Abstract
Background
Low serum magnesium has been implicated in cardiovascular mortality, but results are conflicting and the pathway is unclear. We studied the association of serum magnesium with coronary heart disease (CHD) mortality and sudden cardiac death (SCD) within the prospective population‐based Rotterdam Study, with adjudicated end points and long‐term follow‐up.
Methods and Results
Nine‐thousand eight‐hundred and twenty participants (mean age 65.1 years, 56.8% female) were included with a median follow‐up of 8.7 years. We used multivariable Cox proportional hazard models and found that a 0.1 mmol/L increase in serum magnesium level was associated with a lower risk for CHD mortality (hazard ratio: 0.82, 95% CI 0.70–0.96). Furthermore, we divided serum magnesium in quartiles, with the second and third quartile combined as reference group (0.81–0.88 mmol/L). Low serum magnesium (≤0.80 mmol/L) was associated with an increased risk of CHD mortality (N=431, hazard ratio: 1.36, 95% CI 1.09–1.69) and SCD (N=217, hazard ratio: 1.54, 95% CI 1.12–2.11). Low serum magnesium was associated with accelerated subclinical atherosclerosis (expressed as increased carotid intima‐media thickness: +0.013 mm, 95% CI 0.005–0.020) and increased QT‐interval, mainly through an effect on heart rate (RR‐interval: −7.1 ms, 95% CI −13.5 to −0.8). Additional adjustments for carotid intima‐media thickness and heart rate did not change the associations with CHD mortality and SCD.
Conclusions
Low serum magnesium is associated with an increased risk of CHD mortality and SCD. Although low magnesium was associated with both carotid intima‐media thickness and heart rate, this did not explain the relationship between serum magnesium and CHD mortality or SCD. Future studies should focus on why magnesium associates with CHD mortality and SCD and whether intervention reduces these risks.
Keywords: cardiovascular diseases; death, sudden; epidemiology; mortality; risk factors
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Introduction
Magnesium is the second most abundant intracellular cation and it plays a key role in a wide range of cellular functions.1 Total body magnesium depends on dietary intake and recent studies showed that the vast majority of elderly do not consume the average dietary requirement for magnesium.2 In addition to a magnesium‐insufficient diet, elderly are also at risk for hypomagnesemia due to comorbidities and medication that increase urinary excretion of magnesium.1 The prevalence of hypomagnesemia in the general population is estimated at 2%,3 but it may be as high as 53% in specific high‐risk groups, such as patients with chronic heart failure.4 Although hypomagnesemia may have acute and chronic complications, serum magnesium is still measured relatively infrequently.1
In recent studies, low serum magnesium has been associated with inflammation5 and disturbances in the regulation of vascular tone and endothelial function.6, 7, 8, 9 These mechanisms are thought to contribute to the development and progression of atherosclerosis, potentially worsening coronary heart disease (CHD).10, 11 Magnesium is also known for its role in the electrical stability and energy balance of cardiomyocytes.12 Hypomagnesemia has been associated with atrial and ventricular arrhythmias and low serum magnesium could therefore also be a risk factor for sudden cardiac death (SCD).2, 4, 13
Previous studies have addressed the relationship between serum magnesium and CHD11, 14 and the relationship with SCD,15, 16 but with conflicting results for both outcomes.2 In addition, these studies had few cases or lacked adjudicated end points, which could have resulted in misclassification. We therefore aimed to study the association of serum magnesium levels with both CHD mortality and SCD within the Rotterdam Study, a prospective population‐based cohort study among middle‐aged and elderly persons with adjudicated end points and long‐term follow‐up. Low serum magnesium has been associated with accelerated atherosclerosis.17 We therefore hypothesized that this could be the potential mechanism through which serum magnesium increases the risk of CHD mortality. Since carotid intima media thickness (cIMT) is considered a proxy for accelerated atherosclerosis, we explored the association between serum magnesium and cIMT as a potential mediator for the relationship with CHD mortality. QT prolongation is a well‐established risk factor for SCD18 and serum magnesium was shown to influence the QT interval in a clinical setting.19 We therefore hypothesized that QT prolongation could be the biological pathway through which low serum magnesium is associated with SCD.
Methods
Study Design, Setting, and Population
This study was embedded within the Rotterdam Study, a prospective population‐based cohort study, ongoing since 1990 in a suburb of the city of Rotterdam, The Netherlands. The rationale and design of this study have been described in detail elsewhere.20 In summary, the original cohort consisted of 7983 unselected inhabitants of the study area, aged 55 years or over. This cohort was extended in 2000 with 3011 persons who had become 55 years of age or who had moved into the research area, since the start of the study. In 2006, the cohort was extended again with 3932 persons aged 45 years and older living in the research area who had not yet been invited. This resulted in a total study population of 14 926 individuals, aged 45 years and older. All eligible participants provided written informed consent to participate in the study. Separate consent was asked for follow‐up data collection on clinical outcomes and vital status. The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus Medical Center and by the Dutch Ministry of Health, Welfare and Sport, implementing the “Wet Bevolkingsonderzoek: ERGO (Population Screening Act: Rotterdam Study).”
