Abstract
Background
Basiliximab and anti‐thymocyte globulin are widely used drugs for induction therapy after pediatric heart transplantation. The aim of this study was to determine whether any differences could be observed between basiliximab and anti‐thymocyte globulin, with respect to long‐term mortality, in a population of pediatric cardiac transplant recipients.
Methods and Results
An analysis of pediatric heart transplant patients (aged <18 years) from the United Network for Organ Sharing database was conducted that compared patients receiving basiliximab with those that received anti‐thymocyte globulin for the risk of all‐cause mortality. Secondary endpoints included death attributable to graft failure, cardiovascular causes, infection, or malignancy. Of the 2275 patients, 685 received basiliximab and 1590 anti‐thymocyte globulin. One‐year survival was similar for both groups; however, at 5 and 10 years, basiliximab was associated with poorer long‐term survival (68% versus 76% at 5 years [P<0.001] and 49% versus 65% at 10 years [P<0.001], respectively). Basiliximab was associated with higher risk of death attributable to graft failure (P=0.013), but not death attributable to cardiovascular causes (P=0.444), infection (P=0.095), or malignancy (P=0.392). After multivariate analysis, use of basiliximab (versus use of anti‐thymocyte globulin) remained significantly associated with all‐cause mortality (hazard ratio, 1.27; 95% confidence interval, 1.02–1.57; P=0.030).
Conclusions
In pediatric heart transplant patients, use of basiliximab for induction therapy was associated with an increased risk of mortality, when compared with those receiving anti‐thymocyte globulin.
Keywords: pediatrics, survival, transplantation
Subject Categories: Transplantation, Pediatrics
Introduction
Since the first pediatric heart transplantation in 1967, over 11 000 transplants have been undertaken in children with end‐stage heart disease.1 Survival has improved, mainly because of improved survival during the first 6 months post–heart transplantation.2 Advances in pre– and early post–heart transplantation care, and possibly the introduction of new immunosuppression agents and protocols, have been associated with the decreased mortality of these children.2 Long‐term survival is still unsatisfactory, however, with cardiac allograft vasculopathy and graft failure being the leading causes of death.1
Despite novel drugs and drug combinations, consensus on the optimal immunosuppressive regimen is lacking.3 Induction treatment is immunosuppression that is initiated at high levels in the immediate post‐transplant period, when the risk of graft rejection is the greatest. The goal is to minimize the frequency of acute rejections and allow for the delayed introduction of the nephrotoxic drugs, cyclosporine or tacrolimus.4 Induction treatment is also indicated when complete corticosteroid avoidance is planned after heart transplantation.3, 5 In contrast to the adult population, the use of induction therapy continues to rise among pediatric patients. Today, over 70% of pediatric patients receive induction treatment, comprising 47% anti‐thymocyte globulin (ATG) and 25% interleukin‐2 receptor (IL2‐R) antagonists, such as basiliximab (BAS).1
Studies in adult heart transplant populations have indicated that BAS, compared with ATG, is associated with lower incidence of infectious deaths and other drug‐related adverse effects, and have failed to show unanimously that one drug has an advantage over the other in terms of rejections and patient survival.6, 7, 8, 9 We have recently shown that induction treatment with ATG is associated with better long‐term survival compared with BAS in adult heart transplantation.10 The literature has few data regarding the use of BAS versus ATG in pediatric cardiac transplantation.
BAS offers several potential benefits over ATG, including a more selective mode of immune‐ suppressive action, targeting specifically the T‐cell receptor as opposed to generalized lymphopenia, and an adverse event profile comparable to placebo.9 Although multiple induction protocols with either BAS or ATG have been used after pediatric cardiac transplantation, there is a scarcity of studies that have compared BAS and ATG with regard to long‐term mortality. Because BAS could offer significant clinical advantages, we aimed to determine whether any differences could be observed between BAS and ATG, with respect to long‐term mortality, in a population of pediatric cardiac transplant recipients.
Methods
Patient Population
Deidentified patient data from the United Network for Organ Sharing (UNOS) research database were extracted. UNOS data include US patients who received thoracic organ transplants reported to the organ procurement network. The database contains >400 clinical, demographic, and operative variables. We identified all recipients of orthotopic cardiac transplants patients under the age of 18 years of age, transplanted between January 3, 2001 and September 30, 2013. The latest follow‐up was on December 5, 2013. We chose to include patients transplanted after 2000 because BAS was approved by the US Food and Drug Administration in 1998.11 Using these criteria resulted in 7341 transplant recipients for analysis. The study population was limited only to those patients receiving induction therapy with either BAS (Simulect) or ATG (equine anti‐thymocyte globulin; Atgam, rabbit anti‐thymocyte globulin; Thymoglobulin). Those with missing values in BAS or ATG treatment were excluded. The final cohort consisted of 2311 heart transplants. The Ethics Committee for Clinical Research at Lund University (Lund, Sweden) approved the study protocol and the UNOS Registry approved the protocol and provided data.
