Abstract
Background
Glycine is an amino acid involved in antioxidative reactions, purine synthesis, and collagen formation. Several studies demonstrate inverse associations of glycine with obesity, hypertension, and diabetes mellitus. Recently, glycine‐dependent reactions have also been linked to lipid metabolism and cholesterol transport. However, little evidence is available on the association between glycine and coronary heart disease. Therefore, we assessed the association between plasma glycine and acute myocardial infarction (AMI).
Methods and Results
A total of 4109 participants undergoing coronary angiography for suspected stable angina pectoris were studied. Cox regression was used to estimate the association between plasma glycine and AMI, obtained via linkage to the CVDNOR project. During a median follow‐up of 7.4 years, 616 patients (15.0%) experienced an AMI. Plasma glycine was higher in women than in men and was associated with a more favorable baseline lipid profile and lower prevalence of obesity, hypertension, and diabetes mellitus (all P<0.001). After multivariate adjustment for traditional coronary heart disease risk factors, plasma glycine was inversely associated with risk of AMI (hazard ratio per SD: 0.89; 95% CI, 0.82–0.98; P=0.017). The inverse association was generally stronger in those with apolipoprotein B, low‐density lipoprotein cholesterol, or apolipoprotein A‐1 above the median (all P interaction≤0.037).
Conclusions
Plasma glycine was inversely associated with risk of AMI in patients with suspected stable angina pectoris. The associations were stronger in patients with apolipoprotein B, low‐density lipoprotein cholesterol, or apolipoprotein A‐1 levels above the median. These results motivate further studies to elucidate the relationship between glycine and lipid metabolism, in particular in relation to cholesterol transport and atherosclerosis.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00354081.
Keywords: amino acids, apolipoprotein, atherosclerosis, glycine, lipids and lipoprotein metabolism, myocardial infarction
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Risk Factors
Introduction
Glycine is a nonessential amino acid that can be obtained either via the diet, or synthesized endogenously from serine, threonine, choline, or glyoxylate in the liver and kidney.1 It is a predominant constituent of collagen and is utilized in the synthesis of several biologically important compounds, including glutathione, creatine, purines, and glucose.2, 3 Glycine exerts anti‐inflammatory and antioxidative effects4, 5 and has been inversely associated with several traditional cardiovascular risk factors, including obesity,6 hypertension,7, 8 and diabetes mellitus.9
Increasing evidence suggests that glycine‐dependent reactions are associated with lipid homeostasis and cholesterol transport. More specifically, glycine is utilized to catabolize excess S‐adenosylmethionine by its remethylation into sarcosine via the enzyme glycine‐N‐methyltransferase (GNMT),10 and excess hepatic S‐adenosylmethionine concentrations have been linked to the regulation of apolipoprotein (apo) B mRNA expression and very low‐density lipoprotein formation.11, 12, 13 Disturbances in these reactions have been associated with lipid accumulation both in the liver and in macrophages, which further promotes oxidized low‐density lipoprotein (LDL)‐induced foam cell formation in the artery wall.12 Therefore, glycine availability may affect lipid metabolism and thereby further modulate the risk of coronary artery disease (CAD). However, data on glycine and the risk of atherosclerotic cardiovascular disease in observational studies are sparse.
We investigated the associations between plasma glycine and incident acute myocardial infarction (AMI) in a large cohort of patients with suspected stable angina pectoris, with a particular focus on potential effect modifications by lipid parameters. The results are reported according to the STrengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology (STROBE‐ME) guidelines.14
Materials and Methods
Study Population
The study population has been described previously.15 In brief, a total of 4164 subjects underwent elective coronary angiography for suspected stable angina pectoris during 2000–2004 at Haukeland (n=3413) or Stavanger (n=751) University Hospitals in Western Norway. Among these patients, 85 were taking folic acid supplementation while 583 were taking multivitamins before the study. In addition, 2573 (61.8%) were enrolled in the Western Norway B Vitamin Intervention Trial (WENBIT; ClinicalTrials.gov Identifier: NCT00354081). Patients with missing baseline data on plasma glycine (n=9), lipid parameters (n=1), or glycated hemoglobin (n=45) were excluded, leaving a total of 4109 patients eligible for the current analyses.
The study protocol was in accordance with the Declaration of Helsinki, and was approved by the Regional Committee for Medical and Health Research Ethics, Western Norway, the Norwegian Medicines Agency, and the Norwegian Data Inspectorate. All patients provided written informed consent.
Baseline Data
Clinical information and blood samples were obtained at baseline before coronary angiography. Smoking status was defined according to self‐reports and serum cotinine levels (≥85 nmol/L) as previously described.16 Obesity was defined as body mass index (BMI) ≥30 kg/m2. Diabetes mellitus was classified by self‐reports, glucose measurements (fasting plasma glucose ≥7.0 mmol/L or nonfasting plasma glucose ≥11.1 mmol/L), or by single measurement of glycated hemoglobin ≥6.5% according to the American Diabetes Association guidelines.17 The extent of CAD at angiography was scored 0 to 3 according to the number of significantly stenotic coronary arteries. Left ventricular ejection fraction was determined by echocardiography or ventriculography.
