Abstract
Background
While aldosterone antagonists have proven benefit among post‐myocardial infarction (MI) patients with low ejection fraction (EF), how this treatment is used among older MI patients in routine practice is not well described.
Methods and Results
Using ACTION Registry‐GWTG linked to Medicare data, we examined 12 080 MI patients ≥65 years with EF ≤40% who were indicated for aldosterone antagonist therapy per current guidelines and without documented contraindications. Of these, 11% (n=1310) were prescribed aldosterone antagonists at discharge. Notably, 10% of patients prescribed an aldosterone antagonist were eligible for, but not concurrently treated with, an angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker. Spironolactone was the predominantly prescribed aldosterone antagonist. At 2‐year follow‐up, aldosterone antagonist use was not associated with lower mortality (unadjusted 39% versus 38%; HR 0.99, 95% CI 0.88–1.33 using inverse probability‐weighted propensity adjustment) except in symptomatic HF patients (HR 0.84, 95% CI 0.72–0.99, P interaction=0.009). Risks of hyperkalemia were low at 30 days, but significantly higher among patients prescribed aldosterone antagonists (unadjusted 2.3% versus 1.5%; adjusted HR 2.04, 95% CI 1.16–3.60), as was 2‐year risk of acute renal failure (unadjusted 6.7% versus 4.8%; adjusted HR 1.39, 95% CI 1.01–1.92) compared with patients not prescribed aldosterone antagonists.
Conclusions
Aldosterone antagonist use among eligible older MI patients in routine clinical practice was not associated with lower mortality except in patients with HF symptoms, but was associated with increased risks of hyperkalemia and acute renal failure. These results underscore the importance of close post‐discharge monitoring of this patient population.
Keywords: aldosterone antagonist therapy, heart failure, mortality, older population
Subject Categories: Myocardial Infarction, Quality and Outcomes, Secondary Prevention
Introduction
The American Heart Association (AHA)/American College of Cardiology (ACC) Guidelines provide a Class I recommendation for aldosterone antagonist therapy among eligible post‐myocardial infarction (MI) patients with left ventricular ejection fraction (EF) ≤40% and either symptomatic heart failure (HF) or diabetes mellitus.1, 2 This recommendation is primarily supported by the Eplerenone Post‐Acute MI HF Efficacy and Survival (EPHESUS) trial, which showed reductions in mortality and HF rehospitalization risk with eplerenone therapy in this patient population.3 Yet older adults are often under‐represented in randomized clinical trials, leading to uncertainty about the risk‐benefit treatment balance. Furthermore, aldosterone antagonist use is of particular concern among older adults since age‐related physiologic changes and comorbidities may significantly influence treatment outcomes.
Therefore, we examined a population of patients ≥65 years hospitalized for an acute MI in routine clinical practice who had an indication for aldosterone antagonist therapy. The goals of this study were to describe current patterns of aldosterone antagonist use in routine practice, and to assess the longitudinal effectiveness and safety of aldosterone antagonists among an older MI patient population. We hypothesized that, as seen in EPHESUS, aldosterone antagonist use would be associated with significantly lower risks of mortality and HF rehospitalization, but higher risks of rehospitalization for hyperkalemia or acute renal failure due to underlying comorbidities and lower baseline renal function.
Methods
Study Population
The linkage of Acute Coronary Treatment and Intervention Outcomes Network Registry®‐Get With The Guidelines™ (ACTION Registry‐GWTG) data to longitudinal Medicare claims offered a unique opportunity to study patterns of care and the longitudinal safety and effectiveness of aldosterone antagonists among older MI patients. The ACTION Registry‐GWTG is a nationally representative quality improvement registry of consecutive ST‐segment elevation MI (STEMI) and non‐STEMI (NSTEMI) patients treated at more than 500 hospitals in the United States, collecting detailed information on medical history, symptoms or signs of HF, EF measurements, prescribed medications, and contraindications to therapy via retrospective chart review. In the absence of unique patient identifiers, we used 5 indirect identifiers (date of birth, sex, hospital identifier, date of admission, date of discharge) to link ACTION Registry‐GWTG patients ≥65 years to their Medicare claims record, as previously described.4 This linkage extended the reach of ACTION Registry‐GWTG to permit assessment of post‐discharge longitudinal outcomes among these MI patients.
