, 25 cases of fatal stroke). Table 5 shows mortality risks (hazard ratios [HRs]) associated with the 2 exposures of interest. After adjusting for age, sex, intervention group, and lifestyle variables, including intake of relevant foods and nutrients, participants consuming at least 500 mg/day of EPA plus DHA had borderline significant reduction of SCD risk by 52% and significant reductions of fatal CVD and CHD by 39% and 46%, respectively. If the exposure of interest was meeting the ISSFAL recommendation for dietary ALA intake of 0.7% of daily energy, multivariable‐adjusted risk of all‐cause mortality was significantly reduced by 28%. No significant associations were found for other outcomes. Table 6 presents the risk of all‐cause mortality for meeting none, one, or both ISSFAL recommendations of n‐3 fatty acid intake. Compared with participants meeting neither recommended ALA nor LCn‐3PUFA intake, the highest reduction was observed in those meeting both recommendations (HR 0.626 [95% CI 0.450–0.871], P=0.005).
Table 5.
Risk of All‐Cause and Cardiovascular Mortality for Meeting the ISSFAL Recommendation of a Healthy Intake of ALA (0.7% of Energy) and LCn‐3PUFA Consumption for Primary Cardiovascular Prevention (at Least 500 mg/day)
| End point (No. of Events) | ALA | LCn‐3PUFA | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Total mortality (431) | 0.722 (0.558–0.933) | 0.013 | 0.837 (0.667–1.050) | 0.124 |
| Fatal CVD (104) | 0.953 (0.579–1.566) | 0.848 | 0.614 (0.393–0.960) | 0.032 |
| Fatal CHD (55) | 0.752 (0.359–1.578) | 0.451 | 0.537 (0.292–0.988) | 0.046 |
| Sudden cardiac death (32) | 0.768 (0.288–2.049) | 0.598 | 0.485 (0.223–1.057) | 0.069 |
| Fatal stroke (25) | 1.288 (0.492–3.373) | 0.607 | 0.779 (0.298–2.036) | 0.610 |
Data are given as HRs and 95% CIs, using as reference category the groups not meeting recommended daily ALA or LCn‐3PUFA intake. Multivariable Cox regression model, stratified for recruiting node; adjusted for age, sex, intervention group, body mass index, smoking status (never, former, or current smoker), physical activity (minutes at a given metabolic equivalent level per day), total energy intake (kcal/day), history of diabetes (yes or no), history of hyperlipidemia (yes or no), history of hypertension (yes or no), alcohol intake (g/day), and dietary factors (fiber, vegetables, fruits, and red meat), reciprocally adjusted for meeting recommended ALA or LCn‐3PUFA intake. ALA indicates α‐linolenic acid; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; ISSFAL, International Society for the Study of Fatty Acids and Lipids; LCn‐3PUFA, long‐chain n‐3 (ω‐3) polyunsaturated fatty acids.
Table 6.
Risk of All‐Cause Mortality for Meeting None, One, or Both ISSFAL Recommendations of n‐3 Fatty Acid Intake
| HR (95% CI) | P Value | |
|---|---|---|
| Meeting neither recommended ALA nor LCn‐3PUFA intake (n=1431) | 1.00 | — |
| Only meeting the recommendation of LCn‐3 PUFA intake for primary cardiovascular prevention (at least 500 mg/day) (n=4156) | 0.781 (0.615–0.994) | 0.044 |
| Only meeting the recommendation of a healthy intake of ALA (0.7% of energy) (n=319) | 0.670 (0.551–0.977) | 0.018 |
| Meeting both recommendations (n=1296) | 0.626 (0.450–0.871) | 0.005 |
Data are given as HRs and 95% CIs, using as reference category the group meeting neither recommended ALA nor LCn‐3PUFA intake. A multivariable Cox regression model was stratified for recruiting node and adjusted for age, sex, intervention group, body mass index, smoking status (never, former, or current smoker), physical activity (minutes at a given metabolic equivalent level per day), total energy intake (kcal/day), history of diabetes (yes or no), history of hyperlipidemia (yes or no), history of hypertension (yes or no), alcohol intake (g/day), and dietary factors (fiber, vegetables, fruits, and red meat). ALA indicates α‐linolenic acid; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; ISSFAL, International Society for the Study of Fatty Acids and Lipids; LCn‐3PUFA, long‐chain n‐3 (ω‐3) polyunsaturated fatty acids.