Clinical End Points
Study subjects were followed from enrollment until the onset of AMI, or until the end of 2009. Information on clinical events was collected from the Cardiovascular Disease in Norway (CVDNOR; https://cvdnor.b.uib.no/) project, reporting on patients being discharged with a cardiovascular disease diagnosis from any of 42 Norwegian public hospitals from 1994 and throughout 2009.18, 19 AMI (including fatal and nonfatal AMI) as the primary end point was classified according to the International Statistical Classification of Disease Tenth Revision (ICD‐10) codes I21 and I22, respectively.
Biochemical Analyses
Earlier reports have described the collection and storage of blood samples and the biochemical analyses for relevant clinical indices.15, 16 In addition, plasma glycine was analyzed by gas chromatography–tandem mass spectrometry20 at Bevital A/S, Norway (www.bevital.no).
Statistical Analyses
Baseline categorical variables are reported as frequencies and percentages, while continuous variables are presented as medians with interquartile ranges. Plasma or serum metabolite concentrations were log‐transformed before statistical analysis due to their right‐skewed distributions. Baseline variables across quartiles of plasma glycine of the whole population were assessed by unadjusted median linear or logistic regression for continuous and categorical variables, respectively.
Cox regression analysis was used to estimate the association between plasma glycine and risk of AMI. The risk estimates were reported as fifth versus first plasma glycine quintiles, trends across quintiles, and per 1 SD increment in log‐transformed plasma glycine. A simple survival model (model I) was adjusted for age (continuous) and sex (male/female). Covariates in the multivariate model (model II) included age (continuous), sex (male/female), smoking (yes/no), obesity (yes/no), hypertension (yes/no), diabetes mellitus (yes/no), extent of CAD at angiography (ordinal), statin treatment (yes/no), and estimated glomerular filtration rate, apolipoprotein A1 (apoA‐1) and apoB (all continuous). Since results from experimental studies suggest that glycine may regulate pro‐inflammatory cytokines,21, 22 we additionally included C‐reactive protein (CRP) in an extended model. Fasting status and baseline revascularization procedures had negligible impact on the risk estimates and were excluded in the final model (data not shown). The assumption of proportionality was examined by the Schoenfeld and scaled Schoenfeld residuals. Potential nonlinear dose–response relationships between plasma glycine and risk of incident AMI were visualized by generalized additive model plots for both simple and multivariate models.
Potential effect modifications by prespecified lipid parameters (including serum apoB, LDL cholesterol, apoA1, and high‐density lipoprotein‐cholesterol) were explored according to their median values and tested by including an interaction product term in Cox models adjusted for age, sex, and the use of statins.
The statistical analyses were performed in R (R Core Team, Vienna, Austria; version 3.1.1 & 3.1.2; packages “coin,” “Hmisc,” “survival,” “MASS,” and “mgcv”). All reported P values were 2‐sided, and P<0.05 was considered significant.
Results
Patient Characteristics and Plasma Glycine Levels at Baseline
The median (interquartile range) age for the 4109 patients at baseline was 62 (15) years and 72.0% were males. As outlined in Table 1, persons in the upper glycine quartiles were more likely to be older, females, and smokers as compared to persons in the lower quartiles. However, the association between plasma glycine and smoking status was not significant after the adjustment for BMI (P=0.11). In addition, persons in upper quartiles of plasma glycine also had higher apoA‐1 (P<0.001) and high‐density lipoprotein‐cholesterol levels (P<0.001) and lower serum apoB (P<0.001) and glycine tended to be associated with LDL cholesterol (P=0.064). Notably, after adjusting for statin treatment, plasma glycine was inversely associated with LDL cholesterol (P<0.001).
Table 1.