Outcome Measures
Our primary endpoint was all‐cause cumulative mortality during the study period. Secondary outcomes included mortality attributable to graft failure (primary failure, rejection‐hyperacute, acute or chronic, graft infection, recurrent disease, or nonspecific), cardiovascular causes (myocardial infarction, cardiac arrest, arterial embolism, ventricular failure, coronary artery disease, atherosclerosis, rhythm disorder, carditis, aortic aneurysm, or cardiogenic shock), infection, or malignancy.
Statistical Analysis
Statistical analyses were performed using the Stata MP statistical package (version 13.1, 2013; StataCorp LP, College Station, TX). We compared baseline characteristics between the groups using the t test or Mann–Whitney U test for continuous variables and the chi‐square or Fisher exact test for categorical variables. Cumulative mortality was modeled using the Kaplan–Meier method with statistical differences between the mortality curves assessed using log‐rank test or clog‐log test (at fixed point in time).12 The association between BAS and ATG use and all‐cause cumulative mortality was assessed with multiple Cox proportional hazard regression (CPH) analyses. Any variable from the univariable test (simple CPH) with a P value <0.25 was selected as a candidate for the multivariable analysis. In the iterative process of variable selection, covariates were removed from the model if they were nonsignificant and not a confounder, as described by Hosmer‐Lemeshow,13 resulting in a main effect model. We fitted a Cox regression model in which we accounted for the effect of time‐varying covariates by specifying that the time‐dependent covariates be interacted with the logarithmic function of analysis time.14 Interactions between induction therapy and clinical relevant risk variables were estimated by Cox regression analysis including covariates from the main model. The results are displayed in a forest plot. Hazard ratios (HRs) are presented with 95% confidence intervals (CIs).
Missing values were imputed using the chained‐equations multiple imputation technique as described by White et al15 The imputation method was predictive mean matching. The number of iterations for each chain was 10 and the number of imputed data sets was 10.
Results
The distribution of immunosuppression use over time is shown in Figure 1. Data from 2311 pediatric heart transplants (2275 patients) were available for analysis. Six hundred ninety‐nine transplants (685 patients) received BAS and 1612 transplants (1590 patients) ATG and they accrued 7818 patient‐years of observation. Median follow‐up time was 2.7 (range, 0–12) years. Mean recipient age was 6.9±6.3 years and 48% were female.
Figure 1.
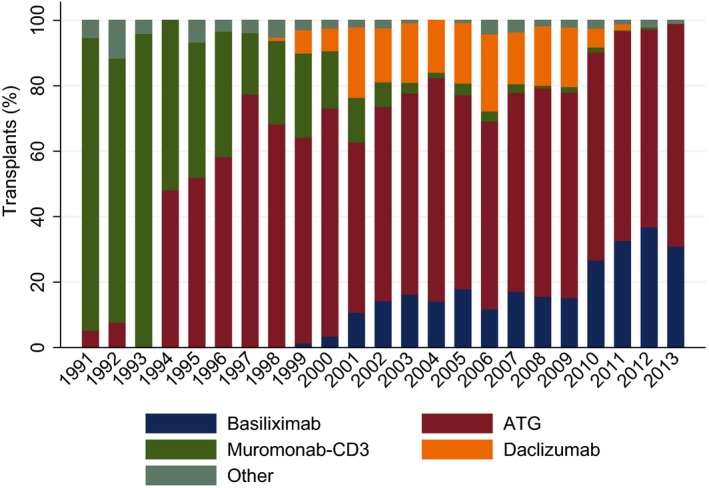
Type of induction therapy for recipients with induction. Distribution is shown by year of transplantation, 1991–2013. ATG equine anti‐thymocyte globulin [Atgam], rabbit anti‐thymocyte globulin [Thymoglobulin/Fresenius‐ATG], or Nashville rabbit antithymocyte globulin/Nashville rabbit antithymocyte serum [NRATG/NRATS]. Other: cyclophosphamide (Cytoxan), methotrexate (Folex PFS, Mexate‐AQ, Rheumatrex), alemtuzumab (Campath), rituximab (Rituxan). ATG indicates anti‐thymocyte globulin.
