Abstract
Background
Heart failure is a highly prevalent cardiovascular complication among patients receiving long‐term hemodialysis, but the benefits of carvedilol, bisoprolol, and metoprolol controlled release/extended release on the outcomes of these patients remain unclear. In this study, we address the use of these 3 β‐blockers and their associations with mortality.
Methods and Results
Long‐term hemodialysis patients, aged ≥35 years, with new‐onset heart failure and receiving various medications were identified through the use of 1999–2010 data from the Taiwan National Health Insurance Research Database. From the total of 4435 heart failure patients, we selected 1700 new users of the 3 β‐blockers (study group) and 1700 nonusers (control group), by using matched cohorts according to their propensity scores, and then compared the 5‐year all‐cause mortality rates by using Cox proportional hazard regressions and time‐dependent covariate adjustment. During 3944 person‐years of follow‐up, 666 (39.2%) deaths occurred within the study group, compared with 918 (54%) deaths during 2893 person‐years of follow‐up in the control group. The 5‐year mortality rate for the study (control) group was 54.5% (70.3%); P<0.001. Adjusted hazard regression analyses revealed that the therapeutic effects of β‐blockers remained significant for all‐cause mortality (hazard ratio 0.80, 95% CI 0.72 to 0.90). Subgroup analyses revealed that patients in the study group receiving β‐blockers plus renin‐angiotensin system antagonists exhibited the lowest mortality rate, while the highest mortality rate was found among patients in the control group receiving neither β‐blockers nor renin‐angiotensin system antagonists.
Conclusions
This study demonstrates that the 3 β‐blockers were associated with improved survival in long‐term hemodialysis patients with heart failure.
Keywords: end‐stage renal disease, heart failure, hemodialysis, mortality, β‐blocker
Subject Categories: Heart Failure, Congenital Heart Disease
Introduction
Heart failure (HF) is known to be a highly prevalent cardiovascular complication among patients receiving long‐term hemodialysis (HD).1 The current treatment guidelines for HF, which include those provided by the American College of Cardiology Foundation/American Heart Association and the European Society of Cardiology, recommend the use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin type II receptor blockers (ARBs) as a means of improving the general survival rates for all patients.2, 3 These 2 sets of guidelines also recommend the use of carvedilol, bisoprolol, or metoprolol controlled release (CR)/extended release (XL), in addition to an ACEI or ARB, as a means of improving HF symptoms and a potential survival benefit.4, 5, 6, 7, 8, 9 Indeed, in patients with HF symptoms, the European Society of Cardiology further recommends administering 1 of the 3 β‐blockers as the initial therapy, followed by the use of an ACEI or ARB.3, 10, 11 Among the general population as a whole, the most compelling evidence on survival in cases of severe chronic HF is provided by the use of carvedilol, followed by bisoprolol and metoprolol CR/XL.4, 5, 6, 7, 8, 9, 10, 11
HF is among the most frequent cardiovascular complications in HD patients, and there is currently insufficient evidence to show the benefits of β‐blockers in long‐term HD patients with HF. This is essentially as a result of the exclusion of this population from large clinical trials. During the past 10 years, several retrospective and observational studies have been carried out with the overall aim of evaluating the therapeutic effects of β‐blockers, although the focus has tended to be on dialysis patients, and not specifically on HF patients.12, 13, 14, 15 The results of these studies have, however, been inconclusive, with only a few providing evidence in support of the use of β‐blockers in HD patients.13, 14, 15 Several factors may have contributed to the mixed results of these studies, including underpowered and misclassified drug exposure over time, as well as a lack of adjustment for nonrandom treatment allocation. Only a study with a small sample size (114 patients) has reported a reduction in all‐cause hospitalization and all‐cause mortality as a result of the addition of carvedilol to ACEI use in long‐term HD patients with HF.16 However, it remains unclear as to whether β‐blockers can be used as the initial therapy regimen for long‐term HD patients with HF.
According to 2005–2012 data reported in the latest Taiwan Renal Registry Data System database, Taiwan has one of the highest prevalence rates (2926/1 million population) and incidence rates (426/1 million population) of end‐stage renal disease in the world.17 Appropriate treatment for long‐term HD patients with HF will prolong their survival and improve their quality of life. To provide more evidence to improve treatment methods for these patients, we set out in the present study to investigate the effects of 3 β‐blockers (carvedilol, bisoprolol, and metoprolol CR/XL) on mortality rates among long‐term HD patients with HF based on real‐world clinical practice information obtained from the Taiwan National Health Insurance (NHI) research database (NHIRD).
Methods and Materials
Study Design and Data Sources
We carried out a nationwide retrospective cohort study based on 1999–2010 data on all patients receiving HD obtained from the NHIRD. The NHIRD provides healthcare utilization data on >99% of the entire 23 million people enrolled in the NHI program and 95% of all hospitals in Taiwan, with the International Classification of Diseases, 9th Revision (ICD‐9) codes being used to define the diseases. The NHIRD are all delinked information and contain all registry and claim data, including the (1) outpatient expenditure, (2) inpatient expenditure, (3) registry for medical personnel with data on each medical professional's date of birth, sex, profession, and specialty, (4) registry of contracted medical facilities with data on each medical institution's accreditation level and geographical location, and (5) registry for patients with catastrophic illness with data for 30 illness and injury categories. The data set has been used for epidemiologic research, and the results have been validated for several diseases, including acute kidney injury, chronic kidney disease, coronary artery disease, congestive HF, and diabetes mellitus.18, 19, 20, 21 This study was approved by the Joint Institutional Review Board of Taipei Medical University, and informed consent was waived because the personal information had been delinked in the NHIRD.