Baseline Characteristics According to Quartiles of Plasma Glycine
| Plasma Glycine Quartiles (μmol/L) | P for Trend | ||||
|---|---|---|---|---|---|
| 1st (<178) | 2nd (178–205) | 3rd (205–243) | 4th (>243) | ||
| Age, y | 61 (14) | 62 (14) | 61 (15) | 63 (16) | 0.037 |
| Male sex, n (%) | 817 (79.5) | 818 (79.7) | 804 (78.2) | 521 (50.7) | <0.001 |
| Current smoking, n (%) | 299 (29.1) | 292 (28.5) | 375 (36.5) | 337 (32.8) | 0.003 |
| Obesity, n (%) | 321 (31.2) | 183 (17.8) | 135 (13.1) | 113 (11.0) | <0.001 |
| Hypertension, n (%) | 561 (54.6) | 483 (47.1) | 451 (43.9) | 434 (42.3) | <0.001 |
| Diabetes mellitus, n (%) | 513 (49.9) | 413 (40.2) | 393 (38.2) | 393 (38.3) | <0.001 |
| HbA1c (%) | 6.23 (1.77) | 6.02 (1.56) | 6.05 (1.33) | 6.08 (1.42) | <0.001 |
| Serum | |||||
| Apolipoprotein A1, mg/dL | 1.24 (0.32) | 1.28 (0.34) | 1.31 (0.34) | 1.37 (0.37) | <0.001 |
| Apolipoprotein B, mg/dL | 0.90 (0.31) | 0.88 (0.30) | 0.85 (0.30) | 0.85 (0.33) | <0.001 |
| HDL cholesterol, mmol/L | 1.10 (0.30) | 1.20 (0.40) | 1.30 (0.42) | 1.40 (0.50) | <0.001 |
| LDL cholesterol, mmol/L | 3.00 (1.30) | 2.99 (1.30) | 2.90 (1.33) | 2.90 (1.40) | 0.064 |
| eGFR, mL/min per 1.73 m2 | 94 (18) | 92 (19) | 91 (20) | 86 (24) | <0.001 |
| Serum CRP, mg/L | 2.48 (3.73) | 1.84 (2.80) | 1.58 (2.36) | 1.35 (2.09) | <0.001 |
| Troponin T, ng/L | 5 (8) | 4 (6) | 5 (7) | 4 (6) | 0.088 |
| Prior MI, n (%) | 438 (42.6) | 436 (42.5) | 437 (42.5) | 347 (33.8) | <0.001 |
| LVEF (%) | 65 (10) | 65 (10) | 65 (10) | 65 (10) | 0.092 |
| Angiographic evidence of CAD, n (%) | |||||
| No significant stenosis | 189 (18.4) | 214 (20.8) | 260 (25.3) | 374 (36.4) | <0.001 |
| Single‐vessel disease | 255 (24.8) | 240 (23.4) | 245 (23.8) | 210 (20.4) | 0.032 |
| Double‐vessel disease | 250 (24.3) | 249 (24.2) | 228 (22.2) | 188 (18.3) | <0.001 |
| Triple‐vessel disease | 334 (32.5) | 323 (31.6) | 295 (28.7) | 255 (24.9) | <0.001 |
| Treatment following baseline coronary angiography, n (%) | |||||
| No or medications only | 413 (40.2) | 424 (41.3) | 468 (45.5) | 565 (55.0) | <0.001 |
| PCI | 374 (36.4) | 357 (34.8) | 337 (32.8) | 283 (27.6) | <0.001 |
| CABG | 229 (22.3) | 230 (22.4) | 209 (20.3) | 157 (15.3) | <0.001 |
| Medications at discharge, n (%) | |||||
| Aspirin | 854 (83.1) | 871 (84.9) | 849 (82.6) | 781 (76.0) | <0.001 |
| Statins | 850 (82.7) | 855 (83.3) | 824 (80.2) | 763 (74.3) | <0.001 |
| Beta blockers | 774 (75.3) | 764 (74.5) | 749 (72.9) | 694 (67.6) | <0.001 |
| ACEIs | 237 (23.1) | 233 (21.7) | 202 (19.6) | 178 (17.3) | <0.001 |
| Loop diuretics | 141 (13.7) | 102 (10.0) | 88 (8.4) | 119 (11.5) | 0.075 |
Variables are given in medians (interquartile ranges) or counts (percentages). ACEIs, angiotensin‐converting‐enzyme inhibitors; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; low‐density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Plasma glycine was negatively associated with obesity, hypertension, diabetes mellitus, serum CRP, and prior AMI. We observed no association of plasma glycine with left ventricular ejection fraction and cardiac high‐sensitive troponin T, but a strong inverse association with the extent of angiographic CAD, the latter also reflected by more use of coronary heart disease medications among patients in the lower plasma glycine quartiles.
Plasma Glycine and Risk of AMI
During a median (interquartile range) follow‐up of 7.4 (2.4) years, 616 patients (15.0%) experienced an AMI. After adjusting for age and sex, higher plasma glycine was associated with a decreased risk of AMI (hazard ratio per SD: 0.88; 95% CI, 0.80–0.95; P=0.003). The association was essentially similar after the multivariate adjustment (hazard ratio per SD: 0.89; 95% CI, 0.82–0.98; P=0.017) (Table 2, Figure 1). However, additional adjustment for plasma CRP slightly attenuated the association between plasma glycine and incident AMI (hazard ratio per SD: 0.92; 95% CI, 0.84–1.01; P=0.085). Hazard ratios for AMI across all quintiles of plasma glycine gave similar results and are given in Table 3.
Table 2.
Association Between Plasma Glycine and Acute Myocardial Infarction
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Model Ia | |||
| Per SD increment | 0.88 | 0.80 to 0.95 | 0.003 |
| Q5 vs Q1 | 0.68 | 0.52 to 0.88 | 0.004 |
| Model IIb | |||
| Per SD increment | 0.89 | 0.82 to 0.98 | 0.017 |
| Q5 vs Q1 | 0.71 | 0.54 to 0.94 | 0.016 |
Adjusted for age and sex.
Adjusted for age, sex, smoking, obesity, hypertension, diabetes mellitus angiographic extent of coronary artery disease, estimated glomerular filtration rate, apolipoprotein A‐1, apolipoprotein B, and statin treatment.
Figure 1.
