Abstract
Background
Coronary heart disease (CHD) represents often the underlying conditions for the development of heart failure (HF). We aimed at exploring the burden and timing of HF complicating an acute myocardial infarction (AMI), using the total population of AMI patients hospitalized during 2001–2009 in Norway.
Methods and Results
A total of 86 771 patients with a first AMI during 2001–2009 and without previous HF were identified in the “Cardiovascular Disease in Norway” project and followed until HF development, death, or December 31, 2009. In 16 219 patients (18.7%), HF was present on admission or developed during hospitalization for the incident AMI. HF occurrence varied according to age (8.9%, 15.2%, and 25.6% among men and 10.2%, 16.8%, and 27.1% among women ages 25–54, 55–74, and 75–85 years). Among 63 853 patients discharged alive without HF, 8058 (12.6%) were hospitalized with or died because of HF during a median follow‐up time of 3.2 years. HF incidence rates (IRs) per 1000 person‐years during follow‐up were 31 (95% CI, 30–32) for men and 46 (95% CI, 44–47) for women (P<0.01). IRs of HF were highest during the first 6 months of follow‐up, after which they leveled off and remained stable until the end of follow‐up.
Conclusions
In this nation‐wide cohort study, we observed that HF remains a frequent complication of the first AMI; both during the acute phase and shortly after the discharge from the hospital.
Keywords: acute myocardial infarction, cardiovascular disease in Norway, epidemiology, heart failure, Norway
Subject Categories: Cardiovascular Disease, Epidemiology, Heart Failure
Introduction
Heart failure (HF) accounts for the majority of hospital admissions among people older than 65 years and carries a poor prognosis.1 Although its etiology encompasses various conditions, coronary heart disease (CHD),2 in particular, acute myocardial infarction (AMI),3 is among the most frequent underlying causes.
Recent changes in diagnostic criteria and advances in treatment have greatly influenced survival of patients suffering an AMI. This, combined with aging of the population, has increased the number of patients living with various degrees of myocardial damage and being at risk of developing HF.4 In this context, HF is considered the “price to pay” for positive changes characterizing coronary care during the last decades. To illustrate, a Global Burden of Disease study reported that despite reductions in AMI incidence and improved survival, the prevalence of HF attributed to ischemic heart disease has increased during 1990–2010.5
Incidence of HF among patients hospitalized for an AMI varies widely among earlier studies, reaching up to 50%.6 Later studies conducted during the revascularization era7, 8, 9 also agree that HF is a common complication of AMI.10
The timing of HF occurrence in relation to the AMI event is important because it can provide information on the mechanisms involved and thereby help reduce the burden of HF. The majority of studies, however, have focused only on early‐phase HF (developed during the hospitalization for the AMI) with only a limited number of studies extending the follow‐up period after discharge from the first AMI.7, 8
Therefore, the purpose of these analyses was to describe the incidence of HF as a complication of the first (index) AMI, focusing on the timing of occurrence by using a nationwide cohort of patients hospitalized in Norway during 2001–2009.
Methods
The “Cardiovascular disease in Norway” (CVDNOR) project (https://cvdnor.b.uib.no) is established in collaboration between the University of Bergen and the Norwegian Knowledge Centre for the Health Services.11 Information on all hospital stays with International Classification of Disease (ICD)‐9 codes 390 to 459 or ICD‐10 codes I00‐I99 were retrieved during 1994–2009 from the electronic patient administrative systems (PAS) in all somatic hospitals in Norway. Information includes patient's sex, age, admission and discharge dates, up to 20 discharge diagnoses, and information on diagnostic/treatment procedures performed during that hospitalization. Information on individuals who died (either in Norway or abroad) was retrieved from the Norwegian Cause of Death Registry.
A unique personal identification number assigned to each Norwegian resident allowed us to trace patients for transfers within or between hospitals and follow them after being discharged from the hospital.
The Study Population and Endpoint
We identified all individuals ages 25 to 85 years who were hospitalized for their first AMI (coded as I21, I22 in ICD‐10) during 2001–2009 (ie, during the previous 7 years they had not been hospitalized with an AMI)12 and did not have previous hospitalizations attributed to HF. They were followed until the study endpoint, death or December 31, 2009 (end of follow‐up), whichever came first.
The study endpoint was a combination of hospitalizations for, or deaths with, HF (I50 in ICD‐10) as the underlying or contributing cause of death.
Based on the timing of the HF occurrence in relation to the index AMI, we distinguished between in‐hospital HF (defined as HF on admission or which developed during the hospitalization for the index AMI) and post‐AMI discharge HF (defined as either a hospitalization with HF or death attributed to HF as underlying or contributing cause, after being discharged from the index AMI hospitalization).
Continuous variables are presented as mean (SD) for normally distributed variables or median (5th–95th percentile) if data distribution was skewed. Categorical variables are presented as numbers and proportions.
