Abstract
Background
Saturated fat (SFA), ω‐6 (n‐6) polyunsaturated fat (PUFA), and trans fat (TFA) influence risk of coronary heart disease (CHD), but attributable CHD mortalities by country, age, sex, and time are unclear.
Methods and Results
National intakes of SFA, n‐6 PUFA, and TFA were estimated using a Bayesian hierarchical model based on country‐specific dietary surveys; food availability data; and, for TFA, industry reports on fats/oils and packaged foods. Etiologic effects of dietary fats on CHD mortality were derived from meta‐analyses of prospective cohorts and CHD mortality rates from the 2010 Global Burden of Diseases study. Absolute and proportional attributable CHD mortality were computed using a comparative risk assessment framework. In 2010, nonoptimal intakes of n‐6 PUFA, SFA, and TFA were estimated to result in 711 800 (95% uncertainty interval [UI] 680 700–745 000), 250 900 (95% UI 236 900–265 800), and 537 200 (95% UI 517 600–557 000) CHD deaths per year worldwide, accounting for 10.3% (95% UI 9.9%–10.6%), 3.6%, (95% UI 3.5%–3.6%) and 7.7% (95% UI 7.6%–7.9%) of global CHD mortality. Tropical oil–consuming countries were estimated to have the highest proportional n‐6 PUFA– and SFA‐attributable CHD mortality, whereas Egypt, Pakistan, and Canada were estimated to have the highest proportional TFA‐attributable CHD mortality. From 1990 to 2010 globally, the estimated proportional CHD mortality decreased by 9% for insufficient n‐6 PUFA and by 21% for higher SFA, whereas it increased by 4% for higher TFA, with the latter driven by increases in low‐ and middle‐income countries.
Conclusions
Nonoptimal intakes of n‐6 PUFA, TFA, and SFA each contribute to significant estimated CHD mortality, with important heterogeneity across countries that informs nation‐specific clinical, public health, and policy priorities.
Keywords: cardiovascular disease, coronary heart disease, dietary fat, ω‐6 polyunsaturated fat, saturated fat, trans fat
Subject Categories: Epidemiology
Introduction
Coronary heart disease (CHD) is the leading cause of death worldwide and accounted for 7 million deaths in 2010.1 The types of dietary fats consumed play an important role in CHD risk, representing key modifiable risk factors.2 In particular, higher intakes of trans fat (TFA)3 and of saturated fat (SFA) replacing ω‐6 (n‐6) polyunsaturated fat (PUFA) are associated with increased CHD,4, 5 whereas higher intake of PUFA replacing either SFA or carbohydrate is associated with lower risk.6
Considerable heterogeneity is evident in intakes of these dietary fats7 and in CHD mortality rates1 globally; however, differences in CHD mortality attributable to nonoptimal intakes of SFA, n‐6 PUFA, and TFA by country, age, and sex are not well established. Furthermore, whereas dietary intakes and CHD rates have changed substantially in recent decades, the regional and country‐level trends in these burdens have not been evaluated in detail. This may be especially relevant for dietary linoleic acid, the predominant n‐6 PUFA, which appears to have similar CHD benefits whether replacing SFA or carbohydrates.6 No prior study has investigated global CHD deaths attributable to higher SFA, insufficient n‐6 PUFA, and higher TFA consumption.
To address these gaps, we used a comparative risk assessment framework to quantify CHD mortality due to nonoptimal intakes of n‐6 PUFA, SFA, and TFA in 186 countries in 1990 and 2010 by age and sex.
Methods
Study Design
To quantify CHD burdens attributable to each dietary fat, we used established methods8 to collect data on (1) population distributions of dietary n‐6 PUFA, SFA, and TFA in 1990 and 2010 by country, age, and sex; (2) age‐specific etiologic effects of these fats on CHD mortality; (3) optimal population intakes of these fats; and (4) total numbers of CHD deaths in 1990 and 2010 by country, age, and sex. These inputs and their uncertainty were incorporated into a comparative risk assessment framework to estimate the proportional and absolute CHD mortality attributable to each dietary fat.
Selection of Dietary Fats
We evaluated 3 dietary fats with probable or convincing evidence for etiologic effects on CHD mortality: insufficient n‐6 PUFA (replacing either SFA or carbohydrates), higher SFA (replacing n‐6 PUFA), and higher TFA (replacing other fats). These dietary factors were selected based on described criteria.2 We did not include seafood ω‐3 PUFA because of its distinct food sources and mechanistic pathways or plant ω‐3 PUFA or total monounsaturated fat (MUFA) because of promising but not yet probable or convincing evidence for causal effects on CHD.9, 10 Our findings for PUFA reflect the estimated CHD burdens related to nonoptimal n‐6 PUFA, not total or ω‐3 PUFA.
Dietary Consumption of Fats
Our methods for estimating intakes of key dietary factors globally have been described.7, 11, 12 Briefly, we systematically searched, identified, and compiled data from nationally representative dietary surveys, large subnational surveys (if national surveys were not available), United Nations food balance sheets, and (for TFA) industry sales data on fats/oils and packaged food to estimate age, sex, and country‐specific intakes of n‐6 PUFA, SFA and TFA among adults in 1990 and 2010 (Table 1). Dietary fat consumption data and their corresponding uncertainty were incorporated into a Bayesian hierarchical model to estimate the mean intake levels and corresponding statistical uncertainty for each age, sex, country, and year stratum, accounting for differences in dietary data, survey methods, representativeness, and sampling and modeling uncertainty.2 The final model estimated dietary SFA, n‐6 PUFA, and TFA in 24 age and sex subgroups (men and women across 12 age categories from 25–30 to ≥80 years) within 186 countries (those with year 2000 population >50 0002) in 1990 and 2010, representing 3.8 billion adults across 21 world regions.