Demographic and clinical data from the patients who received BAS were compared with those who received ATG (Table 1). Recipients receiving BAS were generally older (8.6 versus 6.1 years; P<0.001). Recipient weight and height (P<0.001 and <0.001, respectively), recipient diagnosis (P<0.001), and the proportion of patients in extracorporeal membrane oxygenation (ECMO; P=0.022) differed significantly between the groups. Patients receiving ATG had higher panel reactive antibody class I and II (9.5% versus 4.7%; P<0.001 and 10.6% versus 4.9%; P<0.001, respectively). Donors were older in the BAS group (P<0.001). Significant differences were also observed in donor weight and height (P<0.001 and <0.001, respectively), the proportion of donors with blood group A (P=0.025) and O (P=0.002), and in the proportion of donors with hypertension (P=0.002).
Table 1.
Baseline Characteristics of the 2 Groups
| Variables | N | BAS (n=699) | ATG (n=1612) | P Value |
|---|---|---|---|---|
| Recipient | ||||
| Age, y | 2311 | 8.6±6.3 | 6.1±6.2 | <0.001 |
| Female sex | 2311 | 363 (51.9) | 756 (46.9) | 0.026 |
| Weight, kg | 2305 | 30.8±22.0 | 24.8±23.0 | <0.001 |
| Height, cm | 2307 | 121.0±39.7 | 105.6±42.9 | <0.001 |
| Diagnosis | ||||
| Coronary artery disease | 16 | 3 (0.4) | 13 (0.8) | <0.001 |
| Cardiomyopathy | 949 | 287 (41.1) | 662 (41.1) | |
| Congenital | 868 | 168 (24.0) | 700 (43.4) | |
| Retransplant because of graft failure | 127 | 26 (3.7) | 101 (6.3) | |
| Heart valve disease | 3 | 0 (0) | 3 (0.2) | |
| Miscellaneous | 348 | 215 (30.8) | 133 (8.3) | |
| Blood group | ||||
| A | 2311 | 254 (36.3) | 623 (38.7) | 0.293 |
| AB | 2311 | 35 (5.0) | 57 (3.5) | 0.097 |
| B | 2311 | 95 (13.6) | 206 (12.8) | 0.594 |
| O | 2311 | 315 (45.1) | 726 (45.0) | 0.990 |
| Antiarrhythmics before transpl | 603 | 23 (21.1) | 81 (16.4) | 0.239 |
| Inotrop support before transpl | 2311 | 261 (37.3) | 779 (48.3) | <0.001 |
| Implantable defibrillator | 1963 | 52 (11.0) | 118 (7.9) | 0.040 |
| Obstructive pulmonary disease | 612 | 2 (1.8) | 0 (0) | 0.034 |
| Diabetes (insulin‐treated) | 2301 | 57 (8.3) | 24 (1.5) | <0.001 |
| Hypertension | 698 | 20 (12.2) | 65 (12.2) | 0.994 |
| CMV status | 1949 | 274 (45.9) | 608 (45.0) | 0.705 |
| Dialysis pretransplant | 2307 | 22 (3.2) | 49 (3.0) | 0.885 |
| Oxygen consumption at exercise | 70 | 14.3±4.6 | 13.7±6.8 | 0.767 |
| Medical condition at transplant | ||||
| Home | 805 | 258 (36.9) | 547 (33.9) | 0.139 |
| Hospital | 400 | 137 (19.6) | 263 (16.3) | |
| Intensive care unit | 1106 | 304 (43.5) | 802 (49.8) | |
| Mechanical ventilation | 2311 | 125 (17.9) | 314 (19.5) | 0.369 |
| ECMO | 2311 | 29 (4.2) | 106 (6.6) | 0.022 |
| Ventricular assist device | 2162 | 81 (12.4) | 204 (13.6) | 0.449 |
| Creatinine most recent, μmol/L | 2258 | 53.7±64.6 | 51.5±47.4 | 0.357 |
| PVR (WU) | 678 | 4.2±3.7 | 4.2±3.9 | 0.962 |
| Previous blood transfusion | 1254 | 235 (83.3) | 831 (85.5) | 0.371 |
| Previous transplant | 2308 | 34 (4.9) | 109 (6.8) | 0.082 |
| PRA class 1 | 1679 | 4.7±14.4 | 9.5±22.9 | <0.001 |
| PRA class 2 | 1602 | 4.9±16.1 | 10.6±25.8 | <0.001 |
| Donor | ||||
| Age, y | 2311 | 11.9±11.2 | 8.2±9.3 | <0.001 |
| Female sex | 2311 | 306 (43.8) | 711 (44.1) | 0.883 |
| Weight, kg | 1579 | 50.1±24.5 | 44.2±24.8 | <0.001 |
| Height, cm | 1566 | 147.9±28.3 | 140.6±31.7 | <0.001 |
| Blood group | ||||
| A | 2311 | 194 (27.8) | 523 (32.4) | 0.025 |
| AB | 2311 | 6 (0.9) | 29 (1.8) | 0.097 |
| B | 2311 | 52 (7.4) | 143 (8.9) | 0.255 |
| O | 2311 | 447 (64.0) | 917 (56.9) | 0.002 |
| Diabetes | 2305 | 9 (1.3) | 12 (0.8) | 0.234 |
| CMV status | 2288 | 376 (54.8) | 855 (53.4) | 0.527 |
| Hypertension | 2303 | 24 (3.4) | 23 (1.4) | 0.002 |
| Ischemic time, minute | 2244 | 225 (181–297) | 226 (184–273) | 0.254 |
Qualitative data are expressed as n (%) and quantitative data as mean±SD or median (interquartile range), as appropriate. ATG indicates anti‐thymocyte globulin; BAS, basiliximab; CMV, cytomegalovirus; ECMO, extracorporeal membrane oxygenation; N, number of transplants with nonmissing values; n, total number of transplants; PRA, panel‐reactive antibodies; Previous transplant, previous kidney, liver, pancreas, pancreas islet cells, heart, lung, intestine, and/or bone marrow transplant; PVR, pulmonary vascular resistance; Transpl, transplant; WU, Wood units.