Logistic regression and linear regression analyses were used to compare baseline characteristics between men and women (Table 1) or between patients developing in‐hospital HF or post‐AMI discharge HF and those who did not develop HF during the follow‐up (Table 2).
Table 1.
Baseline Characteristics of Men and Women Hospitalized for the First AMI in Norway During 2001–2009: A CVDNOR Project
| Patient Characteristics | Men (n=57 475) | Women (n=29 296) | P Value |
|---|---|---|---|
| Age, mean (SD) | 65.9 (12.5) | 72.1 (11.2) | <0.001 |
| Age group, n (%) | |||
| 25 to 54 y | 11 518 (20.0) | 2638 (9.0) | |
| 55 to 74 y | 28 075 (48.9) | 11 093 (37.9) | |
| 75 to 85 y | 17 882 (31.1) | 15 565 (53.1) | |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 7366 (12.8) | 4188 (14.3) | 0.002 |
| Stroke | 1647 (2.9) | 1403 (4.8) | <0.001 |
| Hypertension | 15 362 (26.7) | 9270 (31.6) | <0.001 |
| Chronic obstructive pulmonary disease | 4216 (7.3) | 2858 (9.8) | <0.001 |
| Chronic renal failure | 1823 (3.2) | 761 (2.6) | <0.001 |
| Cardiac coexisting conditions/complications, n (%) | |||
| Atrial fibrillation | 6952 (12.1) | 4287 (14.6) | <0.001 |
| Ventricular fibrillation | 1283 (2.2) | 439 (1.5) | <0.001 |
| Atrioventricular block | 755 (1.3) | 439 (1.5) | 0.892 |
| Length of AMI hospitalization (days), median (5th–95th percentile) | 6 (3–27) | 7 (5–31) | <0.001 |
| Procedures during hospitalization for the index AMI, n (%) | |||
| Coronary angiography | 26 787 (46.6) | 10 302 (42.7) | <0.001 |
| Percutaneous coronary intervention | 18 286 (31.8) | 6219 (21.2) | <0.001 |
| Coronary artery bypass grafting | 3837 (6.6) | 1170 (4.0) | <0.001 |
P values are obtained from logistic regression or linear regression analyses comparing men to women and are adjusted for age. AMI indicates acute myocardial infarction; CVDNOR, Cardiovascular Disease in Norway.
Table 2.
Baseline Characteristics of Patients Hospitalized for the First AMI With or Without Heart Failure (HF) During Follow‐up: A CVDNOR Project
| Patient Characteristics | No HF (n=62 494) | In‐Hospital HF (n=16 219) | Post‐AMI Discharge HF (n=8058) |
|---|---|---|---|
| Age, mean (SD) | 66.1 (12.5) | 72.5 (10.9)a | 73.7 (9.9)a |
| Age group, n (%) | |||
| 25 to 54 y | 12 399 (19.8) | 1291 (8.0) | 466 (5.8) |
| 55 to 74 y | 30 169 (48.3) | 6143 (37.8) | 2856 (35.4) |
| 75 to 85 y | 19 926 (31.9) | 8785 (54.2) | 4736 (59.8) |
| Sex (men), n (%) | 42 716 (68.4) | 9872 (60.9) | 4887 (60.6) |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 7239 (11.6) | 2870 (17.7)b | 1445 (17.9)b |
| Stroke | 2044 (3.3) | 675 (4.2) | 331 (4.1) |
| Hypertension | 18 021 (28.8) | 4196 (25.9)b | 2415 (30.0) |
| Chronic obstructive pulmonary disease | 4226 (6.8) | 1864 (11.5)b | 984 (12.2)b |
| Chronic renal failure | 1338 (2.1) | 906 (5.6)b | 340 (4.2)b |
| Cardiac coexisting conditions/complications, n (%) | |||
| Atrial fibrillation | 6038 (9.7) | 3594 (22.2)b | 1607 (19.9)b |
| Ventricular fibrillation | 1070 (1.7) | 538 (3.3)b | 114 (1.4) |
| Atrioventricular blocks | 743 (1.2) | 309 (1.9)b | 142 (1.8)c |
| Length of AMI hospitalization (days), median (p5–p95) | 6 (2–23) | 10 (4–44)b | 7 (3–31)b |
| Procedures during hospitalization for the index AMI, n (%) | |||
| Coronary angiography | 29 186 (46.7) | 5700 (35.2)b | 2203 (27.3)b |
| Percutaneous coronary intervention | 19 741 (31.6) | 3393 (20.9)b | 1371 (17.0)b |
| Coronary artery bypass grafting | 3676 (5.8) | 1046 (6.5)b | 285 (3.6)c |
AMI indicates acute myocardial infarction; CVDNOR, Cardiovascular Disease in Norway.
P<0.001 for comparisons between patients with in‐hospital HF or post‐AMI discharge HF with those without HF during the follow‐up (adjusted for sex).
P<0.001 for comparisons between patients with in‐hospital HF or post‐AMI discharge HF with those without HF during the follow‐up (adjusted for age and sex).
