Abstract
Background
Galectin‐3 may play a role in cardiac and noncardiac fibrosis, and elevated circulating levels of this protein predict adverse outcomes in patients with heart failure who do not have congenital heart disease. We investigated galectin‐3 in adults with single‐ventricle Fontan circulation, patients who are prone to premature clinical deterioration in the context of extensive multiorgan fibrosis.
Methods and Results
We measured plasma galectin‐3 concentrations in 70 ambulatory adult Fontan patients and 21 age‐ and sex‐matched control participants. Galectin‐3 level was significantly higher in the Fontan group (11.85 ng/mL, interquartile range 9.9 to 15.0 ng/mL) versus the control group (9.4 ng/mL, interquartile range 8.2 to 10.8 ng/mL; P<0.001). Among Fontan patients, galectin‐3 was positively correlated with age, uric acid, and high‐sensitivity C‐reactive protein and negatively correlated with estimated glomerular filtration rate. There was no significant relationship between galectin‐3 and oxygen saturation, Fontan type, or ventricular morphology. Over a median follow‐up of 461 days, 15 events occurred among the Fontan patients: 12 nonelective hospitalizations (with 2 subsequent deaths) and 3 deaths without prior hospitalization. Patients with elevated galectin‐3 (n=19, defined as >2 SD above the control group mean value) had a higher risk of nonelective hospitalization or death (hazard ratio 6.0, 95% CI 2.1 to 16.8, P<0.001). This relationship persisted after individual adjustment for covariates including age, New York Heart Association functional class, C‐reactive protein, and estimated glomerular filtration rate and after multivariable adjustment for independently predictive covariates (hazard ratio 9.2, 95% CI 2.4 to 35.2, P=0.001).
Conclusions
Galectin‐3 concentrations are elevated among adults with a Fontan circulation, and elevated galectin‐3 is associated with an increased risk of nonelective cardiovascular hospitalization or death.
Keywords: adult congenital heart disease, biomarker, congenital heart disease, Fontan procedure, galectin‐3
Subject Categories: Congenital Heart Disease, Heart Failure, Biomarkers, Fibrosis
Introduction
The Fontan procedure, which functionally separates pulmonary and systemic circulation, is the most common approach to single‐ventricle palliation. As a result of this procedure, cyanosis and ventricular volume overload are alleviated at the expense of elevated systemic venous pressure and limited stroke volume augmentation. Consequences include decreased aerobic capacity and a wide variety of end‐organ injury that can manifest in both common and esoteric diagnoses such as liver fibrosis and protein‐losing enteropathy.1, 2, 3 It follows that the Fontan circulation is associated with premature morbidity and mortality.4 There is marked variation, however, in the timing, specific manifestations, and extent of clinical deterioration between individual patients. Effective noninvasive strategies to identify Fontan patients at the highest risk for near‐term adverse outcomes could advance efforts to prevent hospitalization and death in this young, high‐risk, poorly understood population.
Both cardiac and noncardiac fibrosis are common and precede adverse outcomes in adulthood5, 6, 7; therefore, markers of fibrosis may prove useful in identifying risk among patients with a Fontan circulation. Galectin‐3 is a lectin, a carbohydrate‐binding protein, with affinity for β‐galactosides, with a wide array of biological effects that include inflammation, cell cycle modulation and cell activation, attraction, and adhesion.8, 9, 10 Reports suggest that it is a mediator of cell growth and behavior and of cancer metastasis.11 Importantly, galectin‐3 is also involved in both cardiac and noncardiac fibrosis.10, 12, 13, 14, 15 It remains to be defined whether galectin‐3 plays an important causal role in the development or maintenance of fibrosis in human disease and whether it may serve as a specific therapeutic target. It is clear, however, that circulating galectin‐3 levels are elevated in various diseases such as heart failure and alcoholic liver cirrhosis,16, 17, 18 that the presence of high galectin‐3 predicts incident heart failure and kidney disease in the general population,17, 19 and that high galectin‐3 is associated with adverse outcomes in adults with noncongenital heart failure.17, 20, 21, 22, 23, 24, 25, 26
Given the extensive multiorgan effects and fibrosis seen in single‐ventricle Fontan patients, we hypothesized that galectin‐3 would be elevated. We further hypothesized that high galectin‐3 levels would be associated with adverse outcomes.
Methods
Study Sample
We enrolled outpatients aged ≥18 years who had previously undergone a Fontan procedure and who consented to participate between February 2012 and June 2014 at Boston Children's Hospital or Brigham and Women's Hospital. None of the patients enrolled had been hospitalized in the prior 30 days. We excluded patients who underwent subsequent 2‐ventricle repair or cardiac transplantation prior to enrollment. Age‐ and sex‐matched comparison group samples, selected based on the distribution of Fontan participants by sex and 10‐year age group rather than matched to individual Fontan participants, were identified; these samples were collected from nonsmokers without diabetes mellitus or known cardiovascular disease who were recruited for this purpose using postings on the Boston Children's Hospital website. The study was approved by the Boston Children's Hospital institutional review board, and informed consent was obtained from all participants; there was a formal reliance agreement between the institutional review boards of Brigham and Women's Hospital and Boston Children's Hospital.
Clinical and Outcomes Assessment
Demographic data, underlying cardiac diagnosis and prior interventions, medication use, and medical comorbidities were extracted from medical records. Available clinical laboratory testing and cardiac imaging results within 2 years of sample collection and invasive hemodynamic data within 5 years of collection were also obtained. Data on qualitative systemic ventricular function and valve regurgitation severity were extracted from clinical reports. The combined outcome of interest was nonelective cardiovascular hospitalization or death. Nonelective cardiovascular hospitalization was defined as an overnight admission for heart failure, arrhythmia or symptoms of arrhythmia, thrombotic or embolic events, cerebral hemorrhage, or a specific Fontan‐related reason (ie, ascites, protein‐losing enteropathy, plastic bronchitis).