Assessment of Serum Magnesium
For our study, we used blood collections from the third visit of the first cohort (1997–1999), and the first visits of the second cohort (2000–2001) and third cohort (2006–2008), as these visits were virtually identical in design. Of the 11 740 eligible participants, serum magnesium was available for 9882 participants. Of these 9882 participants, 59 did not provide consent to collect follow‐up information from their treating physicians and 3 participants were lost to follow‐up at the date of blood collection and therefore did not contribute to our study. This resulted in a study population of 9820 participants. Magnesium was measured in serum by the Department of Clinical Chemistry of the Erasmus Medical Center using a Roche/Hitachi Cobas c501 analyzer. We plotted multivariate adjusted log relative hazard curves using restricted cubic splines, to examine the association between serum magnesium and CHD mortality. We found this association to be linear and therefore used serum magnesium as a continuous parameter when quantifying the association with CHD mortality. The association with SCD was also modeled using restricted cubic splines and found to be U‐shaped. Therefore, we also divided serum magnesium into quartiles and analyzed serum magnesium as a categorical variable in all our analyses. We combined the second and third quartile into a reference group to best fit the observed U‐shape, yielding a low, high, and reference group.21
Assessment of Covariables
The assessment of anthropometrics in the Rotterdam Study has been described previously.20 Body mass index was calculated by dividing body weight in kilograms by height in meters squared. Diabetes mellitus was defined as the use of glucose‐lowering drugs, a fasting glucose level ≥7.0 mmol/L, or a nonfasting glucose level ≥11.1 mmol/L when fasting levels were not available.22 The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation23 and was expressed as mL/min per 1.73 m2 based on serum creatinine values that were measured at the same visit as the serum magnesium levels using an enzymatic assay method. Total cholesterol and high‐density lipoprotein cholesterol were measured at the same visit as the serum magnesium levels using the Roche Modular P800. Information on history of stroke, myocardial infarction, and heart failure was obtained through linkage with medical records kept by general practitioners working in the study area, and subsequently adjudicated by 2 research physicians and confirmed by a neurologist or cardiologist.24 Information on smoking habits and current alcohol consumption was obtained during a home interview. Smoking was categorized into 3 categories: nonsmoker, former smoker, and current smoker. Alcohol consumption was categorized into yes or no. Drug exposure has been monitored continuously since January 1, 1991 through linkage with digital pharmacy records of the pharmacies in the study district. The following Anatomical Therapeutic Chemical (ATC) codes were used to retrieve relevant drug exposure for all participants: verapamil (C08DA01), diltiazem (C08DB01), β‐blocking agents (C07), digoxin (C01AA05), class I/III antiarrhythmic drugs (C01B), and diuretics (C03).25 The use of definite QTc‐prolonging drugs was determined using the University of Arizona list of QTc‐prolonging drugs.26 Participants were classified as being exposed if the date of the blood draw fell within the prescription period. Physical activity was assessed using a validated adapted version of the Zutphen Physical Activity Questionnaire, and expressed in metabolic equivalent of task hours per week.27
Assessment of Outcomes
Follow‐up time was calculated from the date that blood was drawn until the date of death, loss to follow‐up (n=594), or the end of the study period (January 1, 2012 for CHD mortality and January 1, 2011 for SCD). Methods for outcome data collection and definitions have previously been described in more detail.24, 28 In short, CHD mortality was defined as definite fatal myocardial infarction, definite fatal CHD, and possible fatal CHD. Information on the vital status of all participants was obtained on a weekly basis from the central registry of the municipality in Rotterdam and through digital linkage with records from general practitioners working in the study area. The cause of death was established by abstracting information from the medical records of the general practitioners or nursing home physicians and hospital discharge letters. SCD was defined as “natural death due to cardiac causes, heralded by abrupt loss of consciousness within 1 hour from onset of acute symptoms; preexisting heart disease may have been known to be present, but the time and mode of death are unexpected,” according to Myerburgs' definition endorsed by the European Society of Cardiology29, 30 Unwitnessed deaths were coded as SCD if death was unexpected in persons found dead, while they were in a stable medical condition 24 hours before they were found in the absence of evidence of a noncardiac cause.28 Each outcome was adjudicated by 2 research physicians who independently classified information on occurrence, certainty, and date using standardized criteria and subsequently validated by an experienced cardiologist.24, 28 The classification of CHD mortality (as a cause of death) and SCD (as a mode of death) overlap and results on CHD mortality could be driven by the effect on SCD (Figure 1). Therefore, we also defined a “nonsudden CHD mortality” category including participants who were only classified as death from CHD but not as SCD.
Figure 1.
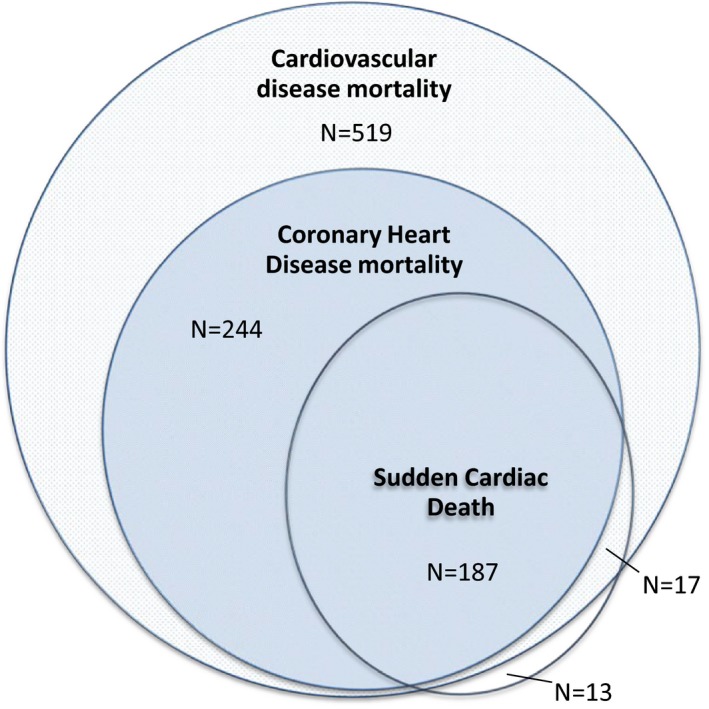
Overlap between the classification of coronary heart disease mortality (as a cause of death) and sudden cardiac death (as a mode of death). Among all 2303 participants who died during follow‐up, 780 died due to cardiovascular disease, of which 431 participants were classified as coronary heart disease mortality. Of these 431 participants, 187 were also classified as sudden cardiac death. Seventeen participants were classified as sudden cardiac death and cardiovascular deaths (but not coronary heart disease) and 13 participants were classified as sudden cardiac death but could otherwise not be classified as cardiovascular deaths.
c‐IMT and ECG Measurements
cIMT correlates with future cardiovascular events and can therefore be used as a subclinical marker of atherosclerosis.31 We used longitudinal 2‐dimensional ultrasound images of the common carotid artery for a 1‐cm length that was proximal to the bulb, obtained with a 7.5 MHz linear array transducer and UltraMark IV (Advanced Technology Laboratories, Bethel, WA), to measure the distance between the lumen intima interface and the media–adventitia interface, which indicates the cIMT. The maximal cIMT, summarized as the mean of the maximal measurements from the near and far walls on both the left and right sides, was used for analysis.32 Each participant also had a standard 12‐lead resting ECG recorded with an ACTA electrocardiograph (ESAOTE, Florence, Italy) at a sampling frequency of 500 Hz, which was stored digitally. All ECGs were processed by the standardized Modular ECG Analysis System (MEANS) to obtain ECG measurements.33 The QT interval represents the interval between the start of ventricular depolarization to the end of ventricular repolarization. MEANS determines this interval from the start of the QRS complex until the end of the T‐wave. Heart rate modifies this interval; therefore QT‐interval measurements are corrected for the individual's RR interval to allow comparison by using Bazett's correction.34 For all participants, cIMT measurements and ECG measurement were performed during the same visit, during which blood was drawn for serum magnesium measurements.
