Abstract
Background
Non–vitamin K antagonist oral anticoagulants are used to prevent thromboembolism in patients with atrial fibrillation. The T‐TAS “Total Thrombus‐formation Analysis System” (Fujimori Kogyo Co Ltd) was developed for quantitative analysis of thrombus formation using microchips with thrombogenic surfaces (collagen, platelet chip [PL] ; collagen plus tissue factor, atheroma chip [AR]). We evaluated the utility of T‐TAS in predicting periprocedural bleeding in atrial fibrillation patients undergoing catheter ablation (CA).
Methods and Results
After exclusion of 20 from 148 consecutive patients undergoing CA, the remaining 128 patients were divided into 2 treatment groups: the warfarin group (n=30) and the non–vitamin K antagonist oral anticoagulants group (n=98). Blood samples obtained on the day of CA (anticoagulant‐free point) and at 3 and 30 days after CA were used in T‐TAS to compute the thrombus formation area under the curve (AUC; AUC for the first 10 minutes for PL tested at flow rate of 24 μL/min [PL 24‐AUC 10]; AUC for the first 30 minutes for AR tested at flow rate of 10 μL/min [AR 10‐AUC 30]). AR 10‐AUC 30 and PL 24‐AUC 10 levels were similar in the 2 groups on the day of CA. Levels of AR 10‐AUC 30, but not PL 24‐AUC 10, were significantly lower in the 2 groups at days 3 and 30 after CA. Multiple logistic regression analyses identified the AR 10‐AUC 30 level on the day of CA as a significant predictor of periprocedural bleeding events (odds ratio 5.7; 95% CI 1.54–21.1; P=0.009). Receiver operating characteristic analysis showed that the AR 10‐AUC 30 level on the day of CA significantly predicted periprocedural bleeding events (AUC 0.859, 95% CI 0.766–0.951; P<0.001). The cutoff AR 10‐AUC 30 level was 1648 for identification of periprocedural bleeding events.
Conclusions
These results suggested that the AR 10‐AUC 30 level determined by T‐TAS is a potentially useful marker for prediction of bleeding events in atrial fibrillation patients undergoing CA.
Keywords: anticoagulants, periprocedural bleeding events, thrombogenicity
Subject Categories: Thrombosis, Vascular Disease
Introduction
Anticoagulants are used to prevent cerebrovascular events in patients with atrial fibrillation (AF). Previous studies reported that warfarin reduces the risk of stroke by 64% compared with placebo.1 Non–vitamin K oral anticoagulants (NOACs) have been used clinically in recent years to prevent cerebrovascular events in patients with nonvalvular AF. Importantly, accumulating clinical and experimental evidence has proved that NOACs, such as dabigatoran,2 apixaban,3 rivaroxaban,4 and edoxaban5 in addition to warfarin, can reduce the likelihood of cerebrovascular events in patients with nonvalvular AF. In fact, a meta‐analysis of randomized clinical trials demonstrated that NOACs had a favorable risk–benefit profile, with significant reductions in stroke, intracranial hemorrhage, and mortality and with risk of major bleeding similar to warfarin, although with increased chance of gastrointestinal bleeding.6
NOACs are innovative drugs that solve complex patient management issues such as frequent blood sampling, dietary restriction, or drug interactions, which were problematic in the warfarin‐only era. There is, however, no definitive tool to monitor the anticoagulant effects of NOACs, although some patients have bleeding complications related to exceedingly high blood concentrations of NOACs.7, 8 Routine tests, such as prothrombin time international normalized ratio (PT‐INR) and activated partial thromboplastin time (APTT), could be problematic for monitoring the anticoagulant effects of NOACs because each NOAC has a different characteristic chemical structure and a different pharmacokinetic profile (eg, plasma half‐life and tissue penetration rate).9
The T‐TAS “Total Thrombus‐formation Analysis System” (Fujimori Kogyo Co Ltd),10, 11 a microchip‐based flow chamber system that can evaluate whole‐blood thrombogenicity, was developed as an easy‐to‐use system for quantitative analysis of thrombus formation. Recently, we demonstrated that T‐TAS might be a useful tool in monitoring the anticoagulant effects of edoxaban, a NOAC.12 The aim of the present study was to evaluate the utility of T‐TAS in monitoring the efficacy of warfarin and NOACs on thrombus formation and to predict the respective risk of bleeding complications in AF patients undergoing radiofrequency catheter ablation (CA).
Methods
Study Population
This retrospective and observational study enrolled 148 consecutive AF patients undergoing CA at Kumamoto University Hospital between September 2013 and August 2015. We excluded patients with cancer (n=4) and inflammatory diseases (n=1) and patients who refused participation (n=15). The remaining 128 paroxysmal/persistent AF patients were divided into 2 groups: patients treated with warfarin (n=30) and those treated with NOACs (n=98) (Figure 1). All procedures were conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol was approved by the human ethics committee of Kumamoto University, and written informed consent was obtained from each patient or the patient's family.
Figure 1.
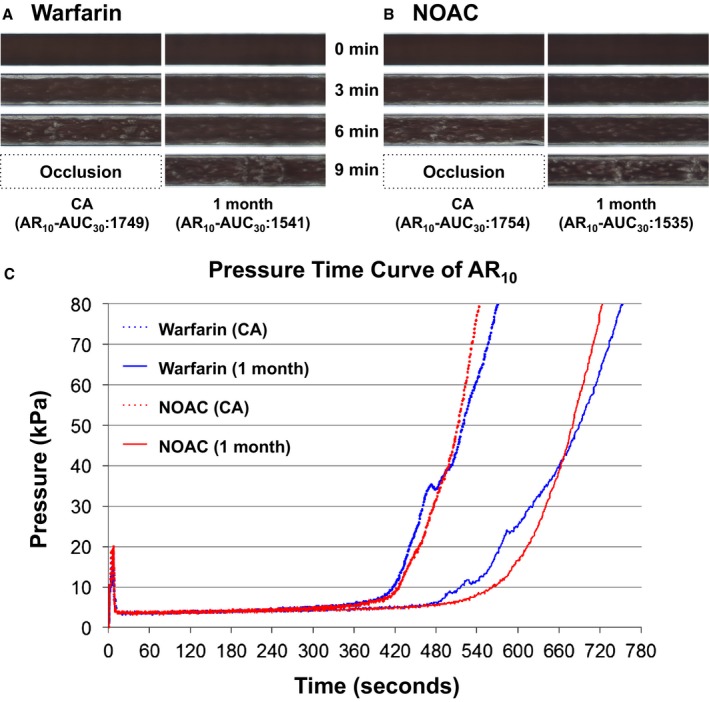
Effects of warfarin and NOACs on total thrombus formation in atheroma chips (AR). Representative microscopic images of thrombus formation under flow condition in AR in warfarin‐treated (A) and NOACs‐treated (B) groups. The time point of 0 minutes indicates initiation of measurements. C, Representative flow pressure changes under flow condition in AR. Warfarin (CA) and warfarin (1 month) represent pressure time curves of AR 10 in a warfarin‐treated patient at the time of CA and at 30 days after CA, respectively. NOAC (CA) and NOAC (1 month) represent pressure time curves of AR in a NOAC‐treated patient at the time of CA and at 30 days after CA, respectively. AR10‐AUC30 indicates area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation; NOACs, non–vitamin K oral anticoagulants; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min.