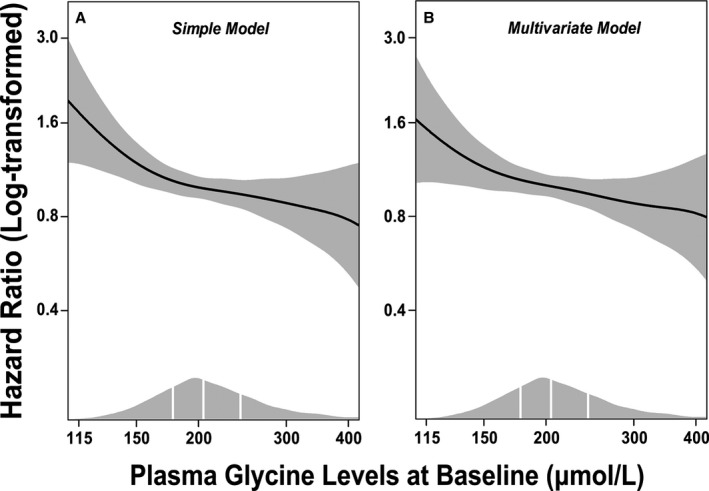
Dose–response associations between (log‐transformed) plasma glycine and risk of acute myocardial infarction. Generalized additive regressions are used with the adjustment for age and sex in the simple model (A), and additional adjustment for smoking, obesity, hypertension, diabetes mellitus, angiographic extent of coronary artery disease (ordinal), estimated glomerular filtration rate, apolipoprotein A‐1, apolipoprotein B, and statin treatment in the multivariate model (B). The solid lines and the shaded areas represent hazard ratios of plasma glycine and their 95% CI, respectively. The areas under the curve along the X‐axes represent the distributions of the plasma glycine concentrations (μmol/L) in the total population. The vertical white lines denote the 25th, 50th, and 75th percentiles of plasma glycine, respectively.
Table 3.
Hazard Ratios (HR) of AMI According to Quintiles of Plasma Glycine Levels
| Model Ia | Model IIb | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Quintiles | ||||
| First | Reference | Reference | ||
| Second | 0.89 (0.71–1.13) | 0.34 | 0.93 (0.73–1.18) | 0.54 |
| Third | 0.76 (0.59–0.97) | 0.025 | 0.81 (0.63–1.04) | 0.10 |
| Fourth | 0.80 (0.63–1.01) | 0.062 | 0.83 (0.64–1.06) | 0.13 |
| Fifth | 0.68 (0.52–0.88) | 0.004 | 0.71 (0.54–0.94) | 0.016 |
| Trend | 0.92 (0.87–0.97) | 0.004 | 0.92 (0.87–0.98) | 0.012 |
Adjusted for age and sex.
Adjusted for age, sex, smoking, obesity, hypertension, diabetes mellitus angiographic extent of coronary artery disease, estimated glomerular filtration rate, apolipoprotein A‐1, apolipoprotein B, and statin treatment.
Notably, nearly 62% of the patients were enrolled in the WENBIT and randomly received treatments with folic acid plus vitamin B12, vitamin B6, or placebo. The risk estimates of plasma glycine in WENBIT were not modified by any intervention treatments (all P interaction≥0.16, Table 4).
Table 4.
Association Between Plasma Glycine and Acute Myocardial Infarction in Different Treatment Arms in WENBIT
| Total | Folic Acid+B12 | Folic Acid+B12+B6 | B6 | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model Ia | ||||||||||
| Per SD increment | 0.88 (0.79–0.97) | 0.012 | 0.89 (0.72–1.11) | 0.31 | 0.94 (0.77–1.16) | 0.56 | 0.87 (0.70–1.08) | 0.20 | 0.80 (0.65–0.97) | 0.025 |
| Q5 vs Q1 | 0.71 (0.52–0.96) | 0.027 | 0.73 (0.39–1.38) | 0.33 | 1.01 (0.55–1.83) | 0.98 | 0.74 (0.39–1.40) | 0.36 | 0.45 (0.24–0.84) | 0.012 |
| Model IIb | ||||||||||
| Per SD increment | 0.89 (0.80–0.99) | 0.046 | 0.94 (0.74–1.19) | 0.61 | 0.97 (0.78–1.21) | 0.80 | 0.88 (0.70–1.11) | 0.29 | 0.82 (0.66–1.02) | 0.07 |
| Q5 vs Q1 | 0.74 (0.53–1.03) | 0.077 | 0.86 (0.44–1.70) | 0.67 | 1.09 (0.57–2.07) | 0.79 | 0.76 (0.37–1.58) | 0.47 | 0.48 (0.25–0.94) | 0.032 |
HR indicates hazard ratio; WENBIT, Western Norway B Vitamin Intervention Trial.
Adjusted for age and sex.
Adjusted for age, sex, smoking, obesity, hypertension, diabetes mellitus angiographic extent of coronary artery disease, estimated glomerular filtration rate, apolipoprotein A‐1, apolipoprotein B, and statin treatment.