Statistical Analyses
We used the cmprsk package in “R” to plot cumulative probability curves for CHD mortality, nonsudden CHD mortality, and SCD adjusted for competing risk of death by other causes as proposed by Fine and Grey.35 We used Cox proportional hazard regression models to examine the relationship between serum magnesium and CHD mortality, nonsudden CHD mortality, and SCD. We analyzed these associations using 2 different models. In the first model we adjusted for age (as a continuous variable) and sex. The second model also included the following continuous variables: eGFR, body mass index, systolic and diastolic blood pressure, total cholesterol/high‐density lipoprotein cholesterol ratio, and the following categorical variables: diabetes mellitus, history of myocardial infarction, stroke, or heart failure, smoking status, alcohol consumption, and use of diuretics. We studied the association between serum magnesium and QTc interval in a multivariable linear model using the same covariables and additionally adjusted for drugs that are known to affect the QT and/or RR interval (see above). Because Bazett's formula is known to overestimate at short RR intervals and underestimate at long RR intervals, we also additionally adjusted for RR interval.36 The association between serum magnesium and heart rate (expressed as RR interval) was also studied using the same covariables. We tested for potential pathways by additionally adjusting the second model for QT and RR interval to account for a potential arrhythmogenic pathway and by including cIMT as marker for subclinical atherosclerosis. The proportional hazard assumption was tested by plotting log‐minus‐log survival versus log of survival time curves and visually examining the curves, which did not indicate that the assumption was violated.37 Results are presented as hazard ratios (HRs) with 95% CIs. Missing data in covariables (0–9.1%) were handled by single imputation using an expectation‐maximization algorithm.38 All analyses were repeated on complete cases to check for potential differences between results based on imputed data and those based on complete cases. With the exception of the baseline characteristics, results are reported for imputed data. We considered a 2‐sided P<0.05 as statistically significant. Data were analyzed using SPSS Statistics (IBM, version 21.0) and R (The R Foundation for Statistical Computing, version 3.1.2).
Sensitivity Analyses
To further distinguish between an effect of serum magnesium on accelerated atherosclerosis or serum magnesium having an anti‐arrhythmogenic effect, we studied the association between serum magnesium and incident myocardial infarction (MI). If the effect of serum magnesium on CHD mortality is mediated through accelerated atherosclerosis, an effect on incident MI would also be expected. We studied the association with incident MI only with serum magnesium as a continuous parameter, as there were only a limited number of MI events and we expected the shape of the association to be similar to CHD mortality.
In a previous study, hypertension and cholesterol levels were found to be intermediates in the association between plasma magnesium and fatal CHD.39 To explore the effect of these factors on our effect estimates, we excluded systolic blood pressure, diastolic blood pressure, and total/high‐density lipoprotein cholesterol ratio from our second model. We then compared effect estimates of the full model to the model without blood pressure and cholesterol to check whether our results had been altered by inclusion of this potential mediator.
To test the robustness of our findings, we also performed 2 additional analyses where we restricted our analysis to participants free of diabetes mellitus and with normal kidney function (defined as eGFR >60 mL/min per 1.73 m2) in order to explore potential effect modification by diabetes mellitus or chronic kidney disease. Furthermore, we additionally adjusted for serum sodium, potassium, and calcium levels to test whether the association of magnesium with any of the outcomes could be explained by a concomitant electrolyte disorder.
We expected physical activity to be a potential confounder of the association between serum magnesium and CHD mortality. However, data on physical activity were only available for a subset of 6385 participants. We therefore performed a sensitivity analysis on this subset to check whether inclusion of physical activity would have influenced our results.
Results
Baseline Characteristics
Baseline characteristics of our study population are shown in Table 1. Among the 9820 participants, the mean age was 65.1 years, 56.8% were women, and 96% were of European descent. The mean serum magnesium level was 0.84 mmol/L (±0.06 mmol/L), with a range of 0.34 to 1.74 mmol/L. Participants in the low magnesium group (N=2351) more often used diuretics, and more often had diabetes mellitus. During a median follow‐up of 8.7 years (80 750 person‐years in total), 2303 of the 9820 participants died (23.5%). In 2213 of the 2303 participants who died, a cause of death could be adjudicated. Among these participants, 780 died of cardiovascular disease, of which 431 were classified as death from CHD. Of the 2303 participants who died, 217 were classified as SCD. Figure 1 shows how the classifications CHD mortality and SCD relate.
Table 1.