Figure shows the multivariate adjusted HRs for total mortality by meeting the ISSFAL recommendation for ALA intake at baseline according to intervention group. Compared with the reference category (participants not meeting target intake who were allocated to the control diet group, n=448), participants meeting target intake who were allocated to the MedDiet with nuts intervention group (n=648) had a borderline decreased risk of 30% (95% CI −6 to 53) for all‐cause mortality, whereas participants meeting target intake who were allocated to the MedDiet supplemented with extra virgin olive oil (n=519) had a significant 42% (95% CI 4–64) reduction in total mortality. No significant associations were found for meeting the ISSFAL recommendation of LCn‐3PUFA by intervention group (data not shown).
Figure 1.
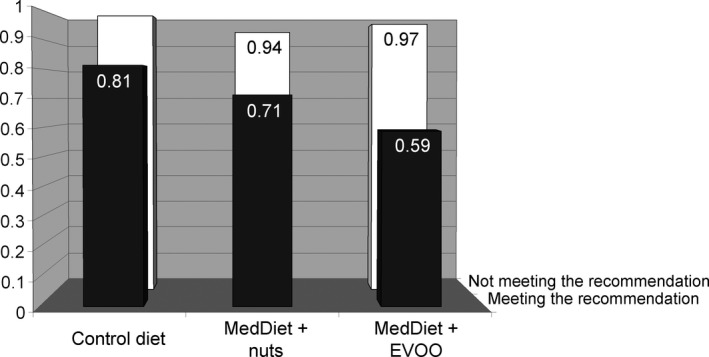
Adjusted hazard ratios of total mortality by meeting the ISSFAL recommendation for a healthy ALA intake and PREvención con DIeta MEDiterránea (PREDIMED) intervention group. Data are given as hazard ratios. A multivariable Cox regression model was stratified for recruiting node and adjusted for age, sex, body mass index, current smoking status (never, former, or current smoker), physical activity (minutes at a given metabolic equivalent per day), total energy intake (kcal/day), history of diabetes (yes or no), history of hyperlipidemia (yes or no), history of hypertension (yes or no), alcohol intake (g/day), dietary factors (fiber, vegetables, fruits, and red meat), and meeting the ISSFAL recommendation of eicosapentaenoic acid and docosahexaenoic acid consumption for primary cardiovascular prevention (yes or no). Values for the “not meeting the recommendation” category are 1.00 (reference) for the low‐fat control diet group, 0.94 (95% CI, 0.73–1.23) for the MedDiet supplemented with nuts (MedDiet plus nuts) group, and 0.97 (95% CI 0.76–1.24) for the MedDiet supplemented with extra virgin olive oil (MedDiet plus EVOO). Values for the “meeting the recommendation” category are 0.81 (95% CI 0.52–1.25) for the control diet group, 0.71 (95% CI 0.47–1.06) for the MedDiet plus nuts group, and 0.59 (95% CI 0.36–0.96) for the MedDiet plus EVOO group. EVOO indicates extra virgin olive oil; ISSFAL, International Society for the Study of Fatty Acids and Lipids; MedDiet, Mediterranean diet.
Discussion
Our findings suggest that in an older population at high cardiovascular risk, consuming at least 500 mg/day of LCn‐3PUFA (mainly from seafood) is associated with a borderline significant reduction in SCD by 52% and significant reductions in fatal CVD and CHD by 39% and 46%, respectively. In addition, dietary ALA (derived mainly from walnuts followed by olive oil) accounting for at least 0.7% of daily energy intake was associated with a 28% reduced risk of all‐cause mortality. Importantly, the beneficial effect of ALA on mortality is observed in the context of a high intake of LCn‐3PUFA.