Subgroup Analyses
Figure 2 demonstrates the risk estimates between plasma glycine and AMI occurrence according to several lipid parameters. We observed a stronger negative association between plasma glycine and incident AMI among patients with high as compared to low serum apoB and LDL cholesterol levels (P interaction=0.037 and 0.012, respectively). Additionally, we observed a stronger risk estimate of plasma glycine in patients with high as compared to low apoA‐1 levels (P interaction=0.027) and a similar trend was also seen for high‐density lipoprotein cholesterol (P interaction=0.16). The estimates were essentially similar after multivariate adjustment (Table 5).
Figure 2.
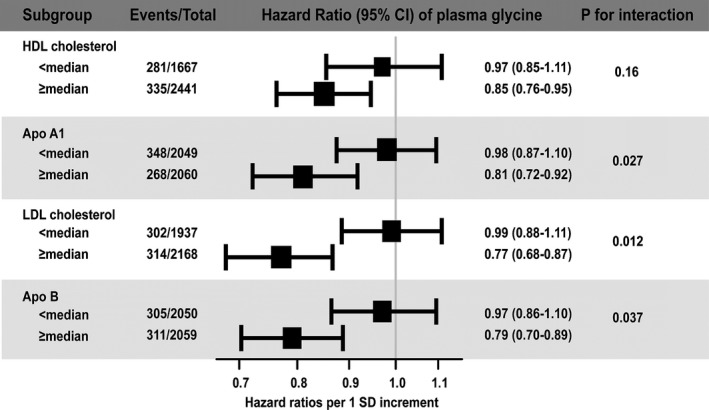
Risk associations between plasma glycine and AMI according to the median values of prespecified lipid parameters. The black squares represent the hazard ratios and their areas are proportional to the subgroup sizes. Horizontal lines represent the 95% CI. AMI indicates acute myocardial infarction; ApoA‐1, apolipoprotein A‐1; ApoB, apolipoprotein B; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 5.
Risk Associations Between Plasma Glycine and AMI According to the Median Values of Prespecified Lipid Parameters
| HR (95% CI)a | P Value | P int | |
|---|---|---|---|
| HDL | |||
| Above median | 0.86 (0.76–0.97) | 0.017 | 0.22 |
| Below median | 0.96 (0.84–1.10) | 0.58 | |
| ApoA‐1 | |||
| Above median | 0.81 (0.71–0.93) | 0.003 | 0.039 |
| Below median | 0.99 (0.87–1.12) | 0.84 | |
| LDL | |||
| Above median | 0.80 (0.70–0.91) | <0.001 | 0.013 |
| Below median | 0.99 (0.87–1.13) | 0.92 | |
| ApoB | |||
| Above median | 0.81 (0.71–0.92) | 0.002 | 0.032 |
| Below median | 0.99 (0.87–1.12) | 0.84 | |
AMI indicates acute myocardial infarction; HDL, high‐density lipoprotein; HR, hazard ratio; LDL, low‐density lipoprotein.
Adjusted for age, sex, smoking, obesity, hypertension, diabetes mellitus angiographic extent of coronary artery disease, estimated glomerular filtration rate, apolipoprotein A‐1, apolipoprotein B, and statin treatment.
Discussion
Principal Findings
In a large cohort of patients undergoing elective coronary angiography for suspected stable angina pectoris, higher plasma glycine was associated with a generally more favorable cardiovascular disease risk factor profile and with a decreased risk of AMI during follow‐up, independent of traditional CAD risk factors. Furthermore, the inverse associations between glycine and AMI were stronger among patients with serum apoB, LDL cholesterol, or apoA‐1 levels above the median.
Plasma Glycine and Cardiovascular Disease
Consistent with our study, glycine has previously been inversely associated with several coronary heart disease risk factors, and in particular those related to insulin sensitivity, glucose homeostasis, and the metabolic syndrome. Population‐based studies have demonstrated a positive association of plasma glycine with estimated glucose disposal rate23 and a negative association with glycated hemoglobin.24 Accordingly, plasma glycine has been inversely associated with obesity,6, 25 hypertension,8 and diabetes mellitus.9, 26 Glycine intake has also been associated with low plasma free fatty acids, cholesterol and triglycerides levels in animal models.7, 27 Although high plasma glycine was related to an overall favorable coronary heart disease risk profile, we somewhat unexpectedly observed a positive association between plasma glycine and smoking status. However, this relationship may be explained by the negative correlation between BMI and smoking behavior,28 as adjusting for BMI rendered the association nonsignificant. The relationships between glycine status and clinical cardiovascular end points have, to the best of our knowledge, not been evaluated previously in large‐scale observational studies; hence the current study extends previous knowledge on cardiovascular prognosis according to glycine status.
Possible Mechanisms
The negative association between plasma glycine and LDL cholesterol in the current study was probably veiled by the intake of statins, since a greater proportion of patients in lower plasma glycine quartiles were prescribed statins. Accordingly, we observed a significant inverse trend between plasma glycine and LDL cholesterol after adjusting for statins, in line with the inverse association with apoB, but positive relationships with apoA‐1and high‐density lipoprotein cholesterol. This suggests an important role of glycine in lipid metabolism.