Study Population and Cohorts
We first defined and identified long‐term HD patients who had undergone ≥26 HD sessions within 3 months of commencing HD. A total of 74 838 patients who received long‐term HD were identified by using the catastrophic illness registry in the NHIRD from 2001 to 2010, with 1999–2010 NHIRD data being used for comorbidity evaluations and follow‐up analysis purposes. We then defined the HF patients based on the ICD‐9 codes. The codes for HF are 401.91, 402.01, 402.11, 404.01, 404.03, 404.11, 404.91, 404.93, and 428. Our sample included new‐onset HF patients after they started to receive HD. The inclusion criteria for HF patients were (1) ≥3 outpatient visit claims with an HF diagnosis within 365 days or (2) 1 claim for incident hospitalization with an HF diagnosis. Figure 1 provides a schematic illustration of the sample selection. The β‐blockers examined in this study were carvedilol, bisoprolol, or metoprolol CR/XL for ≥30 days, because only these 3 β‐blockers are proved to have survival benefits for HF patients. The exclusion criteria were (1) patients diagnosed with HF before HD, (2) patients taking these β‐blockers for <30 days, (3) patients taking β‐blockers within the 3‐month period before HF diagnosis (ie, washout period), (4) patients using β‐blockers other than our 3 focus β‐blockers, and (5) patients who did not take any antihypertensive drug. We also defined comorbidities by using the same criteria, according to the ICD‐9 codes, as shown in Table 1.
Figure 1.

Enrollment of study participants.
Table 1.
Baseline Characteristics of the Full Sample and the Propensity Score–Matched Sample
| Characteristics | Full Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Study Group (n=2095) | Control Group (n=2340) | P Value | Study Group (n=1700) | Control Group (n=1700) | ASD (%) | |
| Sex: male, n (%) | 1051 (50.2) | 1135 (48.5) | 0.25 | 844 (49.7) | 834 (49.1) | 1.2 |
| Age at cohort entry (y), mean (SD) | 65.6 (11.5) | 69.1 (11.4) | <0.001 | 67.3 (11.1) | 67.5 (11.5) | 1.7 |
| 35 to 44 y, n (%) | 81 (3.8) | 71 (3.0) | <0.001 | 54 (3.2) | 60 (3.5) | 2.4 |
| 45 to 54 y, n (%) | 366 (17.5) | 225 (9.6) | 210 (12.4) | 205 (12.1) | ||
| 55 to 64 y, n (%) | 522 (24.9) | 470 (20.1) | 398 (23.4) | 399 (23.5) | ||
| 65 to 74 y, n (%) | 635 (30.3) | 796 (34.0) | 578 (34.0) | 570 (33.5) | ||
| ≥75 y, n (%) | 491 (23.4) | 778 (33.2) | 460 (27.1) | 466 (27.4) | ||
| Charlson comorbidity index, mean (SD)a | 3.64 (3.0) | 3.60 (2.8) | <0.001 | 3.68 (2.9) | 3.64 (2.9) | 1.7 |
| No. of hospitalizations, mean (SD)a | 2.7 (2.6) | 2.3 (2.3) | <0.001 | 2.6 (2.5) | 2.4 (2.3) | 3.5 |
| Duration of dialysis at enrollment (mo), mean (SD) | 32.1 (24.2) | 30.1 (25.5) | <0.01 | 32.5 (24.3) | 30.9 (25.5) | 6.1 |
| Comorbiditiesa (ICD‐9 codes), n (%) | ||||||
| Ischemic heart disease (411, 413, 414) | 814 (38.9) | 831 (35.5) | 0.02 | 630 (37.1) | 630 (37.1) | 0 |
| Myocardial infarction (410, 412) | 99 (4.7) | 98 (4.2) | 0.38 | 85 (5.0) | 79 (4.7) | 1.7 |
| Cardiac dysrhythmia (426, 427)b | 254 (12.1) | 296 (12.6) | 0.61 | 203 (11.9) | 207 (12.2) | 0.7 |
| Cerebrovascular disease (430 to 438) | 359 (17.1) | 531 (22.7) | <0.001 | 324 (19.1) | 328 (19.3) | 0.6 |
| Peripheral artery disease (440.2, 443) | 95 (4.5) | 91 (3.9) | 0.28 | 69 (4.1) | 73 (4.3) | 1.2 |
| Hypertension (401 to 405) | 1621 (77.4) | 1769 (75.6) | 0.14 | 1300 (76.5) | 1285 (75.6) | 2.1 |
| Diabetes mellitus (250) | 1065 (50.8) | 1217 (52.0) | 0.47 | 858 (50.5) | 914 (53.8) | 6.6 |
| COPD (491 to 493, 495 to 496) | 250 (11.9) | 379 (16.2) | <0.001 | 227 (13.4) | 228 (13.4) | 0.2 |
| Cirrhosis of liver (571) | 70 (3.3) | 115 (4.9) | <0.01 | 64 (3.8) | 61 (3.6) | 0.9 |
| Cancer (140 to 208) | 205 (9.8) | 245 (10.5) | 0.46 | 174 (10.2) | 174 (10.2) | 0 |
| Tests or procedures,a n (%) | ||||||
| Echocardiography | 1409 (67.3) | 1452 (62.1) | <0.001 | 1126 (66.2) | 1074 (63.2) | 6.4 |
| Myocardial perfusion scan | 314 (15.0) | 230 (9.8) | <0.001 | 242 (14.2) | 183 (10.8) | 10.5 |
| Coronary angiography | 223 (10.6) | 171 (7.3) | <0.001 | 166 (9.8) | 137 (8.1) | 6.0 |
| Percutaneous coronary intervention | 131 (6.3) | 82 (3.5) | <0.001 | 97 (5.7) | 67 (3.9) | 8.2 |
| Coronary artery bypass graft surgery | 14 (0.7) | 11 (0.5) | 0.38 | 12 (0.7) | 8 (0.5) | 3.1 |
| 24‐Hour electrocardiogram | 219 (10.5) | 240 (10.3) | 0.81 | 180 (10.6) | 178 (10.5) | 0.4 |
| Permanent pacemaker implantation | 15 (0.7) | 26 (1.1) | 0.17 | 13 (0.8) | 17 (1.0) | 2.5 |
ASD indicates absolute standardized difference; COPD, chronic obstructive pulmonary disease; ICD‐9, International Classification of Diseases, 9th Revision.