Dietary changes play a key role in protection against CVD,1 the leading cause of death worldwide.17 In this regard, the intake of >250 mg/day of LCn‐3PUFA (fulfilled by 95% of our population) has been associated with a significant reduction of fatal CHD in healthy populations.18 The association is less clear for randomized controlled trials of supplemental LCn‐3PUFA for secondary CVD prevention,19 although the methodological issues in these trials (in particular, statistical power, the length of intervention, background diet, and drug use) might preclude drawing firm conclusions, as recently noted.20 Based on large prospective population studies and case‐control studies mainly conducted in the United States, ISSFAL recommended in 2004 an intake of at least 500 mg/day of LCn‐3PUFA. This goal can be achieved by following the recommendation of the American Heart Association to consume 2 weekly servings of fish, preferably oily fish.3 In our population, meeting the ISSFAL recommendation for LCn‐3PUFA reduced the risk of fatal CVD; however, observational studies suggest that LCn‐3PUFA protects against fatal CHD, particularly SCD, rather than fatal CVD.21 This effect, observed with even a modest dietary intake of LCn‐3PUFA, is believed to be due to cardiac membrane accretion of EPA and DHA, with ensuing improvement of myocardial oxygen consumption efficiency, which contributes to limit myocardial damage on ischemia.22 In our cohort, LCn‐3PUFA intake related to lower CHD mortality and, in examining the association with SCD, despite the low number of events (n=32), we found a trend (P=0.069) for protection, with a 52% reduced risk. Consequently, our results regarding SCD concur in direction, if not in magnitude, with results from prior larger studies. Importantly, our findings also support the hypothesis that the customarily high intake of fish as part of the MedDiet in Spain might contribute to explain the paradox of low rates of both incident CHD and cardiac death,17, 23, 24 despite a high burden of cardiovascular risk factors.25
Evidence regarding the cardiovascular benefits of marine n‐3 notwithstanding, both low customary fish consumption in most Western societies9 and the unsustainability of fishing prompted the search for alternative dietary sources of n‐3 fatty acids. ALA is an n‐3 that is available from plant sources (mainly flaxseeds, walnuts, soy products, and vegetable oils such as canola and olive oil) and that is inexpensive.26 Although the evidence from large prospective studies and randomized controlled trials is strong on the cardioprotective effects of EPA and DHA, data on ALA are limited.27 Two clinical studies have examined the effects of ALA on hard CVD end points, the Lyon Diet Heart study28 and the Alpha‐Omega trial,29 both conducted in myocardial infarction survivors. A large benefit in reduction of reinfarction in the Lyon study could not be ascribed entirely to ALA, as other dietary changes took place,28 whereas the Alpha‐Omega trial revealed a trend toward CVD protection for ALA in patients receiving up‐to‐date cardiologic treatment.29 There have been no primary prevention trials with ALA, only observational studies. Many of these studies have been carried out in US populations30, 31, 32 that are characterized by low fish consumption,9 and data from the Health Professionals Follow‐up Study suggest that ALA protects against CHD only in this situation.30 In line with this, studies in mice reported that diets rich in ALA are protective against endothelial dysfunction and plaque inflammation,33 although this effect appears to be modest compared with DHA.34 This might explain why, in our population with high fish consumption, we could not detect a cardioprotective effect of ALA; it was probably overrun by the concomitant high intake of EPA and DHA with a stronger benefit against CHD. Alternatively, an eventual protective effect of ALA against fatal cardiovascular outcomes could have been masked by participants who met the ALA recommendation being the healthiest subsample among the study population (Table 2).
In contrast, we found that PREDIMED participants meeting the ISSFAL recommendation for dietary ALA intake at baseline had significantly decreased risk of all‐cause mortality by 28%. These results concur with those of Koh and collaborators35 in a large sample of Chinese adults in whom ALA intake was inversely associated with all‐cause mortality in the setting of moderately high intakes of EPA plus DHA, albeit lower than in our cohort. The highest reduction in all‐cause mortality was observed in participants meeting both ALA and LCn‐3PUFA recommendations (Table 6), suggesting that the 2 n‐3 fatty acids (or their parent foods) are partners in reducing the risk of total mortality. Given that the almost exclusive source of ALA in our population was consumption of walnuts, followed by olive oil, this association may be attributable not only to ALA per se but also to other components of the parent foods, such as polyphenols, tocopherols, and phytosterols, all with salutary properties.36 This notion is consistent with prior evidence on reduced all‐cause mortality associated with the consumption of nuts37, 38, 39 and walnuts, in particular,39 and olive oil.40 Consequently, it is plausible that in a Mediterranean population, reduced all‐cause mortality associated with ALA intake relates to the bioactive components of walnuts and olive oil (including ALA itself), with dietary ALA being a surrogate marker of the consumption of the parent foods. Such a hypothesis is reinforced by the fact that, when considering the 3 intervention groups in the trial, the highest reduction in mortality was observed in the participants allocated to the MedDiet supplemented with extra virgin olive oil, suggesting that phytochemicals with antioxidant and anti‐inflammatory properties contained in extra virgin olive oil might act synergistically with walnut‐derived bioactive compounds.