CA for Patients With AF
CA was performed in fasting and unsedated patients with AF. Briefly, a 6F quadripolar electrode catheter (St. Jude Medical) was inserted percutaneously via the femoral vein (at 3 locations on the right femoral vein and at 1 location on the left femoral vein) and positioned into the right ventricle. A 6F duodecapolar electrode catheter (St. Jude Medical) was inserted percutaneously via a single site of the right internal jugular vein and positioned in the coronary sinus. A single transseptal puncture was performed under fluoroscopic guidance. On completion of the transseptal puncture, patients received intravenous heparin to maintain an activated clotting time of >300 seconds. Protamine was not used in any of the patients for the reversal of heparin‐induced excess anticoagulation after the CA procedure. Patients underwent ablation procedures point by point. These procedures included pulmonary vein isolation with or without additional substrate modification. Irrigated ablation catheters (St. Jude Medical) were used for the ablation.
Periprocedural Anticoagulation Regimen and Collection of Blood Samples
Dabigatran, rivaroxaban, and apixaban were withheld in the morning the day before CA and were bridged with unfractionated heparin until 6 hours before CA. Warfarin was given in the evening once daily, interrupted 4 days before CA, and bridged with unfractionated heparin until 6 hours before CA. All NOACs were restarted the next morning after CA. Warfarin was restarted the next evening after CA and was bridged with unfractionated heparin from the next morning after CA until target PT‐INR of 1.6 to 2.6. Blood samples were obtained on the day of CA (anticoagulant‐free point) and at 3 days (before [trough] taking medication) and 30 days (trough) after CA.
Blood samples were collected, as described in detail previously.13 Briefly, blood samples were collected from the antecubital vein with a 21‐gauge butterfly needle into the following tubes: a hirudin‐containing blood sampling tube (MP0600 [Verum Diagnostia]; final concentration of hirudin 25 μg/mL), blood collection tubes (VP‐CA050K70, Venoject II; Terumo), and a syringe containing 0.11 mL of 3.8% sodium citrate solution. Each sample was immediately centrifuged at 1800g for 10 minutes at room temperature, and plasma was collected for biochemical analysis. Prothrombin time and APTT were measured using commercially available thromboplastin reagents (Coagupia PT‐N for prothrombin time; Coagupia APTT‐N for APTT; Sekisui Medical Co), according to the protocol supplied by the manufacturer.
Measurement of Thrombogenicity by T‐TAS
T‐TAS is an automated microchip‐based flow chamber system developed for easy and quick assessment of platelet thrombus formation under flow conditions.10, 13, 14 We have already reported details of T‐TAS, including appearance and components, and established the validity of the method with NOAC use.12 In brief, this system analyzes different thrombus‐formation processes with a simple procedure using 2 microchips with different thrombogenic surfaces. One chip, the platelet chip (PL), is coated with type I collagen. Inside the microchip, platelets adhere and aggregate on the surface of the collagen, and microchip capillaries are occluded. The other chip, the atheroma chip (AR), is coated with type I collagen plus tissue thromboplastin. Inside the microchip, activation of the platelets and the coagulation system is triggered simultaneously by collagen and tissue thromboplastin, respectively. The process of thrombus formation inside the 2 chips was analyzed by monitoring the flow pressure change. The area under the curve (AUC) for flow pressure was computed to assess platelet thrombogenicity inside the microchips. AUC for the first 10 minutes for the PL tested at flow rate of 24 μL/min is described as PL24‐AUC10, and AUC for the first 30 minutes for the AR tested at flow rate of 10 μL/min is described as AR10‐AUC30.
Efficacy and Safety End Points
The efficacy outcome included stroke or systemic embolism. Periprocedural bleeding events were defined as bleeding complications within 30 days after CA. We defined major and minor bleeding according to the 2012 Heart Rhythm Society, European Heart Rhythm Association, and European Cardiac Arrhythmia Society expert consensus statement on catheter and surgical ablation of AF.15 Major bleeding was defined as severe bleeding requiring blood transfusion or hematoma requiring surgical intervention. Small hematomas and pericardial effusion that did not require any intervention were considered minor bleeding complications. Anticoagulant therapy was immediately withdrawn in patients who developed major bleeding, and their data were excluded from analysis.
Statistical Analysis
Continuous variables with normal distribution were expressed as mean±SD, and categorical variables were expressed as frequencies and percentages, whereas those with skewed distribution were expressed as median values with interquartile range. Continuous variables were analyzed by the unpaired or paired t test for data with normal distribution, as appropriate, and the Mann–Whitney U test or Wilcoxon signed rank test for data with skewed distribution, as appropriate. Age, sex, history of disease, blood counts, blood biochemical and coagulation parameters, and other significant parameters in simple logistic analysis were entered into multiple logistic regression analysis to determine their association with bleeding events using the forced entry method, and the Hosmer‐Lemeshow goodness‐of‐fit statistic was calculated. A receiver operating characteristic curve was generated, and the AUC and its 95% CI were calculated to assess the ability of T‐TAS parameters, prothrombin time, and APTT at the point of CA to predict bleeding events. A 2‐tailed P value of <0.05 denoted the presence of a statistically significant difference. All statistical analyses were performed by using the SPSS software version 22 (IBM Corp).