P<0.01 for comparisons between patients with in‐hospital HF or post‐AMI discharge HF with those without HF during the follow‐up (adjusted for age and sex).
Competing‐risk Cox proportional hazard regression was used to model the effect of age group at the hospitalization for an incident AMI on the risk of post‐AMI discharge HF. Sex‐specific cumulative incidence function curves (subhazard curves) were constructed for 3 age groups; young (25–54 years); middle‐aged (55–74 years); and elderly (75–85 years). This approach was chosen over conventional Kaplan‐Meier survival curves to account for death; an important competing risk for the development of post‐AMI discharge HF in this subset of patients.
The timing of post‐AMI discharge HF development was presented by plotting HF incidence rates (calculated for 6‐month time intervals) against the time from discharge from the index AMI.
Statistical Analysis
Statistical analyses were performed using STATA software (version 13; StataCorp LP, College Station, TX). Two‐sided tests with a 0.05 significance level were used. The informed consent was waived because data were collected from a national registry. The study protocol was approved by the Regional Committee for Medical and Health Research Ethics, Health Region West.
Results
Among 94 883 patients ages 25 to 85 years who were hospitalized for an index AMI in Norway from 2001 through 2009, 8112 had previous hospitalizations for HF and were therefore excluded from the analyses (Figure 1).
Figure 1.
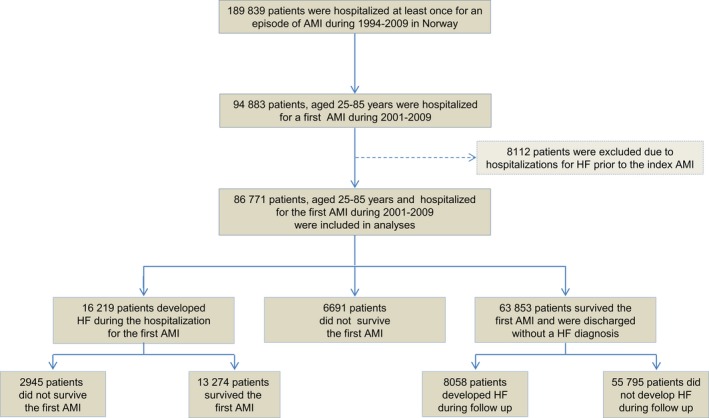
Flow chart showing selection of the study population and development of heart failure in relation to the first (index) acute myocardial infarction: a CVDNOR project. AMI indicates acute myocardial infarction; CVDNOR, Cardiovascular Disease in Norway; HF indicates heart failure.
Compared to men, women were, on average, 6 years older (65.9 vs 72.1 years; P<0.001) and had a higher proportion of diabetes mellitus (DM), hypertension, chronic obstructive pulmonary disease (COPD), and stroke. Renal failure was more frequent among men. A higher proportion of women had atrial fibrillation (AF) whereas ventricular fibrillation (VF) during the index AMI hospitalization was more frequent among men. Women had longer hospital stays, but were less likely to receive diagnostic and revascularization procedures compared to men. These differences in the distribution of the baseline characteristics remained statistically significant after adjusting for age (Table 1).
Overall, 24 277 patients (28%) developed HF either during the hospitalization for the incident AMI or after discharge (total HF). Age group–specific proportions of total HF were 12.2% in young, 22.4% in middle‐aged, and 39.5% in elderly men. The corresponding age group–specific proportions of total HF among women were 13.4%, 24.5%, and 41.5%, respectively.
Presence of HF was associated with a significant increase in mortality. Proportion of deaths among men with HF was 61.4% compared to 28.3% among those without HF. Such differences were observed in each age group (20.1% vs 7.4% among young, 47.8% vs 21.2% among middle‐aged, and 81.8% vs 62.0% among elderly men). A similar association between presence of HF and increased mortality was observed among women, overall (70.0% vs 39.2%) and in each age group (23.2% vs 10.1% among young, 52.2% vs 25.2% among middle‐aged, and 80.1% vs 59.3% among elderly women).
In‐Hospital HF
A total of 16 219 patients (18.7% of the study population) presented with or developed HF during hospitalization for the incident AMI. HF occurrence was largely influenced by age. It varied from 8.9% among young (25–54 years), 15.2% among middle‐aged (55–74), to 25.6% among elderly (75–85 years) men. The corresponding proportions of in‐hospital HF among women were 10.2%, 16.8%, and 27.1%, respectively. Such sex differences in the proportion of in‐hospital HF were statistically significant for all age groups (P=0.03 in young, P<0.01 in middle‐aged, and P=0.02 in elderly patients; Figure 2).
Figure 2.