Our study has several limitations. First, we had a relatively low number of fatal cardiovascular events, resulting in imprecise estimates. Second, nutrient exposures were estimated with a food‐frequency questionnaire, which has the potential of misclassification bias. An objective biomarker (circulating or adipose tissue fatty acids) would have provided more accurate estimation of ALA intake. Third, residual confounding is possible in a longitudinal cohort analysis, but we adjusted for many possible confounders, including reciprocal adjustment for the 2 types of n‐3 fatty acids examined. Fourth, given the advice to increase adherence to a MedDiet (in 2 PREDIMED arms) and the nut supplementation (in 1 arm), cumulative average estimates of exposures of interest would provide a more robust measure than a single baseline assessment. Finally, the generalizability of our results is limited, given that participants were older persons at high cardiovascular risk living in a Mediterranean country. There are also strengths to our study, such as a large sample size, relative homogeneity of participants, a prospective design, thorough ascertainment of mortality, validation of the food‐frequency questionnaire, and adjustment for relevant confounders.
In conclusion, our results add supporting evidence to the notion that, in a population at high cardiovascular risk but no prior CVD with a customarily high consumption of seafood, dietary ALA relates inversely to all‐cause mortality, but protection from cardiovascular and cardiac death is limited to fish‐derived EPA plus DHA. ALA intake was derived mostly from walnuts and olive oil, both sources of phytochemicals with antioxidant and anti‐inflammatory properties besides ALA. The highest reductions in mortality were observed in participants meeting the ISSFAL recommendation for ALA intake allocated to the MedDiet plus extra virgin olive oil arm and in participants meeting the recommendations for both LCn‐3PUFA and ALA intake. This suggests that, together with the MedDiet pattern, marine and vegetable n‐3 fatty acids (or their parent foods) act synergistically and are partners rather than competitors in reducing mortality.
Appendix
Other PREDIMED Investigators
Hospital Clínic, Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain: A. Pérez‐Heras, C. Viñas, R. Casas, L. de Santamaría, S. Romero, E. Sacanella, G. Chiva, P. Valderas, S. Arranz, J.M. Baena, M. García, M. Oller, J. Amat, I. Duaso, Y. García, C. Iglesias, C. Simón, Ll. Quinzavos, Ll. Parra, M. Liroz, J. Benavent, J. Clos, I. Pla, M. Amorós, M.T. Bonet, M.T. Martin, M.S. Sánchez, J. Altirriba, E. Manzano, A. Altés, M. Cofán, C. Valls‐Pedret, M. Doménech, R. Gilabert, and N. Bargalló.
University Rovira i Virgili, Reus, Spain: R. González, C. Molina, F. Márquez, N. Babio, M. Sorli, J. García Roselló, A. Diaz‐López, F. Martin, R. Tort, A. Isach, B. Costa, J.J. Cabré, J. Fernández‐Ballart, N. Ibarrola‐Jurado, C. Alegret, P. Martínez, S. Millán, J.L. Piñol, T. Basora, and J.M. Hernández.
University of Navarra, Primary Care Centres, Pamplona, Spain: E. Toledo, P. Buil‐Cosiales, M. Ruiz‐Canela, B. Sanjulián, J. Díez‐Espino, V. Extremera‐Urabayen, A. García‐Arellano, I. Zazpe, F.J. Basterra‐Gortari, E Goñi, C. Razquin, M. Serrano‐Martínez, M. Bes‐Rastrollo, A. Gea, E.H. Martínez‐Lapiscina, J.M. Nuñez‐Córdoba, C. Arroyo‐Azpa, L. García‐Pérez, J. Villanueva‐Tellería, F. Cortés‐Ugalde, T. Sagredo‐Arce, Mª D. García de la Noceda‐Montoy, Mª D. Vigata‐López, Mª T. Arceiz‐Campo, A. Urtasun‐Samper, Mª V. Gueto‐Rubio, and B. Churio‐Beraza.
School of Pharmacy, University of Barcelona, Barcelona, Spain: Rosa M. Lamuela‐Raventós, A.I. Castellote‐Bargallo, A. Medina‐Remón, and A. Tresserra‐Rimbau.
