Abstract
Background
Cardiovascular disease (CVD) is the leading cause of death in rheumatoid arthritis (RA) patients. This study is the first to report the association of hydroxychloroquine (an antirheumatic medication that has been associated with decreased risk of diabetes, a less atherogenic lipid profile, and antithrombotic properties) with CVD in RA.
Methods and Results
A retrospective incident RA cohort from January 1, 2001, to October 31, 2013, excluding patients with CVD prior to RA diagnosis, was constructed. Patients were categorized as hydroxychloroquine users versus nonusers and were allowed to contribute time to either group according to hydroxychloroquine exposure. The primary outcome was adjudicated incident CVD defined as a composite of coronary artery disease, stroke, transient ischemic attack, sudden cardiac death, and peripheral artery disease with arterial revascularization procedure. The secondary outcome was a composite of incident coronary artery disease, stroke, and transient ischemic attack. Cox time‐varying regression models were used to estimate the association between hydroxychloroquine exposure and development of CVD, after adjusting for propensity score and relevant confounders, including demographics, CVD‐related comorbidities, RA severity, and activity indicators and medications. We included 1266 RA patients, 547 hydroxychloroquine users, and 719 nonusers. During the observation period, 102 CVD events occurred, 3 in hydroxychloroquine users and 99 in nonusers. The fully adjusted Cox model showed a hazard ratio of 0.28 (95% CI 0.12–0.63, P=0.002) for incident CVD and 0.30 (95% CI 0.13–0.68, P=0.004) for incident composite coronary artery disease, stroke, and transient ischemic attack for hydroxychloroquine users versus nonusers, respectively.
Conclusion
In this hypothesis‐generating study, hydroxychloroquine use was associated with a 72% decrease in the risk of incident CVD in RA patients. If these preliminary results are confirmed in larger studies, our findings may be used as a rationale for a randomized study of hydroxychloroquine use for primary prevention of CVD in RA or nonrheumatic high‐risk patients.
Keywords: cardiovascular diseases, hydroxychloroquine, primary prevention, rheumatoid arthritis
Subject Categories: Primary Prevention, Cardiovascular Disease
Introduction
Mortality is increased 2‐fold in patients with rheumatoid arthritis (RA).1 Cardiovascular disease (CVD) due to atherosclerosis is the leading cause of death in these patients.2, 3 Although traditional risk factors for atherosclerosis account for some of this risk, having a diagnosis of RA also puts one at risk, likely due, at least in part, to the adverse effects of chronic inflammation on the vasculature.4, 5 Antirheumatic medications appear to modify the risk of CVD in RA. Methotrexate6 and tumor necrosis factor α inhibitors7, 8 have been associated with decreased clinical CVD in RA, whereas corticosteroid use is associated with increased clinical CVD.9
Hydroxychloroquine has been used in the treatment of RA for decades and is associated with decreased risk of diabetes,10, 11, 12 a less atherogenic lipid profile,13 and antithrombotic properties due to effects on platelet aggregation14 in addition to its disease‐modifying effect in RA. Despite these promising traits, the association of hydroxychloroquine with the risk of CVD in RA has not been studied. The purpose of the present study was to examine the association of hydroxychloroquine use with incident CVD in a retrospective inception cohort of RA patients.
Methods
Geisinger Health System (GHS) is a multispecialty system in central Pennsylvania with fully implemented electronic health records since 2001. For the present study, a retrospective cohort of incident RA patients from January 1, 2001, to October 31, 2013, was assembled (n=1459). RA diagnosis was based on International Classification of Diseases, 9th Revision (ICD‐9) code 714.0 twice by a GHS rheumatologist, and this definition was validated, as reported previously.13 Included patients had a primary care physician within the GHS network to enhance availability of data on laboratory values and comorbidities and to ensure that the majority of CVD events were captured. Patients with CVD (defined as a composite of coronary artery disease [CAD], stroke, transient ischemic attack [TIA], and peripheral arterial disease with revascularization procedure) prior to RA diagnosis were excluded.
