Abstract
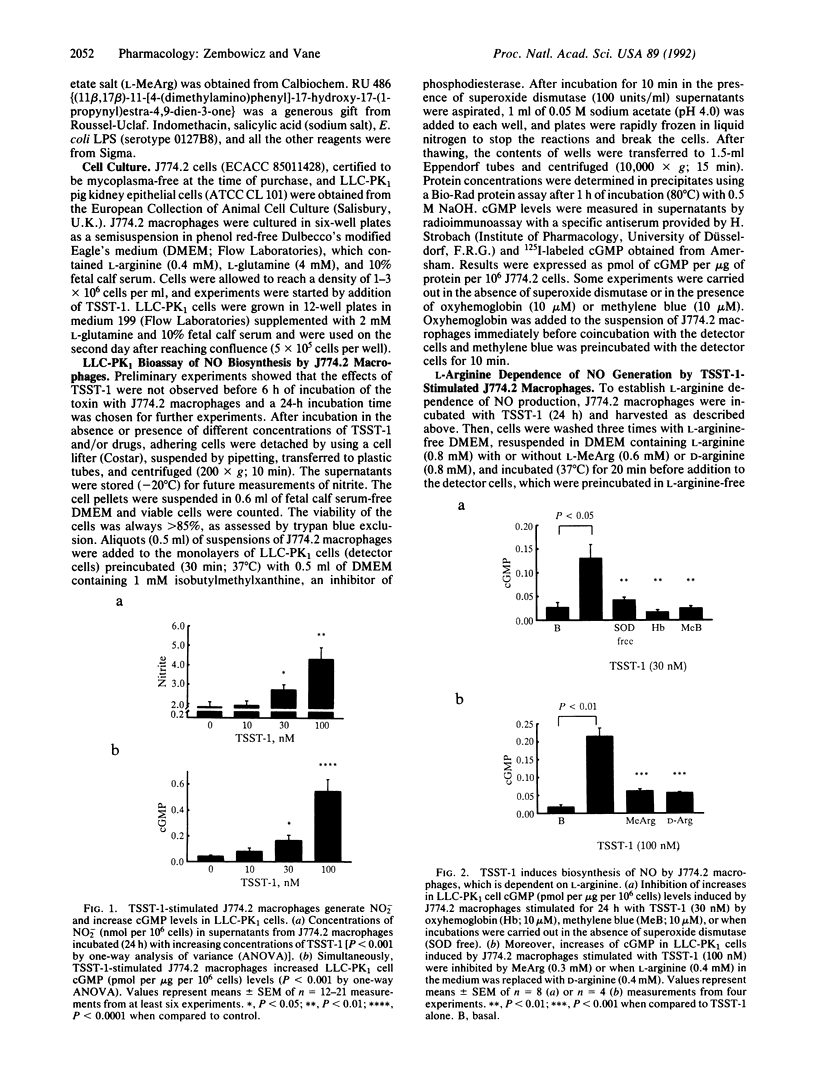
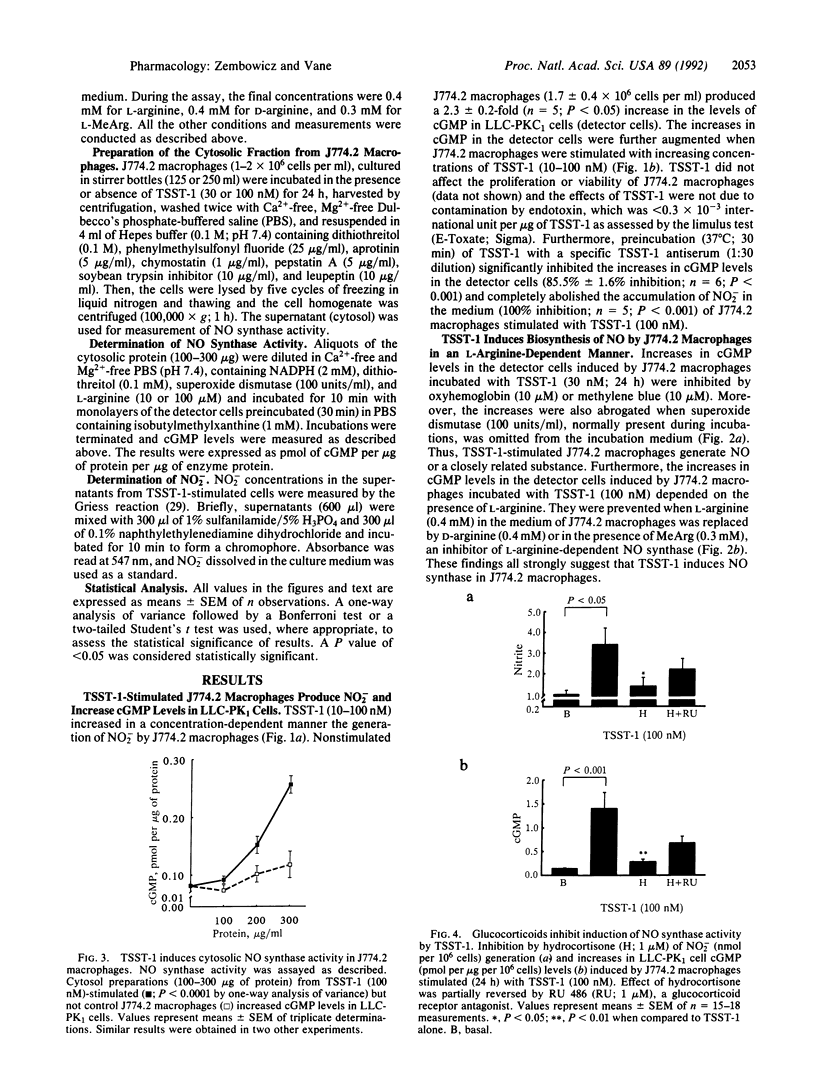
Toxic shock syndrome toxin 1 (TSST-1) is a Mr 22,000 protein produced by Staphylococcus aureus. It is thought to be the cause of toxic shock syndrome. We investigated the hypothesis that TSST-1 induces nitric oxide (NO) synthase and that the NO formed may be involved in the pathogenesis of toxic shock syndrome. We used the murine monocyte-macrophage cell line J744.2 that responds to TSST-1 and also expresses NO synthase activity upon immunological stimulation. J774.2 macrophages stimulated with TSST-1 (10-100 nM) generated nitrite, a breakdown product of NO, and induced concentration-dependent elevations of cGMP in the pig kidney epithelial cell line (LLC-PK1). This latter effect was due to the generation of L-arginine-derived NO for it was (i) abolished by oxyhemoglobin (10 microM), a scavenger of NO, or by methylene blue (10 microM), an inhibitor of NO-activated guanylate cyclase; (ii) potentiated by superoxide dismutase (100 units/ml), which prolongs the life of NO; (iii) inhibited by NG-monomethyl-L-arginine (0.3 mM), an inhibitor of NO synthase; (iv) significantly decreased when L-arginine (0.4 mM) in the medium was replaced by D-arginine (0.4 mM). Moreover, TSST-1 (100 nM) enhanced the activity of cytosolic NO synthase in J774.2 cells. Hydrocortisone (1 microM) but not indomethacin (5 micrograms/ml) or salicylic acid (5 micrograms/ml) prevented the generation of NO2- and the increases in cGMP levels in LLC-PK1 cells induced by J774.2 cells stimulated with TSST-1. The effects of hydrocortisone were partially reversed by coincubation with RU 486 (1 microM), an antagonist of glucocorticoid receptors. Thus, TSST-1 and perhaps other exotoxins produced by Gram-positive bacteria induce NO synthase and the increased NO formation may contribute to toxic shock syndrome and possibly to changes in the immune responses that accompany infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Bergdoll M. S., Crass B. A., Reiser R. F., Robbins R. N., Davis J. P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981 May 9;1(8228):1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Stadler J., Simmons R. L., Murray S. A. Inducible cytosolic enzyme activity for the production of nitrogen oxides from L-arginine in hepatocytes. Biochem Biophys Res Commun. 1990 May 16;168(3):1034–1040. doi: 10.1016/0006-291x(90)91133-d. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Fast D. J., Nelson R. D., Schlievert P. M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Ferrari F. K., Kispert P. H., Stadler J., Stuehr D. J., Simmons R. L., Billiar T. R. Nitric oxide and nitric oxide-generating compounds inhibit hepatocyte protein synthesis. FASEB J. 1991 Apr;5(7):2085–2092. doi: 10.1096/fasebj.5.7.1707021. [DOI] [PubMed] [Google Scholar]