Characteristics of the Study Population
| Total n=9820 | Low n=2351 | Reference n=5170 | High n=2299 | |
|---|---|---|---|---|
| Age, y | 65.1 (9.9) | 65.1 (10.2) | 64.9 (9.7) | 65.6 (10.0) |
| Women, n (%) | 5575 (56.8) | 1368 (58.2) | 2908 (56.2) | 1299 (56.5) |
| Body mass index, kg/m2 | 27.3 (4.2) | 27.8 (4.6) | 27.2 (4.1) | 26.8 (4.0) |
| Systolic blood pressure, mm Hg | 140 (21) | 141 (21) | 139 (21) | 140 (22) |
| Diastolic blood pressure, mm Hg | 79 (12) | 79 (12) | 79 (11) | 79 (12) |
| Smoking, n (%) | ||||
| Never | 2991 (30.8) | 661 (28.4) | 1600 (31.3) | 730 (32.2) |
| Former | 4549 (46.8) | 1094 (47.0) | 2406 (47.0) | 1049 (46.3) |
| Current | 2175 (22.4) | 574 (24.6) | 1112 (21.7) | 489 (21.6) |
| Alcohol use, n (%) | 8274 (85.2) | 1919 (82.5) | 4430 (86.6) | 1925 (84.9) |
| History of diabetes mellitus, n (%) | 1021 (10.4) | 466 (19.9) | 433 (8.4) | 122 (5.3) |
| History of myocardial infarction, n (%) | 494 (5.0) | 134 (5.7) | 237 (4.6) | 123 (5.4) |
| History of stroke, n (%) | 340 (3.5) | 92 (3.9) | 160 (3.1) | 88 (3.8) |
| History of heart failure, n (%) | 257 (2.6) | 69 (2.9) | 118 (2.3) | 70 (3.1) |
| eGFR, mL/min per 1.73 m2 | 81.5 (17.3) | 83.2 (20.0) | 81.8 (16.0) | 79.0 (17.0) |
| Serum magnesium, mmol/L | 0.84 (0.06) | 0.77 (0.04) | 0.85 (0.02) | 0.92 (0.04) |
| Total cholesterol, mmol/L | 5.7 (1.0) | 5.6 (1.0) | 5.7 (1.0) | 5.8 (1.1) |
| HDL cholesterol, mmol/L | 1.40 (0.41) | 1.38 (0.42) | 1.41 (0.40) | 1.40 (0.41) |
| Diuretic use, n (%) | 1017 (10.7) | 325 (14.2) | 418 (8.1) | 233 (10.6) |
Data are shown for nonimputed data, values are counts (valid percentages) or means (SD). Low magnesium group 0.34 to 0.80 mmol/L, reference group 0.81 to 0.88 mmol/L, high magnesium group 0.89 to 1.74 mmol/L. eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein.
Magnesium and Mortality
Figure 2 shows the cumulative probability curves for CHD mortality, nonsudden CHD mortality, and SCD between the different groups of serum magnesium, taking competing risk into account. Table 2 shows the associations between the 3 magnesium groups and mortality. Low serum magnesium was associated with an increased risk for CHD mortality (HR 1.36, 95% CI 1.09–1.69) and an increased risk for SCD (HR 1.54, 95% CI 1.12–2.11). A marginally insignificant association was found with high serum magnesium levels and SCD (HR 1.35, 95% CI 0.96–1.89). Excluding SCD cases from the CHD outcome resulted in an inverse linear association with serum magnesium levels. The risk in participants with low serum magnesium was attenuated (HR 1.27, 95% CI 0.95–1.70) and an association with lower risk was observed in participants with high serum magnesium levels (HR 0.69, 95% CI 0.48–0.98). We tested for a linear association with CHD mortality and nonsudden CHD mortality and found that a 0.1 mmol/L increase in serum magnesium was associated with a decreased risk of CHD mortality (HR 0.82, 95% CI 0.70–0.96), and nonsudden CHD mortality (HR 0.72, 95% CI 0.58–0.88). We found no evidence of effect modification by sex (P for interaction=0.436), and therefore did not stratify by sex.
Figure 2.
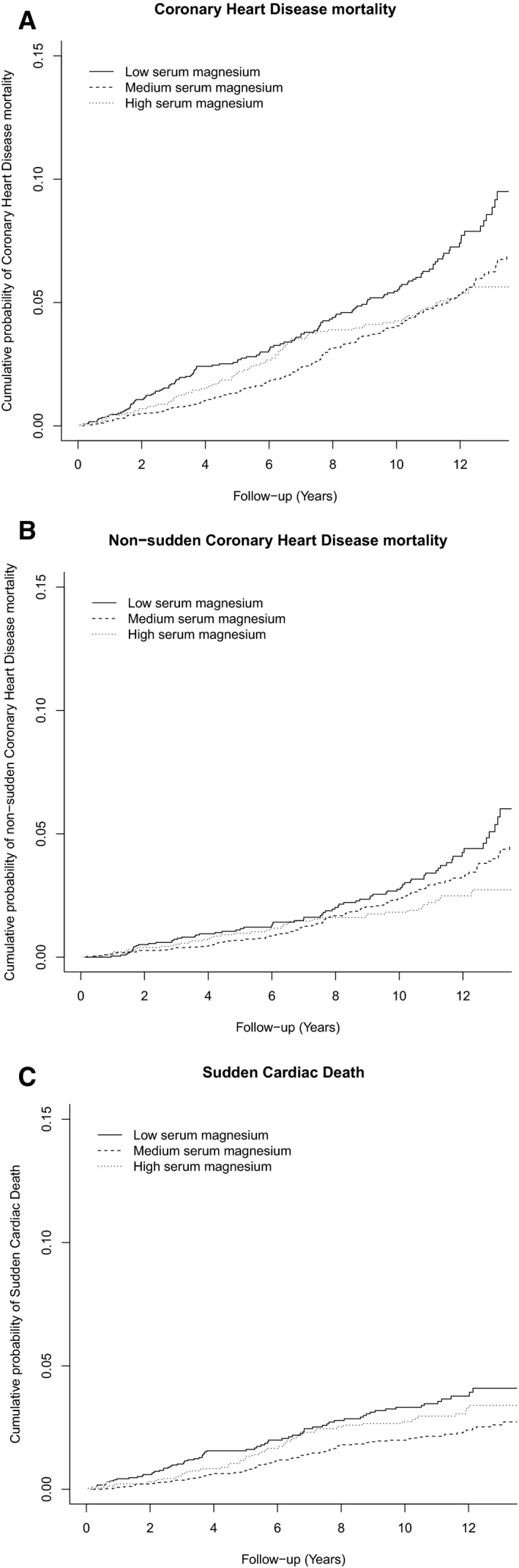
Estimated cumulative probability curves for coronary heart disease mortality, nonsudden coronary heart disease mortality, and sudden cardiac death. Estimated cumulative probability curves are shown for coronary heart disease mortality (A), nonsudden coronary heart disease mortality (B), and sudden cardiac death (C) between different groups of serum magnesium levels, taking into account the competing risk of death by other causes. Low serum magnesium is defined as 0.34 to 0.80 mmol/L, medium serum magnesium (reference) as 0.81 to 0.88 mmol/L, and high serum magnesium as 0.89 to 1.74 mmol/L.
Table 2.