Biomarker Assessment
Blood collected via peripheral venipuncture in an EDTA tube was processed to plasma within 30 minutes of collection and frozen at −80°C. Galectin‐3 concentration was measured with an enzyme‐linked immunoassay (BG Medicine). Characteristics of this assay have been described previously.18 There was no association between galectin‐3 concentration and time between blood draw and assay (β=−0.0016 ng/mL per day, P=0.60). Other laboratory testing was performed on fresh processed samples by a Clinical Laboratory Improvement Amendments–certified laboratory (Laboratory Corporation of America).
Statistical Analyses
The 2‐sided unpaired Student t test or Wilcoxon rank sum test, as appropriate, was used to compare continuous variables. The Fisher exact test was used to analyze categorical variables between groups stratified by galectin‐3 level. Galectin‐3 was normally distributed in the control group (Shapiro–Wilk, P=0.37) but not in the Fontan group (Shapiro–Wilk, P<0.001). The upper limit of normal for galectin‐3 was defined as 2 SD above the control group mean (>14.3 ng/mL). Continuous variables are presented as mean±SD, with addition of median (25th to 75th percentiles) for nonnormally distributed variables. Galectin‐3 was natural log transformed for statistical analyses of galectin‐3 as a continuous variable, but we have reported untransformed mean±SD for ease of interpretation. Pearson's product moment correlation coefficients were computed to assess the relationship between log‐transformed galectin‐3 and continuous clinical characteristics including other laboratory tests. The log‐rank test was used to perform univariate survival analysis, stratified by galectin‐3 level. Cox regression, with galectin‐3 level as the independent variable, was performed to individually adjust for specified covariates; a multivariable model was developed that considered all variables significantly (P<0.05) associated with high galectin‐3 level, using forward selection with P<0.1 as the criterion for entry. Time‐to‐event analyses were conducted from the date of sample collection to the date of the first clinical event, with censoring of event‐free participants at the most recent clinical follow‐up date when event status was known. All analyses were performed using SAS 9.3 (SAS Institute) and GraphPad Prism (GraphPad Software). A 2‐sided P<0.05 was considered statistically significant.
Results
Clinical characteristics of Fontan patients in the study sample (N=70) are shown in Table 1. Compared with age‐ and sex‐matched control participants (n=21), Fontan patients tended to be slightly shorter (167.4±9.7 versus 172.8±10.3 cm, P=0.05) but were similar in terms of age (30.5±10.1 versus 32.4±11.3 years, P=0.56), sex (42.9% versus 52.4% male, P=0.46), weight (70.0±15.3 versus 70.4±17.5 kg, P=0.87), and body mass index (24.7±4.1 versus 23.4±4.2 kg/m2, P=0.11). Galectin‐3 level, however, was significantly higher in the Fontan group than in the comparison sample (13.3±5.4 versus 9.8±2.3 ng/mL; 11.85 [interquartile range (IQR) 9.9 to 15.0] versus 9.4 [IQR 8.2 to 10.8] ng/mL; Wilcoxon rank sum, P<0.001) (Figure 1).
Table 1.
Baseline Demographic and Clinical Data by Galectin‐3 Level Among 70 Patients Aged 18 to 58 Years With a Fontan Circulation
| Galectin‐3 Level, Stratified | P Value | ||
|---|---|---|---|
| ≤14.3 ng/mL (n=51) | >14.3 ng/mL (n=19) | ||
| Age, y | 28.5±8.1 | 36.2±12.7 | 0.004 |
| Male, % | 19 (37.3) | 11 (57.9) | 0.17 |
| Height, cm | 163.2±10.0 | 169.0±9.2 | 0.31 |
| Weight, kg | 70.8±15.4 | 66.6±15.0 | 0.19 |
| BMI, kg/m2 | 24.7±4.3 | 24.8±3.6 | 0.74 |
| Oxygen saturation, % | 94.1±4.4 | 92.9±3.3 | 0.15 |
| Race, % | |||
| White | 45 (93.8) | 18 (100) | 1.0 |
| Black | 1 (2.1) | 0 | |
| Other | 2 (4.2) | 0 | |
| Diagnosis, % | |||
| Tricuspid atresia | 19 (37.3) | 9 (42.1) | 0.63 |
| Double‐inlet LV | 10 (19.6) | 7 (36.8) | |
| HLHS | 11 (21.6) | 3 (15.8) | |
| Unbalanced AV canal | 3 (5.9) | 0 | |
| Double‐outlet RV | 2 (3.9) | 0 | |
| Other | 6 (11.8) | 1 (5.3) | |