Results
Baseline Patient Characteristics
Table 1 shows the baseline characteristics of patients treated with warfarin (n=30) and NOACs (n=98). The NOACs group comprised patients treated with dabigatran (n=21), rivaroxaban (n=52), and apixaban (n=25). There were no significant differences in baseline characteristics among the warfarin and NOACs groups except for a higher rate of patients with a HAS‐BLED score of 0 and use of antiarrhythmic drugs in the NOACs group.
Table 1.
Comparison of Baseline Demographics, Clinical Parameters, and Medication Use Between Patients in the Warfarin and NOACs Groups
| Warfarin (n=30) | NOACs (n=98) | P Value | |
|---|---|---|---|
| Age, y | 64.9±8.9 | 62.2±9.4 | 0.111 |
| Male | 16 (53.3) | 65 (66.3) | 0.196 |
| Body mass index, kg/m2 | 23.7±5.7 | 23.2±3.0 | 0.236 |
| Paroxysmal atrial fibrillation | 22 (73.3) | 71 (72.4) | 0.924 |
| Duration of atrial fibrillation (months) | 64.1±94.3 | 47.5±63.6 | 0.582 |
| Hypertension | 19 (63.3) | 51 (52.0) | 0.277 |
| Age >75 y | 3 (10.0) | 11 (11.2) | 0.576 |
| Diabetes mellitus | 3 (10.0) | 14 (14.3) | 0.398 |
| Stroke | 3 (10.0) | 8 (8.2) | 0.500 |
| CHADS2‐VASc score | |||
| 0 | 4 (13.3) | 23 (23.5) | 0.234 |
| 1 | 5 (16.7) | 22 (22.4) | 0.497 |
| ≥2 | 21 (66.7) | 53 (54.1) | 0.122 |
| HAS‐BLED score | |||
| 0 | 10 (33.3) | 53 (54.1) | 0.047 |
| 1 | 13 (43.3) | 31 (31.6) | 0.238 |
| 2 | 7 (23.3) | 13 (13.3) | 0.149 |
| ≥3 | 1 (3.3) | 3 (3.1) | 0.661 |
| LAD, mm | 36.0±6.0 | 37.1±6.1 | 0.273 |
| LVEF | 63.2±4.9 | 63.0±5.8 | 0.770 |
| Hemoglobin, g/dLa | 13.9±1.6 | 14.1±1.5 | 0.443 |
| Platelet count, 103/μLa | 206.7±47.9 | 206.6±44.6 | 0.740 |
| eGFR, mL/min/1.73 m2 a | 70.2±12.7 | 78.2±58.8 | 0.469 |
| BNP, pg/mLa | 69.5±91.7 | 61.1±70.4 | 0.600 |
| Aspirin | 1 (3.3) | 7 (7.1) | 0.400 |
| CCB | 14 (46.7) | 37 (38.5) | 0.429 |
| β‐blockers | 11 (36.7) | 35 (35.7) | 0.924 |
| ARB/ACE‐I | 10 (33.3) | 40 (41.2) | 0.439 |
| Statins | 8 (26.7) | 27 (27.6) | 0.924 |
| Antiarrhythmic drug | 15 (50.0) | 70 (71.4) | 0.030 |
| PPI | 7 (23.3) | 24 (24.5) | 0.897 |
Data are mean±SD or n (%). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NOACs, non–vitamin K antagonist oral anticoagulants; PPI, proton pump inhibitor.
Data of this parameter were at the point of admission.
Effects of Warfarin and NOACs on T‐TAS Parameters, PT‐INR, and APTT
Figure 1A and 1B show representative microscopic images of thrombus formation under flow condition in AR in warfarin‐ and NOACs‐treated groups. Total thrombus formation (indicated in white) was suppressed in both warfarin‐ and NOACs‐treated groups (Figure 1A and 1B). Figure 1C shows representative flow pressure changes under flow conditions in AR. The pressure time curves of AR10 in the warfarin and NOAC groups shifted to the right, indicating the suppression of thrombus formation 30 days after CA in both groups.
Table 2 and Figure 2 show serial changes in T‐TAS parameters (AR10‐AUC30 and PL24‐AUC10), PT‐INR, and APTT in patients treated with warfarin and NOACs. There were no significant differences in baseline AR10‐AUC30, PL24‐AUC10, PT‐INR, and APTT levels between the 2 groups. In each group, the AR10‐AUC30 levels at 3 and 30 days after CA were significantly lower than those at the day of CA; however, there were no significant differences in PL24‐AUC10 levels among the different time points. The PT‐INR and APTT levels were significantly higher at 3 and 30 days after CA than the day of CA in each group.
Table 2.
Serial Changes in T‐TAS Parameters, PT‐INR, and APTT
| Warfarin (n=30) | NOACs (n=98) | |||||
|---|---|---|---|---|---|---|
| Day of CA | 3 Days After CA | 30 Days After CA | Day of CA | 3 Days After CA | 30 Days After CA | |
| AR10‐AUC30 | 1749 [1655–1839] | 1524 [1251–1627]a | 1639 [1437–1720]a | 1794 [1679–1861] | 1591 [1448–1722]a | 1631 [1482–1734]a |
| PL24‐AUC10 | 415 [383–440] | 388 [357–440] | 411 [370–431] | 407 [369–436] | 419 [380–435] | 398 [375–421] |
| PT‐INR | 1.06 [1.03–1.11] | 1.40 [1.21–1.65]a | 1.78 [1.58–2.09]a | 1.05 [1.01–1.09] | 1.13 [1.06–1.21]a | 1.11 [1.06–1.19]a |
| APTT, s | 31.2 [29.9–34.8] | 51.9 [41.9–62.7]a | 38.6 [36.6–43.6]b | 32.1 [29.5–34.5] | 35.6 [31.6–40.7]a | 35.2 [32.3–39.6]a |
Warfarin was administered with heparin to maintain APTT at 60 to 80 seconds. Values are expressed as median [25–75%]. APTT indicates activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation; NOACs, non–vitamin K antagonist oral anticoagulants; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min.
P≤0.001, compared with the data at the day of CA.
P≤0.05, compared with the data at the day of CA.
Figure 2.