Association Between Magnesium Groups and Different Types of Mortality
| Serum Magnesium Groups | Number of Events | Crude Incidence Rate Per 1000 Person‐Years (95% CI) | Model 1 Hazard Ratio (95% CI)a | Model 2 Hazard Ratio (95% CI)b |
|---|---|---|---|---|
| Coronary heart disease mortality | ||||
| Low | 134 | 7.1 (6.0–8.4) | 1.53 (1.23–1.90) | 1.36 (1.09–1.69) |
| Reference | 206 | 4.8 (4.1–5.5) | 1.00 (Reference) | 1.00 (Reference) |
| High | 91 | 4.9 (3.9–6.0) | 0.97 (0.76–1.24) | 0.94 (0.73–1.21) |
| Nonsudden coronary heart disease mortalityc | ||||
| Low | 74 | 3.9 (3.1–4.9) | 1.39 (1.04–1.85) | 1.27 (0.95–1.70) |
| Reference | 127 | 2.9 (2.4–3.5) | 1.00 (Reference) | 1.00 (Reference) |
| High | 43 | 2.3 (1.7–3.1) | 0.73 (0.52–1.04) | 0.69 (0.48–0.98) |
| Sudden cardiac death | ||||
| Low | 70 | 4.2 (3.2–5.3) | 1.76 (1.29–2.40) | 1.54 (1.12–2.11) |
| Reference | 92 | 2.4 (1.9–2.9) | 1.00 (Reference) | 1.00 (Reference) |
| High | 55 | 3.3 (2.5–4.3) | 1.32 (0.94–1.84) | 1.35 (0.96–1.89) |
Low magnesium group ≤0.80 mmol/L, reference group 0.81 to 0.88 mmol/L, high magnesium group ≥0.89 mmol/L.
Adjusted for age and sex.
Model 1 additionally adjusted for body mass index, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, history of diabetes mellitus, history of myocardial infarction, history of stroke, history of heart failure, smoking status, alcohol consumption, total/high‐density lipoprotein cholesterol ratio, and diuretic use.
This category includes participants who died of coronary heart disease, but who were not classified as sudden cardiac deaths.
IMT, QT‐Interval, and Heart Rate
Table 3 shows the associations between the different magnesium groups and markers of potential pathways that may play a role in the association between serum magnesium and CHD mortality and SCD. Low serum magnesium was associated with a significantly higher cIMT (+0.013 mm, 95% CI 0.005–0.020); no association was found in participants with high serum magnesium. Both low serum magnesium and high serum magnesium were associated with a significantly longer QTc interval (+1.8 ms 95% CI 0.7–2.9 and +2.2 ms 95% CI 1.1–3.3, respectively). When we additionally adjusted this analysis on QTc interval for heart rate, we found that the association remained significant, but the effect sizes became smaller. Therefore, we analyzed the effect of serum magnesium on heart rate by studying the association between serum magnesium and RR interval. We found that both low serum magnesium and high serum magnesium were significantly associated with RR interval (−7.1 ms, 95% CI −13.5 to −0.7 and 7.8 ms, 95% CI −14.1 to −1.4, respectively). Due to the significant associations between serum magnesium and cIMT, QT interval, and heart rate, we hypothesized that cIMT could be a potential mediator for the association with CHD mortality and nonsudden CHD mortality, and QT interval or heart rate could be a mediator in the association with SCD. Table 4 shows the result of additionally adjusting for these potential mediators. We found that additional adjustment for cIMT did not change the association between low serum magnesium and CHD mortality (before adjustment HR 1.36, 95% CI 1.09–1.69; after adjustment HR 1.35, 95% CI1.08–1.68). The same was true for the association with nonsudden CHD (before adjustment HR 1.27, 95% CI 0.95–1.70; after adjustment HR 1.26, 95% CI 0.94–1.69). Additional adjustment for QT interval and heart rate did not change the association between low serum magnesium and SCD (before adjustment HR 1.54, 95% CI 1.12–2.11; after adjustment HR 1.50, 95% CI 1.09–2.06).
Table 3.
Association Between Different Magnesium Groups and Markers of Potential Pathways
| Serum Magnesium Groups | Model 1 Mean Difference (95% CI)a | Model 2 Mean Difference (95% CI)b |
|---|---|---|
| Carotid intima‐media thickness (in millimeters) | ||
| Low | 0.023 (0.015–0.030) | 0.013 (0.005–0.020) |
| Reference | Reference | Reference |
| High | −0.005 (−0.013 to 0.003) | −0.003 (−0.010 to 0.004) |
| QTc interval (in milliseconds)c | ||
| Low | 2.7 (1.6–3.9) | 1.8 (0.7–2.9) |
| Reference | Reference | Reference |
| High | 2.0 (0.9–3.1) | 2.2 (1.1–3.3) |
| QTc interval (in milliseconds), additionally adjusted for RR intervalc | ||
| Low | 2.0 (1.0–3.0) | 1.3 (0.3–2.3) |
| Reference | Reference | Reference |
| High | 1.5 (0.5–2.5) | 1.6 (0.6–2.6) |
| RR interval (in milliseconds)c | ||
| Low | −10.1 (−16.7 to −3.5) | −7.1 (−13.5 to −0.8) |
| Reference | Reference | Reference |
| High | −7.1 (−13.8 to −0.4) | −7.8 (−14.1 to −1.4) |
Low magnesium group ≤0.80 mmol/L, reference group 0.81 to 0.88 mmol/L, high magnesium group ≥0.89 mmol/L.
Adjusted for age and sex.
Model 1 additionally adjusted for body mass index, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, history of diabetes mellitus, history of myocardial infarction, history of stroke, history of heart failure, smoking status, alcohol consumption, total/high‐density lipoprotein cholesterol ratio, and diuretic use.
In the analysis on ECG parameters, model 2 also includes adjustment for verapamil use, diltiazem use, β‐blocker use, digoxin use, anti‐arrhythmic drug use, and QT‐prolonging drug use.
Table 4.