Indeed, considerable evidence suggests that glycine availability may be important in lipid metabolism and atherosclerosis. First, glycine can be methylated into sarcosine via GNMT, which is mainly confined to the liver and kidney29, 30; however, rodent studies have shown that the GNMT is also localized to aortic endothelial cells.12 Impaired GNMT flux was shown to exacerbate lipid accumulation in both the liver and in macrophages, which can further promote oxidized LDL‐induced foam cell formation in the artery wall.12 Furthermore, GNMT flux has also been shown to affect composition of atherosclerotic plaques and regulate inflammation response within atherosclerotic lesions.12 We observed stronger associations of glycine with AMI among those with higher apoB and LDL cholesterol levels in subgroup analyses. This finding may support the hypothesis that GNMT flux plays a role in plaques formation and the progression of atherosclerosis.
In addition, impaired GNMT flux was suggested to interrupt reverse cholesterol transport by downregulating the expression of scavenger receptors class B member 1 and ATP‐binding cassette transporters‐A1 and G1.12 GNMT deficiency has also been associated with hepatic cholesterol accumulation and overt dyslipidemia by downregulation of Niemann‐Pick type C2 protein,31 a regulator of intracellular cholesterol trafficking and homeostasis. In the same study, enhanced GNMT flux was shown to promote cholesterol export from the cells by upregulating Niemann‐Pick type C2, a process requiring the involvement of lipid‐poor apolipoproteins (apoA‐1 and apoE), which may explain the stronger beneficial effect of plasma glycine among patients with higher apoA‐1 levels.
Moreover, reduced flux over the GNMT pathway may cause the accumulation of excess S‐adenosylmethionine,10 which is shown to interrupt hepatic apoB mRNA expression and very low‐density lipoprotein assembly.12, 13 Accordingly, glycine infusion normalized hepatic triglyceride‐rich very low‐density lipoprotein secretion in rats fed a high‐fat diet,32 suggesting the necessity of adequate glycine status in avoiding hepatic lipid accumulation, which is considered an independent coronary heart disease risk factor.33
Plasma and tissue glycine concentrations are regulated by the B6‐dependent glycine cleavage system. Therefore, glycine elevation may reflect B6 deficiency.34 Interestingly, low B6 status is suggested as a risk marker for CAD.35, 36 However, in part of our population who received B vitamin treatments, we did not observe any significant interaction between plasma glycine and vitamin B6 on AMI occurrence. This finding may indicate that the glycine‐related atherogenesis may not be solely dependent on pathways requiring vitamin B6.
Nevertheless, glycine has wide metabolic ramifications, which therefore makes it difficult to make conclusions on any particular pathomechanism involved in the current study. For instance, the negative correlation between plasma glycine and CRP is in line with other studies,37, 38 implying a role of glycine in inflammation. Indeed, glycine has been shown to directly regulate the production of pro‐inflammatory cytokines22, 39 and has been suggested as a modulator of the pro‐inflammatory state.21 These findings may indicate the involvement of glycine‐related inflammation in atherogenesis and can at least partly explain the attenuation of the risk estimate of glycine when including serum CRP in the extended Cox model.
Strengths and Limitations
The strengths of the study include the large sample size, detailed baseline clinical characteristics, and its long‐term prospective design. Notably, a prior study from a subsample of the current cohort showed that plasma glycine has an excellent within‐person reproducibility over time (intraclass correlation coefficient: 0.77 [95% CI: 0.74–0.79]),40 allowing 1‐exposure assessment of biomarker status, as well as low risk of regression‐dilution bias.41
Several metabolic pathways contribute to glycine formation, and their relative quantitative contributions are not fully elucidated. Glycine concentrations among individuals may therefore be influenced by genetic and metabolic traits, as well as dietary habits, which were not evaluated in the current study. Hence, the possibility of residual confounding cannot be excluded.
Conclusions
Plasma glycine was associated with decreased risk of AMI in patients with suspected stable angina pectoris. This association was particularly strong in those with apoB, LDL cholesterol, or apoA‐1 levels above the median. Our findings motivate further studies to elucidate the role of glycine in regulating lipid metabolism and cholesterol transport in patients with atherosclerosis.
Sources of Funding
The present work has been funded by the University of Bergen, the Department of Heart Disease, Haukeland University Hospital, Norway, the Western Norway Health Authority, and the Foundation to Promote Research into Functional Vitamin B12 Deficiency.
Disclosures
None.
Acknowledgments
The authors thank Reinhard Seifert and Arve Ulvik for statistical assistance, and all the recruiting physicians and nurses for collecting the clinical information, laboratory technicians and coworkers for biochemical analyses at Haukeland University Hospital, Norway; Stavanger University Hospital, Norway; and Bevital A/S, Norway. We also thank Tomislav Dimoski at The Norwegian Knowledge Centre for the Health Services, Oslo, Norway for his contribution by developing the software necessary for obtaining data from Norwegian hospitals, conducting the data collection, and quality assurance of data in this project.