Table 1.
Data Sources, Modeling Approaches, and Validation Methods Used to Estimate Adult Dietary Fat Levels and Their Effects on CHD by Country, Age and Sex
| Dietary Fats | Data Sources | Statistical Methods Used for Pooling and Modeling Data From Diverse Global Sources | |||
|---|---|---|---|---|---|
| Individual Level Surveya | National Food Disappearance Sheetsb | Modeling Approach7, 11, 12 | Covariates | Validity | |
| Dietary Fat consumption by country, age, and sex2 | |||||
| n‐6 PUFA consumption | DisMod3, a Bayesian hierarchical method, was used to pool data from multiple sources and model missing data using informative time‐varying covariates, borrowing information across geographical region and time period while also incorporating uncertainty due to measurement error and model specification. Models were fit using a randomized MCMC algorithm based on the Adaptive Metropolis step function. | Both study‐specific and national‐level covariates were incorporated in the model. Study‐level covariates included information on national representativeness of data points and sex. Country‐level covariates were used to inform global and country‐level trend prediction by the model. These nation‐level covariates were based on FAO food balance sheets which capture a country's net annual food availability based on reported local production, imports and exports. We used factor analysis to reduce 22 FAO food items representing the majority of food available for human consumption in 186 countries into 4 factor variables which were included in the model in improve country‐level predictions. In addition, we included lag‐distributed GDP per capita as a model covariate | Models were assessed for convergence of Markov chain Monte Carlo iterations. DisMod3 was validated using goodness‐of‐fit tests and out‐of‐sample predictive validity tests, in which 10% to 20% of data were held‐out of the model. Qualitative evaluation for dietary fats was conducted by comparing the estimated dietary fats with known high‐quality data and assessing their face validity through the contact with country experts. | ||
| Total n‐6 fatty acid intake from all dietary sources (primarily liquid vegetable oils, including soybean oil, corn oil and safflower oil) | A total of 51 surveys (34.6% from multiple dietary recall/record surveys corrected for within‐person variation, 22.6% from food frequency questionnaires, and 42.8% from single dietary recall/record surveys) with 1069 age‐ and sex‐specific data points, 85% nationally representative, were collected from 32 countries and represented 47% of the world's adult population. | Calculated n‐6 PUFA intake (derived from FAO data on cottonseed oil, rape/mustard seed oil, soybean oil, sesame seed oil, rice bran oil, sunflower seed oil, maize germ oil, and groundnut oil) consumed per capita per day in 186 countries in each year 1990–2010 | |||
| SFA consumption | |||||
| Total SFA intake from all dietary sources (primarily meat and dairy products and tropical oils) | A total of 75 surveys (35.6% from multiple dietary recall/record surveys corrected for within‐person variation, 24.4% from food frequency questionnaires, and 40.0% from single dietary recall/record surveys) with 1363 age‐ and sex‐specific data points, 82% nationally representative, were collected from 47 countries and represented 70% of the world's adult population. | Calculated SFA intake (derived from FAO data on coconuts, palm kernel oil, palm oil, coconut oil, butter/ghee, and cream) consumed per capita per day in 186 countries in each year 1990–2010c | |||
| TFA consumption | |||||
| Total TFA intake from all dietary sources (primarily partially hydrogenated vegetables oils and ruminant products) | A total of 56 surveys (39.4% from multiple dietary recall/record surveys corrected for within‐person variation, 23.4% from food frequency questionnaires, 33.8% from single dietary recall/record surveys, and 2.4% from household availability/budget survey) with 411 age‐ and sex‐specific data points, 90.5% nationally representative, were collected from 23 countries and represented 19% of the world's adult population. | Calculated hydrogenated oil net ratio (the ratio of FAO data on hydrogenated oil to total oil crops) in 186 countries in each year 1990–2010d | |||
| Relative risks by age and sex | |||||
| Effects of PUFA replacing SFA on CHD6 | |||||
| Published Meta‐Analysis of Cohort Studies on Linoleic Acid and Coronary Heart Disease | Data were from 10 cohort studies in North America and Europe composing a total of 310 602 participants, 12 479 coronary events and 5882 coronary deaths. | Effect modification by race/ethnicity and sex were assessed but were not found to be statistically significant. | Trends in age‐specific relative risks from pooled analyses were compared with trends in original cohort data to ensure validity of pooled results. The I2 test did not reveal significant heterogeneity between studies for any age group. | ||
| Effects of TFA on CHD20 | |||||
| Published Meta‐Analysis of Prospective Cohort Studies | Data were from 4 cohort studies in America and Europe composing a total of 139 836 participants and 4965 coronary events. | The relative risks from the pooling projects and the meta‐analysis were interpolated and extrapolated into standard age groups using log‐linear models. Age‐specific relative risks were pooled using random‐effects models. | Effect modification by race/ethnicity and sex were assessed but were not found to be statistically significant. | Trends in age‐specific relative risks from pooled analyses were compared with trends in original cohort data to ensure validity of pooled results. The I2 test did not reveal significant heterogeneity between studies for any age group. | |
| Cause‐specific total mortality by country, age, and sex1 | |||||
| Vital registration with medical certification of cause of death | Data represented 2798 site‐years from 130 countries | Cause of Death Ensemble Modeling (CODEm), a modeling strategy encompassing 4 families of statistical models, was used to pools mortality data from diverse sources, aggregate deaths hierarchically and capture uncertainty due to model parameter estimation, model specification, and fundamental sources of error. | Covariates were selected from a database of mortality predictors based on the cause of death being modeled. Covariates were tested for predictive ability prior to inclusion in a given model. | Models were validated using out‐of‐sample predictive validity tests in which 30% of data were withheld from initial model fits. Predicted trends were then compared against trends in the existing held‐out data. | |
| Verbal autopsy (sample registration, demographic surveillance systems) | Data represented 486 site‐years from 66 countries, 10% nationally representative | ||||
| Cancer registries | Data represented 2715 site‐years from 93 countries | ||||
| Survey/census data | Data were from 56 national surveys | ||||
| Sibling history | Data represented 1557 survey‐years from 61 countries | ||||
| Burial/mortuary data | Data represented 32 site‐years from 11 countries | ||||
| Hospital records | Data represented 21 site‐years | ||||
| Police records | Data represented 1129 site‐years from 122 countries | ||||
These primary data were used to compute CHD mortality attributable to different dietary fat consumption by age, sex, and country in 2010 and 1990. CHD indicates coronary heart disease; FAO, United Nations Food and Agriculture Organization; GDP, gross domestic product; MCMC, Markov Chain Monte Carlo; n‐6, ω‐6; PUFA, polyunsaturated fat; SFA, saturated fat; TFA, trans fat.