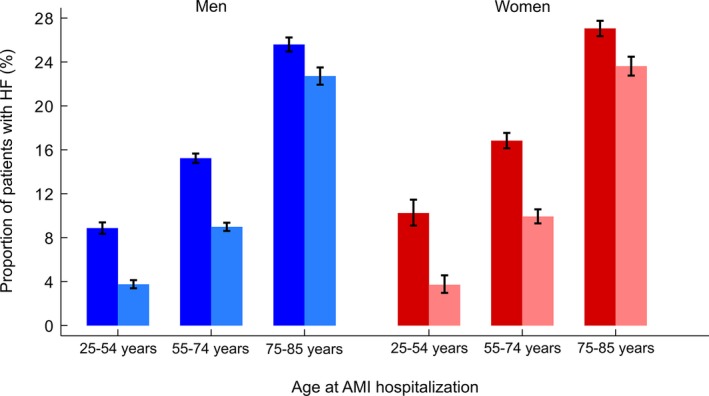
Proportion of patients with acute myocardial infarction (AMI) complicated with in‐hospital (fully colored bars) and post‐AMI discharge (light‐colored bars) heart failure (HF) by sex and age group: a CVDNOR project. CVDNOR indicate Cardiovascular Disease in Norway.
Patients with in‐hospital HF were older and had a longer hospital stay compared to those who were discharged without HF. After adjusting for age, in‐hospital HF patients had more often DM, COPD, renal failure, atrial fibrillation (AF), and adverse cardiac events, such as ventricular fibrillation (VF) and atrioventricular block. They were also less likely to undergo coronary angiography and receive myocardial revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]) compared to those without HF. No differences between groups were observed with regard to stroke whereas hypertension was more prevalent among patients not developing HF (Table 2).
Post‐AMI Discharge HF
Of 63 853 patients discharged alive from the index AMI and without a diagnosis of HF, 8058 (12.6%) developed HF later at some point during a median (interquartile range) follow‐up time of 3.2 (1.2–5.7) years. In 1045 of the AMI cases (13.0%), HF occurred within 30 days and in another 2626 (32.6%) between 30 days and 1 year from the index AMI discharge.
The proportion of patients developing post‐AMI discharge HF increased with age in both men and women. The sex differences in these proportions were statistically significant only among middle‐aged patients (9.0% in men vs 9.9% in women; P=0.01), but not among the young (3.7% in both sexes) and elderly (22.7% in men vs 23.6% in women; P=0.13; Figure 2).
Patients with post‐AMI discharge HF were older, had a longer hospitalization for the index AMI hospitalization, had more comorbidities (DM, COPD, renal failure, and AF), and were less likely to receive coronary angiography and myocardial revascularization compared to those not developing HF (Table 2).
Timing of the Post‐AMI Discharge HF
Figure 3 shows sex‐specific cumulative subhazard risk of developing post‐AMI discharge HF across different age groups of the cohort of AMI patients. Among male patients surviving their index AMI, 6% of young, 15% of middle‐aged, and 40% of elderly were subsequently hospitalized with, or died from, HF during follow‐up. The corresponding proportions of post‐AMI discharge HF among female patients were 7%, 16%, and 40% (Figure 3). No statistically significant sex differences in risk of HF were observed among young (P=0.9) and elderly (P=0.1), whereas a borderline significance level (0.49) was observed among middle‐aged patients.
Figure 3.
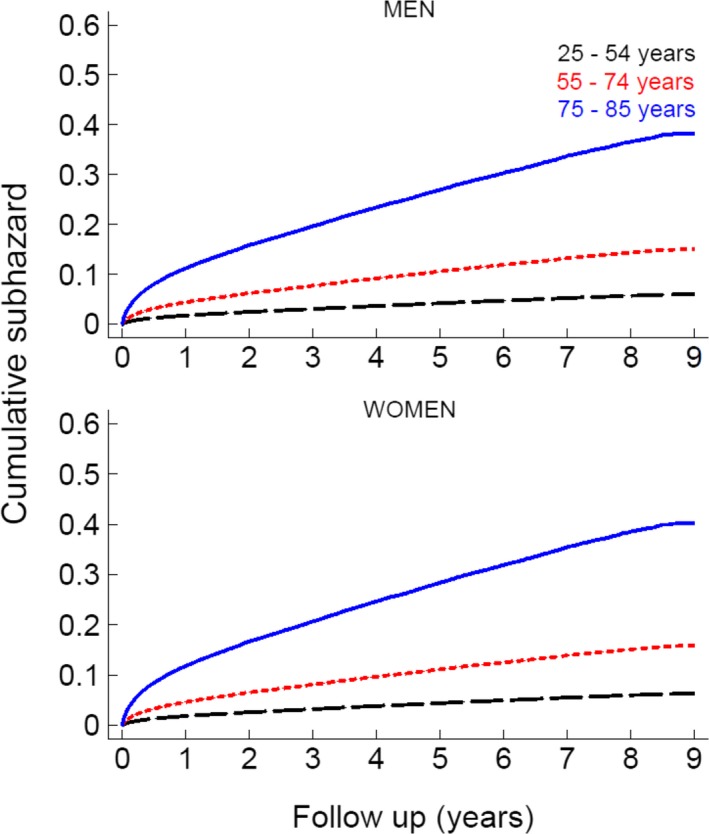
Sex‐specific cumulative incidence of post‐AMI (acute myocardial infarction) discharge heart failure (HF) by age group: a CVDNOR project. CVDNOR indicate Cardiovascular Disease in Norway.