Table 2 shows the use of maintenance immunosuppression therapy at discharge in the BAS and ATG groups. Compared to the patients in the BAS group, patients treated with ATG were less likely to have received maintenance therapy with tacrolimus (TAC; P<0.001), mycophenolate mofetil (MMF; P<0.001), and were more likely to have received cyclosporine (CYA; P<0.001) or azathioprine (AZA; P<0.001).
Table 2.
Maintenance Immunosuppression Therapy in the BAS and ATG Groups
| Variables | N | BAS (n=699) | ATG (n=1612) | P Value |
|---|---|---|---|---|
| Maintenance therapy | ||||
| CYA | 2236 | 130 (19.1) | 499 (32.1) | <0.001 |
| TAC | 2236 | 568 (83.4) | 1087 (69.9) | <0.001 |
| MMF | 2138 | 615 (93.9) | 1260 (85.0) | <0.001 |
| Steroids | 2233 | 657 (95.1) | 934 (60.6) | <0.001 |
| AZA | 2140 | 44 (6.7) | 332 (22.4) | <0.001 |
| Rapamycin | 2278 | 6 (0.9) | 82 (5.2) | <0.001 |
Qualitative data are expressed as n (%).ATG indicates anti‐thymocyte globulin; AZA, azathioprine; BAS, basiliximab; CYA, cyclosporine; MMF, mycophenolate mofetil; N, number of transplants with nonmissing values; n, total number of transplants; TAC, tacrolimus.
For the entire study group the overall 30‐day mortality was 3.8% (95% CI, 3.1–4.6%) and 1‐year mortality 10.5% (95% CI, 9.3–11.8%). A total of 493 (21%) patients died during the follow‐up. As illustrated in Figure 2, patients treated with BAS had similar estimated survival compared with the ATG group at 30 days and at 1 year after transplantation (97% versus 96%; P=0.545, and 90% versus 89%; P=0.727, respectively). However at 5 and 10 years after transplantation, the use of BAS was associated with poorer long term survival (68% versus 76% at 5 years; P<0.001, and 49% versus 65% at 10 years; P<0.001, respectively). As shown in Table 3, this finding was confirmed in a univariable and multivariable Cox regression model. Patients treated with BAS (versus ATG use) had an increased mortality risk of 27% (HR of 1.27; 95% CI, 1.02–1.57; P<0.030). This main model incorporated 11 significant independent covariates and 2 time‐varying covariates.
Figure 2.
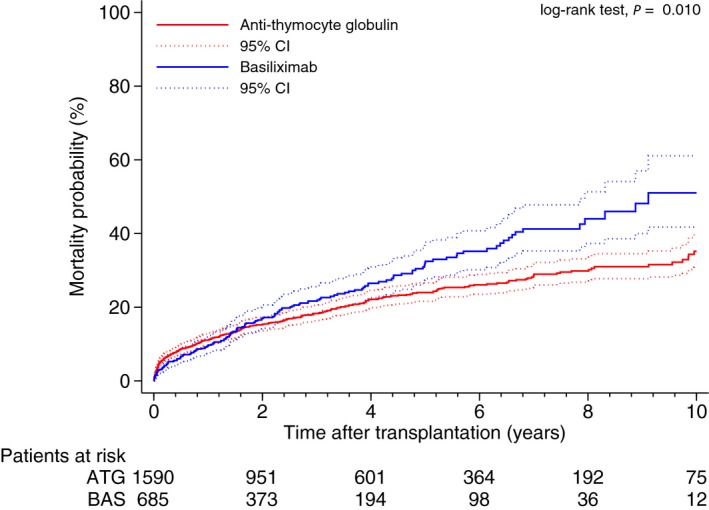
Comparison of all‐cause mortality probability between the basiliximab (BAS) and anti‐thymocyte globulin (ATG) groups (P=0.010, log‐rank test).