The linked database included 79 750 patients ≥65 years hospitalized for STEMI or NSTEMI between January 1, 2007 and December 31, 2010 (Figure 1). Among these, we focused on the 17 393 patients (22%) who met a guideline indication for aldosterone antagonist therapy, defined as in‐hospital EF ≤40% and either: (1) signs or symptoms of HF on admission or during the index MI hospitalization; or (2) in the absence of HF, a history of diabetes. We excluded patients who died during the index hospitalization (n=2507); were transferred out to another hospital and discharge therapy could not be ascertained (n=681); were discharged against medical advice or to end‐of‐life hospice care (n=761); had any contraindication to aldosterone antagonist use documented in the medical record (n=671); had hyperkalemia during the index MI hospitalization (n=275; International Classification of Disease, Ninth Revision, Clinical Modification [ICD‐9‐CM] code 276.7); or had missing data on aldosterone antagonist prescription (n=70). For patients with multiple MI admissions, we used the index admission (excluding 348 subsequent admissions). These exclusions yielded a final analysis population of 12 080 patients treated at 464 ACTION Registry‐GWTG hospitals who met a guideline‐recommended indication for, and were not contraindicated to, aldosterone antagonist therapy.
Figure 1.
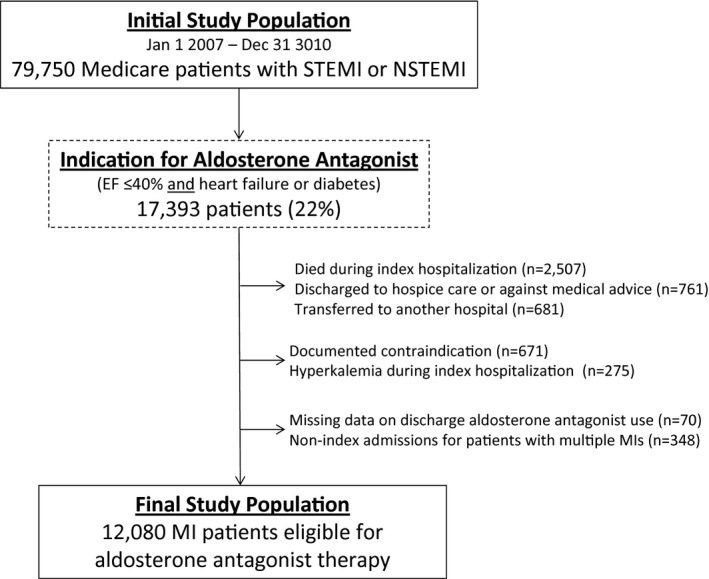
Study population. This figure displays the initial population, through exclusions, to the final study population. EF indicates ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Data Definitions
Discharge prescription of an aldosterone antagonist was denoted on the ACTION Registry‐GWTG data collection form. The primary effectiveness outcome was death within 2 years post‐discharge as ascertained from the Medicare denominator file. Secondary effectiveness outcomes evaluated rehospitalization for a cardiovascular event, rehospitalization for HF, and all‐cause rehospitalization within 2 years post‐discharge. Cardiovascular and HF rehospitalizations were defined using the primary (first) diagnosis code and all procedure codes for subsequent hospitalizations in the Medicare claims file as in previous studies of Medicare data.5 Safety endpoints included rehospitalization for hyperkalemia (ICD‐9‐CM code 276.7) and rehospitalization for acute renal failure (ICD‐9‐CM code 584.x, 586.x, or 788.5).6 To enhance sensitivity for these safety endpoints; ICD‐9‐CM codes in any diagnosis position were included. Safety endpoints were evaluated at 30 days and 2 years post‐discharge. Given concerns for persistent selection bias despite propensity adjustment, we also examined rehospitalization for pneumonia (ICD‐9‐CM codes 481.0–486.0 and 487.0 in the first diagnosis position) within 2 years post‐discharge as a falsification endpoint, which is an endpoint for which the treatment is, a priori, believed to be unlikely to have an effect.7 If the treatment does have a significant effect on these outcomes, then this finding raises concern that there are confounding factors present and, therefore, these outcomes are analogous to “negative controls” in laboratory science.
Statistical Methods
Baseline clinical characteristics and in‐hospital treatment were compared between patients who were and were not discharged on aldosterone antagonist therapy. Continuous variables were expressed as median values with 25th and 75th percentiles, and categorical variables were presented as percentages. Continuous variables were compared using Wilcoxon rank‐sum tests and categorical variables were compared using Pearson's χ2 tests.
The unadjusted cumulative incidence of each outcome was compared using log‐rank tests for mortality, and Gray's method for rehospitalizations to account for mortality as a competing risk.8 We used inverse probability‐weighted methods to study the association of aldosterone antagonist use with each outcome. We created a propensity score model with discharge aldosterone antagonist as the outcome, conditioned on a comprehensive list of baseline clinical covariates captured in the ACTION Registry‐GWTG (Table S1). Using this model, we estimated each patient's probability of receiving treatment conditioned on observed covariates. We assessed the balance of measured covariates between groups after propensity modeling using Cramer's phi for categorical variables and R‐squared measure of association for continuous variables. Then, we assigned a weight as the inverse of that probability for each patient to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox regression modeling for each outcome.9
We conducted a series of secondary analyses to determine whether a differential effect was observed in certain subgroups for mortality and hyperkalemia. For each subgroup, we added the subgroup variable and an interaction term between subgroup and aldosterone antagonist use into each Cox model. If the interaction term was significant (P<0.05), then we determined that a differential relationship between aldosterone antagonist use and the outcome existed between subgroup types. Adjusted HRs and 95% CIs were reported for each outcome using the inverse‐probability‐weighted propensity adjustment method described above.