Within the 2‐year period before the index date.
Cardiac dysrhythmia includes both conduction disorders (ICD‐9 code 426: atrioventricular block, bundle branch block, and anomalous atrioventricular excitation) and cardiac dysrhythmias (ICD‐9 code 427: paroxysmal supraventricular and ventricular tachycardia, atrial fibrillation and flutter, ventricular fibrillation and flutter, cardiac arrest, and premature beats).
The sample patients were further divided into 2 subgroups, the “study group” who were defined as new users of the 3 β‐blockers following their HF diagnosis, and the “control group” who had never used any β‐blockers after their HF diagnosis. We were ultimately left with 2095 patients as the study group and 2340 patients as the control group (Figure 1).
Propensity Score Computation and Matching
We used propensity score (PS) analyses to adjust for any differences in the baseline patient characteristics between the study and control groups and to reduce the biases in the estimation process that may be attributable to such differences.22 The scores were computed by modeling a logistic regression with use of the variables of patient age and sex, HD duration at enrollment, the number of hospitalizations, Charlson comorbidity index, all comorbidities, and performed tests/procedures (Table 1) and medication being consumed at enrollment, as reported in Table 2. Prescription data were classified according to the Anatomical Therapeutic and Chemical Classification System. The logistic regression model used to calculate the PS and the distribution of PS among the treatment and control groups are shown in Tables S1 and S2. The c‐statistic value for the model used to create the PS was 0.66, indicating that the predictive accuracy of the logistic model was fairly good.23 We used the “nearest neighbor matching without replacement on the estimated PS” to match patients.24 Patients receiving the 3 β‐blockers were matched 1:1 to untreated patients with a difference in PS of ≤0.1. Finally, 81% of the treated patients were matched to a control and 1700 patients were identified in each group (Figure 1, Table 1, Table S3, and Table 2).
Table 2.
Concomitant Medication at Enrollment for the Full Sample and the Propensity Score–Matched Samplea
| Variables | Full Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Study Group (n=2095) | Control Group (n=2340) | P Value | Study Group (n=1700) | Control Group (n=1700) | ASD (%) | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| ACEIs or ARBs | 752 (35.9) | 579 (24.7) | <0.001 | 494 (29.1) | 486 (28.6) | 1.0 |
| Calcium channel blockers | 1014 (48.4) | 1011 (43.2) | <0.001 | 767 (45.1) | 781 (45.9) | 1.7 |
| α‐Blockers | 121 (5.8) | 123 (5.3) | 0.44 | 91 (5.4) | 90 (5.3) | 0.3 |
| Hydralazine | 68 (3.3) | 70 (3.0) | 0.62 | 51 (3.0) | 52 (3.1) | 0.3 |
| Nitrates | 784 (37.4) | 779 (33.3) | <0.01 | 602 (35.4) | 596 (35.1) | 0.7 |
| Digoxin | 153 (7.3) | 139 (5.9) | 0.07 | 102 (6.0) | 107 (6.3) | 1.2 |
| Antiarrhythmics | 208 (9.9) | 243 (10.4) | 0.63 | 165 (9.7) | 157 (9.2) | 1.6 |
| Platelet inhibitors | 567 (27.1) | 579 (24.7) | 0.07 | 442 (26.0) | 438 (25.8) | 0.5 |
| Warfarin | 46 (2.2) | 59 (2.5) | 0.48 | 37 (2.2) | 43 (2.5) | 2.3 |
| Statins | 275 (13.1) | 244 (10.4) | <0.01 | 214 (12.6) | 202 (11.9) | 2.2 |
| Fibrates | 100 (4.8) | 125 (5.3) | 0.40 | 80 (4.7) | 107 (6.3) | 7.0 |
| Oral hypoglycemic drugs | 456 (21.8) | 502 (21.5) | 0.78 | 364 (21.4) | 390 (22.9) | 3.7 |
| Insulins | 470 (22.4) | 570 (24.4) | 0.14 | 352 (20.7) | 431 (25.4) | 11.1 |
| H2‐antagonists or PPIs | 525 (25.1) | 747 (31.9) | <0.001 | 419 (24.7) | 537 (31.6) | 15.5 |
| NSAIDs | 953 (45.5) | 1075 (45.9) | 0.80 | 762 (44.8) | 780 (45.9) | 2.1 |
| Benzodiazepinesb | 688 (32.8) | 735 (31.4) | 0.29 | 541 (31.8) | 548 (32.2) | 1.0 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin type II receptor blocker; ASD, absolute standardized difference; NSAID, nonsteroidal anti‐inflammatory drug; PPI, proton pump inhibitor.
Within the 3‐month period before the index date.
Refers to benzodiazepines used as anxiolytics, hypnotics, and sedatives.
Outcome Measures
The main outcome of interest in this study was the all‐cause mortality within the 5‐year period after the index date, with the first β‐blocker prescription after HF diagnosis being defined as the index date for the study group to prevent immortal time bias, and the date of HF diagnosis being defined as the index date for the control group. The HF patients were subsequently followed up for a period of up to 5 years or until the date of death. A death event was identified if the date of death was obtained from the NHIRD or the patients were disenrolled from the NHI program and had not been enrolled in the NHI beneficiary registry files. In Taiwan, patients receiving long‐term HD rarely withdrew from the NHI because the NHI is a compulsory plan. The censor criteria included patients who were followed for up to 5 years until the last day of follow‐up (December 31, 2010) or death (whichever happened first). Patients who switched from or to peritoneal dialysis and received a kidney transplant after starting long‐term HD were already excluded (Figure 1). Further subgroup analysis was carried out according to the use of ACEIs or ARBs during the follow‐up period.