For each individual survey, we obtained and assessed information about survey methods and population characteristics, and extracted or (in most cases) obtained data directly from the survey authors for dietary intakes by age, sex, and time
The country level dietary fat intakes were collected from the FAO annual food disappearance balance sheets for the 186 countries from 1990 to 2010.
The FAO‐based estimate of SFA availability was based on tropical fats/oils, which could lead to underestimation of estimated intake in regions with missing data but in which meat and dairy intake are high; however, individual‐level survey data were available in most regions, minimizing such potential underestimation.
For TFA intake, the hydrogenated oil net ratio corresponded to the net amount of hydrogenated oils available for consumption in each country‐year. Using the FAO data, the numerator of this ratio was calculated based on exported hydrogenated oil (in kcal per capita) and exported oil crops (in kcal per capita) through space‐time with lag‐distributed income as a covariates. The denominator was calculated by adding import values to production values minus the export values, and then applying to the space‐time model. In addition, TFA intake information was also obtained from Euromonitor using total fats/oils sold (per capita) via retail and total packaged foods sold (per capita) via retail.
Etiologic Effects of Dietary Fats on CHD Mortality
Etiologic effects of nonoptimal intakes of these dietary fats on CHD mortality were evaluated, as described previously.2, 11, 13 The relative risk (RR) and its uncertainty for each dietary fat were obtained from published meta‐analyses of prospective cohort studies including multivariable adjustment for age, sex, other cardiovascular risk factors, and often other dietary factors (Tables 1 and 2).4, 6, 14, 15, 16, 17, 18, 19, 20 These RRs represent the best causal estimates for effects of each dietary fat on CHD mortality. Based on these findings, we evaluated the impact of insufficient n‐6 PUFA as an isocaloric replacement for either SFA or carbohydrate, excess SFA as an isocaloric replacement for n‐6 PUFA, and excess TFA as an isocaloric replacement for other fats (equivalent thirds of SFA, MUFA, and PUFA). Notably, depending on levels of SFA and n‐6 PUFA consumption within any age, sex, and country stratum, the CHD burden attributable to insufficient n‐6 PUFA will nearly always include the CHD burden of excess SFA consumption but not vice versa. In sensitivity analyses, we assumed that benefits of reducing SFA also extended to replacement with MUFA, although evidence linking total MUFA to CHD mortality is not well‐established.4, 10 We did not include potential effects on other cardiac, vascular, or other chronic diseases due to insufficient evidence for causal effects. Emerging evidence suggests, for instance, that SFA may protect against stroke,21 certain TFA isomers may increase risk of sudden death and diabetes,3 and n‐6 PUFA may protect against these end points.22 These end points can be reevaluated in future analyses, as more evidence becomes available.
Table 2.
Sources and Magnitudes of the Optimal Levels and Effects of Nonoptimal Intakes of SFA, n‐6 PUFA and TFA on CHD
| Risk Factor | Outcome | Optimal Level | Source of Relative Risk | Unit of Relative Risk | Sex | Age, y | Relative Riska | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Higher SFA intakeb | CHD Deaths | 10%E±1% (7%E±0.7% in sensitivity analysis) | Published meta‐analysis of 10 cohort studies6 | Per 5% of energy increase | Both | 25–34 | 1.19 | 1.09–1.30 |
| 35–44 | 1.18 | 1.08–1.28 | ||||||
| 45–54* | 1.15* | 1.07–1.23* | ||||||
| 55–64 | 1.12 | 1.06–1.19 | ||||||
| 65–74 | 1.10 | 1.05–1.16 | ||||||
| 75+ | 1.08 | 1.04–1.12 | ||||||
| Insufficient n‐6 PUFA intakec | CHD Deaths | 12%E±1.2% | Published meta‐analysis of 10 cohort studies6 | Per 5% of energy increase | Both | 25–34 | 0.84 | 0.77–0.92 |
| 35–44 | 0.85 | 0.78–0.92 | ||||||
| 45–54* | 0.87*, d | 0.81–0.93* | ||||||
| 55–64 | 0.89 | 0.84–0.95 | ||||||
| 65–74 | 0.91 | 0.87–0.95 | ||||||
| ≥75 | 0.93 | 0.90–0.96 | ||||||
| Higher TFA consumptione | CHD Deaths | 0.5%E±0.05% | Published meta‐analysis of 4 cohort studies20 | Per 2% of energy increase | Both | 25–34 | 1.42 | 1.28–1.57 |
| 35–44 | 1.40 | 1.27–1.54 | ||||||
| 45–54 | 1.33 | 1.22–1.45 | ||||||
| 55–64* | 1.27* | 1.18–1.36* | ||||||
| 65–74 | 1.22 | 1.15–1.29 | ||||||
| ≥75 | 1.16 | 1.11–1.21 |
%E indicates percentage of total energy intake; CHD, ischemic heart disease; LA, linoleic acid; n‐6 PUFA, ω‐6 polyunsaturated fat; SFA, saturated fat; TFA, trans fat.