| Ventricular morphology, % | 0.83 | ||
| Right | 14 (27.5) | 4 (21.1) | |
| Left | 36 (70.6) | 15 (79.0) | |
| Other | 1 (2.0) | 0 | |
| Heterotaxy, % | 4 (7.8) | 1 (5.3) | 1.0 |
| Fontan type, % | |||
| Atriopulmonary | 9 (17.7) | 5 (26.3) | 0.23 |
| Lateral tunnel | 34 (66.7) | 8 (42.1) | |
| AV or Bjork | 1 (2.0) | 1 (5.3) | |
| Extracardiac | 7 (13.7) | 5 (26.3) | |
| Patent fenestration, % | 8 (15.7) | 2 (10.5) | 0.72 |
| NYHA functional class I, % | 39 (76.5) | 13 (68.4) | 0.55 |
| History of PLE, % | 2 (3.9) | 1 (5.3) | 1.0 |
| Clinical ascites, % | 2 (3.9) | 6 (31.6) | 0.004 |
| Clinical arrhythmia, % | 26 (52.0) | 14 (73.7) | 0.17 |
| Atrial flutter, %a | 17 (65.4) | 12 (85.7) | 0.27 |
| Atrial fibrillation, %a | 5 (19.2) | 2 (14.3) | 1.0 |
| Pacemaker, % | 20 (39.2) | 12 (63.2) | 0.11 |
| Medication, % | |||
| ACEI/ARB | 30 (58.8) | 10 (52.6) | 0.79 |
| Beta blocker | 14 (27.5) | 4 (21.1) | 0.76 |
| Digoxin | 14 (27.5) | 7 (36.8) | 0.56 |
| Antiarrhythmic | 7 (13.7) | 4 (21.1) | 0.47 |
| Aspirin | 32 (62.8) | 7 (36.8) | 0.06 |
| Warfarin | 20 (39.2) | 12 (63.2) | 0.11 |
| Loop diuretic | 13 (25.5) | 12 (63.2) | 0.005 |
| Potassium‐sparing diuretic | 8 (15.7) | 7 (36.8) | 0.10 |
| Thyroid replacement | 2 (3.9) | 5 (26.3) | 0.01 |
| Systemic ventricular function, % | |||
| Normal | 24 (49.0) | 9 (47.4) | 0.03 |
| Mildly depressed | 23 (46.9) | 5 (26.3) | |
| Moderately or severely depressed | 2 (4.1) | 5 (26.3) | |
| Systemic AVV regurgitation, % | |||
| None | 14 (28.6) | 7 (36.8) | 0.49 |
| Mild | 28 (57.1) | 12 (63.2) | |
| Moderate or severe | 7 (14.3) | 0 | |
| Aortic regurgitation, % | |||
| None | 30 (61.2) | 8 (42.1) | 0.29 |
| Mild | 17 (35.7) | 10 (52.6) | |
| Moderate or severe | 2 (4.1) | 1 (5.3) | |
A subset of patients was missing valid data for some variables (eg, 2 of 51 patients with normal galectin‐3 level had not had relevant imaging in the prior 2 years). Continuous variables are presented as mean±SD, and categorical variables are presented as n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AV, atrioventricular; AVV, atrioventricular valve; BMI, body mass index; HLHS, hypoplastic left heart syndrome; LV, left ventricle; NYHA, New York Heart Association; PLE, protein‐losing enteropathy; RV, right ventricle.
Percentage of patients with clinical arrhythmia with this diagnosis. These diagnoses are not mutually exclusive.
Figure 1.
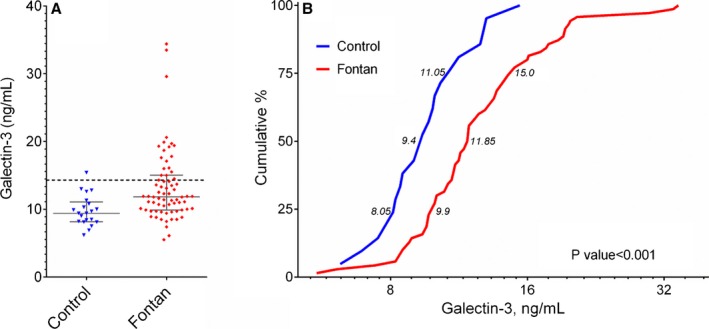
Distribution of galectin‐3 values for Fontan participants and an age‐ and sex‐matched control group. A, Absolute galectin‐3 values for Fontan participants and the control group; the error bars represent the 25th to 75th percentiles. The dashed line designates 14.3 ng/mL, the cutoff used to define elevated galectin‐3 in this study. B, Cumulative distribution of galectin‐3 for control and Fontan participants, logarithmic scale. Numbers adjacent to respective curves designate the median value bounded by the first and third quartiles. The Wilcoxon rank sum test was used to compare galectin‐3 level between groups.
Among Fontan patients, age positively correlated with galectin‐3 (r=0.35, P=0.003) (Table 1, Figure 2A), averaging 11.3±2.7, 13.2±6.4, and 17.2±5.5 ng/mL for those aged <25, 25 to 40, and >40 years, respectively (P<0.001). This relationship was not specifically due to either age at the time of initial Fontan completion or time living with a Fontan circulation. Although there was a modest correlation between age at initial Fontan procedure and galectin‐3 (r=0.26, P=0.03), this was due to secular trends in timing of initial Fontan with earlier Fontan completion in younger patients. There was no appreciable relationship between galectin‐3 and either age at initial Fontan or time since initial Fontan procedure after adjusting for age at sample collection.
Figure 2.
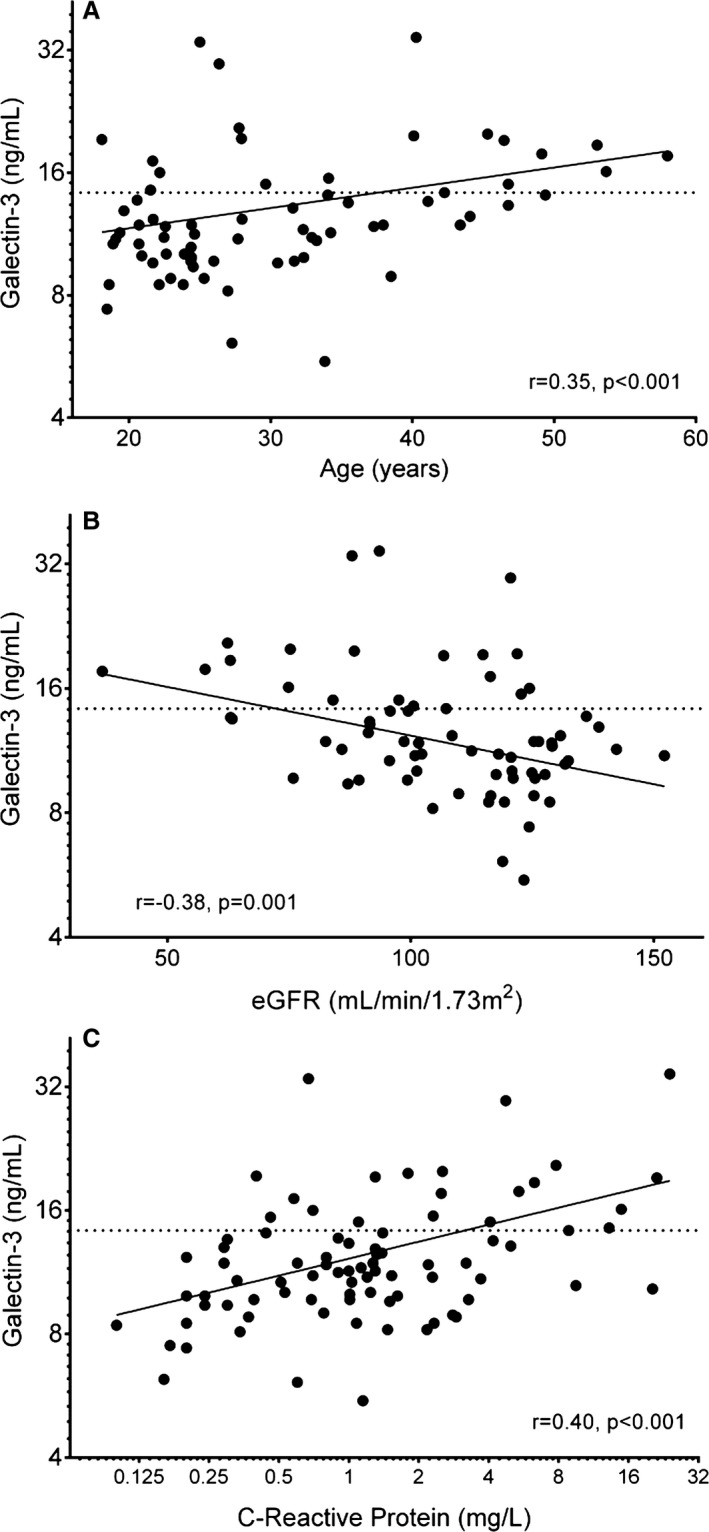