(J Am Heart Assoc. 2016;5:e002621 doi: 10.1161/JAHA.115.002621)
References
- 1. Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45:463–477. [DOI] [PubMed] [Google Scholar]
- 2. Hall JC. Glycine. JPEN J Parenter Enteral Nutr. 1998;22:393–398. [DOI] [PubMed] [Google Scholar]
- 3. Gannon MC, Nuttall JA, Nuttall FQ. The metabolic response to ingested glycine. Am J Clin Nutr. 2002;76:1302–1307. [DOI] [PubMed] [Google Scholar]
- 4. Senthilkumar R, Sengottuvelan M, Nalini N. Protective effect of glycine supplementation on the levels of lipid peroxidation and antioxidant enzymes in the erythrocyte of rats with alcohol‐induced liver injury. Cell Biochem Funct. 2004;22:123–128. [DOI] [PubMed] [Google Scholar]
- 5. McCarty MF, DiNicolantonio JJ. The cardiometabolic benefits of glycine: is glycine an ‘antidote’ to dietary fructose? Open Heart. 2014;1:e000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oberbach A, Bluher M, Wirth H, Till H, Kovacs P, Kullnick Y, Schlichting N, Tomm JM, Rolle‐Kampczyk U, Murugaiyan J, Binder H, Dietrich A, von Bergen M. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res. 2011;10:4769–4788. [DOI] [PubMed] [Google Scholar]
- 7. El Hafidi M, Perez I, Zamora J, Soto V, Carvajal‐Sandoval G, Banos G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose‐fed rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:1387–1393. [DOI] [PubMed] [Google Scholar]
- 8. El Hafidi M, Perez I, Banos G. Is glycine effective against elevated blood pressure? Curr Opin Clin Nutr Metab Care. 2006;9:26–31. [DOI] [PubMed] [Google Scholar]
- 9. De Luca G, Calpona PR, Caponetti A, Macaione V, Di Benedetto A, Cucinotta D, Di Giorgio RM. Preliminary report: amino acid profile in platelets of diabetic patients. Metabolism. 2001;50:739–741. [DOI] [PubMed] [Google Scholar]
- 10. Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, Vance DE, Wagner C. Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr. 2007;85:19–25. [DOI] [PubMed] [Google Scholar]
- 11. Liu SP, Li YS, Chen YJ, Chiang EP, Li AF, Lee YH, Tsai TF, Hsiao M, Huang SF, Chen YM. Glycine N‐methyltransferase−/− mice develop chronic hepatitis and glycogen storage disease in the liver. Hepatology. 2007;46:1413–1425. [DOI] [PubMed] [Google Scholar]
- 12. Chen CY, Ching LC, Liao YJ, Yu YB, Tsou CY, Shyue SK, Chen YM, Lee TS. Deficiency of glycine N‐methyltransferase aggravates atherosclerosis in apolipoprotein E‐null mice. Mol Med. 2012;18:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez‐Una M, Varela‐Rey M, Mestre D, Fernandez‐Ares L, Fresnedo O, Fernandez‐Ramos D, Juan VG, Martin‐Guerrero I, Garcia‐Orad A, Luka Z, Wagner C, Lu SC, Garcia‐Monzon C, Finnell RH, Aurrekoetxea I, Buque X, Martinez‐Chantar ML, Mato JM, Aspichueta P. S‐Adenosylmethionine increases circulating very‐low density lipoprotein clearance in non‐alcoholic fatty liver disease. J Hepatol. 2014;62:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JP, Kirsch‐Volders M, Matullo G, Phillips DH, Schoket B, Stromberg U, Vermeulen R, Wild C, Porta M, Vineis P. STrengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology (STROBE‐ME): an extension of the STROBE statement. Eur J Clin Invest. 2012;42:1–16. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen ER, Tuseth N, Eussen SJ, Ueland PM, Strand E, Svingen GF, Midttun O, Meyer K, Mellgren G, Ulvik A, Nordrehaug JE, Nilsen DW, Nygard O. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2015;35:455–462. [DOI] [PubMed] [Google Scholar]
- 16. Svingen GF, Ueland PM, Pedersen EK, Schartum‐Hansen H, Seifert R, Ebbing M, Loland KH, Tell GS, Nygard O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33:2041–2048. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes A . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sulo G, Igland J, Nygard O, Vollset SE, Ebbing M, Tell GS. Favourable trends in incidence of AMI in Norway during 2001–2009 do not include younger adults: a CVDNOR project. Eur J Prev Cardiol. 2014;21:1358–1364. [DOI] [PubMed] [Google Scholar]
- 19. Sulo G, Igland J, Vollset SE, Nygård O, Øyen N, Tell GS. Cardiovascular disease and diabetes mellitus in Norway during 1994–2009 CVDNOR—a nationwide research project. Norsk Epidemiol. 2013;23:101–107. [Google Scholar]
- 20. Windelberg A, Arseth O, Kvalheim G, Ueland PM. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography‐mass spectrometry. Clin Chem. 2005;51:2103–2109. [DOI] [PubMed] [Google Scholar]
- 21. Garcia‐Macedo R, Sanchez‐Munoz F, Almanza‐Perez JC, Duran‐Reyes G, Alarcon‐Aguilar F, Cruz M. Glycine increases mRNA adiponectin and diminishes pro‐inflammatory adipokines expression in 3T3‐L1 cells. Eur J Pharmacol. 2008;587:317–321. [DOI] [PubMed] [Google Scholar]