Table 3.
Cox Multivariate Logistic Regression Analysis (n=2311)
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| BAS vs ATG unadjusted | 1.27 | 1.06 to 1.55 | 0.011 |
| BAS vs ATG adjusted for age and sex | 1.23 | 1.01 to 1.49 | 0.035 |
| BAS vs ATG adjusted for 11 covariates and timea | 1.27 | 1.02 to 1.57 | 0.030 |
ATG indicates anti‐thymocyte globulin; BAS, basiliximab; HR, hazard ratio.
Adjusted for previous cardiac surgery, pulmonary systolic artery pressure, infection within 2 weeks, recipient age, underlying diagnosis, panel reactive antibodies, recipient diabetes, recipient on dialysis, recipient on ventilator, maintenance drug tacrolimus, and maintenance drug azathioprine. Time‐varying variables: recipient age and induction therapy.
We examined the cumulative incidence of death, based on specific causes of death in the BAS and ATG groups, censoring other causes of death. As illustrated in Figure 3A through 3D, BAS was associated with higher risk of death due to graft failure (P=0.013), but not death due to cardiovascular event (P=0.444), infection (P=0.095) or malignancy (P=0.392).
Figure 3.
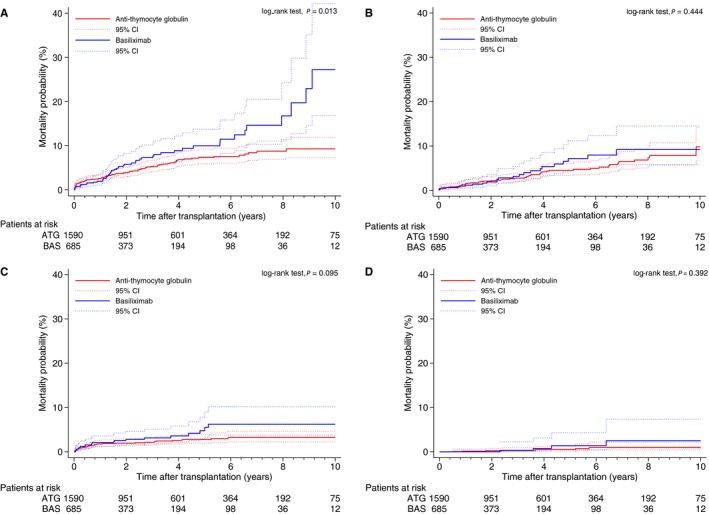
Mortality probability curves by treatment group: (A) graft failure–related death (P=0.013, log‐rank test); (B) cardiovascular‐related death (P=0.444, log‐rank test); (C) infection‐related death (P=0.095, log‐rank test); and (D) malignancy‐related death (P=0.392, log‐rank test).
Subgroup analyses with interaction testing were performed to determine whether the increase in the HR for death (adjusting for the same covariates as in the main Cox regression model) after induction treatment with BAS was consistent across 18 clinical important subgroups. No significant interactions were observed except for one subgroup. As shown in Figure 4B, patients treated with BAS who did not receive corticosteroids had more than double the risk for death compared with those who received corticosteroids. Furthermore there was no interaction with any of the 11 UNOS geographic regions.
Figure 4.