Associations between galectin‐3 and age (A), estimated glomerular filtration rate (B), and C‐reactive protein (C) among patients with a Fontan circulation. Galectin‐3 is plotted on a natural logarithmic scale, as is C‐reactive protein in (C). The horizontal dotted line signifies a galectin‐3 level of 14.3 ng/mL. eGFR indicates estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula.27
A subset of Fontan patients (17.1%) had ≥1 chronic medical comorbidity: 2.9% were current tobacco users, 4.3% were former tobacco users, 4.3% had systemic hypertension, 7.1% had obstructive sleep apnea, 2.9% had dyslipidemia, 1.4% had a history of subacute bacterial endocarditis, and 1.4% had asthma. No patient had a diagnosis of diabetes mellitus, atherosclerotic coronary artery disease, or chronic obstructive pulmonary disease. There was no statistically significant association between the presence of chronic comorbidities and galectin‐3 level.
There was no statistically significant difference in galectin‐3 levels by sex (13.4±6.2 versus 13.1±4.1 in women and men, respectively; P=0.70). Galectin‐3 was not associated with height (r=−0.08, P=0.49), weight (r=−0.06, P=0.61), body mass index (r=−0.03, P=0.79), or oxygen saturation (r=0.07, P=0.59). There was also no significant relationship between galectin‐3 and underlying diagnosis, ventricular morphology, heterotaxy syndrome, current Fontan type, or patent fenestration (Table 1). A Fontan revision or conversion had been performed prior to enrollment in 15 patients. Most (n=11, 73.3%) of these patients initially had an atriopulmonary Fontan procedure; the most common revision or conversion was to an extracardiac Fontan procedure (n=11, 73.3%). Patients who initially underwent an atriopulmonary Fontan procedure (n=24) were more likely to have high galectin‐3 than the 41 patients with a primary lateral tunnel Fontan procedure (univariate odds ratio 3.5, 95% CI 1.1 to 10.9, P=0.03; 51.6% versus 27.5% of patients with elevated and normal galectin‐3, respectively); however, this was due to the older age of patients who had initially had such a Fontan procedure (adjusted for age, odds ratio 1.8, 95% CI 0.4 to 7.9, P=0.44). Most Fontan participants in this study were classified as New York Heart Association (NYHA) functional class I (74.3%); this proportion was similar for those with normal and elevated galectin‐3 levels.
Cardiac catheterization was performed for clinical reasons in a minority of patients in each group (n=23 and n=7 for those with normal and high galectin‐3 levels, respectively). Fontan pressure tended to be slightly higher in the high galectin‐3 group (16.5 [IQR 16 to 17] versus 14 [IQR 12 to 16 mm Hg]; Wilcoxon rank sum, P=0.06), as did pulmonary vascular resistance (1.9 [IQR 1.7 to 2.7] versus 1.6 [IQR 1.4 to 2.1] WU×m2; P=0.07). Cardiac index was similar in those with high and normal galectin‐3 (2.5 [IQR 2.5 to 2.7] versus 2.9 [IQR 2.5 to 3.2] L/min per m2; P=0.23); this was also true for pulmonary artery occlusion pressure (10 [IQR 8 to 13] versus 10 [IQR 8 to 12] mm Hg; P=0.96) and mean arterial blood pressure (77 [IQR 63 to 90] versus 73 [IQR 67 to 88] mm Hg; P=0.88).
Notably, those with high galectin‐3 levels were much more likely to have required either paracentesis or an increase in diuretic dose for treatment of ascites (31.6% versus 3.9%; P=0.004) (Table 1). The 8 patients who had such intervention tended to have higher galectin‐3 levels (15.6±3.5 versus 13.0±5.5 ng/mL; Wilcoxon rank sum, P=0.03). Those with high galectin‐3 were more likely to be prescribed loop and potassium‐sparing diuretics. There was also a trend toward higher warfarin use (with, conversely, lower aspirin use). Only 1 patient, who had elevated galectin‐3, was prescribed an oral pulmonary vasodilator medication. Those with higher galectin‐3 were more likely to be taking thyroid replacement therapy. Notably, 5 of the 7 patients on thyroid hormone had been prescribed amiodarone at some point. At the time of galectin‐3 measurement, 2 were still taking amiodarone; only 2 of the 63 patients not taking thyroid replacement were on amiodarone at the time of galectin‐3 measurement (28.6% versus 3.2%; Fisher exact test, P=0.047).