Eligible patients (n=1266) were divided into hydroxychloroquine users and nonusers, according to hydroxychloroquine use throughout the observation period. Patients were allowed to contribute time to either group according to hydroxychloroquine exposure. Any ≤30‐day gap between hydroxychloroquine exposures was considered continuous hydroxychloroquine use; therefore, events occurring within the first 30 days of hydroxychloroquine start were attributed to the nonuser group. Accordingly, in case of hydroxychloroquine discontinuation, events occurring within 30 days of discontinuation were attributed to the hydroxychloroquine user group. Additional sensitivity analysis was performed treating hydroxychloroquine use as continuous for gaps of 90 days in accordance with the delayed effect of this disease‐modifying antirheumatic drug.
The primary outcome was incident CVD defined as a composite of CAD (myocardial infarction, unstable angina, or cardiac revascularization procedure), sudden death due to cardiac etiology, stroke, TIA, and peripheral arterial disease with arterial revascularization procedure. Incident CVD cases were identified electronically based on a broad range of ICD‐9 codes to ensure that all prevalent CVD cases were excluded and possible incident CVD cases were captured. Electronically identified incident CVD cases were subsequently reviewed and validated by an internal medicine hospitalist and 1 of the authors (D.V.) who was blinded to the study hypothesis using prespecified criteria. The sudden death cases were reviewed and validated by another author (T.S.S.). The secondary outcome was a composite of incident CAD, stroke, and TIA to capture only ischemic events in accordance with the Framingham Risk Score.15 Covariates included demographics such as age, sex, and ethnicity. Comorbidities included diabetes mellitus, hypertension, hyperlipidemia, smoking, body mass index (in kg/m2), and low‐density lipoprotein. Measures of RA severity or activity included rheumatoid factor, anti–cyclic citrullinated peptide antibodies, and erythrocyte sedimentation rate. Medications included corticosteroids, nonsteroidal anti‐inflammatory drugs, methotrexate, tumor necrosis factor α inhibitors, and statins (Data S1). In the GHS electronic health records, medications were reconciled by a nurse and a physician at each visit.
Statistical Analysis
Patient follow‐up started at the time of RA diagnosis and continued until the first CVD event, death, or end of the observation period. At GHS, inpatient sudden deaths are coded by cause of death, so a sudden death caused by CVD would have been coded using one of the CVD‐related ICD‐9 codes.
The primary analysis was the comparison of incident CVD risk between hydroxychloroquine users and nonusers. The secondary analysis was the comparison between hydroxychloroquine users and nonusers and incident composite CAD, stroke, and TIA. Adjustment for covariates was done at baseline with the exception of medications (time varying). Rates of CVD were determined for each medication group and expressed as the number of events per 1000 person‐years. Results were expressed using a Poisson regression model to estimate the event rates across groups and expressed as incident rate ratios. A propensity score for the probability of each patient receiving hydroxychloroquine was calculated using a logistic regression model that adjusted for age, sex, body mass index, anti–cyclic citrullinated peptide antibodies, rheumatoid factor, corticosteroids, methotrexate, and tumor necrosis factor α inhibitors. A box plot was used to investigate differences in the propensity score between hydroxychloroquine users and nonusers. If balancing was achieved, the distributions of propensity scores for user and nonuser groups within each quintile were considered to be similar. We found that within‐strata box plots showed balanced distribution between groups.
Time‐dependent Cox regression models were used to calculate the hazard ratio (HR; and 95% CI) of CVD for the hydroxychloroquine users compared with nonusers, adjusting for age, sex, body mass index, low‐density lipoprotein, smoking, diabetes, hypertension, anti–cyclic citrullinated peptide antibodies, rheumatoid factor, erythrocyte sedimentation rate, methotrexate, and tumor necrosis factor α inhibitor use. Kaplan–Meier analysis was used to compare the CVD‐free probability over time in the hydroxychloroquine users versus nonusers.