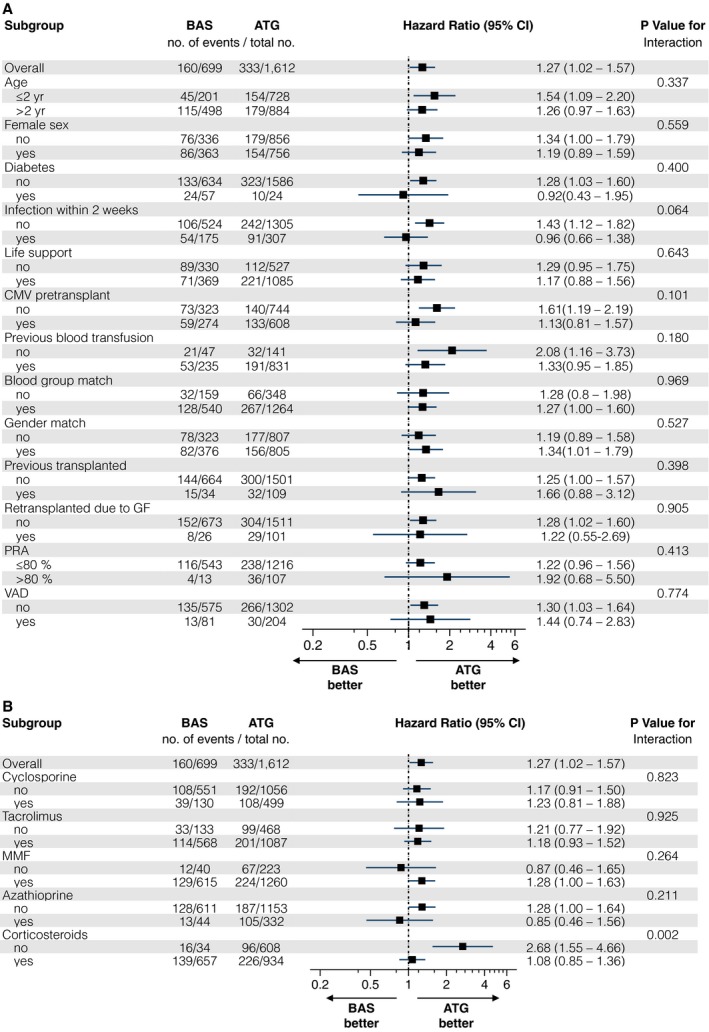
A and B, Subgroup analyses of the primary endpoint of death from any cause. Squares represent the adjusted hazard ratio (HR) for the treatment effect, basiliximab (BAS) vs anti‐thymocyte globulin (ATG) for different subgroups. Lines represent the 95% confidence intervals. The P value for interaction represents the likelihood of an interaction between the subgroup variable and the treatment effect. The overall effect included no interaction terms. The adjusted HR was calculated using the same covariate as presented in Table 3. CMV indicates cytomegalovirus; GF, graft failure; MMF, mycophenolate mofetil; PRA, panel‐reactive antibodies; Previously transplanted, previously kidney, liver, pancreas, pancreas islet cells, heart, lung, intestine, or/and bone marrow transplantation; VAD, ventricular assist device.
Discussion
This study has demonstrated that BAS is associated with higher long‐term mortality compared with ATG after pediatric heart transplantation. The discrepancy in mortality appeared towards the end of the follow‐up.
Approximately 30% of the patients in recent years received BAS in our study population. This rate is similar to the 25% rate, of those receiving any induction, in patients receiving interleukin‐2 receptor antagonists reported by the Registry of the International Society for Heart and Lung Transplantation.1 Our data also showed that the use of BAS has risen. In the unadjusted analysis, there was a marked separation between the survival curves and use of BAS, which was confirmed after multivariable adjustment. There was no interaction with any of the relevant clinical variables, suggesting that in no subgroup in particular would BAS use be preferred over ATG.
The exact mechanism of BAS is not known. BAS is a chimeric (mouse/human) monoclonal antibody that targets specifically the IL‐2 receptor, which is expressed on activated T‐cells in response to an antigenic stimulus.16 This specific binding of BAS to the IL‐2 receptor competitively inhibits the subsequent binding of interleukin‐2, which signals T‐cell proliferation. ATG binds to numerous receptors crucial during the T‐cell activating cascade, leading to the elimination of T‐cells from the circulation through complement dependent lysis.17 It also inhibits B‐cell differentiation and function.18 The higher selectivity of BAS versus ATG, with regard to the immune system, is in line with one prospective randomized study in an adult heart population that showed lower incidence of adverse events by 6 months post‐transplant.8 BAS has also been shown to be tolerated with a similar safety profile to placebo in adult heart transplant recipients.9 Findings from a previous smaller study of 29 patients suggested that BAS was well tolerated and associated with low incidence of rejection in a group of critically ill children undergoing heart transplantation.19 Another study in pediatric heart transplantation reported that thymoglobulin versus no induction was associated with lower incidence of lymphoma, whereas IL2‐R antagonists versus no induction was not associated with an increased lymphoma risk.20 Several studies have suggested that ATG use in pediatric heart transplantation is effective in terms of rejection rate and safe in terms of infections and malignancy.21, 22, 23 None of these studies, however, compared BAS with ATG.
In our study, the distinct immunosuppressive mechanisms of the 2 drugs did not translate into differences in mortality related to potential drug‐induced adverse effects (ie, cardiovascular disease, infection, or malignancy). We have previously shown a trend for a lower rate of malignancy in BAS‐treated patients late after adult heart transplantation.10 It is possible that the relative rareness of cancers in childhood stopped us from observing even large increases in risk.