Finally, 4677 patients (40% of the final study population) were enrolled in the Medicare Prescription Drug Plan (Part D) prior to index hospital discharge, which presented an opportunity to examine rates of aldosterone antagonist initiation and persistence post‐discharge. The comparison of Medicare patients enrolled and not enrolled in part D have been described previously.10 Among patients not prescribed aldosterone antagonist at index discharge, we reviewed medication fill records to calculate the proportion of patients who filled an aldosterone antagonist prescription within 14 days (EPHESUS treatment window) and 6 months post‐discharge. Among those prescribed an aldosterone antagonist, we examined the type of medication filled (eplerenone versus spironolactone), and the persistence of treatment at 6 months (persistence defined as prescription claims showing continuous supply of the prescribed medication without a treatment gap >60 days). We conducted multivariable Cox regression using post‐discharge aldosterone antagonist use as a time‐dependent covariate. In this “on‐treatment” comparison, patients were considered to be on‐treatment until the first day of a >60 day gap. We adjusted for all covariates (Table S1) to compare 2‐year risks of mortality and rehospitalization for hyperkalemia between patients who received aldosterone antagonist therapy and those who did not.
This study was supported by a Centers for Education and Research on Therapeutics grant (U19HS021092) from the Agency for Healthcare Research and Quality. The Duke University Medical Center Institutional Review Board granted a waiver of the informed consent and authorization for this study, and all analyses were conducted by the Duke Clinical Research Institute using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
Patterns of Aldosterone Antagonist Use
Among MI patients ≥65 years who met indications and were eligible for treatment, 1310 (10.8%) were prescribed an aldosterone antagonist at discharge. Clinical comorbidities were prevalent in this older population (median age: 77 years). Patients prescribed an aldosterone antagonist were more likely to have presented with STEMI and to have cardiogenic shock or symptomatic HF during the MI hospitalization than patients discharged on no aldosterone antagonist (Table 1). While all patients in our study population had an EF ≤40%, patients prescribed an aldosterone antagonist were more likely to have worse left ventricular function (EF <25%: 30% versus 17%, P<0.001). Patients prescribed an aldosterone antagonist at discharge were more likely to have been on the medication prior to admission (24% versus 1%, P<0.001).
Table 1.
Patient Characteristics
| Aldosterone Antagonist at Discharge | P Value | ||
|---|---|---|---|
| Prescribed (n=1310) | Not Prescribed (n=10 770) | ||
| Demographics | |||
| Age, y | 76 (70, 83) | 77 (71, 84) | 0.001 |
| Female sex | 45.0% | 44.0% | 0.46 |
| Race | |||
| White | 86.8% | 86.8% | 0.60 |
| Black | 7.7% | 7.8% | |
| Other | 5.5% | 5.4% | |
| Clinical characteristics | |||
| Prior HF | 40.0% | 31.8% | <0.001 |
| Prior MI | 38.2% | 35.9% | 0.10 |
| Prior CABG | 25.6% | 25.8% | 0.84 |
| Prior PCI | 27.9% | 27.2% | 0.61 |
| Diabetes | 51.6% | 59.3% | <0.001 |
| Prior stroke | 15.3% | 14.0% | 0.19 |
| Peripheral arterial disease | 17.9% | 18.4% | 0.67 |
| Medication use prior to MI admission | |||
| ACEI/ARB | 52.5% | 51.0% | 0.26 |
| Beta‐blocker | 55.5% | 52.2% | 0.03 |
| Aldosterone antagonist | 24.3% | 1.3% | <0.001 |
| In‐hospital characteristics | |||
| STEMI (vs NSTEMI) presentation | 34.7% | 29.2% | <0.001 |
| Body mass index, kg/m2 | 27.1 (23.8, 30.9) | 27.1 (23.8, 31.1) | 0.95 |
| Cardiogenic shock during hospitalization | 15.7% | 12.1% | <0.001 |
| Signs of HF during hospitalizationa | 80.0% | 65.6% | <0.001 |
| Systolic BP on admission, mm Hg | 135 (115, 152) | 140 (119, 161) | <0.001 |
| EF <25% | 29.5% | 16.6% | <0.001 |
| Dialysis | 0.4% | 4.1% | <0.001 |
| CrCl (among non‐dialysis, mL/min) | 52.9 (39.0, 72.1) | 50.5 (35.3, 69.3) | <0.001 |
| Cr >2.5 for men or >2.0 for women | 4.1% | 9.9% | <0.001 |
Continuous variables presented as median (25th, 75th percentile). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CABG, coronary artery bypass grafting; CrCl, creatinine clearance; EF, ejection fraction; HF, heart failure; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Defined as documentation of symptoms of heart failure (eg, dyspnea on light exertion, recurrent dyspnea in the supine position, fluid retention); or the description of rales, jugular venous distension, pulmonary edema on physical exam, or pulmonary edema on chest x‐ray attributed to cardiac dysfunction.