The overall incidence rates (IR) of post‐AMI discharge HF per 1000 person‐years during the study period was 31 (95% CI, 30–32) among men and 46 (95% CI, 44–47) among women (P<0.01). Age group–specific overall incidence rates (IRs) were 9 (95% CI, 8–10), 24 (95% CI, 22–25), and 81 (95% CI 78–84) among men and 9 (95% CI, 7–11), 26 (95% CI, 24–28), and 81 (95% CI, 78–85) among women ages 25 to 54, 55 to 74, and 75 to 85 years, respectively. Statistically significant sex differences in IRs were observed only among middle‐aged patients (P=0.02), but not among the young (P=0.41) or elderly (P=0.45).
Incidence of post‐AMI discharge HF was highest during the first months and dropped at 1 year after the index AMI. After that, IRs remained stable. Despite age differences in rates, the described time‐dependent distribution of events was very similar between younger, middle‐aged, and elderly patients among both men and women (Figure 4).
Figure 4.
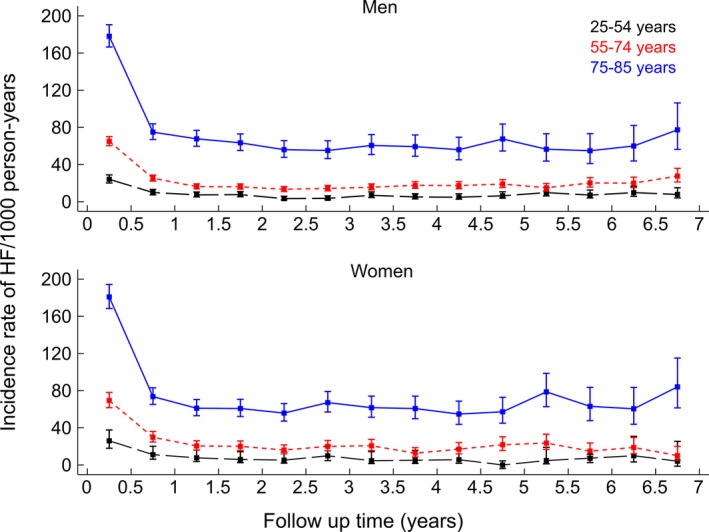
Sex‐ and age group–specific incidence rates of post‐AMI (acute myocardial infarction) discharge heart failure (HF) among patients hospitalized for the first acute myocardial infarction: a CVDNOR project. CVDNOR indicate Cardiovascular Disease in Norway.
Discussion
We observed that HF remains still a common complication among patients hospitalized with their first AMI and follows a time‐dependent pattern with high IRs during the first months up to 1 year post‐AMI discharge, dropping and remaining stable thereafter.
Previous knowledge on HF complicating an AMI mostly comes from studies focusing on in‐hospital HF. Among 483 incident AMI cases recruited during 1992–1996, 4% presented with signs of HF on admission and another 39% developed HF during MI hospitalization.13 Data from the Global Registry of Acute Coronary Events (GRACE) revealed that that among 13 707 ACS patients hospitalized during 1999–2001, 13% had HF on admission and another 5.6% developed HF during the hospital stay.14 Spencer et al. found that 20.4% of 123 938 AMI patients hospitalized during 1994–2000 presented with signs of HF whereas another 8.6% developed HF during hospitalization.15 A more recent study including 187 803 AMI patients hospitalized during 2007–2011 found that 12% of patients presented with signs of HF at admission and another 4% developed HF during hospitalization.8
Similar to ours, some other studies have reported the total proportion of patients developing HF during a hospitalization for an AMI, without distinguishing between cases in whom HF was present on admission or those who developed it during the hospitalization. Velazquez and Pfeffer16 reported that among 5566 patients hospitalized for an AMI during 1999–2001, 42% were complicated with HF during AMI hospitalization. An analysis using data from the Euro Heart Survey reported that 26% of 9587 ACS patients hospitalized during 2000–2001 presented with HF at admission or developed it during the hospital stay.17 Data from the FAST‐MI registry in France showed that 32% of 3059 AMI patients enrolled in the study during 2005 presented or developed HF during the AMI hospitalization.7 Our results are in line with recent register‐based studies including similar patients to ours8, 18 with regard to development of in‐hospital HF.