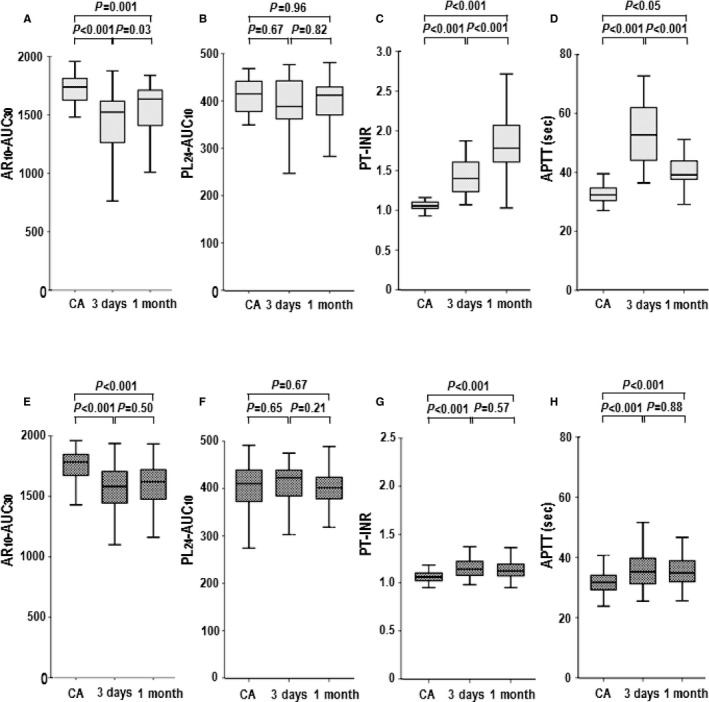
Serial changes in T‐TAS parameters, PT‐INR, and APTT: AR 10‐AUC 30 (A), PL 24‐AUC 10 (B), PT‐INR (C), and APTT (D) levels in the warfarin group; AR 10‐AUC 30 (E), PL 24‐AUC 10 (F), PT‐INR (G), and APTT (H) levels in the NOACs group. Summary data of each parameter by the box‐and‐whisker plot. In these plots, the lines within the boxes represent the median values. The upper and lower lines of the boxes represent the 25th and 75th percentiles, respectively. The upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively. 1 month indicates 30 days after catheter ablation; 3 days, 3 days after catheter ablation; APTT, activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation; NOACs, non–vitamin K oral anticoagulants; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min; PT‐INR, prothrombin time international normalized ratio.
Table 3 shows serial changes in T‐TAS parameters, PT‐INR, and APTT according to the type of NOACs (dabigatran, rivaroxaban, and apixaban). There were no significant differences in baseline AR10‐AUC30, PL24‐AUC10, PT‐INR, and APTT levels among the 3 NOACs groups except for a higher APTT level in the dabigatran group compared with the rivaroxaban group. In all 3 subgroups, AR10‐AUC30 levels were significantly lower at 3 and 30 days after CA than those at the day of CA, although the AR10‐AUC30 levels at each sampling point were identical among the 3 NOACs groups.
Table 3.
Serial Changes in T‐TAS Parameters, PT‐INR, and APTT According to the Type of NOACs
| Day of CA | 3 Days After CA | 30 Days After CA | |
|---|---|---|---|
| Dabigatran (n=21) | |||
| AR10‐AUC30 | 1786 [1693–1865] | 1589 [1413–1726]a | 1621 [1452–1715]a |
| PL24‐AUC10 | 417 [379–446] | 427 [398–446] | 395 [370–436] |
| PT‐INR | 1.03 [0.99–1.06] | 1.12 [1.06–1.15]a, b | 1.10 [1.06–1.14]a, c |
| APTT, s | 34.5 [32.1–38.1]c | 40.8 [37.8–45.7]a, b, c | 42.7 [39.2–46.1]a, b, c |
| Rivaroxaban (n=52) | |||
| AR10‐AUC30 | 1812 [1677–1860] | 1629 [1474–1797]a | 1645 [1540–1740]a |
| PL24‐AUC10 | 401 [363–437] | 411 [377–435] | 405 [380–421] |
| PT‐INR | 1.06 [1.01–1.09] | 1.11 [1.04–1.19]a, b | 1.11 [1.05–1.21]a |
| APTT, s | 30.8 [28.6–33.0] | 33.3 [30.6–38.6]a | 33.5 [30.8–36.5]a |
| Apixaban (n=25) | |||
| AR10‐AUC30 | 1781 [1673–1861] | 1541 [1435–1674]a | 1612 [1460–1734]a |
| PL24‐AUC10 | 395 [373–432] | 418 [356–431] | 392 [372–412] |
| PT‐INR | 1.05 [1.01–1.09] | 1.23 [1.13–1.27]a | 1.16 [1.11–1.22]a |
| APTT, s | 31.8 [30.2–34.3] | 33.7 [31.6–37.7]a | 32.9 [31.8–37.5]a |
APTT indicates activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation; NOACs, non–vitamin K antagonist oral anticoagulants; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min.
P<0.01, compared with the data at the day of CA.
P<0.01, compared with the data of the same sampling point in the apixaban group.
P<0.05, compared with the data of the same sampling point in the apixaban group.
P<0.01, compared with the data at the same sampling point in rivaroxaban group.
Effects of Anticoagulants on Thrombotic and Bleeding Complications and on Serial Changes in T‐TAS Parameters, PT‐INR, and APTT
One of the 128 AF patients treated with warfarin developed cerebral infarction at 3 days after CA. Periprocedural bleeding events were observed in 21 (16.4%) of the 128 AF patients (4 [13.3%] of 30 treated with warfarin and 17 [17.3%] of 98 treated with NOACs) who underwent CA (Table 4). Major bleeding events were observed in 3 patients (2.4%; 2 puncture related [femoral bleeding] and 1 not puncture related [leg hematoma]), whereas minor bleeding events were observed in 18 patients (14.1%; 15 puncture related [femoral bleeding], 3 not puncture related [1 gingival, 1 menstrual, and 1 leg hematoma]). There were no significant differences in the clinical features of patients with and without bleeding events except for a lower platelet count at the point of admission and a higher rate of patients with HAS‐BLED score 2 in the group with bleeding events (Table 4). Three of 21 and 5 of 107 patients with and without bleeding events, respectively, were being treated with aspirin. None of the patients was treated with other antiplatelet drugs apart from aspirin. Furthermore, there were no significant differences in procedure time, activated clotting time levels at the end of CA, and total dose of heparin during CA between the groups with and without periprocedural bleeding events.
Table 4.