Patients prescribed an aldosterone antagonist had a median creatinine clearance of 53 (25th, 75th percentiles: 39, 72); those not prescribed had a creatinine clearance of 50 (25th, 75th percentiles: 35, 69; P<0.001). The EPHESUS trial excluded men with creatinine levels >2.5 mg/dL and women with creatinine >2.0 mg/dL.3 In our study, 4% of patients prescribed an aldosterone antagonist at discharge met this criteria, and 0.4% were on dialysis.
Rates of reperfusion for STEMI and percutaneous coronary intervention for NSTEMI were not significantly different between groups. Patients prescribed an aldosterone antagonist at discharge were less likely to have had in‐hospital coronary artery bypass graft surgery (Table 2). Approximately one‐third of patients prescribed an aldosterone antagonist at discharge received the medication within the first 24 hours of admission. Patients prescribed an aldosterone antagonist were also more likely to be prescribed an angiotensin‐converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) and a beta‐blocker at discharge. Notably, 10% of patients prescribed an aldosterone antagonist at discharge were not concurrently treated with ACEI/ARB, even in the absence of therapy contraindication.
Table 2.
In‐Hospital Treatment Among Patients Without Contraindications
| Aldosterone Antagonist at Discharge | P Value | ||
|---|---|---|---|
| Prescribed (n=1310) | Not Prescribed (n=10 770) | ||
| Management strategy | |||
| Primary PCI (among STEMI) | 81.4% | 78.5% | 0.12 |
| Fibrinolysis (among STEMI) | 12.0% | 11.9% | 0.89 |
| Diagnostic cath (among NSTEMI) | 68.1% | 66.4% | 0.32 |
| PCI (among NSTEMI) | 33.6% | 31.9% | 0.30 |
| CABG | 6.0% | 11.3% | <0.001 |
| Acute medications (treated within 24 hours of admission) | |||
| Aldosterone antagonist | 32.7% | 1.0% | <0.001 |
| ACEI/ARB | 56.4% | 51.8% | 0.004 |
| Beta‐blocker | 93.4% | 89.5% | <0.001 |
| Aspirin | 96.3% | 96.3% | 0.81 |
| Thienopyridine | 66.5% | 60.9% | <0.001 |
| Statin | 62.0% | 59.9% | 0.16 |
| Discharge medications | |||
| ACEI/ARB | 89.2% | 80.2% | <0.001 |
| Beta‐blocker | 97.3% | 96.5% | 0.16 |
| Both ACEI/ARB and beta‐blocker | 87.2% | 79.2% | <0.001 |
| Aspirin | 98.1% | 96.9% | 0.006 |
| Thienopyridine | 78.2% | 71.6% | <0.001 |
| Statin | 88.5% | 85.3% | 0.002 |
Continuous variables presented as median (25th, 75th percentile). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; NSTEMI, non–ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Effectiveness and Safety Outcomes
Unadjusted cumulative incidence curves for mortality, all‐cause rehospitalization, and rehospitalization for cardiovascular events or HF are shown in Figure 2, stratified by discharge aldosterone antagonist prescription. Two‐year mortality rates approached 40%, but were not significantly different between treatment groups. We used inverse probability‐weighted propensity methods to compare outcomes between patients with and without discharge aldosterone antagonist prescription and observed good balance of measured covariates between groups after propensity modeling (Figure S1). We observed similar risk‐adjusted mortality between patients treated with and without aldosterone antagonists: adjusted HR 0.99, 95% CI 0.88 to 1.13. In both unadjusted and propensity‐adjusted analyses, discharge aldosterone antagonist use was not associated with significant differences in all‐cause rehospitalization, rehospitalization for cardiovascular events, or rehospitalization for HF (Table 3).
Figure 2.