There was a modest relationship of more systemic ventricular dysfunction in those with high galectin‐3, but most of these patients (14 of 19) had normal or only mildly reduced systemic ventricular function. There was no statistically significant association between galectin‐3 and aortic or atrioventricular valve regurgitation. A history of clinical arrhythmia was very common, especially atrial flutter (intra‐atrial reentrant tachycardia); arrhythmia was present in a greater proportion of those with high galectin‐3, although the difference was not statistically significant.
Patients with elevated galectin‐3 had, on average, lower estimated glomerular filtration rate (eGFR) and higher uric acid and high‐sensitivity C‐reactive protein (hsCRP) (Table 2, Figure 2B and 2C). Galectin‐3, however, was not associated with red cell indices, platelet count, hepatic markers, albumin, or serum sodium concentration. B‐type natriuretic peptide was measured only for clinical indications in a minority of participants (24 of 70) within 2 years of galectin‐3 sample collection. Among this subset, there was a positive correlation between galectin‐3 and B‐type natriuretic peptide (r=0.45, P=0.03; both variables log transformed), although there was no significant difference in B‐type natriuretic peptide between those with normal and elevated galectin‐3 levels (24 [IQR 10 to 96] versus 87 [IQR 24 to 174]; Wilcoxon rank sum, P=0.20).
Table 2.
Relationship of Galectin‐3 to Common Clinical Laboratory Tests Among 70 Patients With a Fontan Circulation
| Galectin‐3 Level, Stratified | Galectin‐3, Continuousa | ||||
|---|---|---|---|---|---|
| ≤14.3 ng/mL (n=51) | >14.3 ng/mL (n=19) | P Value | r | P Value | |
| Hemoglobin, g/dL | 15.2±1.3 | 15.1±2.4 | 0.86 | 0.05 | 0.66 |
| MCV, fL/cell | 88.1±5.7 | 89.6±5.4 | 0.30 | 0.10 | 0.43 |
| RDW, % | 14.3±1.2 | 14.6±0.9 | 0.30 | 0.20 | 0.10 |
| Platelet count, ×103/μL | 175±52 | 206±72 | 0.50 | 0.10 | 0.43 |
| AST/SGOT, U/L | 26.6±11.6 | 26.2±7.6 | 0.89 | −0.02 | 0.89 |
| ALT/SGPT, U/L | 28.2±14.6 | 27.9±11.2 | 0.93 | −0.08 | 0.52 |
| Total bilirubin, mg/dL | 1.0±0.9 | 0.8±0.5 | 0.48 | −0.13 | 0.29 |
| Albumin, g/dL | 4.5±0.6 | 4.6±0.7 | 0.69 | 0.03 | 0.78 |
| Creatinine, mg/dL | 0.84±0.2 | 0.95±0.2 | 0.06 | 0.30 | 0.01 |
| eGFR, mL/min per 1.73 m2 | 111.4±19.6 | 92.1±25.6 | 0.001 | −0.38 | 0.001 |
| Sodium, mEq/L | 138.3±2.3 | 137.9±3.4 | 0.56 | −0.17 | 0.17 |
| Uric acid, mg/dL | 5.9±1.7 | 7.2±2.2 | 0.01 | 0.36 | 0.003 |
| hsCRP, mg/La | 1.7±1.9 | 6.1±7.1 | <0.001 | 0.40 | <0.001 |
Data are presented as mean±SD. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula27; hsCRP, high‐sensitivity C‐reactive protein; MCV, mean corpuscular volume; RDW, red cell distribution width; SGOT, serum glutamic‐oxaloacetic transaminase; SGPT, serum glutamic‐pyruvic transaminase.
Statistical tests performed using natural log‐transformed values.
Outcomes Analysis
The median follow‐up duration was 461 days (IQR 319 to 650 days). There were 15 events: 12 nonelective hospitalizations (2 patients subsequently died) and 3 deaths without preceding nonelective hospitalization. Median time to event was 340 days (IQR 139 to 465 days). Of the 12 incident nonelective cardiovascular hospitalizations, 6 were related to heart failure or other volume retention including ascites, 4 were for arrhythmia (3 atrial flutter, 1 atrial fibrillation), 1 was for hemoptysis related to pulmonary collateral vessels, and 1 was for presyncope related to hypotension. Two of the hospitalized patients later died: 1 had presumed sudden cardiac arrest, and the other had a sinus pause followed by pulseless electrical activity after an elective cardioversion for atrial flutter, could not be resuscitated, and died after prolonged support with extracorporeal membrane oxygenation. There were 3 patients who died without preceding nonelective cardiovascular hospitalization. Two experienced sudden death without autopsy, and the third died as the result of sepsis due to recurrent cellulitis in the context of chronic volume retention and severe edema. Of note, this patient did not have an active infection at the time of baseline blood draw and died about 1 year after enrollment.
Higher galectin‐3 was associated with greater hazard for the combined outcome (hazard ratio [HR] 2.32, 95% CI 1.35 to 3.97; per 1 SD increase in log‐transformed galectin‐3, P=0.002). Fontan patients with high galectin‐3 levels were at increased risk for the combined outcome (HR 6.0, 95% CI 2.1 to 16.8; P<0.001) (Figure 3). Adjustment for age did not affect the predictive value of elevated galectin‐3 (HR 6.5, 95% CI 2.1 to 20.0; P=0.001; age, P=0.69; results were equivalent for adjustment for age at Fontan completion and time since initial Fontan procedure and are not presented). In addition, the predictive value of elevated galectin‐3 was not affected by adjustment for eGFR (HR 7.5, 95% CI 2.4 to 23.2, P<0.001; eGFR, P=0.37), exclusion of patients with eGFR <90 mL/min per 1.73 m2 (HR 6.0, 95% CI 1.6 to 22.8, P=0.008; n=54), adjustment for hsCRP (HR 3.8, 95% CI 1.2 to 11.7, P=0.02; log hsCRP, P=0.04), or adjustment for NYHA functional class (HR 6.6, 95% CI 2.3 to 18.9, P<0.001; NYHA functional class, P=0.01; NYHA functional class I: HR 4.8, 95% CI 1.1 to 21.8, P=0.04; NYHA functional class II: HR 7.7 95% CI 1.8 to 33.3, P=0.006). Omitting the 3 participants with the highest galectin‐3 levels also did not affect the results (HR 5.6, 95% CI 1.9 to 16.2, P=0.002). High galectin‐3 remained a significant predictor of outcome after multivariable adjustment (HR 9.2, 95% CI 2.4 to 35.2, P=0.001; covariates included in final model were hsCRP and eGFR). The 1‐year Kaplan–Meier estimates for the combined outcome were 38.1% versus 4.4% for those with elevated and normal galectin‐3, respectively; the corresponding 2‐year Kaplan–Meier estimates were 82.3% versus 17.8%, respectively.