Finally, a fixed‐effect Cox regression model in which hydroxychloroquine use was taken as a time‐fixed binary variable (ie, ever versus never use) was applied as a sensitivity test.
Analysis was performed using the SAS (version 9.4) statistical package (SAS Institute, Inc). A 2‐sided test was used for reported P values with a significance level of 0.05.
Institutional review board approval was obtained, and informed consent was waived for this observational study.
Results
We identified 1459 eligible patients with RA. After excluding 192 patients with prevalent CVD and 1 patient with medical record inconsistencies, 1266 patients were included in the final analysis, with 547 hydroxychloroquine users and 719 nonusers. Median observation time was 6.0 years (25th–75th percentiles 3.1–9.9 years). The median time for hydroxychloroquine exposure was 2.3 years (25th–75th percentiles 0.95–4.8 years). The median time for hydroxychloroquine onset after the RA diagnosis was 1.76 years (25th–75th percentiles 0.63–4.62 years). Patients were predominantly female (80%), 97% white, and 51% rheumatoid factor positive, with a mean age of 56.3 years (±13.9 years). The average dose of hydroxychloroquine was 400 mg/day.13 Patient characteristics according to hydroxychloroquine exposure are shown in Table 1.
Table 1.
Characteristics According to Hydroxychloroquine Ever Use and Never Usea
| Variables | Hydroxychloroquine Ever Use (n=547) | Hydroxychloroquine Never Use (n=719) |
|---|---|---|
| Demographics | ||
| Age at RA diagnosis, y | 55±13 | 57±14 |
| Female (%) | 443 (80) | 510 (71) |
| White (%) | 526 (96) | 701 (97) |
| CVD risk factors | ||
| Smoking (%) | 160 (29) | 225 (31) |
| BMI, kg/m2 |
30.8±9.4 n=533 |
29.8 (8.0) n=707 |
| Hypertension (%) | 224 (41) | 255 (31) |
| Hyperlipidemia (%) | 161 (29) | 189 (26) |
| Diabetes (%) | 88 (16) | 94 (13) |
| LDL, mg/dL |
108±34 n=502 |
112±33 n=626 |
| RA‐related measures | ||
| ESR, mm/h |
24 (12–40) n=523 |
26 (14–43) n=676 |
| RF positive (%) |
302 (55) n=389 |
338 (47) n=574 |
| ACPA positive (%) |
177 (32) n=342 |
198 (28) n=569 |
| Medicationsa | ||
| NSAIDs (%) | 437 (80) | 538 (75) |
| Corticosteroids (%) | 468 (86) | 596 (83) |
| Methotrexate (%) | 339 (62) | 532 (74) |
| TNF‐α inhibitors (%) | 224 (41) | 268 (37) |
| Statins (%) | 214 (39) | 280 (39) |
ACPA indicates anti–cyclic citrullinated peptide antibody; BMI, body mass index; CVD, cardiovascular disease; ESR, erythrocyte sedimentation rate; LDL, low‐density lipoprotein; NSAIDs, nonsteroidal anti‐inflammatory drugs; RA, rheumatoid arthritis; RF, rheumatoid factor; TNF‐α, tumor necrosis factor α.
Classification of hydroxychloroquine and all medication use as ever and never is for descriptive purposes only. All medication use was treated as time varying in the analysis. Demographics, CVD risk factors, and RA‐related variables were measured as the closest values at RA diagnosis. Values are the number (percentage), mean±SD, or median (25th–75th percentiles), unless indicated otherwise.