Statistical Analysis
The first of the analyses in this study involved a comparison between the characteristics of the study group and those of the control group at the baseline. We estimated absolute standardized differences for all the covariates after matching to assess postmatch balance. Absolute standardized differences directly quantify balance in the means of covariates across the groups. The differences are displayed as percentages of pooled standardized differences. An absolute standardized difference of 0% on a covariate indicates no between‐group imbalance for that covariate, and values <10% indicate inconsequential imbalance.25 We used an independent‐sample Student t test to analyze the continuous variables, with the categorical variables being analyzed by using the Pearson χ2 test. We then charted the survival curves by using the Kaplan–Meier method and subsequently examined the treatment effect with use of the log‐rank test.
Finally, we applied Cox regression univariate and multivariable analyses with and without adjustment for the demographic variables (sex and age), the clinically relevant variables (diabetes, ischemic heart disease, duration of dialysis at enrollment, number of hospitalizations, and the Charlson comorbidity index), the procedures (myocardial perfusion scan, coronary angiography, and percutaneous coronary intervention), and medication at enrollment (fibrates, insulins, H2‐antagonists, and proton pump inhibitors) to assess the therapeutic effects on the probability of death. The proportional hazards assumption was also tested. The difference between the 2 groups was considered significant if the 2‐sided P<0.05. All of the analyses in this study were carried out by using SAS 9.3 software (SAS Institute Inc).
Sensitivity Analysis
In addition to the main analysis, we performed additional analyses to assess the reliability of our results. The US National Kidney Foundation provided a Kidney Disease Outcomes Quality Initiative evidence‐based clinical practice guideline for long‐term HD patients with cardiovascular disease in 2005.26 The guideline suggested the treatment of HF in patients on long‐term HD with conventional therapies according to expert opinion, and the evidence for the use of a β‐blocker was moderately strong (only carvedilol). To evaluate the effect of the initiative guideline on the therapeutic results of our 10‐year cohort study, we first conducted discrete analyses for patients with index dates from 2001 to 2005 and from 2006 to 2010 (before and after publication of the guideline).
Second, persistence in therapy is a potential confounding factor in this study. The patients who received β‐blockers, ACEIs, or ARBs might not continue therapy during the entire follow‐up period. Because the consumption of β‐blocker would accumulate over time, when we followed the death event of the HF patients, the exposure duration by month of β‐blocker therapy was treated as a time‐dependent covariate to reflect its changing nature. In this analysis, we used the counting process method by allowing our β‐blocker therapy variable to vary over time. The time‐dependent variable of each individual was followed and updated at every monthly interval. For example, if an individual started β‐blocker therapy for 10 months, quit for 3 months, then restarted for 2 months before dying, we would have 2 changes of β‐blocker therapy status. We then set these 3 observations equal to 10 months with β‐blocker therapy, 3 months without β‐blocker therapy, and 2 months with β‐blocker therapy then censored. Similarly, the exposure duration of ACEI or ARB therapy was treated as a time‐dependent covariate because its use also has survival benefits for the HF patients.21
In addition, we conducted analysis by defining the observation period beginning at intervals of 30 and 45 days after the initial HF diagnosis to assess the therapeutic effects on the probability of death and to minimize the risk of potential bias.
Results
Demographic Characteristics
A total of 3400 long‐term HD patients with HF were included in the PS matching in this study (Figure 1). The baseline patient characteristics before and after cohort matching are reported in Table 1, which shows that there were no significant differences in sex, age, comorbidities, tests, and procedures between the 2 groups after the cohort matching analyses, with the exceptions of myocardial perfusion scan. Table 2 also shows that concomitant medications at the time of enrollment were similar in the 2 groups, with the exceptions of insulins and H2‐antagonists or proton pump inhibitors.
Outcomes
The primary outcome in this study is all‐cause mortality during a follow‐up period of up to 5 years. Among the matched cohort patients of the study group, classified by their first use of β‐blockers during the follow‐up period, 1008 (59.3%) patients had taken carvedilol, 629 (37%) patients had taken bisoprolol, and 63 (3.7%) patients had taken metoprolol CR/XL. The respective mean daily doses of carvedilol, bisoprolol, and metoprolol CR/XL were 16.4, 4.4, and 65.4 mg.
The respective mean (SD) durations of carvedilol, bisoprolol, and metoprolol CR/XL use were 104.5 (93.5) days/person‐year, 102.2 (101.1) days/person‐year, and 82.5 (69.2) days/person‐year. During the 3944 person‐year follow‐up period, 666 deaths (39.2%) occurred in the study group, while in the 2893 person‐year follow‐up period in the control group, there was a substantially higher total of 918 deaths (54.0%). The respective all‐cause mortality rates at the 12‐, 24‐, 36‐, 48‐, and 60‐month follow‐up periods were 19.1%, 29.1%, 38.5%, 46.2%, and 54.5%, for the study group, while the respective mortality rates for the control group over the same periods were 34.2%, 48.7%, 58.6%, 65.4%, and 70.3%. The incident rate ratios of all‐cause mortality for the study and control groups from 2001 to 2005 and from 2006 to 2010 are shown in Table S4.
The Kaplan–Meier analyses of the survival proportion of the study and control groups are illustrated in Figure 2A, which shows that the study group had significantly higher survival benefits than the control group (log‐rank test, P<0.001). We further identified the individual survival benefits of carvedilol, bisoprolol, and metoprolol CR/XL. As we can see from Figure 2B, each of the 3 β‐blockers showed significant survival benefits compared with the control group, but there were no discernible differences in survival benefits between any 2 of the 3 β‐blockers.
Figure 2.

Kaplan–Meier estimates of hemodialysis patient survival rates in a propensity‐matched inception cohort of patients with heart failure. A, Survival of patients with/without β‐blockers. B, Survival of patients receiving carvedilol, bisoprolol, metoprolol CR/XL, or no β‐blockers. No differences are discernible between the survival benefits for any 2 of these 3 β‐blockers.