Figure 3.
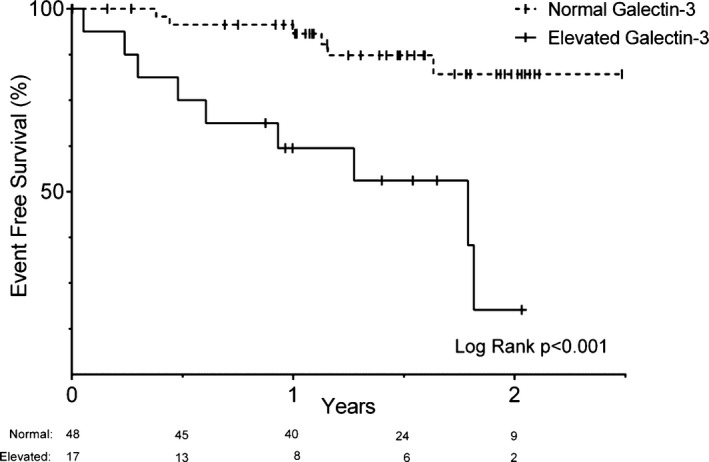
Kaplan–Meier plot of event‐free survival stratified by galectin‐3 level ≤14.3 or >14.3 ng/mL. A Kaplan–Meier plot of risk in each group from time of blood draw is listed below the graph; hash marks designate censored observations. Event‐free survival refers to survival without nonelective cardiovascular or Fontan‐related hospitalization. Number of participants at risk in each group is listed below the x‐axis. A small subset of participants (3 of 51 and 2 of 19 in the normal and elevated galectin‐3 groups, respectively) had no follow‐up time.
Having any of the comorbidities listed (current or prior tobacco use, systemic hypertension, obstructive sleep apnea, dyslipidemia, history of subacute bacterial endocarditis, or asthma) tended to be associated with a higher risk of the combined outcome, although the association was not statistically significant (HR 2.5, 95% CI 0.9 to 7.4, P=0.09). High galectin‐3 remained a significant predictor of outcome after excluding participants with a chronic comorbidity (HR 6.1, 95% CI 1.7 to 21.6, P=0.005; n=58) or adjusting for presence of a chronic comorbidity (HR 5.8, 95% CI 2.0 to 16.4, P=0.001; HR for having a comorbidity 2.3, 95% CI 0.8 to 6.9, P=0.13).
There were 5 deaths over the follow‐up period. There was no statistically significant difference in galectin‐3 level between the 5 patients who died and those who survived (14.4±4.7 versus 12.9±5.0 ng/mL; 11.9 [IQR 11.0 to 19.2] versus 11.7 [IQR 9.7 to 14.5]; P=0.37). Death occurred during follow‐up in 10.5% of the patients with high galectin‐3 compared with 5.9% of those with normal galectin‐3. In the context of a small number of events, high galectin‐3 was not a statistically significant predictor of death in survival analysis (HR 2.0, 95% CI 0.4 to 11.8, P=0.46).
Discussion
These data suggest that among patients with a Fontan circulation, galectin‐3 (1) is elevated compared with age‐ and sex‐matched controls, (2) increases with age and is associated with elevated markers of inflammation and worse kidney function but is not significantly related to congenital anatomy or Fontan type, and (3) is associated with adverse outcomes.
Prior research on circulating biomarkers in the Fontan circulation has focused on catecholamines and natriuretic peptides28, 29, 30, 31; studies have not demonstrated any single circulating biomarker to be strongly associated with mortality or other specific longitudinal outcomes.31, 32, 33 This makes the strong, independent association between high galectin‐3 level and adverse outcomes especially noteworthy. The small number of specific types of adverse event, such as arrhythmic death, precludes extensive exploration to determine whether galectin‐3 is associated with a particular subset of adverse outcomes. This will be an important question to address in future studies.
We did, however, observe a strong association between clinical ascites and high galectin‐3 level. The pattern of medication use, more common use of loop and potassium‐sparing diuretics, further supports a relationship between galectin‐3 and clinical volume retention. Although it may be that galectin‐3 simply reflects disease severity in these patients (eg, a marker of cumulative fibrosis or ongoing inflammation), there are several mechanisms by which elevated galectin‐3 may have a causal role in Fontan deterioration. A study reported, for example, that galectin‐3 knockout mice demonstrated less peritoneal inflammation in response to intraperitoneal thioglycollate broth injection.34 Recurrent ascites is a common issue for adult Fontan patients; often it is not readily explained by hemodynamic findings, and the cause is unknown. Could galectin‐3 be involved in the pathogenesis of Fontan‐related recurrent ascites? If so, compounds under development to inhibit the actions of galectin‐3 might be a promising therapy for this challenging clinical syndrome.
The relationship between galectin‐3 and hsCRP, a nonspecific marker of inflammation, is also noteworthy. Prior studies have also reported a positive association between galectin‐3 and hsCRP, although the strength of association has generally been weaker than seen in our study (r≈0.13 to 0.25).24, 25, 35 Further investigation is warranted regarding why these patients develop an inflammatory response, the typical pattern of immune activation, and the role of inflammation in the diverse causes of premature Fontan failure and subsequent death.