We could identify only 2 studies extending their investigation beyond hospitalization for the index AMI and reporting on post‐AMI discharge HF.4, 9 One study included 896 AMI patients hospitalized in the UK in 1998 and reported a proportion of in‐hospital and post‐AMI discharge HF of 46% and 33%, respectively.9 The other study included 7733 patients over 65 years hospitalized for their first AMI between 1994 and 2000 in Canada, of whom 37% were diagnosed with HF during the index AMI and, among the remaining, another 64% developed HF within 1 year of discharge.
Differences in findings from various studies are largely influenced by differences in patient populations. That includes selection of only incident13 versus mixed AMI cases,7, 8, 15 exclusion13, 14, 15 or not9, 19 of patients with a history of HF, and differences in the definition of the study endpoint (mild, severe, or any type of HF). Results of these studies taken together indicate a redistribution of HF occurrence in time. The proportion of cases presenting with signs of HF at the time of admission for an AMI is increasing (from 4% during 1992–199613 to 12%–13% during 2001–20118, 14), whereas the proportion developing HF during AMI hospitalization has greatly decreased from 39%13 to 4% to 8%.8, 14 HF being present on admission for an AMI is an indicator of coronary heart disease severity in terms of disease anatomical extension and myocardial involvement.20, 21 Such an increase may reflect the decline in the proportion of AMI patients dying outside hospitals that Norway,12 like other countries22, 23, 24 are experiencing. Thus, it is feasible that, currently, more‐severe cases reach the hospital alive and are at greater risk of presenting signs of HF on admission than in former time periods. On the other hand, the reduction in the proportion of AMI patients developing HF during AMI hospitalization can, to a great extent, be explained by better access to revascularization (including early revascularization), leading to myocardial salvage.
Another important factor to be considered is changes in characteristics of the AMI population. Since the troponin inclusion in diagnostic algorithm of AMI, patients are older and presenting more often with non‐ST elevation myocardial infarction (NSTEMI). Besides being a strong predictor of HF in and of itself,25 older age is associated with more comorbidities and influences treatment decisions.
Other factors influencing treatment approaches are AMI subtype and anatomical characteristics of atherosclerotic plaques and time from symptom onset to hospitalization. Such factors might help explain, in part, the undertreatment observed among older AMI patients during their hospital stay26, 27, 28 and upon discharge.29 Regardless of the cause, lack of revascularization leads to more patients leaving the hospital with myocardial damage and therefore being at high risk of developing HF.
Strength and Limitations
This is the first study describing the burden and timing of HF in a nation‐wide, unselected cohort of patients hospitalized with their first AMI. Our study population was selected after introduction of troponin in AMI diagnostic algorithms,30 reducing the effect of the well‐known diagnostic shift in AMI patient characteristics. Through record linkages with hospital discharge diagnoses and the Cause of Death Registry, we were able to conduct a complete follow‐up of the cohort. Exclusion of prevalent AMI cases minimized the effect of a mixture of incident and prevalent cases. We also excluded patients previously hospitalized with HF, maximizing the probability that the diagnosed HF occurring during follow‐up was a complication of the index AMI.
Our study carries also some limitations. We were unable to distinguish between AMI patients presenting with signs of HF on admission and those developing it during the hospital stay. Therefore, the 2 groups are combined under the in‐hospital HF category in the analyses. Data from general practitioners or hospital outpatient visits were not available; hence, our analyses were restricted to more‐severe cases requiring hospitalization or resulting in death. Furthermore, the ICD‐10 codes obtained from hospital PAS do not distinguish between ST‐elevation (STEMI) and NSTEMI. However, previous studies did not find any differences in the occurrence of HF between STEMI and NSTEMI.8, 14
Another limitation of this study is the lack of information on evidence‐based therapy (including use of thrombolysis) applied to AMI patients during hospitalization and at discharge.
Although information on invasive myocardial revascularization procedures (PCI and CABG) was available, we did not know the chronology of the events among cases developing in‐hospital HF (ie, HF present on admission render the patient not a good candidate for PCI or lack of PCI in a timely manner led to HF) and therefore could not speculate on the direction of the association. PAS do not provide information on whether there were medical conditions, severity of disease, contraindications, or other reasons (eg, lack of proper communication between medical staff and patients or patient refusal to undergo such procedure) that led to the patient not receiving such treatment.
Although the data in the CVDNOR project have been previously investigated and found to be of good quality,31, 32 we have not specifically checked the quality of coding for HF and that is a limitation of our study. A previous meta‐analysis has shown that the quality of HF coding in PAS vary widely from study to study.33 Such variation is dependent on several factors including the gold standard used for comparison, the ICD version used, and the study population. A study conducted in Sweden reported that the quality of HF diagnosis from the discharge registers is slightly inferior to AMI and stroke diagnoses.34 Furthermore, another study conducted in the UK showed that hospital discharge codes underestimate the true number of patients hospitalized with or complicated by HF.35 However, prevalence of HF based on both ICD‐9 and ICD‐10 codes among AMI patients was shown to be in agreement with chart reviews.36
Last, our definition of incident AMI is based on the absence of a previous AMI hospitalization for the same individual during a retrospective search of 7 years. Therefore, there is a small chance that some of the AMI cases defined by us as “incident” could be recurrences.