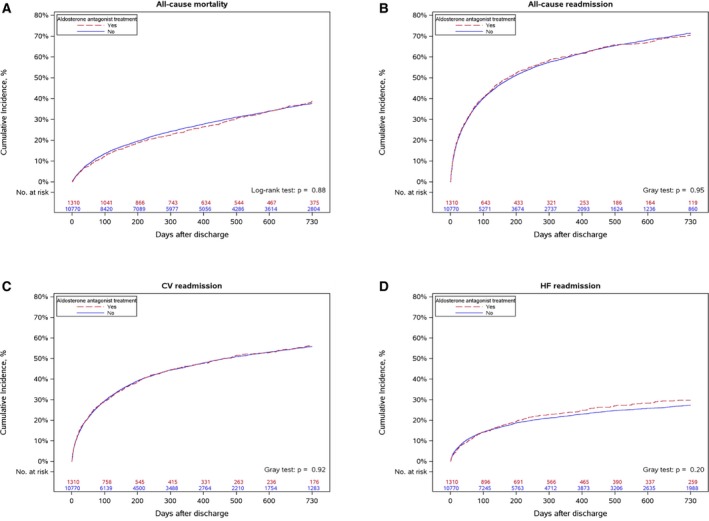
Cumulative incidence of adverse events. Cumulative incidence of: (A) all‐cause mortality, (B) all‐cause readmission, (C) cardiovascular readmission, and (D) HF readmission, comparing MI patients prescribed and not prescribed aldosterone antagonist at discharge. CV indicates cardiovascular; HF, heart failure.
Table 3.
Unadjusted and Adjusted Outcomes Comparing Older MI Patients Prescribed and Not Prescribed Aldosterone Antagonist at Discharge
| Outcome | Unadjusted Event Rates | Adjusted HR (95% CI) | |
|---|---|---|---|
| Prescribed at Discharge | Not Prescribed at Discharge | ||
| Effectiveness outcomes (2 years) | |||
| Mortality | 391 (38.7%) | 3192 (37.7%) | 0.99 (0.88–1.13) |
| All‐cause rehospitalization | 784 (70.4%) | 6534 (71.4%) | 0.96 (0.87–1.06) |
| Rehospitalization for CV events | 613 (56.3%) | 5065 (55.9%) | 0.96 (0.86–1.06) |
| Rehospitalization for HF | 320 (29.7%) | 2458 (27.3%) | 0.93 (0.79–1.09) |
| Safety outcomes | |||
| Hyperkalemia at 30 days | 29 (2.3%) | 164 (1.5%) | 2.04 (1.16–3.60) |
| Hyperkalemia at 2 years | 76 (7.0%) | 565 (6.7%) | 1.28 (0.94–1.74) |
| Acute renal failure at 30 days | 19 (1.5%) | 94 (0.9%) | 1.75 (0.98–3.10) |
| Acute renal failure at 2 years | 71 (6.7%) | 397 (4.8%) | 1.39 (1.01–1.92) |
CI indicates confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial Infarction.
In this older MI population, the unadjusted rate of rehospitalization with hyperkalemia was 2% within 30 days post‐discharge, but rates of rehospitalization for hyperkalemia or acute renal failure were as high as 7% by 2 years (Figure 3, Table 3). After multivariable adjustment, aldosterone antagonist prescription at discharge was associated with a 2‐fold higher risk of hyperkalemia at 30 days (Table 3), and a 40% higher risk for acute renal failure hospitalization at 2 years.
Figure 3.
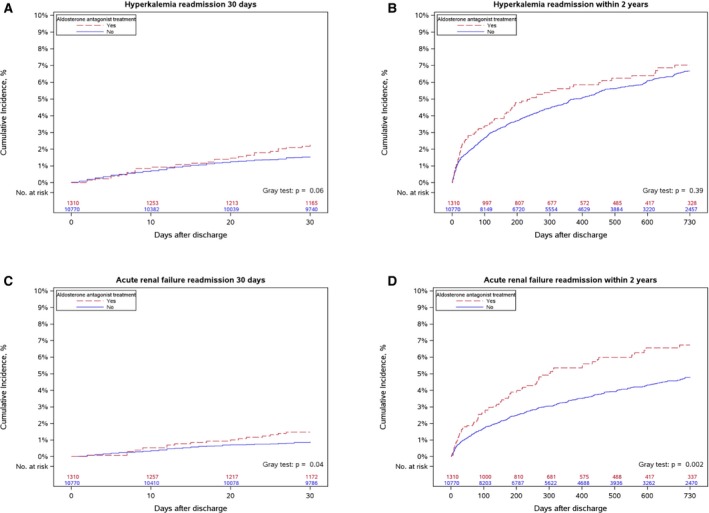
Cumulative incidence of readmission. Cumulative incidence of readmission for hyperkalemia within: (A) 30 days and (B) 2 years, and acute renal failure within (C) 30 days and (D) 2 years, comparing MI patients prescribed and not prescribed aldosterone antagonist at discharge. MI indicates myocardial infarction.
Given concerns for persistent selection bias despite propensity adjustment, we examined the association of aldosterone antagonist use with risk of rehospitalization for pneumonia (ICD‐9‐CM codes 481.0–486.0 and 487.0) as a falsification endpoint. The risk of rehospitalization for pneumonia was not significantly different after propensity adjustment: adjusted HR 0.88, 95% CI 0.62 to 1.24.