The bold relative risks corresponded to the original relative risk in the meta‐analysis (for TFA, the original relative risk was determined by subtraction of the summary coefficients for TFA replacing carbohydrates derived from the Nurses Health Study, the Health Professional Follow‐up Study, the Finnish ATBC study and the Zutphen Elderly Study and the coefficients for other dietary fats replacing carbohydrates derived from the Nurses Health Study and the Health Professional Follow‐up Study). The relative risks of other age groups were extrapolated based on a log‐linear relationship derived from metabolic risk factors (Singh et al23).
Higher SFA intake defined as higher SFA (>10%E) intake replacing n‐6 PUFA (<12%E) intake.
Insufficient n‐6 PUFA intake defined as lower n‐6 PUFA (<12%E) intake replacing either carbohydrates or SFA.
Although potential harms of high n‐6 PUFA consumption have been theorized,14, 15, 16 randomized controlled trials demonstrate no evidence linking dietary LA to increased levels of inflammation.17 LA improves all major lipid and lipoprotein risk factors18 and both total n‐6 PUFA and LA are associated with lower risk of clinical CHD events.4, 6 Indeed, higher blood biomarker levels of arachidonic acid, the prototypical n‐6 PUFA considered to be harmful, are actually linked to significantly lower risk of CHD.19 Thus, the American Heart Association, US Dietary Guidelines Advisory Committee, and United Nations have each concluded that higher LA consumption is beneficial for health.4, 6, 19 In observational cohorts and controlled trials of clinical events, levels of dietary LA linked to lower risk range from ≈7%E to 10%E and 9%E to 30%E, respectively.
Higher TFA consumption defined as higher TFA (>0.5%E) intake replacing SFA or n‐6 PUFA or monounsaturated fats.
Based on our prior work, proportional effects of dietary factors on CHD mortality were generally similar by sex,5 thus we assumed no heterogeneity in RRs by sex. Conversely, proportional effects (RRs) of major CHD risk factors decline with age in an approximately log‐linear relationship23; we applied this age‐varying RR pattern to distributions of RRs for dietary fats. We did not identify sufficient evidence for effect modification by other factors, such as total diet quality or obesity.
Optimal Intake Distribution of Dietary Fats
Optimal intakes of each dietary fat were determined using reported methods,11, 13 based on (1) observed levels associated with lowest CHD mortality in meta‐analyses, (2) observed highest (for n‐6 PUFA) or lowest (for SFA and TFA) consumption levels in at least 2 to 3 countries globally, and (3) general consistency with national and international dietary guidelines.24, 25 Using these methods, we identified optimal intake levels of 12%E (percentage of total energy intake) for n‐6 PUFA, 10%E for SFA, and 0.5%E for TFA. For n‐6 PUFA and SFA (Table 2), we recognized that optimal intakes were further dependent on the replacement nutrient: Benefits of reducing SFA were considered only when replaced by n‐6 PUFA (up to 12%E), and benefits of increasing n‐6 PUFA were considered only when replacing SFA (down to 10%E) or carbohydrate.4, 6, 10 For each fat, we assumed no additional health benefits accrued beyond the optimal intake level and nutrient replacement scenario within each age, sex, and country stratum. In sensitivity analyses, we evaluated potential harms of SFA down to an optimal intake level of 7%E.
CHD Deaths by Country, Age, and Sex
Data on country‐, age‐, and sex‐specific CHD mortalities were obtained from the 2010 Global Burden of Diseases study.1 Briefly, causes of death were collected in 186 countries from 1980 to 2010 based on vital registration, verbal autopsy, mortality surveillance, population census, surveys, hospital and police records, and mortuaries; completeness, diagnostic accuracy, missing data, stochastic variations, and probable cause of death were assessed (Table 1). CHD mortality was estimated using statistical modeling strategies including different permutations of covariates. Model performance was assessed with rigorous out‐of‐sample testing of prediction error and the validity of the 95% uncertainty interval (UI). CHD death was defined as International Classification of Diseases, 10th revision, codes I20–I25.
Statistical Analysis
The population‐attributable fraction (PAF) due to nonoptimal intakes of dietary fat was calculated using the following equation:
PAFi is a age‐, sex‐ and country‐specific population attributable fraction; x is the level of dietary fat; Pi(x) is the age‐, sex‐, and country‐specific actual distribution of dietary fat; P′i(x) is the age‐ and sex‐specific optimal distribution of dietary fat; RRi(x) is the age‐ and sex‐specific multivariable‐adjusted RR of mortality at level x; and m is the optimal level of dietary fat.