Conclusion
HF remains a frequent complication of AMI both during the acute phase and soon after discharge. The risk of HF can be influenced by a combination of factors related to patient characteristics, such as age, comorbidities, and AMI type, as well as to underuse of early revascularization procedures, especially among elderly patients.
Sources of Funding
The CVDNOR project has received funding from Nasjonalforeningen for folkehelsen.
Disclosures
None.
Acknowledgments
The authors thank Tomislav Dimoski at The Norwegian Knowledge Center for Health Services (Oslo, Norway) for his contribution by developing the software necessary for obtaining data from Norwegian hospitals, conducting the data collection, and quality assurance of data in this project.
(J Am Heart Assoc. 2016;5:e002667 doi: 10.1161/JAHA.115.002667)
References
- 1. Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 2. Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, Turner RM, Poole‐Wilson PA, Davies SW, Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–236. [DOI] [PubMed] [Google Scholar]
- 3. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L; EuroHeart Survey I, Heart Failure Association ESoC . EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 4. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in‐hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 5. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS, Roger VL. Heart failure after myocardial infarction: a review. Am J Med. 2002;113:324–330. [DOI] [PubMed] [Google Scholar]
- 7. Juilliere Y, Cambou JP, Bataille V, Mulak G, Galinier M, Gibelin P, Benamer H, Bouvaist H, Meneveau N, Tabone X, Simon T, Danchin N; Investigators F‐M . Heart failure in acute myocardial infarction: a comparison between patients with or without heart failure criteria from the FAST‐MI registry. Rev Esp Cardiol. 2012;65:326–333. [DOI] [PubMed] [Google Scholar]
- 8. Shah RV, Holmes D, Anderson M, Wang TY, Kontos MC, Wiviott SD, Scirica BM. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: insights from the National Cardiovascular Data ACTION Registry. Circ Heart Fail. 2012;5:693–702. [DOI] [PubMed] [Google Scholar]
- 9. Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, Caplin JL, Rigby AS, Goode K. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. 2008;29:859–870. [DOI] [PubMed] [Google Scholar]
- 10. Jhund PS, McMurray JJ. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118:2019–2021. [DOI] [PubMed] [Google Scholar]
- 11. Sulo G, Igland J, Vollset SE, Nygård O, Øyen N, Tell GS. Cardiovascular disease and diabetes mellitus in Norway during 1994–2009: CVDNOR–a nationwide research project. Nor Epidemiol. 2013;23:101–107. [Google Scholar]
- 12. Sulo G, Igland J, Nygard O, Vollset SE, Ebbing M, Tell GS. Favourable trends in incidence of AMI in Norway during 2001–2009 do not include younger adults: a CVDNOR project. Eur J Prev Cardiol. 2014;21:1358–1364. [DOI] [PubMed] [Google Scholar]
- 13. Ali AS, Rybicki BA, Alam M, Wulbrecht N, Richer‐Cornish K, Khaja F, Sabbah HN, Goldstein S. Clinical predictors of heart failure in patients with first acute myocardial infarction. Am Heart J. 1999;138:1133–1139. [DOI] [PubMed] [Google Scholar]
- 14. Steg PG, Dabbous OH, Feldman LJ, Cohen‐Solal A, Aumont MC, Lopez‐Sendon J, Budaj A, Goldberg RJ, Klein W, Anderson FA Jr; Global Registry of Acute Coronary Events I . Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109:494–499. [DOI] [PubMed] [Google Scholar]
- 15. Spencer FA, Meyer TE, Gore JM, Goldberg RJ. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: the National Registry of Myocardial Infarction. Circulation. 2002;105:2605–2610. [DOI] [PubMed] [Google Scholar]
- 16. Velazquez EJ, Pfeffer MA. Acute heart failure complicating acute coronary syndromes: a deadly intersection. Circulation. 2004;109:440–442. [DOI] [PubMed] [Google Scholar]
- 17. Haim M, Battler A, Behar S, Fioretti PM, Boyko V, Simoons ML, Hasdai D. Acute coronary syndromes complicated by symptomatic and asymptomatic heart failure: does current treatment comply with guidelines? Am Heart J. 2004;147:859–864. [DOI] [PubMed] [Google Scholar]
- 18. Nunez‐Gil IJ, Garcia‐Rubira JC, Luaces M, Vivas D, De Agustin JA, Gonzalez‐Ferrer JJ, Bordes S, Macaya C, Fernandez‐Ortiz A. Mild heart failure is a mortality marker after a non‐ST‐segment acute myocardial infarction. Eur J Intern Med. 2010;21:439–443. [DOI] [PubMed] [Google Scholar]