University of Valencia, Valencia, Spain: P. Carrasco, C. Ortega‐ Azorín, E.M. Asensio, R. Osma, R. Barragán, F. Francés, M. Guillén, J.I. González, C. Saiz, O. Portolés, F.J. Giménez, O.Coltell, P. Guillem‐Saiz, L. Quiles, V. Pascual, C. Riera, M.A. Pages, D. Godoy, A. Carratalá‐Calvo, M.J. Martín‐Rillo, E. Llopis‐Osorio, J. Ruiz‐ Baixauli, and A. Bertolín‐Muñoz.
University Hospital of Alava, Vitoria, Spain: I. Salaverría, T. del Hierro, J. Algorta, S. Francisco, A. Alonso, J. San Vicente, E. Sanz, I. Felipe, A. Alonso Gómez, and A. Loma‐Osorio.
Institute of Health Sciences IUNICS, University of Balearic Islands, and Hospital Son Espases, Palma de Mallorca, Spain: M. García‐Valdueza, M. Moñino, A. Proenza, R. Prieto, G. Frontera, M. Ginard, F. Fiol, A. Jover, and J. García.
Institut de Recerca Hospital del Mar, Barcelona, Spain: M.I. Covas, S. Tello, J. Vila, H. Schröder, R. De la Torre, D. Muñoz‐Aguayo, R. Elosúa, J. Marrugat, and M. Ferrer.
University of Las Palmas de Gran Canaria, Las Palmas, Spain: J. Álvarez‐Pérez, E. DíazBenítez, I. Bautista‐Castaño, I. Maldonado‐Díaz, A. Sánchez‐Villegas, I. Castro, P. Henríquez, C. Ruano, A. P. Ortiz, F. Sarmiendo de la Fe, C. Simón‐García, I. Falcón‐Sanabria, B. Macías‐Gutiérrez, and A.J. Santana‐Santana.
University of Málaga, Málaga, Spain: E. Gomez‐Gracia, J. Fernández‐Crehuet, R. Benítez Pont, M. Bianchi Alba, J. Wärnberg, R. Gómez‐Huelgas, J. Martínez‐González, V. Velasco García, J. de Diego Salas, A. Baca Osorio, J. Gil Zarzosa, J.J. Sánchez Luque, and E. Vargas López.
Instituto de la Grasa, Consejo Superior de Investigaciones Científicas, Sevilla, Spain: V. Ruiz‐Gutierrez, E. Jurado Ruiz, E. Montero Romero, and M. García García.
Department of Family Medicine, Primary Care Division of Sevilla, Sevilla, Spain: J. Lapetra, M. Leal, E. Martínez, J.M. Santos, M. Ortega‐Calvo, P. Román, F. José García, P. Iglesias, Y. Corchado, E. Mayoral, and C. Lama.
Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain: X. Pintó, E. de la Cruz, A. Galera, Y. Soler, F. Trias, I. Sarasa, E. Padres, R. Figueras, X. Solanich, R. Pujol, and E. Corbella.
Primary Care Division, Catalan Institute of Health, Barcelona, Spain: C. Cabezas, E. Vinyoles, M.A. Rovira, L. García, G. Flores, J.M. Verdú, P. Baby, A. Ramos, L. Mengual, P. Roura, M.C. Yuste, A. Guarner, A. Rovira, M.I. Santamaría, M. Mata, C. de Juan, and A. Brau.
Other investigators of the PREDIMED network: A. Marti (University of Navarra), M.T. Mitjavila (University of Barcelona), M.P. Portillo (University of Basque Country), G. Sáez (University of Valencia), and J. Tur (University of Balearic Islands).
Sources of Funding
This study was funded in part by Instituto de Salud Carlos III (ISCIII) (Spanish Ministry of Economy) through grants RTIC G03/140, RTIC RD 06/0045, Centro Nacional de Investigaciones Cardiovasculares CNIC 06/2007, ISCIII FIS PS09/01292, the Spanish Ministry of Science and Innovation (MICINN) AGL2010‐22319‐C03‐02 and AGL2009‐13906‐C02‐02, and an unrestricted grant from the California Walnut Commission. Sala‐Vila holds a Miguel Servet I fellowship from the Ministry of Economy and Competitiveness through the ISCIII.
Disclosures
Salas‐Salvadó has received research funding and is a non‐paid member of the scientific advisory committee of the International Nut Council. Hu and Ros have received research funding through their institutions from the California Walnut Commission; Ros is also a non‐paid member of its scientific advisory committee. No other authors declare a conflict of interest.
Acknowledgments
CIBERobn is an initiative of ISCIII, Spain.