Higher galectin‐3 was also associated with lower eGFR, as has been reported in prior studies of other groups of patients. This may be because high galectin‐3 plays a causal role in progressive kidney disease, because Fontan dysfunction is independently associated with both low eGFR and high galectin‐3, or because galectin‐3 handling is affected by kidney disease.19, 36 Because very few patients had low eGFR (n=2, <60 mL/min per 1.73 m2), the last reason is unlikely to explain our findings. Importantly, the relationship between galectin‐3 and outcomes was not mediated by eGFR.
Galectin‐3 was higher in Fontan patients than in an age‐ and sex‐matched comparison group, although the absolute values were lower in both groups compared with those reported in most prior studies, including those in the general adult population and in acquired heart failure. Median galectin‐3, for example, was 13.1 ng/mL in men and 14.3 ng/mL in women in a sample of 3353 community participants in the Framingham Heart Study with an average age of 59 years.17 Christenson and colleagues reported 20.3 ng/mL as the 95th percentile for galectin‐3 in a diverse population‐based sample of 1092 ostensibly healthy participants aged 55 to 80 years.18 The younger age of our sample likely explains the lower values. Although reference values for other age groups are not yet available, we observed that galectin‐3 increased with age in Fontan participants; this pattern has also been reported in the general population and in acquired heart failure.17
There was an association between use of thyroid replacement medication and high galectin‐3. This association may be spurious related to the low absolute number of patients on thyroid medication, or it may be related to an independent relationship between thyroid disease and galectin‐337, 38 or to amiodarone toxicity. This observation deserves consideration in future studies.
Limitations
These results must be interpreted in light of the observational study design. The lack of relationship between underlying congenital diagnosis, ventricular morphology, or Fontan type may be related to the relatively low sample size. Results from analyses of incomplete data related to clinical practice patterns (ie, catheterization and B‐type natriuretic peptide results) are particularly susceptible to unmeasured confounding and bias. Furthermore, although there was no statistically significant association between galectin‐3 and hemodynamic variables, this study may have been underpowered to detect even a clinically relevant association. The small number of events precluded comprehensive multivariable adjustment, and our findings may be attributable to confounders such as chronic kidney disease. The age‐ and sex‐matched comparison group represents a convenience sample and may not derive from an equivalent at‐risk population such as the Fontan participants. We did not measure other established or promising biomarkers for prediction of outcomes in acquired heart failure, such as N‐terminal B‐type natriuretic peptide or ST2.39, 40 A multimarker approach with pathophysiologically complementary biomarkers may provide additional information, as it may in patients with acute coronary syndrome or heart failure.41, 42 We also did not measure galectin‐3 longitudinally, and it is possible that a change in levels over time could improve the prognostic value compared with a single measurement and may even serve as a surrogate marker of response to therapy. The current results hint that galectin‐3 may be a useful clinical predictor in Fontan patients; however, further study is required not only to validate the robustness of our findings but also to demonstrate that galectin‐3 provides information beyond that available from alternative biomarkers and other clinical variables. In addition, the relationship between age and galectin‐3 suggests that a given absolute level of galectin‐3 may have different implications for younger and older patients.
Conclusions
Plasma galectin‐3 concentration is elevated in patients with a Fontan circulation. In these patients, high galectin‐3 is associated with an increased incidence of adverse outcomes, incident nonelective cardiovascular hospitalization or death.
Sources of Funding
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. Cheng is supported by the Ellison Foundation and NIH grant R00HL107642. Opotowsky, Landzberg, Wu, Valente, and Singh are supported by the Dunlevie Family Fund.
Disclosures
None.
Acknowledgments
We appreciate Dan Halpern's insightful review of this manuscript.
(J Am Heart Assoc. 2016;5:e002706 doi: 10.1161/JAHA.115.002706)
References
- 1. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348–1352. [DOI] [PubMed] [Google Scholar]
- 2. Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein‐losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg. 1998;115:1063–1073. [DOI] [PubMed] [Google Scholar]
- 3. Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross‐sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. [DOI] [PubMed] [Google Scholar]
- 4. Fontan F, Kirklin JW, Fernandez G, Costa F, Naftel DC, Tritto F, Blackstone EH. Outcome after a “perfect” Fontan operation. Circulation. 1990;81:1520–1536. [DOI] [PubMed] [Google Scholar]
- 5. Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol. 2010;55:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemmer JH, Coran AG, Behrendt DM, Heidelberger KP, Stern AM. Liver fibrosis (cardiac cirrhosis) five years after modified Fontan operation for tricuspid atresia. J Thorac Cardiovasc Surg. 1983;86:757–760. [PubMed] [Google Scholar]
- 7. Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, Cope R, Sheron N, Millward‐Sadler H, Veldtman GR, Iredale JP. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol. 2008;61:504–508. [DOI] [PubMed] [Google Scholar]
- 8. Henderson NC, Sethi T. The regulation of inflammation by galectin‐3. Immunol Rev. 2009;230:160–171. [DOI] [PubMed] [Google Scholar]
- 9. Dumic J, Dabelic S, Flogel M. Galectin‐3: an open‐ended story. Biochim Biophys Acta. 2006;1760:616–635. [DOI] [PubMed] [Google Scholar]
- 10. Li LC, Li J, Gao J. Functions of galectin‐3 and its role in fibrotic diseases. J Pharmacol Exp Ther. 2014;351:336–343. [DOI] [PubMed] [Google Scholar]
- 11. Newlaczyl AU, Yu LG. Galectin‐3–a jack‐of‐all‐trades in cancer. Cancer Lett. 2011;313:123–128. [DOI] [PubMed] [Google Scholar]