Subgroup Analyses
We examined several subgroups to determine whether certain patients were more likely to demonstrate lower 2‐year mortality or higher 30‐day risk of hyperkalemia with aldosterone antagonist use. The relationship between aldosterone antagonist use and mortality did not vary with older age, race, gender, or MI type (STEMI versus NSTEMI; Figure 4A). However, aldosterone antagonist use at discharge was associated with significantly lower mortality among patients with in‐hospital symptoms or signs of HF; this association was not observed among patients without HF, but among those who met indication for therapy because of low EF and diabetes (P interaction=0.009). Aldosterone antagonist use was not associated with mortality among patients who were newly initiated on aldosterone antagonist therapy, nor in those already on aldosterone antagonist therapy pre‐admission (Figure 4A). Subgroup analyses examining the association of aldosterone antagonist use with the risk of hyperkalemia did not find any statistically significant associations when stratified by age, renal function, or concurrent ACEI/ARB therapy (Figure 4B).
Figure 4.
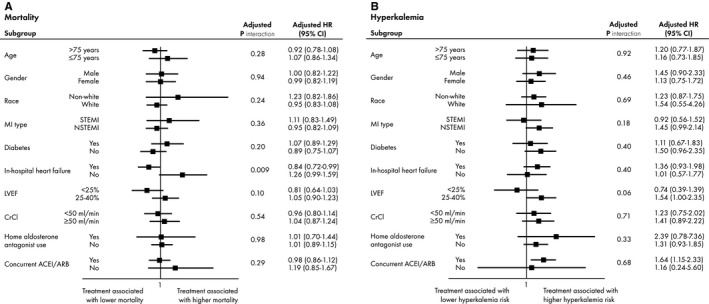
Aldosterone antagonist use. Association between aldosterone antagonist use and: (A) mortality and (B) hyperkalemia, in high‐risk subgroups. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CrCl, creatinine clearance; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Aldosterone Antagonist Use Post‐Index Hospital Discharge
Among the subset of 4677 patients who were discharged home alive and enrolled in the Medicare Part D Prescription Drug Plan prior to index hospital discharge, 482 patients (10.3%) were discharged on an aldosterone antagonist, which was consistent with our observation for the overall study population. Among patients discharged on an aldosterone antagonist, most filled a spironolactone prescription, with only a minority (2.7%) filling an eplerenone prescription. However, 36.0% were no longer persistent with aldosterone antagonist therapy (ie, treatment gap >60 days) by 6 months post‐discharge. Among patients not discharged on an aldosterone antagonist, 5.1% were started on an aldosterone antagonist within 14 days post‐discharge, and 11.6% of patients were on aldosterone antagonist therapy by 6 months post‐discharge.
In a secondary “on‐treatment” analysis examining post‐discharge aldosterone antagonist use as a time‐dependent covariate, we found similar results for 2‐year risks of mortality (adjusted HR 0.98, 95% CI 0.84–1.16) and rehospitalization for hyperkalemia (adjusted HR 1.87, 95% CI 0.36–2.58).
Discussion
This study represents one of the largest analyses of aldosterone antagonist use among post‐MI patients ≥65 years. We had several major findings. First, the overall use of aldosterone antagonists was low among patients indicated and eligible for treatment. Second, we observed aldosterone antagonist use in routine practice that was inconsistent with trial criteria and guideline recommendations. Third, overall risks of hyperkalemia and acute renal failure were low in this older patient population, but significantly higher among patients prescribed an aldosterone antagonist. Finally, we observed no significant differences in the risks of mortality or HF rehospitalization associated with aldosterone antagonist use among patients ≥65 years. These findings were consistent among multiple subgroups; however, those with symptomatic HF during the MI hospitalization had lower mortality associated with aldosterone antagonist use.
EPHESUS first reported that, compared with optimal medical therapy, eplerenone use led to a 15% mortality reduction in post‐MI patients with low EF and either HF symptoms or diabetes,3 and a 15% reduction in the secondary endpoint of HF rehospitalization, forming the basis for an AHA/ACC recommendation for aldosterone antagonist use in post‐MI patients.1, 2 Yet since the dissemination of EPHESUS results, there has only been a modest increase in aldosterone antagonist use in this population.11, 12 Among older patients, the incremental benefit margin of aldosterone antagonists may be limited in someone already receiving optimal medical therapy (ACEIs/ARBs or beta‐blockers), and may present additional safety challenges, including greater susceptibility to renal dysfunction and metabolic disturbances. Combination medical therapy can have unintended anti‐hypertensive effects, increasing the risk of hemodynamic instability and falls. Drug‐drug interaction risk is also considerably higher in this older population. As the risk versus benefit of aldosterone antagonist use is often deliberated for older adults given these age‐related physiologic changes and comorbidities, the objective of this study was to assess the longitudinal effectiveness and safety of aldosterone antagonists in a large community‐based sample of MI patients ≥65 years with an indication for aldosterone antagonist therapy.