(J Am Heart Assoc. 2016;5:e002543 doi: 10.1161/JAHA.115.002543)
References
- 1. Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Caterina R. n‐3 Fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. [DOI] [PubMed] [Google Scholar]
- 3. Kris‐Etherton PM, Harris WS, Appel LJ. Omega‐3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. [DOI] [PubMed] [Google Scholar]
- 4. Burdge GC. Metabolism of alpha‐linolenic acid in humans. Prostaglandins Leukot Essent Fatty Acids. 2006;75:161–168. [DOI] [PubMed] [Google Scholar]
- 5. Barceló‐Coblijn G, Murphy EJ. Alpha‐linolenic acid and its conversion to longer chain n‐3 fatty acids: benefits for human health and a role in maintaining tissue n‐3 fatty acid levels. Prog Lipid Res. 2009;48:355–374. [DOI] [PubMed] [Google Scholar]
- 6. Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. Alpha‐linolenic acid and risk of cardiovascular disease: a systematic review and meta‐analysis. Am J Clin Nutr. 2012;96:1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welch AA, Lund E, Amiano P, Dorronsoro M, Brustad M, Kumle M, Rodriguez M, Lasheras C, Janzon L, Jansson J, Luben R, Spencer EA, Overvad K, Tjønneland A, Clavel‐Chapelon F, Linseisen J, Klipstein‐Grobusch K, Benetou V, Zavitsanos X, Tumino R, Galasso R, Bueno‐De‐Mesquita HB, Ocké MC, Charrondière UR, Slimani N. Variability of fish consumption within the 10 European countries participating in the European Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2002;5:1273–1285. [DOI] [PubMed] [Google Scholar]
- 8. Saadatian‐Elahi M, Slimani N, Chajès V, Jenab M, Goudable J, Biessy C, Ferrari P, Byrnes G, Autier P, Peeters PH, Ocké M, Bueno de Mesquita B, Johansson I, Hallmans G, Manjer J, Wirfält E, González CA, Navarro C, Martinez C, Amiano P, Suárez LR, Ardanaz E, Tjønneland A, Halkjaer J, Overvad K, Jakobsen MU, Berrino F, Pala V, Palli D, Tumino R, Vineis P, Santucci de Magistris M, Spencer EA, Crowe FL, Bingham S, Khaw KT, Linseisen J, Rohrmann S, Boeing H, Noethlings U, Olsen KS, Skeie G, Lund E, Trichopoulou A, Oustoglou E, Clavel‐Chapelon F, Riboli E. Plasma phospholipid fatty acid profiles and their association with food intakes: results from a cross‐sectional study within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:331–346. [DOI] [PubMed] [Google Scholar]
- 9. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group NutriCoDE . Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country‐specific nutrition surveys. BMJ. 2014;348:g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, Gómez‐Gracia E, Ruiz‐Gutiérrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez‐González MA; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 11. Martínez‐González MÁ, Corella D, Salas‐Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz‐Gutiérrez V, Lamuela‐Raventós RM, Lapetra J, Muñoz MÁ, Martínez JA, Sáez G, Serra‐Majem L, Pintó X, Mitjavila MT, Tur JA, Portillo MP, Estruch R; PREDIMED Study Investigators . Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41:377–385. [DOI] [PubMed] [Google Scholar]
- 12. Elosua R, Marrugat J, Molina L, Pons S, Pujol E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol. 1994;139:1197–1209. [DOI] [PubMed] [Google Scholar]
- 13. Fernández‐Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez‐Bauer M, Martínez‐González MA, Salas‐Salvadó J, Martín‐Moreno JM. Relative validity of a semi‐quantitative food‐frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103:1808–1816. [DOI] [PubMed] [Google Scholar]
- 14. Schröder H, Fitó M, Estruch R, Martínez‐González MA, Corella D, Salas‐Salvadó J, Lamuela‐Raventós R, Ros E, Salaverría I, Fiol M, Lapetra J, Vinyoles E, Gómez‐Gracia E, Lahoz C, Serra‐Majem L, Pintó X, Ruiz‐Gutierrez V, Covas MI. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–1145. [DOI] [PubMed] [Google Scholar]
- 15. Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, Poppas A, Prystowsky EN, Schoenfeld MH, Zimetbaum PJ, Heidenreich PA, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF; American College of Cardiology; American Heart Association Task Force on Clinical Data Standards; (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology) . ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). J Am Coll Cardiol. 2006;48:2360–2396. [DOI] [PubMed] [Google Scholar]
- 16. ISSFAL, Intake of PUFA in Healthy Adults , http://www.issfal.org/statements/pufa-recommendations/statement-3. Accessed December 30 2015.