During the observation period, there were 102 CVD events (66 CAD, 30 stroke or TIA, 6 peripheral artery disease with associated revascularization procedure), with incidence rates of 12.8 (95% CI 10.5–15.5) per 1000 person‐years for CVD and 12.8 (95% CI 10.5–15.6) per 1000 person‐years for composite CAD, stroke, and TIA. Of these events, 3 occurred in the hydroxychloroquine users (observation time of 2881 person‐years) and 99 in the nonusers (observation time of 3975.4 person‐years). CVD incidence rates for users and nonusers was 4.7 (95% CI 1.5–14.4) and 13.5 (95% CI 11.1–16.4) per 1000 person‐years, respectively, with an incidence rate ratio of 0.35 (95% CI 0.11–1.09, P=0.07) for users compared with nonusers. Of the 96 composite incident CAD, stroke, and TIA events, 2 occurred in users and 94 in nonusers. The composite CAD, stroke, and TIA incidence rates for users and nonusers were 3.1 (95% CI 0.8–12.4) and 12.8 (95% CI 10.5–15.7) per 1000 person‐years, respectively, with an incidence rate ratio of 0.24 (95% CI 0.06–0.98, P=0.04) for users compared with nonusers. Stratification of hydroxychloroquine onset time after RA diagnosis by CVD events showed no association between time of hydroxychloroquine onset and events.
The fully adjusted, time‐dependent Cox model showed HRs of 0.28 (95% CI 0.12–0.63, P=0.002) for incident CVD and 0.30 (95% CI 0.13–0.68, P=0.004) for composite incident CAD, stroke, and TIA for hydroxychloroquine users versus nonusers, respectively. Details of the fully adjusted multivariable Cox model are shown in Table 2. Results of the univariable analysis are included in Table S1 for reference.
Table 2.
Risk of Incident CVD and Composite CAD, Stroke, and TIA in RA Patients According to Hydroxychloroquine Use
| Variable | Hydroxychloroquine Users vs Nonusers | |||
|---|---|---|---|---|
| CVD HR (95% CI) | P Value | Composite CAD/Stroke/TIA HR (95% CI) | P Value | |
| Unadjusted | ||||
| Hydroxychloroquine | 0.28 (0.12–0.63) | 0.002 | 0.30 (0.13–0.68) | 0.004 |
| Fully adjusted multivariable Cox regression model, hydroxychloroquine as a fixed variable | ||||
| Hydroxychloroquine ever use | 0.60 (0.41–0.94) | 0.02 | 0.67 (0.42–1.07) | 0.09 |
| Fully adjusted multivariable time‐dependent Cox regression model | ||||
| Age, y | 1.05 (1.03–1.07) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| Female | 1.14 (0.71–1.82) | 0.6 | 1.05 (0.65–1.71) | 0.84 |
| Smoking (Yes) | 2.9 (1.75–4.80) | <0.001 | 2.39 (1.42–4.01) | <0.001 |
| Diabetes | 2.41 (1.45–4.01) | <0.001 | 2.10 (1.25–3.53) | 0.005 |
| Hydroxychloroquine | 0.31 (0.13–0.71) | 0.006 | 0.32 (0.14–0.75) | 0.008 |
Adjustment for confounders is at baseline except for all medication use that is time‐varying. CAD indicates coronary artery disease; CVD, cardiovascular disease; HR, hazard ratio; RA, rheumatoid arthritis; TIA, transient ischemic attack.
Sensitivity analyses for CVD analyzing 90‐day gaps in treatment with hydroxychloroquine as continuous showed an HR of 0.30 (95% CI 0.13–0.71, P=0.006) for hydroxychloroquine users versus nonusers, respectively. For the composite incident CAD, stroke, and TIA, analysis of 90‐day gaps in treatment with hydroxychloroquine as continuous showed an HR of 0.32 (95% CI 0.13–0.75, P=0.008) for hydroxychloroquine users versus nonusers, respectively. Finally, analysis of hydroxychloroquine use as a time‐fixed binary variable showed HRs of 0.6 (95% CI 0.41–0.94, P=0.02) and 0.67 (95% CI 0.42–1.07, P=0.09) for incident CVD and for composite incident CAD, stroke, and TIA, respectively.