We observed low rates of aldosterone antagonist use among eligible patients ≥65 years; this rate (11%) was only modestly lower compared with a recently described all‐aged MI population (15%).12 Patients with STEMI presentation, prior history of HF, substantially depressed EF, or symptomatic HF during the MI hospitalization were more likely to receive aldosterone antagonists. While overall use rates were low, we also observed use inconsistent with trial eligibility criteria and guideline recommendations: 4% of patients discharged on an aldosterone antagonist had significant renal dysfunction and 10% received an aldosterone antagonist without concurrent ACEI/ARB therapy in the absence of contraindications. While EPHESUS demonstrated the benefit of eplerenone use in this patient population, spironolactone appears to be predominantly filled when an aldosterone antagonist is prescribed.
In our study of >12 000 MI patients ≥65 years of age, we observed no association between aldosterone antagonist use and mortality or HF rehospitalizations. In contrast to EPHESUS, our population of community‐treated patients was substantially older (mean age 77 versus 64 years), had a higher proportion of female and black patients, and a greater prevalence of comorbidities, such as prior HF, diabetes, and renal insufficiency.3 Subgroup analyses found no significant difference in mortality based on age, sex, or race, but did show an association between aldosterone antagonist use and lower mortality among patients who presented or developed in‐hospital symptoms or signs of HF. Since EPHESUS randomized patients up to 14 days post‐MI, we considered whether aldosterone antagonist initiation may be deferred to the post‐discharge setting, and showed that only 5% of patients who were not prescribed an aldosterone antagonist at discharge filled a prescription for it in the next 14 days. However, more than one‐third of patients discharged on an aldosterone antagonist were no longer taking it 6 months post‐discharge, whereas in EPHESUS, only 16% of aldosterone antagonist‐treated patients discontinued the study drug over the mean duration of 16‐month follow‐up. Therefore, non‐persistence may be an explanation for the lack of benefit associated with aldosterone antagonist use in our study. An “on‐treatment” analysis again showed no significant association between aldosterone antagonist use and mortality. We applied robust methodologies adjusting for any differences in captured patient characteristics with a good observed balance of covariates using propensity methods, but we cannot exclude measured and unmeasured biases inherent to these non‐randomized comparisons that may explain the discrepancy between our study and EPHESUS results.
Among older adults, the potential treatment benefit may be outweighed by the risk associated with aldosterone antagonist use in the absence of strict trial protocols for patient follow‐up and dose adjustment. A post‐hoc analyses of EPHESUS revealed a 6% risk of hyperkalemia (4.4% with K+>5.5 meq/L and 1.6% with K+≥6 meq/L), although no attributable increase in mortality was observed.13 While overall rates were low (<3%), we observed a 2‐fold higher incidence of rehospitalization for hyperkalemia within 30 days in older patients discharged on aldosterone antagonists in routine clinical practice. Subgroup analyses stratified by age, sex, baseline renal function, and concurrent ACEI/ARB therapy use did not reveal any particular groups at higher risk of hyperkalemia development. This discordance between reported results from clinical trials and observational data from routine clinical practice echoes previous experience with aldosterone antagonist therapy after broader uptake into clinical practice. After the Randomized Aldactone Evaluation Study (RALES) trial proved the benefit of aldosterone antagonist therapy in severe HF patients, an abrupt increase in hyperkalemia‐associated morbidity and mortality (more significant than that suggested by RALES) was observed in community practice.14, 15 This has been partially attributed to a lower intensity of follow‐up, and serum potassium or creatinine measurement, than specified in the clinical trial.16, 17
Limitations
Our study had several limitations. First, although we excluded patients with contraindications to aldosterone antagonist therapy, contraindications may have been missed if not clearly documented in the medical record. Second, we cannot distinguish intentional non‐use from errors of omission, since the database does not collect whether aldosterone antagonists were considered for each patient by the treating provider. Third, we rely on inpatient administrative data for long‐term outcomes, and may have underestimated outcomes like hyperkalemia if its severity did not warrant hospitalization or inclusion as a diagnosis during hospitalization. Hyperkalemia and renal dysfunction may be under‐identified among patients not on aldosterone antagonist therapy since laboratory surveillance in routine practice is likely less frequent in these patients than among those prescribed aldosterone antagonist therapy. Furthermore, deaths were unadjudicated in this claims database, so we cannot ascertain the mortality attributable to hyperkalemia. Fourth, while we were able to examine therapy persistence in the subset of patients enrolled in the Medicare Part D Medication Prescription Plan, we could not estimate the contribution of medication non‐persistence to outcomes, since the reasons for therapy discontinuation are not captured in these pharmacy claims. Finally, in this observational study design, residual unmeasured confounding exists despite the use of robust comparative methods, and causal inferences should not be drawn from our results. These results are intended to supplement randomized trial evidence with “real world” patient experience.