Influence of Potential Pathway Markers on the Association Between Magnesium Groups and Cardiovascular Outcomes
| Serum Magnesium Groups | Model 2 Hazard Ratio (95% CI)a | Model 3 Hazard Ratio (95% CI) b | Model 4 Hazard Ratio (95% CI)c |
|---|---|---|---|
| Coronary heart disease mortality | |||
| Low | 1.36 (1.09–1.69) | 1.34 (1.08–1.68) | 1.35 (1.08–1.68) |
| Reference | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High | 0.94 (0.73–1.21) | 0.94 (0.73–1.20) | 0.95 (0.74–1.22) |
| Nonsudden coronary heart disease mortality | |||
| Low | 1.27 (0.95–1.70) | 1.27 (0.95–1.71) | 1.26 (0.94–1.69) |
| Reference | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High | 0.69 (0.48–0.98) | 0.69 (0.48–0.97) | 0.70 (0.49–0.99) |
| Sudden cardiac death | |||
| Low | 1.54 (1.12–2.11) | 1.50 (1.09–2.06) | 1.53 (1.11–2.10) |
| Reference | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High | 1.35 (0.96–1.89) | 1.32 (0.94–1.85) | 1.35 (0.96–1.89) |
Equal to model 2 from Table 2; adjusted for age, sex, body mass index, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, history of diabetes mellitus, history of myocardial infarction, history of stroke, history of heart failure, smoking status, alcohol consumption, total/high‐density lipoprotein cholesterol ratio, and diuretic use.
Model 2+adjustment for QT interval and RR interval.
Model 2+adjustment for carotid intima‐media thickness.
Sensitivity Analyses
When studying the association between serum magnesium and incident MI, we found that in our age‐ and sex‐adjusted model a 0.1 mmol/L increase in serum magnesium level was associated with a decreased risk of MI (HR 0.84, 95% CI 0.72–0.99). However, this association was no longer statistically significant after further adjustment for potential confounders (HR 0.90, 95% CI 0.76–1.06, data not shown). To study whether blood pressure and cholesterol could be potential intermediates in the association between serum magnesium and CHD mortality, we excluded systolic blood pressure, diastolic blood pressure, and total/high‐density lipoprotein cholesterol ratio from our model. We found that by removing these variables, our analysis was not appreciably altered. When restricting all analyses to participants free of diabetes mellitus and chronic kidney disease (eGFR >60 mL/min), the relationship found with CHD mortality and SCD was not altered (Table 5). Additional adjustments for serum sodium, potassium, and calcium to investigate whether the effect of serum magnesium on mortality is mediated through other electrolytes did not alter the point estimates. In the subset of 6385 participants where data on physical activity was available, the inclusion of physical activity to the full model did not change the association between serum magnesium and CHD mortality, nonsudden CHD mortality, and SCD (data not shown).
Table 5.
Sensitivity Analysis on Subset of Participants Free of Diabetes Mellitus and With Normal Kidney Function and Analysis on the Influence of Other Electrolytes
| Serum Magnesium Groups | Model 2 Hazard Ratio (95% CI)a N=9280 | Subset of Participants Free of Diabetes Mellitus and With Normal Kidney Function Hazard Ratio (95% CI)b N=7843 | Including Electrolytes Hazard Ratio (95% CI)c N=9280 |
|---|---|---|---|
| Coronary heart disease mortality | |||
| Low | 1.36 (1.09–1.69) | 1.44 (1.06–1.95) | 1.33 (1.06–1.66) |
| Reference | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High | 0.94 (0.73–1.21) | 0.93 (0.67–1.29) | 0.97 (0.76–1.25) |
| Sudden cardiac death | |||
| Low | 1.54 (1.12–2.11) | 1.67 (1.11–2.51) | 1.56 (1.13–2.14) |
| Reference | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High | 1.35 (0.96–1.89) | 1.19 (0.77–1.85) | 1.35 (0.96–1.90) |
Low magnesium group ≤0.80 mmol/L, reference group 0.81 to 0.88 mmol/L, high magnesium group ≥0.89 mmol/L.
Equal to model 2 from Table 2; adjusted for age, sex, body mass index, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate (eGFR), history of diabetes mellitus, history of myocardial infarction, history of stroke, history of heart failure, smoking status, alcohol consumption, total/high‐density lipoprotein cholesterol ratio, and diuretic use.
Model 2 in subset of participants free of diabetes mellitus and with normal kidney function (defined as an eGFR >60 mL/min per 1.73 m2).
Model 2 additionally adjusted for serum sodium, serum potassium, and serum calcium in all participants.
Discussion
In this prospective population‐based cohort study among 9820 participants with a median follow‐up of 8.7 years, we found that low serum magnesium was associated with an increased risk of CHD mortality and SCD. When we excluded SCD from the CHD mortality end point, we found that higher serum magnesium levels were associated with a lower risk of nonsudden CHD mortality. Even though serum magnesium was associated with QTc, heart rate, and cIMT, this did not explain the relationship between serum magnesium and CHD mortality or SCD.