Kaplan–Meier plots comparing the probability of developing CVD over time in the hydroxychloroquine users versus nonusers are shown in Figure.
Figure 1.
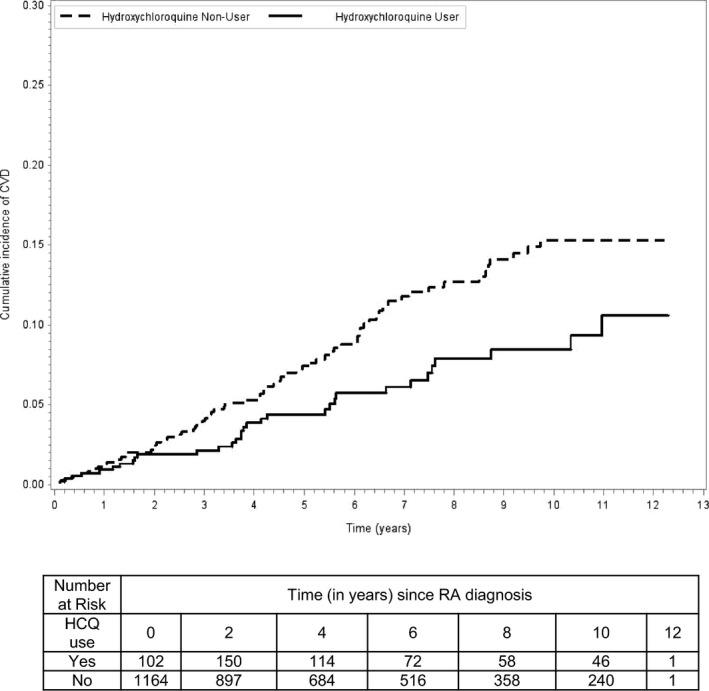
Probability of incident CVD according to HCQ use. Kaplan–Meier survival curves: A solid curve represents the HCQ users, and a broken curve represents nonusers. CVD indicates cardiovascular disease; HCQ, hydroxychloroquine; RA, rheumatoid arthritis.
Discussion
In this inception cohort of RA patients, treatment with hydroxychloroquine was independently associated with a 72% reduction in all incident CVD events and a 70% reduction in the risk of incident composite CAD, stroke, and TIA.
This study is the first to report such an association. Hydroxychloroquine, as part of a triple‐therapy regimen, was associated with decreased CVD risk in RA in a case–control study of 613 RA patients.16 A similar protective association, with an odds ratio of 0.32 (95% CI 0.14–0.74) for thrombovascular events, was found in a nested case–control study of lupus patients after adjustment for disease severity, duration, and calendar year.17
The biological plausibility of this protective association is supported by the favorable associations of hydroxychloroquine with glucose and lipids in RA patients and with thrombosis in lupus patients and nonrheumatic patients. Wasko et al10 reported a decreased risk of incident diabetes in hydroxychloroquine users compared with nonusers in RA, and this reduction increased with prolonged use, suggesting a causative effect. This observation was validated in subsequent studies.11, 12 Hydroxychloroquine use was also associated with decreased hemoglobin A1c in 45 diabetic patients with rheumatologic diseases18 and with improved insulin sensitivity in nondiabetic obese patients with systemic inflammatory conditions.19
Hydroxychloroquine also has been associated with beneficial changes in lipid profiles, including decrease in low‐density lipoprotein and total cholesterol, which result in a less atherogenic lipid profile.13, 20 Finally, prior to the advances in current antithrombotic therapies, hydroxychloroquine was used perioperatively for deep venous thrombosis prophylaxis in abdominal and orthopedic surgeries.21, 22, 23 More recently, hydroxychloroquine use was associated with reduced risk of thrombotic events in patients with lupus and antiphospholipid syndrome.24 In addition, a recent study showed a platelet inhibitory effect after only 7 days of hydroxychloroquine use in healthy adults.14 We postulate that the antiplatelet effect of hydroxychloroquine in part explains the protective CVD association seen in our study.