The age‐, sex‐, and country‐specific absolute CHD mortality attributable to each dietary fat was quantified by multiplying the age‐, sex‐, and country‐specific PAFi by the total CHD deaths in the corresponding stratum. Absolute attributable mortalities were summed across strata to estimate national, regional, and global absolute burdens, and divided by total CHD deaths within these strata for corresponding proportional burdens. To evaluate changes between 1990 and 2010, attributable CHD mortalities in 1990 were age‐standardized to 2010 nation‐specific age distributions. Statistical uncertainty was quantified using the Markov chain Monte Carlo algorithm, drawing randomly 1000 times from the 95% uncertainty distributions of the estimated dietary fat intake, its etiologic effect on CHD (RR), and total CHD mortality within each age, sex, and country stratum. The central PAF was derived from the mean value of these 1000 estimations, and its 95% UI was derived from the 2.5th and 97.5th percentiles. All analyses were performed using R software version 3.0.2 (R Foundation for Statistical Computing).
The study obtained institutional review board approval and informed consent from participants. The paper was also approved by an institutional review committee.
Results
Detailed findings on dietary n‐6 PUFA, SFA, and TFA by age, sex, country, and region in 1990 and 2010 have been reported7 (Tables 3, 4, S1, and S2). In 2010, national mean intakes across 186 nations ranged from 1.2%E to 12.5%E for n‐6 PUFA, 2.3%E to 27.5%E for SFA, and 0.2%E to 6.5%E for TFA.
Table 3.
Global and Regional CHD Mortality Attributable to SFA, n‐6PUFA, and TFA in 2010
| Population (Millions) | Total CHD Deaths (Thousands) | Mean Dietary Consumption (95% UI)a | Attributable CHD Deaths/Year (Thousands) (95% UI) | Attributable CHD Deaths/Year Per Million Adults (95% UI) | Proportional Attributable CHD Deaths (% of Total CHD Deaths) (95% UI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFA (%E) | n‐6PUFA (%E) | TFA (%E) | Higher SFAb (>10.0%E) | Insufficient n‐6 PUFAc (<12.0%E) | Higher TFAd(>0.5%E) | Higher SFAb (>10.0%E) | Insufficient n‐6 PUFAc (<12.0%E) | Higher TFAd(>0.5%E) | Higher SFAb (>10.0%E) | Insufficient n‐6 PUFAc (<12.0%E) | Higher TFAd(>0.5%E) | |||
| Global | ||||||||||||||
| Both sexes | ||||||||||||||
| Age 25–69, y | 3460 | 2366 | 9.3 (9.3–9.4) | 6.0 (5.9–6.0) | 1.4 (1.4–1.4) | 103.2 (97.2–109.7) | 316.4 (296.9–337.9) | 244.0 (234.1–253.6) | 30 (28–32) | 91 (86–98) | 71 (68–73) | 4.4 (4.2–4.5) | 13.4 (12.9–13.8) | 10.3 (10.1–10.5) |
| Age ≥70, y | 348 | 4566 | 9.9 (9.8–10.1) | 5.9 (5.8–6.0) | 1.3 (1.3–1.3) | 147.7 (136.1–161.0) | 395.3 (369.9–423.7) | 293.1 (275.9–311.3) | 425 (391–463) | 1137 (1064–1219) | 843 (794–896) | 3.2 (3.1–3.4) | 8.7 (8.2–9.1) | 6.4 (6.2–6.7) |
| All ages, y | 3808 | 6933 | 9.4 (9.0–9.7) | 6.0 (5.6–6.3) | 1.4 (1.3–1.5) | 250.9 (236.9–265.8) | 711.8 (680.7–745.0) | 537.2 (517.6–557.0) | 66 (62–70) | 187 (179–196) | 141 (136–146) | 3.6 (3.5–3.7) | 10.3 (9.9–10.6) | 7.7 (7.6–7.9) |
| Female | ||||||||||||||
| Age 25–69, y | 1723 | 730 | 9.5 (9.4–9.6) | 6.0 (5.9–6.1) | 1.4 (1.4–1.4) | 31.0 (28.7–33.6) | 95.1 (86.6–104.5) | 77.6 (72.5–82.6) | 18 (17–20) | 55 (50–61) | 45 (42–48) | 4.2 (4.0–4.4) | 13.0 (12.4–13.6) | 10.6 (10.3–11.0) |
| Age ≥70, y | 200 | 2515 | 10.2 (10.1–10.4) | 6.0 (5.8–6.1) | 1.3 (1.3–1.3) | 85.6 (75.0–97.4) | 210.9 (191.2–231.8) | 159.0 (144.7–174.0) | 428 (375–488) | 1055 (956–1160) | 796 (724–871) | 3.4 (3.1–3.7) | 8.4 (7.8–9.0) | 6.3 (5.9–6.7) |