Clinical Characteristics of Patients With and Without Periprocedural Bleeding Events
| Without Bleeding Events (n=107) | With Bleeding Events (n=21) | P Value | |
|---|---|---|---|
| Age, y | 62.5±9.5 | 64.6±8.8 | 0.360 |
| Male | 69 (64.5) | 12 (57.1) | 0.523 |
| Body mass index, kg/m2 | 23.3±3.8 | 23.5±4.2 | 0.792 |
| Paroxysmal atrial fibrillation | 76 (71.0) | 17 (81.0) | 0.351 |
| Duration of atrial fibrillation (months) | 43.0±64.8 | 95.4±92.2 | 0.060 |
| Hypertension | 59 (55.1) | 11 (52.4) | 0.816 |
| Age >75 y | 10 (9.3) | 4 (19.0) | 0.175 |
| Diabetes mellitus | 14 (13.1) | 3 (14.3) | 0.557 |
| Stroke | 9 (8.4) | 2 (9.5) | 0.569 |
| CHADS2‐VASc score | |||
| 0 | 24 (22.4) | 3 (14.3) | 0.304 |
| 1 | 23 (21.5) | 4 (19.0) | 0.532 |
| ≥2 | 60 (56.1) | 14 (66.7) | 0.369 |
| HAS‐BLED score | |||
| 0 | 54 (50.5) | 9 (42.9) | 0.524 |
| 1 | 40 (37.4) | 4 (19.0) | 0.106 |
| 2 | 10 (9.3) | 6 (28.6) | 0.026 |
| ≥3 | 2 (1.9) | 2 (9.5) | 0.126 |
| LAD, mma | 36.5±6.0 | 38.7±6.2 | 0.105 |
| LVEF a | 62.8±5.9 | 64.2±4.0 | 0.349 |
| Hemoglobin, g/dLa | 14.1±1.5 | 13.7±1.6 | 0.126 |
| Platelet count, 103/μLa | 211.6±42.9 | 181.4±49.4 | 0.004 |
| eGFR, mL/min/1.73 m2 a | 77.5±55.1 | 68.5±12.5 | 0.292 |
| BNP, pg/mLa | 65.4±79.3 | 50.5±53.2 | 0.951 |
| Aspirin | 5 (4.7) | 3 (14.3) | 0.124 |
| CCB | 42 (40.0) | 8 (40.0) | 1.000 |
| β‐blockers | 36 (34.0) | 9 (42.9) | 0.436 |
| ARB/ACE‐I | 40 (37.7) | 9 (45.0) | 0.541 |
| Statins | 25 (23.6) | 9 (42.9) | 0.068 |
| Antiarrhythmic drug | 71 (67.0) | 13 (61.9) | 0.653 |
| PPI | 26 (24.5) | 4 (19.0) | 0.411 |
| NOACs | 81 (75.7) | 17 (81.0) | 0.419 |
| Procedure time, min | 155.0 [114.5–187.5] | 175.0 [118.5–198.0] | 0.604 |
| ACT level at end of CA, s | 293.0 [274.0–322.0] | 289.0 [266.5–305.5] | 0.518 |
| Total dose of heparin, units | 14 000 [11 000–16 000] | 13 000 [8750–16 750] | 0.432 |
Data are mean±SD, n (%), or median [25–75%]. ACE‐I indicates angiotensin‐converting enzyme inhibitor; ACT, activated clotting time; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CA, catheter ablation; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NOACs, non–vitamin K antagonist oral anticoagulants; PPI, proton pump inhibitor.
At the point of admission.
Table 5 and Figure 3 show the levels of T‐TAS parameters (AR10‐AUC30 and PL24‐AUC10), PT‐INR, and APTT in patients with and without periprocedural bleeding events. The AR10‐AUC30 and PL24‐AUC10 levels were significantly lower in the group with periprocedural bleeding events than the group without such events at the day of and 3 days after CA. In contrast, PT‐INR and APTT levels were identical between the groups with and without periprocedural bleeding events at the day of and 3 days after CA.
Table 5.
T‐TAS Parameters, PT‐INR, and APTT in Patients With and Without Periprocedural Bleeding Events
| Without Bleeding Events (n=107) | With Bleeding Events (n=21) | ||||
|---|---|---|---|---|---|
| Day of CA | 3 Days After CA | Day of CA | 3 Days After CA | At Event | |
| AR10‐AUC30 | 1805 [1702–1861] | 1616 [1455–1722] | 1525 [1447–1713]a | 1437 [1205–1540]a | 1390 [1246–1504] |
| PL24‐AUC10 | 411 [383–441] | 422 [384–440] | 364 [333–432]b | 367 [307–421]b | 363 [261–404] |
| PT‐INR | 1.05 [1.01–1.09] | 1.15 [1.07–1.25] | 1.07 [1.03–1.10] | 1.19 [1.09–1.33] | 1.13 [1.08–1.21] |
| APTT, s | 31.7 [29.5–34.4] | 36.9 [31.7–43.7] | 32.5 [30.7–35.1] | 37.3 [34.0–43.8] | 37.3 [33.6–41.6] |
Values are expressed as median [25–75%]. APTT indicates activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min.
P<0.001, without bleeding events vs with bleeding events at the day of CA or 3 days after CA.
P<0.05, without bleeding events vs with bleeding events at the day of CA or 3 days after CA.
Figure 3.