- 12. Lin YH, Lin LY, Wu YW, Chien KL, Lee CM, Hsu RB, Chao CL, Wang SS, Hsein YC, Liao LC, Ho YL, Chen MF. The relationship between serum galectin‐3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–99. [DOI] [PubMed] [Google Scholar]
- 13. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. [DOI] [PubMed] [Google Scholar]
- 14. Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez‐Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, Lopez‐Andres N. Galectin‐3 mediates aldosterone‐induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. [DOI] [PubMed] [Google Scholar]
- 15. Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, Shirai K. Role of galectin‐3 in human pulmonary fibrosis. Allergol Int. 2007;56:57–65. [DOI] [PubMed] [Google Scholar]
- 16. Wanninger J, Weigert J, Wiest R, Bauer S, Karrasch T, Farkas S, Scherer MN, Walter R, Weiss TS, Hellerbrand C, Neumeier M, Schaffler A, Buechler C. Systemic and hepatic vein galectin‐3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine. 2011;55:435–440. [DOI] [PubMed] [Google Scholar]
- 17. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin‐3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christenson RH, Duh SH, Wu AH, Smith A, Abel G, deFilippi CR, Wang S, Adourian A, Adiletto C, Gardiner P. Multi‐center determination of galectin‐3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43:683–690. [DOI] [PubMed] [Google Scholar]
- 19. O'Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin‐3 precedes the development of CKD. J Am Soc Nephrol. 2013;24:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin‐3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val‐HeFT. Eur J Heart Fail. 2013;15:511–518. [DOI] [PubMed] [Google Scholar]
- 21. Stolen CM, Adourian A, Meyer TE, Stein KM, Solomon SD. Plasma galectin‐3 and heart failure outcomes in MADIT‐CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Card Fail. 2014;20:793–799. [DOI] [PubMed] [Google Scholar]
- 22. Lok DJ, Lok SI, Bruggink‐Andre de la Porte PW, Badings E, Lipsic E, van Wijngaarden J, de Boer RA, van Veldhuisen DJ, van der Meer P. Galectin‐3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol. 2013;102:103–110. [DOI] [PubMed] [Google Scholar]
- 23. Lopez‐Andres N, Rossignol P, Iraqi W, Fay R, Nuee J, Ghio S, Cleland JG, Zannad F, Lacolley P. Association of galectin‐3 and fibrosis markers with long‐term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE‐HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail. 2012;14:74–81. [DOI] [PubMed] [Google Scholar]
- 24. de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin‐3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah RV, Chen‐Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin‐3, cardiac structure and function, and long‐term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin‐3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL‐HF study. Clin Res Cardiol. 2010;99:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowenthal A, Camacho BV, Lowenthal S, Natal‐Hernandez L, Liszewski W, Hills NK, Fineman JR, Bernstein HS. Usefulness of B‐type natriuretic peptide and N‐terminal pro‐B‐type natriuretic peptide as biomarkers for heart failure in young children with single ventricle congenital heart disease. Am J Cardiol. 2012;109:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernstein HS, Nawaytou H. Biomarkers of cardiac function and outcome in univentricular congenital heart disease. Curr Biomark Find. 2014;4:53–59. [Google Scholar]
- 30. Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos‐Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012;60:2140–2149. [DOI] [PubMed] [Google Scholar]
- 31. Ohuchi H, Diller GP. Biomarkers in adult congenital heart disease heart failure. Heart Fail Clin. 2014;10:43–56. [DOI] [PubMed] [Google Scholar]
- 32. Inai K, Nakanishi T, Nakazawa M. Clinical correlation and prognostic predictive value of neurohumoral factors in patients late after the Fontan operation. Am Heart J. 2005;150:588–594. [DOI] [PubMed] [Google Scholar]
- 33. Motoki N, Ohuchi H, Miyazaki A, Yamada O. Clinical profiles of adult patients with single ventricular physiology. Circ J. 2009;73:1711–1716. [DOI] [PubMed] [Google Scholar]
- 34. Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung‐Leung WP, Liu FT. Targeted disruption of the galectin‐3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bozcali E, Polat V, Aciksari G, Opan S, Bayrak IH, Paker N, Karakaya O. Serum concentrations of galectin‐3 in patients with cardiac syndrome X. Atherosclerosis. 2014;237:259–263. [DOI] [PubMed] [Google Scholar]
- 36. Meijers WC, van der Velde AR, Ruifrok WP, Schroten NF, Dokter MM, Damman K, Assa S, Franssen CF, Gansevoort RT, van Gilst WH, Sillje HH, de Boer RA. Renal handling of galectin‐3 in the general population, chronic heart failure, and hemodialysis. J Am Heart Assoc. 2014;3:e000962 doi: 10.1161/JAHA.114.000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saussez S, Glinoer D, Chantrain G, Pattou F, Carnaille B, Andre S, Gabius HJ, Laurent G. Serum galectin‐1 and galectin‐3 levels in benign and malignant nodular thyroid disease. Thyroid. 2008;18:705–712. [DOI] [PubMed] [Google Scholar]
- 38. Mehrotra P, Okpokam A, Bouhaidar R, Johnson SJ, Wilson JA, Davies BR, Lennard TW. Galectin‐3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology. 2004;45:493–500. [DOI] [PubMed] [Google Scholar]
- 39. Shah RV, Januzzi JL Jr. Soluble ST2 and galectin‐3 in heart failure. Clin Lab Med. 2014;34:87–97, vi–vii. [DOI] [PubMed] [Google Scholar]
- 40. Bayes‐Genis A, de Antonio M, Vila J, Penafiel J, Galan A, Barallat J, Zamora E, Urrutia A, Lupon J. Head‐to‐head comparison of 2 myocardial fibrosis biomarkers for long‐term heart failure risk stratification: ST2 versus galectin‐3. J Am Coll Cardiol. 2014;63:158–166. [DOI] [PubMed] [Google Scholar]
- 41. Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multimarker approach to risk stratification in non‐ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C‐reactive protein, and B‐type natriuretic peptide. Circulation. 2002;105:1760–1763. [DOI] [PubMed] [Google Scholar]
- 42. Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, Goldberg LR, Jessup M, Cappola TP. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]