We observed a 36% increased risk of CHD mortality in participants with low serum magnesium. The association between serum magnesium and CHD mortality has been studied previously in a cohort of Finnish men but no significant association was found, possibly because of a low number of cases (n=230).14 The National Health and Nutrition Examination Survey Epidemiologic Follow‐up Study (NHEFS) did find a significant association, but only when comparing the third quartile of serum magnesium to the first quartile.11 The Nurses' Health Study analyzed the association between dietary magnesium and CHD mortality.39 A significant association was identified when comparing the highest with the lowest quintile of dietary magnesium intake, but they were unable to study the association between serum magnesium and fatal CHD due to a lack of cases.40 A meta‐analysis on the association between low serum magnesium and fatal CHD, which included the 2 studies discussed above,11, 14 found a nonsignificant trend towards an increased risk.9 However, this meta‐analysis also included 2 studies with SCD as outcome of interest which, judging from the Forest plot, were mainly responsible for this observed inverse effect.2
We analyzed SCD independently of CHD and found a significantly elevated risk for SCD in participants with low serum magnesium levels. Investigators from the Framingham Heart Study did not observe an association between low serum magnesium and SCD.16 However, this study included only 29 cases of SCD (compared to 217 cases in our study), and may therefore have lacked power. Investigators from the Atherosclerosis Risk in Communities Study (ARIC) and from the Nurses' Health Study also analyzed the association between serum magnesium and SCD and both reported a lower risk for SCD when comparing the highest magnesium quartile with the lowest magnesium quartile.15, 40 The difference in risk in participants with high serum magnesium between our study and the other 2 studies could be the result of differences in kidney function. In comparison to baseline kidney function from the ARIC Study and the Nurses' Health Study, participants within the Rotterdam Study had on average a 10 mL/min per 1.73 m2 lower eGFR at baseline than the participants from the ARIC Study and Nurses' Health Study. The significantly elevated risk of SCD in the high serum magnesium category could therefore still be the result of residual confounding by kidney function, assuming that a single eGFR measurement does not capture all changes associated with a decreased kidney function. We considered QT prolongation as a potential pathway through which serum magnesium may affect SCD risk, since prolongation and shortening of the QT interval is associated with SCD.18, 41 However, adjustments for QT and RR interval did not fully explain the observed associations. An explanation for this limited change in effect estimate may be that a single measurement of QT interval does not fully capture all the electrophysiological changes caused by low serum magnesium. Another possibility could be that the effect of serum magnesium on QT interval is too small to have impact on the risk of SCD, as it is currently assumed that a minimal increase of 5 ms is necessary to increase the risk of torsade de pointes.42
A substantial overlap between participants classified as CHD mortality and SCD exists. CHD mortality is a mixture of modes of death ranging from ischemia‐induced ventricular arrhythmia to chronic ischemic heart failure. SCD is a particular mode of death that is predominantly caused by arrhythmias.30 When we limited our outcome to nonsudden CHD deaths, we still observed a trend toward increased risk in the low magnesium group. We also observed a potential protective effect of high serum magnesium on nonsudden CHD mortality, which has been observed previously in NHEFS.11 Although this observation combined with the significant effect on cIMT supports a role for magnesium in atherosclerosis, it does not appear to be the main pathway since the association between serum magnesium and nonsudden CHD death remained after adjustment for cIMT. This could be due to the small effect size of serum magnesium on cIMT. In previous studies it was shown that per 0.17 mm increase in cIMT, the risk for acute MI increased by 17%.44 However, in our study, we only found an increase of 0.013 mm when comparing participants with low serum magnesium with the reference group. It is therefore unlikely that this small increase would have a large influence on our outcome. Furthermore, no association was found between serum magnesium and incident MI, which would have been expected if the main effect of serum magnesium is through accelerated atherosclerosis. This lack of association with incident ischemic heart disease has been observed previously in the National Health and Nutrition Examination Survey study.11 The discrepancy between an effect on CHD mortality but not on incident MI argues against a direct role of serum magnesium on accelerated atherosclerosis, making an (acute) arrhythmogenic effect of serum magnesium more plausible.
Our study has several strengths. First, we did not use total cardiovascular mortality but studied CHD mortality, nonsudden CHD mortality, and SCD separately. The classification of cardiovascular mortality is heterogeneic and often also includes nonatherosclerotic outcomes such as hemorrhagic stroke. This heterogeneity makes it difficult to study associations, since there is also heterogeneity in the underlying pathophysiology of the various types of cardiovascular disease. Another strength of our study is the high case load, and, more importantly, the availability of adjudicated end points that were blinded from the laboratory results, reducing potential bias due to differential misclassification.
The main limitation of our study was the fact that we only have a single serum magnesium measurement at baseline and no follow‐up serum magnesium measurements. We therefore cannot adjust for intraindividual variability over time. However, a recent study showed that there is a strong correlation between serum magnesium concentrations measured 1 year apart,5 suggesting the possibility of an individual setpoint for serum magnesium, similar to what has been shown for serum sodium.44, 45 In observational studies, reversed causality remains a limitation. However, the long‐term follow‐up after the serum magnesium measurement decreases the probability of reversed causality in our study. Another limitation of our study is the potential heterogeneity of SCD cases, which is inherent to the definition of SCD.46 Generalization to other ethnic groups is also a limitation, since the participants in the Rotterdam Study are predominantly of European descent (96%). Finally, residual confounding can always play a role in observational studies. We tried to address this by adjusting for a large number of potential confounders and performing various sensitivity analyses, which did not indicate substantial amounts of confounding. A potential confounder for which we were unable to adjust is diet. Higher dietary magnesium intake could be a proxy for a healthy diet, therefore resulting in a lower risk of CHD mortality. We were unable to adjust for this confounder, as dietary assessment was available for only 2504 participants. Since there is only a weak correlation between serum magnesium and dietary magnesium (R=0.06), it is unlikely that confounding by dietary magnesium intake could explain the observed associations.
In conclusion, low serum magnesium is associated with an increased risk of CHD mortality and SCD. Although low magnesium has a significant effect on subclinical atherosclerosis and heart rate, this did not explain the observed associations with CHD mortality or SCD. The results from this and previous studies may provide a rationale to design intervention studies to analyze whether magnesium supplementation could prove to be effective in lowering the burden of CHD mortality and SCD.
Sources of Funding
The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organisation for Scientific Research (NWO); the Netherlands Organisation for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. None of the funders had any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
Kieboom, Niemeijer, van den Berg, Deckers, Hofman, Zietse, and Stricker state that they have no financial conflict of interest. Leening has received a Prins Bernhard Cultuurfonds Fellowship; and grants from the De Drie Lichten Foundation; the American Heart Association; the Netherlands Epidemiology Society; and the European Society of Cardiology, which had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the study. Franco reports working in ErasmusAGE, a center for aging research across the life course, funded by Nestlé Nutrition (Nestec Ltd); Metagenics Inc; and AXA. Nestlé Nutrition (Nestec Ltd); Metagenics Inc; and AXA had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the study. Hoorn is supported by grants from the Netherlands Organisation for Scientific Research (NWO, Veni 916.12.140) and the Dutch Kidney Foundation (KSP‐14OK19).
Acknowledgments
The dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists of the Ommoord district to the Rotterdam Study are gratefully acknowledged.