Multivariable Analysis
The Cox proportional hazard regressions on all‐cause mortality are shown in Table 3. In the final model, the study group was found to have an 20% lower risk of all‐cause mortality than the control group (hazard ratio [HR], 0.80, 95% CI, 0.72 to 0.90; P<0.001) after adjustment for the exposure duration of β‐blocker therapy and the exposure duration of ACEI or ARB therapy. The survival curves of the final model after adjustment for the exposure duration of β‐blocker therapy and the exposure duration of ACEI or ARB therapy are shown in Figure 3. Detailed results from 2001 to 2005 and from 2006 to 2010 can be found in Table S5 and Figures S1 and S2.
Table 3.
Cox Proportional Hazard Regression on All‐Cause Mortality for the Study Group Versus the Control Group
| Models and Adjustments | HR | 95% CI | P Value |
|---|---|---|---|
| Univariate model | 0.56 | 0.51 to 0.62 | <0.001 |
| Multivariate model | |||
| Adjusted for diabetes | 0.57 | 0.51 to 0.63 | <0.001 |
| Adjusted for ischemic heart disease | 0.56 | 0.51 to 0.62 | <0.001 |
| Adjusted for duration of dialysis at enrollment | 0.57 | 0.51 to 0.63 | <0.001 |
| Adjusted for No. of hospitalization | 0.55 | 0.50 to 0.61 | <0.001 |
| Adjusted for Charlson comorbidity index | 0.56 | 0.51 to 0.62 | <0.001 |
| Adjusted for various proceduresa | 0.55 | 0.50 to 0.61 | <0.001 |
| Adjusted for medication at enrollmentb | 0.58 | 0.52 to 0.64 | <0.001 |
| Final modelc | 0.56 | 0.50 to 0.62 | <0.001 |
| Final model adjusted with time‐dependent covariates | |||
| Adjusted for the exposure duration of β‐blocker therapy | 0.76 | 0.68 to 0.85 | <0.001 |
| Adjusted for the exposure duration of β‐blocker therapy and the exposure duration of ACEI or ARB therapy | 0.80 | 0.72 to 0.90 | <0.001 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; HR, hazard ratio.
The procedures include myocardial perfusion scan, coronary angiography, and percutaneous coronary intervention.
The medications include fibrates, insulins, H2‐antagonists, and proton pump inhibitors.
The control variables include in the final model demographic variables (sex and age), clinically relevant variables (diabetes, ischemic heart disease, duration of dialysis at enrollment, No. of hospitalizations, and Charlson comorbidity index), procedures (myocardial perfusion scan, coronary angiography, and percutaneous coronary intervention), and medications at enrollment (fibrates, insulins, H2‐antagonists, and proton pump inhibitors).
Figure 3.

The survival curves of hemodialysis patients with heart failure in a propensity‐matched inception cohort after adjustment for the exposure duration of β‐blocker therapy and the exposure duration of ACEI or ARB therapy. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin type II receptor blocker.
The interactions between the therapeutic effect and the clinical parameters are illustrated in Figure 4, which also reveals significant interactions when considering the sex, Charlson comorbidity index, and HD sessions per month.
Figure 4.
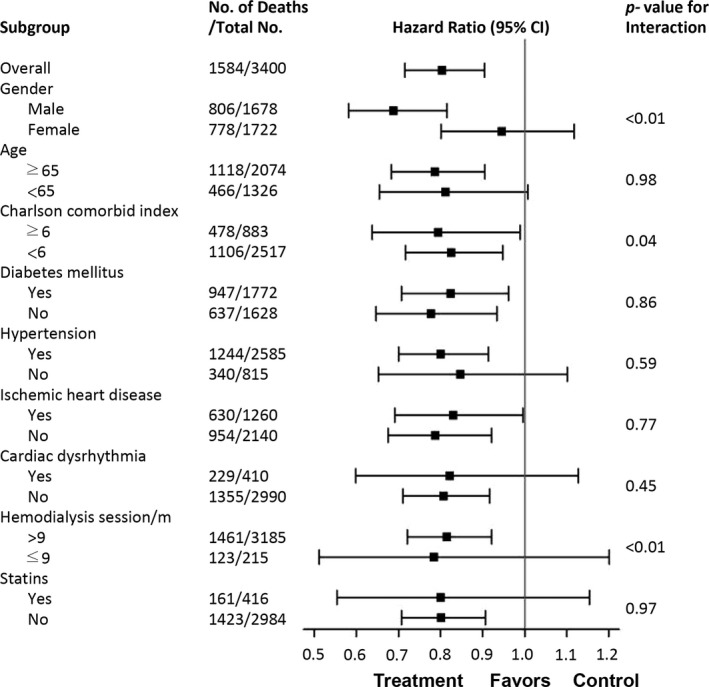
Hazard ratios for all‐cause mortality from the final multivariate model and interaction term for selected subgroups.
HR by Subgroup
The medications used at enrollment (including ACEIs or ARBs, calcium channel blockers, hydralazine, and nitrates) were similar in both groups after cohort matching. To clarify their therapeutic effects, we further analyzed ACEI or ARB use during the follow‐up period. As shown in Table 4 and Figure 5, the patients in the study group who received β‐blockers plus ACEIs or ARBs had the best survival benefits, followed by those who received β‐blockers alone. The patients in the control group who took neither of these 2 groups of drugs had the highest mortality rate.
Table 4.
Hazard Ratios for All‐Cause Mortality by Medication During the Follow‐up Period
| Subgroups | Total No. | Exposure Time, Person‐Years | Death No. | Final Model | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | ||||
| Control group | 1700 | 2893 | 918 | |||
| No β‐Blockers, ACEIs, or ARBs | 689 | 958 | 398 | 1.74 | 1.44 to 2.11 | <0.001 |
| ACEIs or ARBs | 1011 | 1935 | 520 | 1.08 | 0.90 to 1.31 | 0.42 |
| Study group | 1700 | 3944 | 666 | |||
| β‐Blockers alone | 366 | 597 | 146 | As reference | ||
| β‐Blockers plus ACEIs or ARBs | 1334 | 3347 | 520 | 0.67 | 0.55 to 0.81 | <0.001 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin type II receptor blocker; HR, hazard ratio.