| All ages, y | 1923 | 3247 | 9.6 (9.1–10.1) | 6.0 (5.5–6.5) | 1.4 (1.3–1.5) | 116.6 (105.6–129.1) | 306.0 (284.3–329.3) | 236.6 (221.5–252.0) | 61 (55–67) | 159 (148–171) | 123 (115–131) | 3.6 (3.4–3.8) | 9.4 (9.0–9.8) | 7.3 (7.1–7.5) |
| Male | ||||||||||||||
| Age 25–69, y | 1737 | 1637 | 9.2 (9.1–9.2) | 5.9 (5.8–6.0) | 1.4 (1.4–1.4) | 72.1 (67.0–78.2) | 221.4 (204.8–240.0) | 166.4 (158.2–174.6) | 42 (39–45) | 127 (118–138) | 96 (91–101) | 4.4 (4.2–4.6) | 13.5 (12.9–14.2) | 10.2 (9.8–10.5) |
| Age ≥70, y | 148 | 2049 | 9.6 (9.5–9.7) | 5.8 (5.7–6.0) | 1.3 (1.3–1.3) | 62.1 (56.7–68.1) | 184.4 (169.8–201.9) | 134.1 (125.5–143.6) | 421 (384–461) | 1249 (1150–1367) | 908 (850–972) | 3.0 (2.8–3.3) | 9.0 (8.3–9.7) | 6.5 (6.2–6.9) |
| All ages, y | 1884 | 3687 | 9.2 (8.7–9.7) | 5.9 (5.4–6.4) | 1.4 (1.2–1.5) | 134.3 (126.4–142.1) | 405.8 (383.8–430.2) | 300.5 (288.1–313.1) | 71 (67–75) | 215 (204–228) | 159 (153–166) | 3.6 (3.5–3.8) | 11.0 (10.5–11.5) | 8.2 (7.9–8.4) |
| Income level | ||||||||||||||
| High income | 755 | 1794 | 11.7 (11.7–11.8) | 5.5 (5.5–5.6) | 1.6 (1.6–1.7) | 68.1 (62.4–74.0) | 183.2 (169.7–197.1) | 171.2 (157.0–186.4) | 90 (83–98) | 243 (225–261) | 227 (208–247) | 3.8 (3.6–3.9) | 10.2 (9.9–10.5) | 9.5 (9.2–9.9) |
| Upper‐middle income | 1528 | 2703 | 9.0 (8.9–9.1) | 7.6 (7.5–7.7) | 1.2 (1.2–1.2) | 97.0 (86.9–109.1) | 214.6 (198.9–231.0) | 160.2 (152.5–168.4) | 64 (57–71) | 140 (130–151) | 105 (100–110) | 3.6 (3.4–3.8) | 7.9 (7.5–8.5) | 5.9 (5.7–6.2) |
| Lower‐middle income | 1212 | 2183 | 8.6 (8.5–8.7) | 4.5 (4.4–4.7) | 1.5 (1.5–1.5) | 76.9 (70.7–83.9) | 279.4 (256.6–304.6) | 189.1 (178.9–199.5) | 63 (58–69) | 230 (212–251) | 156 (148–165) | 3.5 (3.3–3.8) | 12.8 (12.0–13.6) | 8.7 (8.3–9.0) |
| Low income | 313 | 255 | 8.7 (8.6–8.9) | 4.5 (4.3–4.6) | 1.2 (1.1–1.3) | 8.8 (8.1–9.5) | 34.6 (32.9–36.2) | 16.7 (15.0–18.3) | 28 (26–30) | 111 (105–116) | 53 (48–59) | 3.4 (3.3–3.6) | 13.5 (13.0–14.0) | 6.5 (5.9–7.1) |
| Regional | ||||||||||||||
| Australasia | 17 | 38 | 13.6 (13.4–13.8) | 5.0 (5.0–5.1) | 1.3 (1.2–1.3) | 2.2 (1.8–2.6) | 4.1 (3.3–5.0) | 2.2 (1.9–2.5) | 126 (101–151) | 238 (191–286) | 128 (110–146) | 5.8 (5.3–6.4) | 10.9 (10.0–11.9) | 5.9 (5.5–6.3) |
| Canada and United States | 226 | 620 | 11.7 (11.6–11.9) | 6.5 (6.4–6.6) | 2.9 (2.8–3.0) | 19.9 (15.4–25.2) | 56.8 (45.7–68.9) | 110.5 (96.8–126.1) | 88 (68–111) | 251 (202–304) | 488 (428–557) | 3.2 (2.8–3.6) | 9.2 (8.3–10.0) | 17.9 (16.8–18.9) |
| East/Central Eurasia | 273 | 1641 | 13.3 (13.0–13.7) | 7.8 (7.5–8.0) | 1.0 (1.0–1.0) | 82.1 (71.4–94.6) | 122.2 (109.7–136.1) | 73.1 (68.5–78.0) | 301 (262–347) | 448 (402–499) | 268 (251–286) | 5.0 (4.6–5.4) | 7.4 (7.0–7.9) | 4.5 (4.3–4.6) |
| East/Southeast Asia | 1354 | 1530 | 10.1 (10.0–10.2) | 6.8 (6.7–6.9) | 1.0 (1.0–1.0) | 71.6 (67.0–76.2) | 146.8 (135.0–159.5) | 67.5 (62.0–73.1) | 53 (49–56) | 108 (100–118) | 50 (46–54) | 4.7 (4.4–4.9) | 9.6 (9.0–10.2) | 4.4 (4.2–4.7) |
| Latin America/Caribbean | 316 | 465 | 8.2 (8.1–8.3) | 6.1 (6.0–6.1) | 1.9 (1.8–1.9) | 6.5 (6.0–7.0) | 48.5 (45.6–51.6) | 49.6 (46.7–52.4) | 21 (19–22) | 154 (144–163) | 157 (148–166) | 1.4 (1.3–1.5) | 10.4 (9.9–11.0) | 10.7 (10.2–11.1) |
| North Africa/Middle East | 225 | 410 | 10.3 (10.1–10.5) | 5.9 (5.8–6.1) | 2.3 (2.3–2.4) | 9.1 (8.4–9.9) | 46.2 (43.5–49.0) | 73.9 (70.1–78.1) | 41 (37–44) | 205 (194–218) | 329 (312–347) | 2.2 (2.1–2.4) | 11.2 (10.7–11.8) | 18.0 (17.3–18.7) |
| South Asia | 776 | 1270 | 4.2 (4.1–4.2) | 4.8 (4.5–5.0) | 1.7 (1.6–1.7) | 17.5 (14.0–22.0) | 175.0 (153.6–199.7) | 113.9 (104.6–123.3) | 23 (18–28) | 226 (198–258) | 147 (135–159) | 1.4 (1.1–1.7) | 13.7 (12.5–15.0) | 8.9 (8.4–9.4) |