(J Am Heart Assoc. 2016;5:e002707 doi: 10.1161/JAHA.115.002707)
References
- 1. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. [DOI] [PubMed] [Google Scholar]
- 2. Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta‐analysis of prospective studies. Am J Clin Nutr. 2013;98:160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126:256–263. [DOI] [PubMed] [Google Scholar]
- 4. Adamopoulos C, Pitt B, Sui X, Love TE, Zannad F, Ahmed A. Low serum magnesium and cardiovascular mortality in chronic heart failure: a propensity‐matched study. Int J Cardiol. 2009;136:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishimura E, Okuno S, Yamakawa T, Inaba M, Nishizawa Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 6. Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102:2353–2358. [DOI] [PubMed] [Google Scholar]
- 7. Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Magnesium deficiency and hypertension: correlation between magnesium‐deficient diets and microcirculatory changes in situ. Science. 1984;223:1315–1317. [DOI] [PubMed] [Google Scholar]
- 8. Maier JA, Malpuech‐Brugere C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689:13–21. [DOI] [PubMed] [Google Scholar]
- 9. Bernardini D, Nasulewic A, Mazur A, Maier JA. Magnesium and microvascular endothelial cells: a role in inflammation and angiogenesis. Front Biosci. 2005;10:1177–1182. [DOI] [PubMed] [Google Scholar]
- 10. Amighi J, Sabeti S, Schlager O, Mlekusch W, Exner M, Lalouschek W, Ahmadi R, Minar E, Schillinger M. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke. 2004;35:22–27. [DOI] [PubMed] [Google Scholar]
- 11. Ford ES. Serum magnesium and ischaemic heart disease: findings from a national sample of US adults. Int J Epidemiol. 1999;28:645–651. [DOI] [PubMed] [Google Scholar]
- 12. Parikka H, Toivonen L, Naukkarinen V, Tierala I, Pohjola‐Sintonen S, Heikkila J, Nieminen MS. Decreases by magnesium of QT dispersion and ventricular arrhythmias in patients with acute myocardial infarction. Eur Heart J. 1999;20:111–120. [DOI] [PubMed] [Google Scholar]
- 13. Martin BJ, Black J, McLelland AS. Hypomagnesaemia in elderly hospital admissions: a study of clinical significance. Q J Med. 1991;78:177–184. [PubMed] [Google Scholar]
- 14. Reunanen A, Knekt P, Marniemi J, Maki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50:431–437. [PubMed] [Google Scholar]
- 15. Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;160:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan AM, Sullivan L, McCabe E, Levy D, Vasan RS, Wang TJ. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J. 2010;160:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orimo H, Ouchi Y. The role of calcium and magnesium in the development of atherosclerosis. Experimental and clinical evidence. Ann N Y Acad Sci. 1990;598:444–457. [DOI] [PubMed] [Google Scholar]
- 18. Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. [DOI] [PubMed] [Google Scholar]
- 19. McBride BF, Min B, Kluger J, Guertin D, Henyan NN, Coleman CI, Silver BB, White CM. An evaluation of the impact of oral magnesium lactate on the corrected QT interval of patients receiving sotalol or dofetilide to prevent atrial or ventricular tachyarrhythmia recurrence. Ann Noninvasive Electrocardiol. 2006;11:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofman A, Brusselle GG, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, Tiemeier HW, Uitterlinden AG, Vernooij MW. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol. 2015;30:661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all‐cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17:308–314. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of WHO/IDF Consultation. Geneva: World Health Organization; 2006. [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leening MJ, Kavousi M, Heeringa J, van Rooij FJ, Verkroost‐van Heemst J, Deckers JW, Mattace‐Raso FU, Ziere G, Hofman A, Stricker BH, Witteman JC. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . ATC/DDD index. Available at: www.whocc.no/atc_ddd_index. Accessed November 20, 2014.
- 26. Woosley R. Drugs that prolong the QTc interval and/or induce torsade de pointes. Available at: http://www.crediblemeds.org/everyone/composite-list-all-qtdrugs. Accessed July 30, 2015.
- 27. Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol. 1991;133:1078–1092. [DOI] [PubMed] [Google Scholar]
- 28. Niemeijer MN, van den Berg ME, Leening MJ, Hofman A, Franco OH, Deckers JW, Heeringa J, Rijnbeek PR, Stricker BH, Eijgelsheim M. Declining incidence of sudden cardiac death from 1990–2010 in a general middle‐aged and elderly population: the Rotterdam Study. Heart Rhythm. 2015;12:123–129. [DOI] [PubMed] [Google Scholar]
- 29. Myerburg RJ, Interian A Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. [DOI] [PubMed] [Google Scholar]
- 30. Priori SGA, Aliot E, Blomstrom‐Lundqvist C, Boassaert L, Breithardt G. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J. 2001;22:1374–1450. [DOI] [PubMed] [Google Scholar]
- 31. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 32. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. [DOI] [PubMed] [Google Scholar]
- 33. van Bemmel JH, Kors JA, van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med. 1990;29:346–353. [PubMed] [Google Scholar]
- 34. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 35. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 36. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H; American Heart Association E, Arrhythmias Committee CoCC, American College of Cardiology F, Heart Rhythm S . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–e250. [DOI] [PubMed] [Google Scholar]
- 37. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. [DOI] [PubMed] [Google Scholar]
- 38. Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via EM algorithm. Journal of the Royal Statistical Society. Series B (Methodological). 1977;39:1–38. [Google Scholar]
- 39. Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc. 2013;2:e000114 doi: 10.1161/JAHA.113.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiuve SE, Korngold EC, Januzzi JL Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL; American College of Cardiology/American Heart Association Task F, European Society of Cardiology Committee for Practice G, European Heart Rhythm A, Heart Rhythm S . ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. [DOI] [PubMed] [Google Scholar]
- 42. Roden DM. Drug‐induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 43. Bots ML, Grobbee DE, Hofman A, Witteman JC. Common carotid intima‐media thickness and risk of acute myocardial infarction: the role of lumen diameter. Stroke. 2005;36:762–767. [DOI] [PubMed] [Google Scholar]
- 44. Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YD, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Kao WH, Witteman JC, Gudnason V, Siscovick DS, Fox CS, Kottgen A; Genetic Factors for Osteoporosis C, Meta Analysis of G, Insulin Related Traits C . Genome‐wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. 2010;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Z, Duckart J, Slatore CG, Fu Y, Petrik AF, Thorp ML, Cohen DM. Individuality of the plasma sodium concentration. Am J Physiol Renal Physiol. 2014;306:F1534–F1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. [DOI] [PubMed] [Google Scholar]