- 19. Wu AH, Parsons L, Every NR, Bates ER; Second National Registry of Myocardial I . Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI‐2). J Am Coll Cardiol. 2002;40:1389–1394. [DOI] [PubMed] [Google Scholar]
- 20. Sanz G, Castaner A, Betriu A, Magrina J, Roig E, Coll S, Pare JC, Navarro‐Lopez F. Determinants of prognosis in survivors of myocardial infarction: a prospective clinical angiographic study. N Engl J Med. 1982;306:1065–1070. [DOI] [PubMed] [Google Scholar]
- 21. Nicod P, Gilpin E, Dittrich H, Polikar R, Henning H, Ross J Jr. Long‐term outcome in patients with inferior myocardial infarction and complete atrioventricular block. J Am Coll Cardiol. 1988;12:589–594. [DOI] [PubMed] [Google Scholar]
- 22. Dudas K, Lappas G, Stewart S, Rosengren A. Trends in out‐of‐hospital deaths due to coronary heart disease in Sweden (1991 to 2006). Circulation. 2011;123:46–52. [DOI] [PubMed] [Google Scholar]
- 23. Capewell S, MacIntyre K, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, McMurray JJ. Age, sex, and social trends in out‐of‐hospital cardiac deaths in Scotland 1986–95: a retrospective cohort study. Lancet. 2001;358:1213–1217. [DOI] [PubMed] [Google Scholar]
- 24. Salomaa V, Ketonen M, Koukkunen H, Immonen‐Raiha P, Jerkkola T, Karja‐Koskenkari P, Mahonen M, Niemela M, Kuulasmaa K, Palomaki P, Mustonen J, Arstila M, Vuorenmaa T, Lehtonen A, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesaniemi YA, Pyorala K. Decline in out‐of‐hospital coronary heart disease deaths has contributed the main part to the overall decline in coronary heart disease mortality rates among persons 35 to 64 years of age in Finland: the FINAMI study. Circulation. 2003;108:691–696. [DOI] [PubMed] [Google Scholar]
- 25. Torabi A, Cleland JG, Rigby AS, Sherwi N. Development and course of heart failure after a myocardial infarction in younger and older people. J Geriatr Cardiol. 2014;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avezum A, Makdisse M, Spencer F, Gore JM, Fox KA, Montalescot G, Eagle KA, White K, Mehta RH, Knobel E, Collet JP, Investigators G. Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2005;149:67–73. [DOI] [PubMed] [Google Scholar]
- 27. Nauta ST, Deckers JW, Akkerhuis KM, van Domburg RT. Age‐dependent care and long‐term (20 year) mortality of 14,434 myocardial infarction patients: changes from 1985 to 2008. Int J Cardiol. 2013;167:693–697. [DOI] [PubMed] [Google Scholar]
- 28. Rathore SS, Mehta RH, Wang Y, Radford MJ, Krumholz HM. Effects of age on the quality of care provided to older patients with acute myocardial infarction. Am J Med. 2003;114:307–315. [DOI] [PubMed] [Google Scholar]
- 29. Salomaa V, Paakkonen R, Hamalainen H, Niemi M, Klaukka T. Use of secondary preventive medications after the first attack of acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2007;14:386–391. [DOI] [PubMed] [Google Scholar]
- 30. Langorgen J, Ebbing M, Igland J, Vollset SE, Nordrehaug JE, Tell GS, Nygard O. Implications of changing definitions of myocardial infarction on number of events and all‐cause mortality: the WHO 1979, ESC/ACC 2000, AHA 2003, and Universal 2007 definitions revisited. Eur J Prev Cardiol. 2014;21:1349–1357. [DOI] [PubMed] [Google Scholar]
- 31. Igland J, Tell GS, Ebbing M, Nygård O, Vollset SE, Dimoski T. The CVDNOR project: cardiovascular disease in Norway 1994–2009. Description of data and data quality. Available at: http://cvdnor.b.uib.no/about-cvdnor/publications/. Accessed February, 23, 2015.
- 32. Clench‐Aas J, Helgeland J, Dimoski T, Gulbrandsen DH, Holmboe O, Movinckel P, Rønning OM. Methodological developement and evaluation of 30‐day mortality as quality indicator for Norwegian hospitals. Available at: http://www.kunnskapssenteret.no/Publikasjoner/Methodological+development+and+evaluation+of+30-day+mortality+as+quality+indicator+for+Norwegian+hospitals.1246.cms. Accessed January 12, 2015. [PubMed]
- 33. McCormick N, Lacaille D, Bhole V, Avina‐Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta‐analysis. PLoS One. 2014;9:e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. [DOI] [PubMed] [Google Scholar]
- 35. Khand AU, Shaw M, Gemmel I, Cleland JG. Do discharge codes underestimate hospitalisation due to heart failure? Validation study of hospital discharge coding for heart failure. Eur J Heart Fail. 2005;7:792–797. [DOI] [PubMed] [Google Scholar]
- 36. So L, Evans D, Quan H. ICD‐10 coding algorithms for defining comorbidities of acute myocardial infarction. BMC Health Serv Res. 2006;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]