Acute early rejection is known to be a risk factor for mortality in pediatric heart transplantation.1 Experience in adult heart transplantation has demonstrated an advantage of ATG, compared with BAS, in preventing early post‐transplant rejection episodes. Carrier et al7 reported that compared with patients receiving ATG, those receiving BAS showed a higher incidence of rejections at 6 months. Similarly Flaman et al6found that rabbit ATG, compared with BAS, provided better protection against acute cellular rejection within the first 3 months post‐transplantation. Although we can only speculate on the immunological pathways, our study shows that BAS and ATG also seem to differ in their impact on chronic rejection. Interestingly, differences in mortality were found only for mortality related to graft failure, and not for the other causes of death. Also worth mentioning is the fact that Daclizumab, another IL2‐R antagonist, was found to be associated with an increase in mortality in a randomized, double‐blind, placebo‐controlled trial,24 and its production was discontinued for the US market in 2009 after a diminished market demand.25
Our study was limited by its retrospective nature and the inherent limitations of using a public registry database, in which the completeness and accuracy of the information cannot readily be verified. Patients in the 2 groups were not randomized to receive the respective induction therapy; therefore, differences in baseline clinical characteristics and immunosuppression treatment may have influenced our results. We aimed to correct for these differences by performing a multivariable analysis that included a wide range of variables. Head‐to‐head drug comparisons are best performed in randomized, control trials. Although randomized, controlled trials eliminate bias and confounding, they may have limited generalizability and may be complemented by rigorous registry studies with greater power.
In conclusion, our results demonstrate that the use of BAS in pediatric transplantation is associated with higher long‐term mortality, as compared with ATG. BAS use, compared with ATG use, increased the risk of chronic rejection, but no significant differences were noted for mortality potentially related to drug side effects, including cardiovascular disease, infection, and malignancy.
Sources of Funding
This work was supported by the Swedish Heart‐Lung Foundation, Swedish Society of Medicine, Government Grant for Clinical Research, Region Skåne Research Funds, and the Crafoord Foundation.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002790 doi: 10.1161/JAHA.115.002790)
References
- 1. Dipchand AI, Kirk R, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Lund LH, Rahmel AO, Yusen RD, Stehlik J; International Society for Heart and Lung Transplantation . The registry of the international society for heart and lung transplantation: sixteenth official pediatric heart transplantation report—2013; focus theme: age. J Heart Lung Transplant. 2013;32:979–988. [DOI] [PubMed] [Google Scholar]
- 2. Singh TP, Edwards LB, Kirk R, Boucek MM. Era effect on post‐transplant survival adjusted for baseline risk factors in pediatric heart transplant recipients. J Heart Lung Transplant. 2009;28:1285–1291. [DOI] [PubMed] [Google Scholar]
- 3. Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales‐Stawinski G, Martinelli L, McGiffin D, Smith J, Taylor D, Meiser B, Webber S, Baran D, Carboni M, Dengler T, Feldman D, Frigerio M, Kfoury A, Kim D, Kobashigawa J, Shullo M, Stehlik J, Teuteberg J, Uber P, Zuckermann A, Hunt S, Burch M, Bhat G, Canter C, Chinnock R, Crespo‐Leiro M, Delgado R, Dobbels F, Grady K, Kao W, Lamour J, Parry G, Patel J, Pini D, Towbin J, Wolfel G, Delgado D, Eisen H, Goldberg L, Hosenpud J, Johnson M, Keogh A, Lewis C, O'Connell J, Rogers J, Ross H, Russell S, Vanhaecke J; International Society for Heart and Lung Transplantation . The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. [DOI] [PubMed] [Google Scholar]
- 4. Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet. 1999;353:1083–1091. [DOI] [PubMed] [Google Scholar]
- 5. Singh TP, Faber C, Blume ED, Worley S, Almond CS, Smoot LB, Dillis S, Nasman C, Boyle GJ. Safety and early outcomes using a corticosteroid‐avoidance immunosuppression protocol in pediatric heart transplant recipients. J Heart Lung Transplant. 2010;29:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flaman F, Zieroth S, Rao V, Ross H, Delgado DH. Basiliximab versus rabbit anti‐thymocyte globulin for induction therapy in patients after heart transplantation. J Heart Lung Transplant. 2006;25:1358–1362. [DOI] [PubMed] [Google Scholar]
- 7. Carrier M, Leblanc M‐H, Perrault LP, White M, Doyle D, Beaudoin D, Guertin M‐C. Basiliximab and rabbit anti‐thymocyte globulin for prophylaxis of acute rejection after heart transplantation: a non‐inferiority trial. J Heart Lung Transplant. 2007;26:258–263. [DOI] [PubMed] [Google Scholar]
- 8. Mattei MF, Redonnet M, Gandjbakhch I, Bandini AM, Billes A, Epailly E, Guillemain R, Lelong B, Pol A, Treilhaud M, Vermes E, Dorent R, Lemay D, Blanc AS, Boissonnat P. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti‐thymocyte globulin as induction therapy. J Heart Lung Transplant. 2007;26:693–699. [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR, Zucker MJ, Wagoner L, Michler R, Boehmer J, Kovarik J, Vasquez A. A multicenter, prospective, randomized, double‐blind trial of basiliximab in heart transplantation. J Heart Lung Transplant. 2005;24:1297–1304. [DOI] [PubMed] [Google Scholar]
- 10. Ansari D, Lund LH, Stehlik J, Andersson B, Hoglund P, Edwards L, Nilsson J. Induction with anti‐thymocyte globulin in heart transplantation is associated with better long‐term survival compared with basiliximab. J Heart Lung Transplant. 2015;34:1283–1291. [DOI] [PubMed] [Google Scholar]
- 11. Us Food and Drug Administration . Basiliximab product approval information—licensing action. Available at: http://www.Fda.Gov/downloads/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/ucm113356.Pdf. Accessed June 22, 2015.