Figure 5.
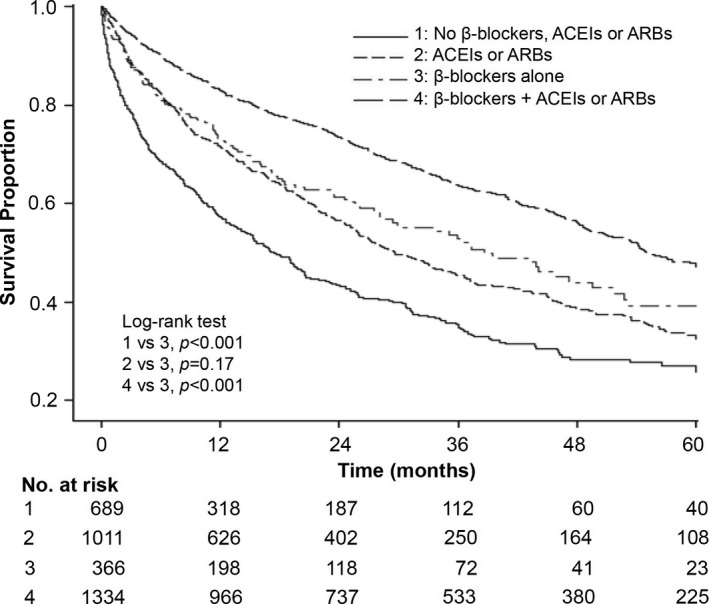
Kaplan–Meier estimates of hemodialysis patients’ survival rates in a propensity‐matched inception cohort of patients with heart failure, by β‐blocker, ACEI, or ARB use in the follow‐up period. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin type II receptor blocker.
Sensitivity Analyses for All‐Cause Death
We further analyzed the risk of all‐cause mortality with observation period beginning at intervals of 30 and 45 days after initial HF diagnosis. The results showed that the patients in the study group still had a lower risk of all‐cause mortality than those in the control group with an observation period beginning at intervals of 30 and 45 days after initial HF diagnosis (Table S6).
Discussion
The clinical therapeutic guidelines published by the American Heart Association and the European Society of Cardiology recommend carvedilol, bisoprolol, and metoprolol CR/XL for the general treatment of HF, but the therapeutic effects of the 3 β‐blockers in HF patients receiving long‐term HD have not been rigorously studied.2, 3 In our population‐based study, we examine whether therapy with the 3 β‐blockers could enhance the survival rate of long‐term HD patients with HF.
We demonstrate that therapy based on these 3 β‐blockers is associated with a 20% lower risk of all‐cause mortality in long‐term HD patients with HF (HR 0.80, 95% CI 0.72 to 0.90; P<0.001). To reduce any potential bias, we used propensity scores to identify matched cohorts for further analyses, with the results being found to be robust (P<0.001) even after adjustment for clinical variables, the exposure duration of β‐blocker therapy and the exposure duration of ACEI or ARB therapy (Table 3). We have also found that patients in the study group who received β‐blockers plus ACEIs or ARBs had the best survival rates (Table 4).
Treatment for HF in the General Population
HF with preserved ejection fraction (HFpEF) may be as common as HF with reduced ejection fraction (HFrEF). Almost 47% of community patients with HF have HFpEF,27 whereas in Chinese dialysis patients with underlying HF, 55% have evidence of HFpEF.28 According to epidemiological studies, the identification of HF is validated by using ICD‐9 codes27, 29; however, identifying HFpEF or HFrEF on the basis of ICD‐9 codes in this study was impossible because of a lack of detailed echocardiography information.
The benefits of the 3 β‐blockers, ACEIs, and ARBs in the general population with HFrEF are indicated based on improved cardiac performance, cardiac remodeling, the number of hospitalizations, and survival.2, 3 A prospective study of 41 791 patients treated with ACEIs or ARBs from the Swedish Heart Failure Registry also revealed an HR of 0.91 (95% CI 0.85 to 0.98) for community patients with HFpEF30; thus, ACEIs or ARBs may be used in both HFpEF or HFrEF patients.
β‐Blockers are also found to have the benefits of lowering blood pressure, intervening with sympathetic activation, reducing sudden death, and promoting antiremodeling effects.31, 32, 33 However, no prospective or randomized studies have yet been able to convincingly demonstrate that β‐blockers reduce morbidity and mortality in a general population with HFpEF.2, 3 The results of a recent meta‐analysis of 21 206 patients enrolled in 12 clinical studies demonstrated that β‐blocker exposure in patients with HFpEF was associated with a 9% reduction in all‐cause mortality (95% CI 0.87 to 0.95)34; based on these findings, carvedilol, bisoprolol, and metoprolol CR/XL could be used in patients with HFpEF or HFrEF.
Treatment for HF in HD Patients
Compared with the general population, HD patients have a more activated sympathetic nervous system, a higher prevalence of HF and ischemic heart disease, and a higher risk of sudden cardiac arrest35, 36, 37; however, the benefits of ACEIs, ARBs, and β‐blockers in HD patients with HFrEF have rarely been evaluated. Only one small prospective study evaluating the use of carvedilol in addition to an ACEI in 114 patients suggested that the treatment had improved survival benefits.16 Our previous population‐based study (involving 4771 patients) showed that ACEIs or ARBs improved the survival benefits of long‐term HD patients with HF regardless of whether they were HFpEF or HFrEF (HR 0.80, 95% CI 0.72 to 0.89).21 In HD patients with HFrEF, the only β‐blocker to be studied, and found to have survival benefits, was carvedilol; no studies on either bisoprolol or metoprolol CR/XL treatment have been reported.16 Similarly, no studies have been carried out to evaluate the benefits of ACEIs, ARBs, and β‐blockers in HD patients with HFpEF. We have shown that the 3 β‐blockers evaluated in the present study could improve survival in long‐term HD patients after time‐dependent adjustment for the exposure duration of β‐blocker therapy and the exposure duration of ACEI or ARB therapy (HR 0.80, 95% CI 0.72 to 0.90). In summary, the results support our hypothesis and show that β‐blockers can have survival benefits on HD patients with HF, as demonstrated by the 20% reduction in all‐cause mortality.