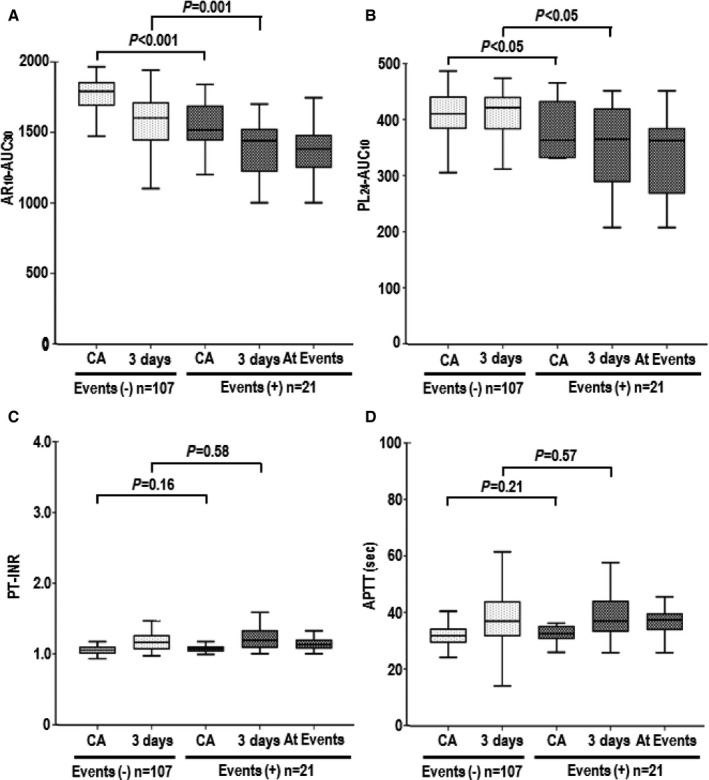
Serial changes in T‐TAS parameters, PT‐INR, and APPT in atrial fibrillation patients with (+) and without (−) bleeding events: AR 10‐AUC 30 (A), PL 24‐AUC 10 (B), PT‐INR (C), and APTT (D) levels. Box‐and‐whisker plots are described in Figure 2. 3 days indicates 3 days after catheter ablation; APTT, activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min; PT‐INR, prothrombin time international normalized ratio.
Predictors of Periprocedural Bleeding Events
We also analyzed the predictors of periprocedural bleeding events in AF patients who underwent CA. Simple logistic regression analysis demonstrated that platelet count and AR10‐AUC30 level at the day of CA correlated with periprocedural bleeding events (Table 6). There were weak positive correlations of AR10‐AUC30 with hemoglobin levels (r=0.244, P=0.006), platelet count (r=0.325, P=0.006), use of NOACs (r=0.174, P=0.049), PL24‐AUC10 levels (r=0.368, P<0.001), and PT‐INR (r=0.226, P=0.012). Multiple logistic regression analyses using 3 forced inclusion models that included various clinical parameters such as platelet count, AR10‐AUC30, PT‐INR, and APTT levels identified AR10‐AUC30 level at the day of CA as a significant predictor of periprocedural bleeding events in all 3 models (Table 7), with a Hosmer‐Lemeshow goodness‐of‐fit chi‐square of 2.195 (P=0.33) in model 1), 0.608 (P=0.74) in model 2, and 0.315 (P=0.854) in model 3.
Table 6.
Results of Simple Logistic Regression Analyses for Periprocedural Bleeding Events
| Simple Regression Analysis | |||
|---|---|---|---|
| OR | 95% CI | P Value | |
| Age (>75 y) | 2.28 | 0.64–8.12 | 0.202 |
| Male (yes) | 0.73 | 0.28–1.90 | 0.524 |
| Body mass index (>25 kg/m2) | 2.19 | 0.80–5.95 | 0.125 |
| Paroxysmal atrial fibrillation (yes) | 1.73 | 0.54–5.57 | 0.355 |
| Hypertension (yes) | 0.90 | 0.35–2.29 | 0.816 |
| Diabetes mellitus (yes) | 1.11 | 0.29–4.25 | 0.882 |
| Stroke (yes) | 1.15 | 0.23–5.73 | 0.868 |
| CHA2DS2‐VASc score ≥2 | 0.93 | 0.31–2.77 | 0.890 |
| HAS‐BLED score ≥3 | 5.53 | 0.73–41.7 | 0.097 |
| LAD (>40 mm) | 2.02 | 0.77–5.29 | 0.154 |
| LVEF (%) | 0.63 | 0.24–1.67 | 0.352 |
| Hemoglobin, g/dLa | 1.61 | 0.62–4.35 | 0.314 |
| Platelet count, 103/La | 5.43 | 1.71–17.2 | 0.004 |
| eGFR, <60 mL/min per 1.73 m2 | 1.15 | 0.30–4.47 | 0.838 |
| BNP, pg/mLb | 2.15 | 0.74–6.24 | 0.161 |
| Aspirin (yes) | 3.40 | 0.75–15.5 | 0.114 |
| CCB (yes) | 0.98 | 0.37–2.59 | 0.962 |
| β‐blockers (yes) | 1.42 | 0.55–3.68 | 0.471 |
| ARB/ACE‐I (yes) | 1.32 | 0.50–3.54 | 0.575 |
| Statins (yes) | 2.34 | 0.89–6.17 | 0.087 |
| Antiarrhythmic drug (yes) | 0.79 | 0.30–2.08 | 0.633 |
| PPI (yes) | 0.70 | 0.22–2.25 | 0.547 |
| NOACs (yes) | 1.36 | 0.42–4.42 | 0.605 |
| AR10‐AUC30 a | 7.96 | 2.21–28.64 | 0.002 |
| PL24‐AUC10 a | 2.29 | 0.85–6.12 | 0.100 |
| PT‐INRb | 1.79 | 0.66–4.84 | 0.253 |
| APTT, sb | 1.62 | 0.61–4.29 | 0.332 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; APTT indicates activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CA, catheter ablation; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NOACs, non–vitamin K antagonist oral anticoagulants; OR, odds ratio; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min; PPI, proton pump inhibitor.
Data of this parameter was lower than the median value at the point of CA.
Data of this parameter was higher than the median value at the point of CA.
Table 7.
Results of Multiple Logistic Regression Analyses for Periprocedural Bleeding Events
| Multiple Regression Analysis | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| AR10‐AUC30 a | 5.7 (1.54–21.1), P=0.009 | 7.1 (1.92–26.1), P=0.003 | 7.2 (1.96–26.2), P=0.003 |
| Platelet count (103/L)a | 3.8 (1.14–12.5), P=0.030 | ||
| PT‐INRb | 1.2 (0.43–3.59), P=0.680 | ||
| APTT, sb | 1.3 (0.45–3.54), P=0.651 | ||
Values are expressed as OR (95% CI), P value. APTT indicates activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; CA, catheter ablation.
Data of this parameter were lower than the median value at the point of CA.
Data of this parameter were higher than the median value at the point of CA.