Conclusions
Limited evidence exists to support the benefit of aldosterone antagonist use among older patients with left ventricular dysfunction after acute MI who potentially meet guideline‐recommended treatment indications. In this large nationally representative cohort, aldosterone antagonist use was not associated with significant differences in mortality or rehospitalization risk among patients ≥65 years, except in those with symptomatic HF. However, we did note a significant safety signal in the risk of rehospitalization for hyperkalemia associated with aldosterone antagonist use. Future studies are needed to validate our study results. Our findings suggest a stronger recommendation for aldosterone antagonist use among older MI patients with low EF and in‐hospital HF, but perhaps more cautious use in those with low EF and diabetes only without HF. This study also underscores the importance of close post‐discharge monitoring of renal function and electrolytes for older MI patients who are prescribed aldosterone antagonist therapy.
Sources of Funding
This study was supported by a Centers for Education and Research on Therapeutics grant (U19HS021092) from the Agency for Healthcare Research and Quality.
Disclosures
Dr Wang reports institutional research grant support from Eli Lilly, Daiichi Sankyo, Gilead Sciences, Glaxo Smith Kline, AstraZeneca, and the American College of Cardiology; honoraria from AstraZeneca and the American College of Cardiology. Dr Vora has no relevant conflicts to disclose. Mr Peng has no relevant conflicts to disclose. Dr Fonarow reports consulting for Novartis (significant) and Medtronic (modest). Dr Das has no relevant disclosures to report. Dr de Lemos reports grant support from Abbott Diagnostics; consulting income for participation in a DSMB for St. Jude Medical. Dr Peterson reports research funding to Duke Clinical Research Institute from the American College of Cardiology, American Heart Association, Eli Lilly & Company, and Janssen Pharmaceuticals; consulting (including CME) for Merck & Co, Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, and Sanofi‐Aventis.
Supporting information
Table S1. Covariates Entered Into the Propensity Model for Aldosterone Antagonist Use Versus Not Figure S1. Balance of covariates before (circle) and after (solid dot) inverse probability‐weighted propensity adjustment. A, Categorical variables. B, Continuous variables.
Acknowledgments
The authors would like to thank Erin Hanley, MS for her editorial contributions to this manuscript. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
(J Am Heart Assoc. 2016;5:e002612 doi: 10.1161/JAHA.115.002612)
Accompanying Table S1 and Figure S1 are available at http://jaha.ahajournals.org/content/5/1/e002612/suppl/DC1
References
- 1. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179–e347. [DOI] [PubMed] [Google Scholar]
- 2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW; American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions . 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 4. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarrett N, Lux L, West S. Systematic evaluation of health outcome of interest definitions in observational studies and clinical definitions for the observational medical outcomes partnership: acute renal failure. Report prepared for the Foundation for the National Institutes of Health via the Observational Medical Outcomes Partnership (OMOP). Research Triangle Park, NC: RTI International; 2009. [Google Scholar]
- 7. Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. [DOI] [PubMed] [Google Scholar]
- 8. Gray RJ. A class of k‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1998;16:1141–1154. [Google Scholar]
- 9. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 10. Goyal A, de Lemos J, Peng SA, Thomas L, Amsterdam E, Hockenberry J, Peterson E, Wang T. The association of patient enrollment in Medicare Part D with outcomes after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2015;8:567–575. [DOI] [PubMed] [Google Scholar]
- 11. Rassi AN, Cavender MA, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Rosas SE, Zhao X, Schwamm LH, Bhatt DL. Temporal trends and predictors in the use of aldosterone antagonists post‐acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. [DOI] [PubMed] [Google Scholar]
- 12. Rao KK, Enriquez JR, de Lemos JA, Alexander KP, Chen AY, McGuire DK, Fonarow GC, Das SR. Use of aldosterone antagonists at discharge after myocardial infarction: results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry‐Get With The Guidelines (GWTG). Am Heart J. 2013;166:709–715. [DOI] [PubMed] [Google Scholar]
- 13. Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R; EPHESUS Investigators . Serum potassium and clinical outcomes in the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation. 2008;118:1643–1650. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 15. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med. 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 16. Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–214. [DOI] [PubMed] [Google Scholar]
- 17. Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Covariates Entered Into the Propensity Model for Aldosterone Antagonist Use Versus Not Figure S1. Balance of covariates before (circle) and after (solid dot) inverse probability‐weighted propensity adjustment. A, Categorical variables. B, Continuous variables.