The present study has multiple methodological advantages such as inclusion of patients with incident RA without preexisting CVD, thus eliminating chronic RA and CVD burden, which are hard to quantitate; primary care physicians at GHS, ensuring that the majority of CVD events were captured; physician‐entered diagnoses, physician‐reconciled medications at each visit, and available laboratory values for low‐density lipoprotein, erythrocyte sedimentation rate (as a surrogate measure of RA activity) and rheumatoid factor (as a surrogate measure of disease severity); and independently adjudicated CVD outcomes by an investigator blinded to the study hypotheses.
Limitations of the study include the potential for confounding by indication, which we attempted to minimize with the use of a propensity score for the probability of hydroxychloroquine use and the use of a multivariable regression model that adjusted for relevant confounders; however, we lacked data on use of low‐dose aspirin, family history of CVD, physical activity level, and dose of corticosteroids. Furthermore, the lack of data on RA disease activity such as tender and swollen joint counts or physician and patient global assessment is a limitation of the study because it is difficult to discern if the observed association is caused by hydroxychloroquine use or lower inflammatory burden.
Some other findings of our study warrant discussion, although they are not the primary focus of the study. In the univariable analysis (Table S1), there was a positive association of statin use with incident CVD events, but this is likely due to the high prevalence of CVD risk factors in statin users (hyperlipidemia, diabetes, or hypertension were present in 79.6% of the patients on a statin). In addition, a reverse association was shown between nonsteroidal anti‐inflammatory drug use and CVD events, which is at odds with published data.25 Although this is likely an incidental finding because this was not a study aim, it is possible that the exclusion of patients with prevalent CVD from the study attenuated a harmful association; nonsteroidal anti‐inflammatory drug use has been shown to confer the greatest risk in patients at high risk for or with preexisting CVD.26
In conclusion, in this incident RA cohort, use of hydroxychloroquine was associated with a 72% reduction in the risk of incident CVD. Given the small number of CVD events in the hydroxychloroquine user group, the nature of the study should be considered hypothesis generating. If confirmed in larger studies, our findings should be taken into consideration in treatment strategies used in RA, which is a systemic autoimmune disease with high cardiovascular mortality. Given the relative safety and low cost of hydroxychloroquine, our findings may ultimately serve to support investigation of this agent in a randomized study for prophylaxis against CVD in nonrheumatic high‐risk patients.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods: Details on the process of identification and validation of incident cardiovascular disease cases.
Table S1. Risk of Incident Cardiovascular Disease and Composite Coronary Artery Disease, Stroke, and Transient Ischemic Attack in Rheumatoid Arthritis Patients According to Hydroxychloroquine Use: Univariable Analysis
(J Am Heart Assoc. 2016;5:e002867 doi: 10.1161/JAHA.115.002867)
Accompanying Data S1 and Table S1 are available at http://jaha.ahajournals.org/content/5/1/e002867/suppl/DC1
References
- 1. Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. [DOI] [PubMed] [Google Scholar]
- 2. Avina‐Zubieta JA, Abrahamowicz M, De Vera MA, Choi HK, Sayre EC, Rahman MM, Sylvestre MP, Wynant W, Esdaile JM, Lacaille D. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population‐based study. Rheumatology (Oxford). 2013;52:68–75. [DOI] [PubMed] [Google Scholar]
- 3. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. [DOI] [PubMed] [Google Scholar]
- 4. del Rincon I, Freeman GL, Haas RW, O'Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–3423. [DOI] [PubMed] [Google Scholar]
- 5. Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, Farkouh ME, Setoguchi S, Greenberg JD. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. See comment. Lancet. 2002;359:1173–1177. [DOI] [PubMed] [Google Scholar]