| Sub‐Saharan Africa | 320 | 209 | 11.3 (11.1–11.6) | 4.2 (4.1–4.3) | 0.8 (0.8–0.9) | 8.1 (7.6–8.6) | 30.6 (29.4–31.8) | 7.7 (7.3–8.1) | 25 (24–27) | 95 (92–99) | 24 (23–25) | 3.9 (3.7–4.1) | 14.6 (14.2–15.1) | 3.7 (3.5–3.9) |
| Western Europe | 301 | 745 | 12.6 (12.5–12.7) | 5.2 (5.1–5.3) | 1.1 (1.1–1.1) | 33.8 (30.4–37.5) | 81.5 (74.7–88.9) | 38.7 (36.2–41.5) | 112 (101–125) | 271 (248–295) | 129 (120–138) | 4.5 (4.3–4.8) | 10.9 (10.5–11.4) | 5.2 (5.0–5.4) |
List of countries within each region: Australasia: Australia, New Zealand; Canada and United States: Canada, United States; East/Central Eurasia: Albania, Armenia, Azerbaijan, Bulgaria, Bosnia and Herzegovina, Belarus, Czech Republic, Estonia, Georgia, Croatia, Hungary, Kazakhstan, Kyrgyzstan, Lithuania, Latvia, Moldova, Macedonia, Montenegro, Mongolia, Poland, Romania, Russian Federation, Serbia, Slovakia, Slovenia, Tajikistan, Turkmenistan, Ukraine, Uzbekistan; East/Southeast Asia: Darussalam, China, Fiji, Micronesia, Indonesia, Cambodia, Kiribati, Republic of Korea, Lao People's Democratic Republic, Sri Lanka, Maldives, Marshall Islands, Myanmar, Malaysia, Philippines, Papua New Guinea, Solomon Islands, Thailand, Timor‐Leste, Tonga, Taiwan, Vietnam, Vanuatu, Samoa, Brunei, Japan, Democratic People's Republic of Korea, Singapore; Latin America/Caribbean: Argentina, Antigua and Barbuda, Bahamas, Belize, Bolivia, Brazil, Barbados, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, Grenada, Guatemala, Guyana, Honduras, Haiti, Jamaica, Saint Lucia, Mexico, Nicaragua, Panama, Peru, Paraguay, El Salvador, Suriname, Trinidad and Tobago, Uruguay, Saint Vincent and the Grenadines, Venezuela; North Africa/Middle East: United Arab Emirates, Bahrain, Algeria, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libyan Arab Jamahiriya, Morocco, Oman, Occupied Palestinian Territory, Qatar, Saudi Arabia, Syrian Arab Republic, Tunisia, Turkey, Yemen; South Asia: Bangladesh, Bhutan, India, Nepal, Pakistan; sub‐Saharan Africa: Angola, Burundi, Benin, Burkina Faso, Botswana, Central African Republic, Côte d'Ivoire, Cameroon, Democratic republic of the Congo, Congo, Comoros, Cape Verde, Djibouti, Eritrea, Ethiopia, Gabon, Ghana, Guinea, Gambia, Guinea‐Bissau, Equatorial Guinea, Kenya, Liberia, Lesotho, Madagascar, Mali, Mozambique, Mauritania, Mauritius, Malawi, Namibia, Niger, Nigeria, Rwanda, Sudan, Senegal, Sierra Leone, Somalia, São Tomé and Príncipe, Swaziland, Seychelles, Chad, Togo, United Republic of Tanzania, Uganda, South Africa, Zambia, Zimbabwe; Western Europe: Andorra, Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Ireland, Iceland, Israel, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, United Kingdom. %E indicates percentage of total energy intake; CHD, ischemic heart disease; n‐6 PUFA, ω‐6 polyunsaturated fat; SFA, saturated fat; TFA, trans fat; UI, uncertainly interval.
Mean national consumption levels among adults, (95%UI) based on intakes in each country‐, age‐, and sex‐specific stratum weighted by the number of adults in that stratum in 2010.
Burdens due to higher SFA (>10%E), based on benefits if isocalorically replaced with n‐6 PUFA (up to 12%E).
Burdens due to insufficient n‐6 PUFA (<12%E), based on benefits if isocalorically replacing either SFA (down to 10%E) or carbohydrates.
Burdens due to higher TFA (>0.5%E), based on benefits if isocalorically replaced with other dietary fats.
Table 4.