- 22. Almanza‐Perez JC, Alarcon‐Aguilar FJ, Blancas‐Flores G, Campos‐Sepulveda AE, Roman‐Ramos R, Garcia‐Macedo R, Cruz M. Glycine regulates inflammatory markers modifying the energetic balance through PPAR and UCP‐2. Biomed Pharmacother. 2010;64:534–540. [DOI] [PubMed] [Google Scholar]
- 23. Thalacker‐Mercer AE, Ingram KH, Guo F, Ilkayeva O, Newgard CB, Garvey WT. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes. 2014;63:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou Y, Qiu L, Xiao Q, Wang Y, Meng X, Xu R, Wang S, Na R. Obesity and diabetes related plasma amino acid alterations. Clin Biochem. 2013;46:1447–1452. [DOI] [PubMed] [Google Scholar]
- 25. Tastesen HS, Keenan AH, Madsen L, Kristiansen K, Liaset B. Scallop protein with endogenous high taurine and glycine content prevents high‐fat, high‐sucrose‐induced obesity and improves plasma lipid profile in male C57BL/6J mice. Amino Acids. 2014;46:1659–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang‐Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, Grallert H, Xu T, Bader E, Huth C, Mittelstrass K, Döring A, Meisinger C, Gieger C, Prehn C, Roemisch‐Margl W, Carstensen M, Xie L, Yamanaka‐Okumura H, Xing G, Ceglarek U, Thiery J, Giani G, Lickert H, Lin X, Li Y, Boeing H, Joost H‐G, de Angelis MH, Rathmann W, Suhre K, Prokisch H, Peters A, Meitinger T, Roden M, Wichmann HE, Pischon T, Adamski J, Illig T. Novel biomarkers for pre‐diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park T, Lee K. Dietary taurine supplementation reduces plasma and liver cholesterol and triglyceride levels in rats fed a high‐cholesterol or a cholesterol‐free diet. Adv Exp Med Biol. 1998;442:319–325. [DOI] [PubMed] [Google Scholar]
- 28. Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. JAMA. 1989;261:1169–1173. [PubMed] [Google Scholar]
- 29. Kerr SJ. Competing methyltransferase systems. J Biol Chem. 1972;247:4248–4252. [PubMed] [Google Scholar]
- 30. Yeo EJ, Wagner C. Tissue distribution of glycine N‐methyltransferase, a major folate‐binding protein of liver. Proc Natl Acad Sci USA. 1994;91:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao Y, Chen T, Lee T, Wang H, Wang C, Liao L, Liu R, Huang S, Chen YA. Glycine N‐methyltransferase deficiency affects Niemann‐Pick type C2 protein stability and regulates hepatic cholesterol homeostasis. Mol Med. 2012;18:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yue JT, Mighiu PI, Naples M, Adeli K, Lam TK. Glycine normalizes hepatic triglyceride‐rich VLDL secretion by triggering the CNS in high‐fat fed rats. Circ Res. 2012;110:1345–1354. [DOI] [PubMed] [Google Scholar]
- 33. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF III. Dietary vitamin B‐6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr. 2005;81:648–655. [DOI] [PubMed] [Google Scholar]
- 35. Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, Rubba P, Palma‐Reis R, Meleady R, Daly L, Witteman J, Graham I. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97:437–443. [DOI] [PubMed] [Google Scholar]
- 36. Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, Pizzolo F, Faccini G, Beltrame F, Corrocher R. Low plasma vitamin B‐6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79:992–998. [DOI] [PubMed] [Google Scholar]
- 37. Suliman ME, Qureshi AR, Stenvinkel P, Pecoits‐Filho R, Barany P, Heimburger O, Anderstam B, Rodriguez Ayala E, Divino Filho JC, Alvestrand A, Lindholm B. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. Am J Clin Nutr. 2005;82:342–349. [DOI] [PubMed] [Google Scholar]
- 38. Wheeler MD, Ikejema K, Enomoto N, Stacklewitz RF, Seabra V, Zhong Z, Yin M, Schemmer P, Rose ML, Rusyn I, Bradford B, Thurman RG. Glycine: a new anti‐inflammatory immunonutrient. Cell Mol Life Sci. 1999;56:843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cruz M, Maldonado‐Bernal C, Mondragón‐Gonzalez R, Sanchez‐Barrera R, Wacher NH, Carvajal‐Sandoval G, Kumate J. Glycine treatment decreases proinflammatory cytokines and increases interferon‐γ in patients with type 2 diabetes. J Endocrinol Invest. 2008;31:694–699. [DOI] [PubMed] [Google Scholar]
- 40. Midttun O, Townsend MK, Nygard O, Tworoger SS, Brennan P, Johansson M, Ueland PM. Most blood biomarkers related to vitamin status, one‐carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within‐person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J Nutr. 2014;144:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frost C, Thompson SG. Correcting for regression dilution bias: comparison of methods for a single predictor variable. J R Stat Soc Ser A. 2000;163:173–189. [Google Scholar]