- 7. Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL, Kirchner HL, Wasko MC. Tumor necrosis factor alpha inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66:355–363. [DOI] [PubMed] [Google Scholar]
- 8. Dixon WG, Watson KD, Lunt M, Hyrich KL; British Society for Rheumatology Biologics Register Control Centre Consortium , Silman AJ, Symmons DP; British Society for Rheumatology Biologics Register . Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti‐tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56:2905–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis JM III, Maradit Kremers H, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, Roger VL, Gabriel SE. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population‐based cohort study. Arthritis Rheum. 2007;56:820–830. [DOI] [PubMed] [Google Scholar]
- 10. Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, Ward MM. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–193. [DOI] [PubMed] [Google Scholar]
- 11. Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease‐modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–2531. [DOI] [PubMed] [Google Scholar]
- 12. Bili A, Sartorius JA, Kirchner HL, Morris SJ, Ledwich LJ, Antohe JL, Dancea S, Newman ED, Wasko MC. Hydroxychloroquine use and decreased risk of diabetes in rheumatoid arthritis patients. J Clin Rheumatol. 2011;17:115–120. [DOI] [PubMed] [Google Scholar]
- 13. Morris SJ, Wasko MC, Antohe JL, Sartorius JA, Kirchner HL, Dancea S, Bili A. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2011;63:530–534. [DOI] [PubMed] [Google Scholar]
- 14. Achuthan S, Ahluwalia J, Shafiq N, Bhalla A, Pareek A, Chandurkar N, Malhota S. Hydroxychloroquine's efficacy as an antiplatelet agent study in healthy volunteers: a proof of concept study. J Cardiovasc Pharmacol Ther. 2015;20:174–180. [DOI] [PubMed] [Google Scholar]
- 15. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 16. van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease‐modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung H, Bobba R, Su J, Shariati‐Sarabi Z, Gladman DD, Urowitz M, Lou W, Fortin PR. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62:863–868. [DOI] [PubMed] [Google Scholar]
- 18. Rekedal LR, Massarotti E, Garg RK, Bhatia R, Lu B, Solomon DH. The effect of hydroxychloroquine and methotrexate on glycated hemoglobin. Arthritis Rheum. 2009;60(suppl 10):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mercer E, Rekedal L, Garg R, Lu B, Massarotti EM, Solomon DH. Hydroxychloroquine improves insulin sensitivity in obese non‐diabetic individuals. Arthritis Res Ther. 2012;14:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kerr G, Aujero M, Richards J, Sayles H, Davis L, Cannon G, Caplan L, Michaud K, Mikuls T. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66:1619–1626. [DOI] [PubMed] [Google Scholar]
- 21. Wu TK, Tsapogas MJ, Jordan FR. Prophylaxis of deep venous thrombosis by hydroxychloroquine sulfate and heparin. Surg Gynecol Obstet. 1977;145:714–718. [PubMed] [Google Scholar]
- 22. Carter AE, Eban R. Prevention of postoperative deep venous thrombosis in legs by orally administered hydroxychloroquine sulphate. Br Med J. 1974;3:94–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen EH, Jessing P, Lindewald H, Ostergaard P, Olesen T, Malver EI. Hydroxychloroquine sulphate in prevention of deep venous thrombosis following fracture of the hip, pelvis, or thoracolumbar spine. J Bone Joint Surg Am. 1976;58:1089–1093. [PubMed] [Google Scholar]
- 24. Petri M. Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody‐positive patients. Curr Rheumatol Rep. 2011;13:77–80. [DOI] [PubMed] [Google Scholar]
- 25. Coxib and traditional NSAID Trialists' (CNT) Collaboration , Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association . Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods: Details on the process of identification and validation of incident cardiovascular disease cases.
Table S1. Risk of Incident Cardiovascular Disease and Composite Coronary Artery Disease, Stroke, and Transient Ischemic Attack in Rheumatoid Arthritis Patients According to Hydroxychloroquine Use: Univariable Analysis