- 17. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. [DOI] [PubMed] [Google Scholar]
- 19. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 20. Harris WS. Are n‐3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care. 2013;16:141–149. [DOI] [PubMed] [Google Scholar]
- 21. Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. [DOI] [PubMed] [Google Scholar]
- 22. McLennan PL. Cardiac physiology and clinical efficacy of dietary fish oil clarified through cellular mechanisms of omega‐3 polyunsaturated fatty acids. Eur J Appl Physiol. 2014;114:1333–1356. [DOI] [PubMed] [Google Scholar]
- 23. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35:2950–2959. pii: ehu299. [DOI] [PubMed] [Google Scholar]
- 24. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabriel R, Alonso M, Segura A, Tormo MJ, Artigao LM, Banegas JR, Brotons C, Elosua R, Fernández‐Cruz A, Muñiz J, Reviriego B, Rigo F; ERICE Cooperative Group . Prevalence, geographic distribution and geographic variability of major cardiovascular risk factors in Spain. Pooled analysis of data from population‐based epidemiological studies: the ERICE Study. Rev Esp Cardiol. 2008;61:1030–1040. [PubMed] [Google Scholar]
- 26. Rajaram S. Health benefits of plant‐derived alpha‐linolenic acid. Am J Clin Nutr. 2014;100:443S–448S. [DOI] [PubMed] [Google Scholar]
- 27. Sanders TA. Plant compared with marine n‐3 fatty acid effects on cardiovascular risk factors and outcomes: what is the verdict? Am J Clin Nutr. 2014;100:453S–458S. [DOI] [PubMed] [Google Scholar]
- 28. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. [DOI] [PubMed] [Google Scholar]
- 29. Kromhout D, Giltay EJ, Geleijnse JM. n‐3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 30. Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, Hennekens CH, Willett WC. Dietary intake of alpha‐linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69:890–897. [DOI] [PubMed] [Google Scholar]
- 32. Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Willett WC, Hu FB. Dietary alpha‐linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–3238. [DOI] [PubMed] [Google Scholar]
- 33. Winnik S, Lohmann C, Richter EK, Schäfer N, Song WL, Leiber F, Mocharla P, Hofmann J, Klingenberg R, Borén J, Becher B, Fitzgerald GA, Lüscher TF, Matter CM, Beer JH. Dietary alpha‐linolenic acid diminishes experimental atherogenesis and restricts T cell‐driven inflammation. Eur Heart J. 2011;32:2573–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Degirolamo C, Kelley KL, Wilson MD, Rudel LL. Dietary n‐3 LCPUFA from fish oil but not alpha‐linolenic acid‐derived LCPUFA confers atheroprotection in mice. J Lipid Res. 2010;51:1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koh AS, Pan A, Wang R, Odegaard AO, Pereira MA, Yuan JM, Koh WP. The association between dietary omega‐3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol. 2015;22:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation. 2013;128:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause‐specific mortality. N Engl J Med. 2013;369:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grosso G, Yang J, Marventano S, Micek A, Galvano F, Kales SN. Nut consumption on all‐cause, cardiovascular, and cancer mortality risk: a systematic review and meta‐analysis of epidemiologic studies. Am J Clin Nutr. 2015;101:783–793. [DOI] [PubMed] [Google Scholar]
- 39. Guasch‐Ferré M, Bulló M, Martínez‐González MÁ, Ros E, Corella D, Estruch R, Fitó M, Arós F, Wärnberg J, Fiol M, Lapetra J, Vinyoles E, Lamuela‐Raventós RM, Serra‐Majem L, Pintó X, Ruiz‐Gutiérrez V, Basora J, Salas‐Salvadó J; PREDIMED study group . Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guasch‐Ferré M, Hu FB, Martínez‐González MA, Fitó M, Bulló M, Estruch R, Ros E, Corella D, Recondo J, Gómez‐Gracia E, Fiol M, Lapetra J, Serra‐Majem L, Muñoz MA, Pintó X, Lamuela‐Raventós RM, Basora J, Buil‐Cosiales P, Sorlí JV, Ruiz‐Gutiérrez V, Martínez JA, Salas‐Salvadó J. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]