β‐Blockers as the Initial Therapy for HD Patients With HF
In our real‐world clinical analysis, as many as 59.3% of patients received carvedilol to treat their HF, followed by bisoprolol (in 37% of patients). A meta‐analysis previously carried out on a general population with HF evaluated the prognostic benefit of β‐blockers in patients not receiving ACEIs, with the results revealing that in the absence of an ACEI or ARB at baseline, the risk ratio for β‐blockers vis‐à‐vis placebo was 0.73 (95% CI 0.53 to 1.02), compared with a risk ratio of 0.76 (95% CI 0.71 to 0.83) when these agents were present.11 The results of the subgroup analysis undertaken in the present study (reported in Table 4 and illustrated in Figure 5) demonstrate that patients who received β‐blockers, but not ACEIs or ARBs, exhibited a similar prognostic benefit for all‐cause mortality compared with the ACEI or ARB users in the control group. These findings indicate that these 3 β‐blockers could be used as the initial therapy for long‐term HD patients with HF.
The effects of β‐blocker dialyzability on mortality in long‐term HD patients were studied recently.38 Weir et al38 examined the dialyzability of β‐blockers and mortality among older patients receiving HD, although not specifically HF patients, with the results showing that compared with a β‐blocker with low dialyzability (carvedilol or bisoprolol), a β‐blocker with high dialyzability (metoprolol) was associated with a higher rate of mortality. However, our subgroup analysis results revealed that each of these 3 β‐blockers showed significant survival benefits, with no difference in such benefits being discernible between any 2 of the 3 β‐blockers (Figure 2B). The possible explanation was that the metoprolol prescribed in the study by Weir et al38 is a short‐acting β‐blocker and cannot be extrapolated to the metoprolol CR/XL we studied, which is an extended‐release formulation.7
Limitations
The implementation of a large, randomized trial would be a significant challenge, essentially because almost 50% of long‐term HD patients with new‐onset HF are already being treated with β‐blockers (Figure 1). For this reason, the matched cohort with time‐dependent covariate adjustment used in this study was notable for its national sample size with the use of real‐world data to confirm the benefits of β‐blockers against new‐onset HF in long‐term HD patients.
Nevertheless, we caution against any attempt at generalizing our results, essentially because our study has a number of limitations. First, the major drawback of our study is its observational nature, which meant that we could not determine the underlying kidney and heart diseases and randomly assign the patients. Although we have used matched cohorts, there may still be residual confounding factors.
Second, we could not use the assigned ICD‐9 codes to identify HF severity (New York Heart Association functional class) or any detailed information on echocardiography (left ventricular end‐diastolic and end‐systolic volume, ejection fraction, and diastolic function), all of which are associated with mortality. The occurrence of HF was based on the ICD‐9 codes registered by the physicians responsible for the treatment of patients. It was verified in general population and our prior study in long‐term HD patients with HF.21, 29 However, dyspnea caused by HF or just fluid overload in long‐term HD patients made a differential diagnosis difficult if the patient was diagnosed clinically without echocardiography. We also lacked important clinical characteristics and laboratory data on the study population (eg, history of smoking and alcohol consumption, blood pressure, heart rate, electrolytes, and nutrition status). The proportion of patients undergoing echocardiography (coronary angiography) was 66% (10%), and, indeed, those who did not undergo echocardiography may have been clinically diagnosed with HF.
Third, to avoid hypotension during HD, the predialysis dosage of cardiovascular medicine before HD therapy is sometimes reduced in patients with long‐term HD; therefore, the β‐blockers used in this study may not reflect actual use.
Conclusion
The findings of this study provide clinicians with additional evidence on the therapeutic effects of carvedilol, bisoprolol, and metoprolol CR/XL in long‐term HD patients with HF. In the absence of data from large randomized trials, the findings of this nationwide retrospective cohort study demonstrate that the 3 β‐blockers were associated with improved survival in long‐term HD patients with HF.
Sources of Funding
This work was supported by grants provided by Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan (grants 100wf‐eva‐15, 102TMU‐WFH‐08 and 103TMU‐WFH‐15).
Disclosures
The authors declare that they have no competing interests.
Supporting information
Table S1. Logistic Regression Model Used to Calculate the Propensity Score
Table S2. Distribution of Propensity Score Among the Treat and Control Groups of the Full and Matched Cohorts
Table S3. Baseline Characteristics of the Excluded and Included Patients in the Study Group
Table S4. Incident Rate Ratios of All‐Cause Mortality for the Study Group Versus the Control Group
Table S5. Cox Proportional Hazard Regression on All‐Cause Mortality for the Study Group Versus the Control Group
Table S6. Cox Proportional Hazard Regression on All‐Cause Mortality for the Study Group Versus the Control Group With Observation Period Beginning at Different Intervals After Initial Heart Failure Diagnosis
Figure S1. The survival curves of hemodialysis patients with heart failure in a propensity‐matched inception cohort, 2001–2005.
Figure S2. The survival curves of hemodialysis patients with heart failure in a propensity‐matched inception cohort, 2006–2010.