- 12. Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–4519. [DOI] [PubMed] [Google Scholar]
- 13. Hosmer D Jr, Lemeshow S, Sturdivant R. Model‐Building Strategies and Methods for Logistic Regression, in Applied Logistic Regression. 3rd ed Hoboken, NJ: John Wiley & Sons, Inc; 2013. [Google Scholar]
- 14. Aydemir U, Aydemir S, Dirschedl P. Analysis of time‐dependent covariates in failure time data. Stat Med. 1999;18:2123–2134. [DOI] [PubMed] [Google Scholar]
- 15. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 16. Novartis Pharmaceuticals Corporation . Simulect (basiliximab), prescribing information. Available at: http://www.Pharma.Us.Novartis.Com/info/products/brands/simulect.Jsp. Accessed June 22, 2015.
- 17. Popow I, Leitner J, Grabmeier‐Pfistershammer K, Majdic O, Zlabinger GJ, Kundi M, Steinberger P. A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant. 2013;13:3103–3113. [DOI] [PubMed] [Google Scholar]
- 18. Klaus P, Heine G, Rasche C, Worm M. Low‐dose anti‐thymocyte globulin inhibits human B‐cell differentiation into antibody‐secreting cells. Acta Derm Venereol. 2015;95:676–680. [DOI] [PubMed] [Google Scholar]
- 19. Ford KA, Cale CM, Rees PG, Elliott MJ, Burch M. Initial data on basiliximab in critically ill children undergoing heart transplantation. J Heart Lung Transplant. 2005;24:1284–1288. [DOI] [PubMed] [Google Scholar]
- 20. Gajarski RJ, Blume ED, Urschel S, Schechtman K, Zheng J, West LJ, Altamirano L, Miyamoto S, Naftel DC, Kirklin JK, Zamberlan MC, Canter CE; Pediatric Heart Transplant Study I . Infection and malignancy after pediatric heart transplantation: the role of induction therapy. J Heart Lung Transplant. 2011;30:299–308. [DOI] [PubMed] [Google Scholar]
- 21. Boucek RJ Jr, Naftel D, Boucek MM, Chinnock R, Morrow RW, Pahl E, DiSano S. Induction immunotherapy in pediatric heart transplant recipients: a multicenter study. J Heart Lung Transplant. 1999;18:460–469. [DOI] [PubMed] [Google Scholar]
- 22. Parisi F, Danesi H, Squitieri C, Di Chiara L, Rava L, Di Donato RM. Thymoglobuline use in pediatric heart transplantation. J Heart Lung Transplant. 2003;22:591–593. [DOI] [PubMed] [Google Scholar]
- 23. Di Filippo S, Boissonnat P, Sassolas F, Robin J, Ninet J, Champsaur G, Bozio A. Rabbit antithymocyte globulin as induction immunotherapy in pediatric heart transplantation. Transplantation. 2003;75:354–358. [DOI] [PubMed] [Google Scholar]
- 24. Hershberger RE, Starling RC, Eisen HJ, Bergh CH, Kormos RL, Love RB, Van Bakel A, Gordon RD, Popat R, Cockey L, Mamelok RD. Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med. 2005;352:2705–2713. [DOI] [PubMed] [Google Scholar]
- 25. Us Food and Drug Administration . Doctor letter. Available at: http://www.Fda.Gov/downloads/drugs/drugsafety/drugshortages/ucm194907.Pdf. Accessed October 10, 2015.