Receiver Operating Characteristic Analysis for Periprocedural Bleeding Events and T‐TAS Parameters, PT‐INR, and APTT
Receiver operating characteristic curves were constructed to assess the ability of T‐TAS parameters, PT‐INR, and APTT at the point of CA and 3 days after CA to predict periprocedural bleeding events. The AUC of AR10‐AUC30 for detection of periprocedural bleeding events was 0.859 (95% CI 0.766–0.951; P<0.001) at the point of CA (Figure 4A) and 0.759 (95% CI 0.657–0.862; P<0.001) at 3 days after CA (Figure 4B). In contrast, the AUCs of PL24‐AUC10, PT‐INR, and APTT for the detection of periprocedural bleeding events were 0.657 (95% CI 0.513–0.802; P=0.02), 0.601 (95% CI 0.464–0.738; P=0.15), and 0.592 (95% CI 0.463–0.720; P=0.20), respectively, at the point of CA and 0.705 (95% CI 0.577–0.833; P=0.007), 0.547 (95% CI 0.402–0.692; P=0.54), and 0.547 (95% CI 0.414–0.680; P=0.54), respectively, at 3 days after CA. Using the cutoff value for AR10‐AUC30 at the day of CA (1648) and 3 days after CA (1582), the sensitivity and specificity for detection of a bleeding event were 71% and 90%, respectively, at the point of CA and 89% and 57%, respectively, at 3 days after CA.
Figure 4.
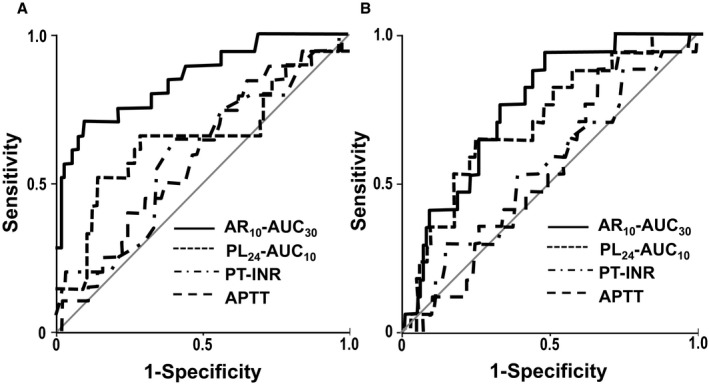
Receiver‐operating characteristic curves for T‐TAS parameters, PT‐INR, and APTT to predict periprocedural bleeding events at the point of CA (A) and at day 3 (B). CA indicates catheter ablation; APTT, activated partial thromboplastin time; AR10‐AUC30, area under the curve for the first 30 minutes for the atheroma chip tested at flow rate of 10 μL/min; PL24‐AUC10, area under the curve for the first 10 minutes for the platelet chip tested at flow rate of 24 μL/min; PT‐INR, prothrombin time international normalized ratio.
Discussion
The main feature of the present study was assessment of whole blood thrombogenicity using T‐TAS in patients treated with anticoagulants who underwent CA for AF. The main findings were as follows: (1) Treatment with anticoagulants, including warfarin and NOACs, significantly decreased AR10‐AUC30 levels; (2) the AR10‐AUC30 level at the day of CA and 3 days after CA was a significant predictor of periprocedural bleeding events in patients who underwent CA for AF, whereas PL24‐AUC10, PT‐INR, and APTT levels did not correlate with bleeding events; and (3) the cutoff levels of AR10‐AUC30 for periprocedural bleeding events were 1648 at the day of CA and 1582 at 3 days after CA. To the best of our knowledge, this report is the first to describe the usefulness of T‐TAS, a novel quantitative assessment of whole‐blood thrombogenicity, as a monitoring tool for the efficacy and safety of anticoagulant therapy, including NOACs, in AF patients undergoing CA.
It is difficult sometimes to monitor the anticoagulant effects of dabigatran,16 rivaroxaban,17 apixaban,18 or edoxaban19 by measuring routinely used parameters such as PT‐INR and APTT. Furthermore, although NOACs are easy to use in general, their concentrations in peripheral blood could reach relatively high levels, placing some patients at high risk of cerebral bleeding.7, 8 In the present study, we found significantly low levels of AR10‐AUC30 at the time of bleeding and demonstrated the usefulness of AR10‐AUC30 levels with T‐TAS as a significant predictor of periprocedural bleeding events in patients who underwent CA for AF. These results indicate that T‐TAS is a potentially suitable monitoring system for the assessment of the anticoagulant effects in AF patients treated with NOACs. In the present study, major and all bleeding events were observed in 2.4% and 16.4%, respectively, of the total study population. These rates are almost identical to those described in previous studies after CA for AF of 1.2% to 3.5% and 5% to 13.9%.20, 21, 22
T‐TAS, the microchip‐based flow chamber apparatus used for evaluation of the extent of thrombus formation, is novel and easy to use for quantitatively monitoring the levels of thrombogenesis under a whole blood flow state. In the present study, we used PL, which can assess thrombogenicity associated with platelets, and AR, which can assess thrombogenicity under the condition of platelets and coagulation–fibrinolysis factors. In this study, AR10‐AUC30 levels decreased significantly after anticoagulation with warfarin or NOACs, whereas PL24‐AUC10 was not suitable for assessment of the effects of anticoagulant therapy. Furthermore, the AR10‐AUC30 levels at all sampling points were identical among the 3 NOACs (dabigatran, rivaroxaban, and apixaban) subgroups. Based on these findings, we believe that AR (AR10‐AUC30 levels) is potentially suitable for monitoring the effects of any anticoagulant therapy (eg, warfarin, heparin, and NOACs), whereas PL (PL24‐AUC10) is perhaps useful for clinical studies monitoring antiplatelet therapy, including aspirin, clopidogrel, or prasugrel in patients with coronary artery disease.
It was demonstrated previously that inhibition of factors VIII and IX effectively reduced the growth of thrombi in AR under lower shear conditions (110 s−1), whereas no significant change was observed under pathological high‐shear conditions (1100 s−1).23 Although lower shear conditions (ie, ≤110 s−1) may be suitable for specific assessment of thrombogenicity in the left atrium, in which the coagulation system plays a predominant role, it is anticipated that the T‐TAS parameter AR10‐AUC30 (with a low‐ to midshear rate of 600 s−1), which simulates the shear conditions at small veins and large arteries, can also be used to evaluate thrombus formation in the left atrium. We previously demonstrated the usefulness of AR10‐AUC30 in distinguishing the pharmacological effects of edoxaban, a NOAC, in patients undergoing total knee arthroplasty.12 The present data also showed low AR10‐AUC30 levels at 3 and 30 days post‐CA compared with those on the day of CA, indicating that anticoagulant therapy with warfarin or NOACs significantly decreased AR10‐AUC30 levels. Based on these findings, we believe that AR10‐AUC30 levels measured by T‐TAS could be useful for monitoring the efficacy and safety of warfarin and NOACs in AF patients. Further prospective large‐population studies are needed to establish the relationship between AR10‐AUC30 levels and future thromboembolic events.