(J Am Heart Assoc. 2016;5:e002584 doi: 10.1161/JAHA.115.002584)
Accompanying Tables S1 through S6 and Figures S1 and S2 are available at http://jaha.ahajournals.org/content/5/1/e002584/suppl/DC1
References
- 1. Stack AG, Bloembergen WE. A cross‐sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. 2001;38:992–1000. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 6. Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann‐Zalan I, DeMets DL. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 7. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT‐HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 8. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT‐HF). MERIT‐HF Study Group. JAMA. 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 9. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 10. Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, Krum H, Ponikowski P, Skene A, van de Ven L, Verkenne P, Lechat P. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426–2435. [DOI] [PubMed] [Google Scholar]
- 11. Krum H, Haas SJ, Eichhorn E, Ghali J, Gilbert E, Lechat P, Packer M, Roecker E, Verkenne P, Wedel H, Wikstrand J. Prognostic benefit of beta‐blockers in patients not receiving ACE‐inhibitors. Eur Heart J. 2005;26:2154–2158. [DOI] [PubMed] [Google Scholar]
- 12. Wetmore JB, Shireman TI. The ABCs of cardioprotection in dialysis patients: a systematic review. Am J Kidney Dis. 2009;53:457–466. [DOI] [PubMed] [Google Scholar]
- 13. Foley RN, Herzog CA, Collins AJ. Blood pressure and long‐term mortality in united states hemodialysis patients: USRDS waves 3 and 4 study. Kidney Int. 2002;62:1784–1790. [DOI] [PubMed] [Google Scholar]
- 14. Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. Beta‐blocker use in long‐term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164:2465–2471. [DOI] [PubMed] [Google Scholar]
- 15. Berger AK, Duval S, Krumholz HM. Aspirin, beta‐blocker, and angiotensin‐converting enzyme inhibitor therapy in patients with end‐stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. [DOI] [PubMed] [Google Scholar]
- 16. Cice G, Ferrara L, D'Andrea A, D'Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabro R. Carvedilol increases two‐year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo‐controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. [DOI] [PubMed] [Google Scholar]
- 17. Lin YC, Hsu CY, Kao CC, Chen TW, Chen HH, Hsu CC, Wu MS. Incidence and prevalence of ESRD in Taiwan renal registry data system (TWRDS): 2005–2012. Acta Nephrol. 2014;28:65–69. [Google Scholar]
- 18. Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ. Long‐term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu TW, Liu JS, Hung SC, Kuo KL, Chang YK, Chen YC, Hsu CC, Tarng DC. Renoprotective effect of renin‐angiotensin‐aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174:347–354. [DOI] [PubMed] [Google Scholar]
- 20. Chang YT, Wu JL, Hsu CC, Wang JD, Sung JM. Diabetes and end‐stage renal disease synergistically contribute to increased incidence of cardiovascular events: a nationwide follow‐up study during 1998–2009. Diabetes Care. 2014;37:277–285. [DOI] [PubMed] [Google Scholar]
- 21. Tang CH, Chen TH, Wang CC, Hong CY, Huang KC, Sue YM. Renin‐angiotensin system blockade in heart failure patients on long‐term haemodialysis in Taiwan. Eur J Heart Fail. 2013;15:1194–1202. [DOI] [PubMed] [Google Scholar]
- 22. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 23. Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed Hoboken, New Jersey: John Wiley & Sons; 2013. [Google Scholar]
- 24. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 25. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 26. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–S153. [PubMed] [Google Scholar]
- 27. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 28. Wang AY, Wang M, Lam CW, Chan IH, Lui SF, Sanderson JE. Heart failure in long‐term peritoneal dialysis patients: a 4‐year prospective analysis. Clin J Am Soc Nephrol. 2011;6:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Svanstrom H, Pasternak B, Hviid A. Association of treatment with losartan vs candesartan and mortality among patients with heart failure. JAMA. 2012;307:1506–1512. [DOI] [PubMed] [Google Scholar]
- 30. Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin‐angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA. 2012;308:2108–2117. [DOI] [PubMed] [Google Scholar]
- 31. Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin‐Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (SOLVD). Circulation. 1990;82:1724–1729. [DOI] [PubMed] [Google Scholar]
- 32. Goldberger JJ. Prevention of sudden cardiac death. Heart Dis. 2000;2:305–313. [PubMed] [Google Scholar]
- 33. Sharpe N, Doughty RN. Left ventricular remodelling and improved long‐term outcomes in chronic heart failure. Eur Heart J. 1998;19(suppl B):B36–B39. [PubMed] [Google Scholar]
- 34. Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta‐blockers on heart failure with preserved ejection fraction: a meta‐analysis. PLoS One. 2014;9:e90555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006;70:1905–1913. [DOI] [PubMed] [Google Scholar]
- 36. Alpert MA. Sudden cardiac arrest and sudden cardiac death on dialysis: epidemiology, evaluation, treatment, and prevention. Hemodial Int. 2011;15(suppl 1):S22–S29. [DOI] [PubMed] [Google Scholar]
- 37. Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end‐stage renal disease. Circulation. 2002;105:1354–1359. [DOI] [PubMed] [Google Scholar]
- 38. Weir MA, Dixon SN, Fleet JL, Roberts MA, Hackam DG, Oliver MJ, Suri RS, Quinn RR, Ozair S, Beyea MM, Kitchlu A, Garg AX. Beta‐blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol. 2015;26:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Logistic Regression Model Used to Calculate the Propensity Score
Table S2. Distribution of Propensity Score Among the Treat and Control Groups of the Full and Matched Cohorts
Table S3. Baseline Characteristics of the Excluded and Included Patients in the Study Group
Table S4. Incident Rate Ratios of All‐Cause Mortality for the Study Group Versus the Control Group
Table S5. Cox Proportional Hazard Regression on All‐Cause Mortality for the Study Group Versus the Control Group
Table S6. Cox Proportional Hazard Regression on All‐Cause Mortality for the Study Group Versus the Control Group With Observation Period Beginning at Different Intervals After Initial Heart Failure Diagnosis
Figure S1. The survival curves of hemodialysis patients with heart failure in a propensity‐matched inception cohort, 2001–2005.
Figure S2. The survival curves of hemodialysis patients with heart failure in a propensity‐matched inception cohort, 2006–2010.