- Dembińska-Kieć A., Zmuda A., Marcinkiewicz J., Sinzinger H., Gryglewski R. J. Influence of no-donor (SIN-1) on functions of inflammatory cells. Agents Actions. 1991 Jan;32(1-2):37–40. doi: 10.1007/BF01983305. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Hoekstra J. W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981 Jul;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Fisher C. J., Jr, Horowitz Z., Albertson T. E. Cardiorespiratory failure in toxic shock syndrome: effect of dobutamine. Crit Care Med. 1985 Mar;13(3):160–165. doi: 10.1097/00003246-198503000-00004. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Korbut R., Kalecinska A., Zembowicz A. Interaction between stimulators of adenylate and guanylate cyclases in human leukocytes, platelets and arteries. Int J Tissue React. 1989;11(6):269–275. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hirose A., Ikejima T., Gill D. M. Established macrophagelike cell lines synthesize interleukin-1 in response to toxic shock syndrome toxin. Infect Immun. 1985 Dec;50(3):765–770. doi: 10.1128/iai.50.3.765-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Billiar T. R., Curran R. D., Simmons R. L. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J Immunol. 1990 Oct 1;145(7):2220–2226. [PubMed] [Google Scholar]
- Igarashi H., Fujikawa H., Usami H. Effects of drugs on the pyrogenicity of toxic shock syndrome toxin 1 and its capacity to enhance susceptibility to the lethal effects of endotoxic shock in rabbits. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S210–S213. doi: 10.1093/clinids/11.supplement_1.s210. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Heme-dependent activation of soluble guanylate cyclase by nitric oxide: regulation of enzyme activity by porphyrins and metalloporphyrins. Semin Hematol. 1989 Jan;26(1):63–76. [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J Infect Dis. 1988 Nov;158(5):1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- Jupin C., Anderson S., Damais C., Alouf J. E., Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988 Mar 1;167(3):752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Gilker C., Griffith O. W., Matthews D. E., Stuehr D. J. L-citrulline production from L-arginine by macrophage nitric oxide synthase. The ureido oxygen derives from dioxygen. J Biol Chem. 1990 Aug 15;265(23):13442–13445. [PubMed] [Google Scholar]
- Lepoivre M., Chenais B., Yapo A., Lemaire G., Thelander L., Tenu J. P. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990 Aug 25;265(24):14143–14149. [PubMed] [Google Scholar]
- Lin Y. S., Patel M. R., Linna T. J., Rogers T. J. Suppression of cytolytic T-cell activity by staphylococcal enterotoxin B-induced suppressor cells: role of interleukin 2. Cell Immunol. 1986 Nov;103(1):147–159. doi: 10.1016/0008-8749(86)90076-6. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micusan V. V., Desrosiers M., Gosselin J., Mercier G., Oth D., Bhatti A. R., Heremans H., Billiau A. Stimulation of T cells and induction of interferon by toxic shock syndrome toxin 1. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S305–S312. doi: 10.1093/clinids/11.supplement_1.s305. [DOI] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M. Inhibition of the induction of nitric oxide synthase by glucocorticoids: yet another explanation for their anti-inflammatory effects? Trends Pharmacol Sci. 1991 Apr;12(4):130–131. doi: 10.1016/0165-6147(91)90528-z. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Hauser A. R., Kim M. H., Schlievert P. M., Nelson K., Selander R. K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ohara O., Teraoka H., Arita H. Glucocorticoids suppress group II phospholipase A2 production by blocking mRNA synthesis and post-transcriptional expression. J Biol Chem. 1990 Jul 25;265(21):12745–12748. [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Olken N. M., Rusche K. M., Richards M. K., Marletta M. A. Inactivation of macrophage nitric oxide synthase activity by NG-methyl-L-arginine. Biochem Biophys Res Commun. 1991 Jun 14;177(2):828–833. doi: 10.1016/0006-291x(91)91864-9. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Parker M. M., Parrillo J. E. Septic shock. Hemodynamics and pathogenesis. JAMA. 1983 Dec 23;250(24):3324–3327. [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A. Production of tumor necrosis factor by human monocytes in response to toxic-shock-syndrome toxin-1. J Infect Dis. 1988 Nov;158(5):1026–1033. doi: 10.1093/infdis/158.5.1026. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Richter A. G., Pier G. B. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun. 1987 May;55(5):1070–1076. doi: 10.1128/iai.55.5.1070-1076.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Suppression of immunoglobulin-secreting cells from human peripheral blood by toxic-shock-syndrome toxin-1. J Infect Dis. 1986 Apr;153(4):772–779. doi: 10.1093/infdis/153.4.772. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Arko R. J., Chandler F. W., Bridges N. B. Affinity purification of staphylococcal toxic shock syndrome toxin 1 and its pathologic effects in rabbits. Infect Immun. 1986 Feb;51(2):431–439. doi: 10.1128/iai.51.2.431-439.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982 Apr;36(1):123–128. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Todd J. K., Ressman M., Caston S. A., Todd B. H., Wiesenthal A. M. Corticosteroid therapy for patients with toxic shock syndrome. JAMA. 1984 Dec 28;252(24):3399–3402. [PubMed] [Google Scholar]
- Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978 Nov 25;2(8100):1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Sanduja R., Tsai A. L., Ferhanoglu B., Loose-Mitchell D. S. Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2384–2387. doi: 10.1073/pnas.88.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azavedo J. C. Animal models for toxic shock syndrome: overview. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S205–S209. doi: 10.1093/clinids/11.supplement_1.s205. [DOI] [PubMed] [Google Scholar]