The present study has several limitations. First, this single‐center observational study included a relatively small number of patients; however, the strength of the study is the enrollment of consecutive patients. In this sense, the study population represents real‐world patients with AF referred for CA. Second, we did not follow the serial changes in T‐TAS parameters and coagulant markers in the non‐AF control patients. Third, there was no significant difference in the frequency of periprocedural bleeding complications between the warfarin and NOACs groups; this finding is probably related to the small sample size. Further large‐population studies are needed to examine the relationship between AR10‐AUC30 measured by T‐TAS and the rates of periprocedural bleeding complications in patients treated with warfarin and NOACs.
In conclusion, our results demonstrated that AR10‐AUC30 level measured by T‐TAS was an independent and significant tool for assessment of the efficacy of warfarin and NOACs in AF patients who underwent CA and that this marker could be a useful predictor of periprocedural bleeding events in such patients.
Sources of Funding
This study was supported in part by Grants‐in‐Aid for Scientific Research (#15K09089) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosures
None.
Acknowledgments
We thank Kazuya Hosokawa and Tomoko Ohnishi from the Research Institute, Fujimori Kogyo Co, Yokohama, Kanagawa, Japan, for the excellent technical support in the measurement of T‐TAS.
(J Am Heart Assoc. 2016;5:e002744 doi: 10.1161/JAHA.115.002744)
References
- 1. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 7. Komori M, Yasaka M, Kokuba K, Matsuoka H, Fujimoto S, Yoshida M, Kameda K, Shono T, Nagata S, Ago T, Kitazono T, Okada Y. Intracranial hemorrhage during dabigatran treatment. Circ J. 2014;78:1335–1341. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki S, Sagara K, Otsuka T, Kano H, Matsuno S, Takai H, Uejima T, Oikawa Y, Koike A, Nagashima K, Kirigaya H, Yajima J, Tanabe H, Sawada H, Aizawa T, Yamashita T. “Blue letter effects”: changes in physicians' attitudes toward dabigatran after a safety advisory in a specialized hospital for cardiovascular care in Japan. J Cardiol. 2013;62:366–373. [DOI] [PubMed] [Google Scholar]
- 9. Ogawa S, Koretsune Y, Yasaka M, Aizawa Y, Atarashi H, Inoue H, Kamakura S, Kumagai K, Mitamura H, Okumura K, Sugi K, Yamashita T. Antithrombotic therapy in atrial fibrillation: evaluation and positioning of new oral anticoagulant agents. Circ J. 2011;75:1539–1547. [DOI] [PubMed] [Google Scholar]
- 10. Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, Tanaka KA. A novel automated microchip flow‐chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9:2029–2037. [DOI] [PubMed] [Google Scholar]
- 11. Hosokawa K, Ohnishi T, Sameshima H, Miura N, Ito T, Koide T, Maruyama I. Analysing responses to aspirin and clopidogrel by measuring platelet thrombus formation under arterial flow conditions. Thromb Haemost. 2013;109:102–111. [DOI] [PubMed] [Google Scholar]
- 12. Sueta D, Kaikita K, Okamoto N, Arima Y, Ishii M, Ito M, Oimatsu Y, Iwashita S, Takahashi A, Nakamura E, Hokimoto S, Mizuta H, Ogawa H. A novel quantitative assessment of whole blood thrombogenicity in patients treated with a non‐vitamin K oral anticoagulant. Int J Cardiol. 2015;197:98–100. [DOI] [PubMed] [Google Scholar]
- 13. Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, Murata M. Studies of a microchip flow‐chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res. 2013;132:263–270. [DOI] [PubMed] [Google Scholar]
- 14. Hosokawa K, Ohnishi T, Fukasawa M, Kondo T, Sameshima H, Koide T, Tanaka KA, Maruyama I. A microchip flow‐chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc Res. 2012;83:154–161. [DOI] [PubMed] [Google Scholar]
- 15. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 16. van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. [DOI] [PubMed] [Google Scholar]
- 17. Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59‐7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–421. [DOI] [PubMed] [Google Scholar]
- 18. Kanemoto M, Kuhara H, Ueda T, Shinohara T, Oda T, Nakao F, Kamei T, Ikeda Y, Fujii T. Association of apixaban therapy and prothrombin time in patients with atrial fibrillation. Circ J. 2014;78:2651–2656. [DOI] [PubMed] [Google Scholar]
- 19. Samama MM, Mendell J, Guinet C, Le Flem L, Kunitada S. In vitro study of the anticoagulant effects of edoxaban and its effect on thrombin generation in comparison to fondaparinux. Thromb Res. 2012;129:e77–e82. [DOI] [PubMed] [Google Scholar]
- 20. Lakkireddy D, Reddy YM, Di Biase L, Vanga SR, Santangeli P, Swarup V, Pimentel R, Mansour MC, D'Avila A, Sanchez JE, Burkhardt JD, Chalhoub F, Mohanty P, Coffey J, Shaik N, Monir G, Reddy VY, Ruskin J, Natale A. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59:1168–1174. [DOI] [PubMed] [Google Scholar]
- 21. Lakkireddy D, Reddy YM, Di Biase L, Vallakati A, Mansour MC, Santangeli P, Gangireddy S, Swarup V, Chalhoub F, Atkins D, Bommana S, Verma A, Sanchez JE, Burkhardt JD, Barrett CD, Baheiry S, Ruskin J, Reddy V, Natale A. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2014;63:982–988. [DOI] [PubMed] [Google Scholar]
- 22. Armbruster HL, Lindsley JP, Moranville MP, Habibi M, Khurram IM, Spragg DD, Berger RD, Calkins H, Marine JE. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann Pharmacother. 2015;49:278–284. [DOI] [PubMed] [Google Scholar]
- 23. Ogawa S, Szlam F, Dunn AL, Bolliger D, Ohnishi T, Hosokawa K, Tanaka KA. Evaluation of a novel flow chamber system to assess clot formation in factor VIII‐deficient mouse and anti‐factor IXa‐treated human blood. Haemophilia. 2012;18:926–932. [DOI] [PubMed] [Google Scholar]