Global and Regional CHD Mortality Attributable to SFA, n‐6PUFA, and TFA in 1990
| Population (Million) | Total CHD Deaths (Thousands) | Mean Intake Level (95% UI)a | CHD Deaths (Thousand) Due to (95% UI) | CHD Deaths/1 Million Population Due to (95% UI) | Proportion of CHD Deaths (%) Due to (95% UI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFA (%E) | n‐6 PUFA (%E) | TFA (%E) | Higher SFAb (>10.0%E) | Insufficient n‐6 PUFAc (<12.0%E) | Higher TFAd (>0.5%E) | Higher SFAb (>10.0%E) | Insufficient n‐6 PUFAc (<12.0%E) | Higher TFAd(>0.5%E) | Higher SFAb (>10.0%E) | Insufficient N‐6 PUFAc (<12.0%E) | Higher TFAd (>0.5%E) | |||
| Global | ||||||||||||||
| Both sexes | ||||||||||||||
| Age 25–69, y | 2343 | 1908 | 9.5 (9.5–9.6) | 5.5 (5.5–5.6) | 1.3 (1.2–1.3) | 99.7 (94.8–105.2) | 271.5 (259.5–284.1) | 175.2 (169.0–181.3) | 40 (38–42) | 119 (113–125) | 78 (75–81) | 5.2 (5.0–5.4) | 14.2 (13.7–14.7) | 9.2 (9.0–9.4) |
| Age ≥70, y | 204 | 3219 | 10.3 (10.1–10.4) | 5.5 (5.4–5.6) | 1.2 (1.2–1.3) | 135.1 (124.3–145.6) | 307.8 (289.8–326.3) | 208.1 (195.1–222.1) | 610 (563–653) | 1504 (1422–1593) | 999 (938–1061) | 4.2 (4.0–4.4) | 9.6 (9.1–10.1) | 6.5 (6.2–6.7) |
| All ages, y | 2547 | 5127 | 9.6 (9.2–9.9) | 5.5 (5.2–5.8) | 1.3 (1.2–1.3) | 234.8 (222.6–247.1) | 579.3 (558.2–601.8) | 383.3 (369.2–398.9) | 92 (87–96) | 245 (236–255) | 162 (156–168) | 4.6 (4.4–4.7) | 11.3 (10.9–11.7) | 7.5 (7.3–7.7) |
| Female | ||||||||||||||
| Age 25–69, y | 1166 | 635 | 9.7 (9.6–9.8) | 5.6 (5.5–5.7) | 1.3 (1.3–1.3) | 32.3 (30.0–34.9) | 87.5 (81.7–94) | 58.0 (54.8–61.4) | 25 (24–27) | 77 (72–83) | 52 (49–55) | 5.1 (4.8–5.3) | 13.8 (13.1–14.4) | 9.1 (8.8–9.4) |
| Age ≥70, y | 121 | 1857 | 10.7 (10.5–10.9) | 5.5 (5.4–5.7) | 1.2 (1.2–1.3) | 82.9 (72.9–93.4) | 173.3 (158.1–189.1) | 117.7 (106.4–129.6) | 616 (546–693) | 1415 (1294–1535) | 943 (857–1033) | 4.5 (4.1–4.8) | 9.3 (8.7–10) | 6.3 (5.9–6.7) |
| All ages, y | 1287 | 2493 | 9.8 (9.3–10.4) | 5.6 (5.1–6.1) | 1.3 (1.2–1.4) | 115.2 (105.2–125.8) | 260.8 (245.1–277.6) | 175.6 (164.1–187.7) | 87 (79–95) | 216 (203–230) | 145 (135–154) | 4.6 (4.4–4.8) | 10.5 (10–10.9) | 7.0 (6.8–7.3) |
| Male | ||||||||||||||
| Age 25–69, y | 1178 | 1273 | 9.3 (9.2–9.4) | 5.5 (5.4–5.6) | 1.2 (1.2–1.3) | 67.4 (63.3–72.1) | 184.0 (173.2–194.9) | 117.2 (112.1–122.6) | 54 (51–57) | 160 (150–169) | 104 (99–108) | 5.3 (5.0–5.6) | 14.5 (13.8–15.1) | 9.2 (8.9–9.5) |
| Age ≥70, y | 83 | 1362 | 9.7 (9.6–9.8) | 5.4 (5.2–5.5) | 1.2 (1.2–1.3) | 52.2 (47.8–56.7) | 134.5 (124.9–144.8) | 90.5 (84.1–97.8) | 601 (551–652) | 1625 (1508–1746) | 1074 (999–1161) | 3.8 (3.6–4.1) | 9.9 (9.1–10.6) | 6.6 (6.3–7.0) |
| All ages, y | 1260 | 2635 | 9.3 (8.8–9.8) | 5.5 (5.0–6.0) | 1.2 (1.1–1.3) | 119.6 (113.1–126.1) | 318.5 (304.1–333.1) | 207.7 (198.8–217.1) | 97 (92–102) | 275 (262–288) | 180 (172–188) | 4.5 (4.3–4.8) | 12.1 (11.5–12.7) | 7.9 (7.6–8.2) |
| Income level | ||||||||||||||
| High income | 604 | 2017 | 11.9 (11.8–12.0) | 5.3 (5.2–5.3) | 1.7 (1.6–1.7) | 93.3 (86.7–100.1) | 220.0 (206.7–233.8) | 203.8 (191.0–217.8) | 179 (166–192) | 442 (413–470) | 404 (378–432) | 4.6 (4.4–4.8) | 10.9 (10.5–11.3) | 10.1 (9.8–10.5) |
| Upper‐middle income | 1026 | 1703 | 9.2 (9.1–9.3) | 6.7 (6.7–6.8) | 1.1 (1.1–1.1) | 84.7 (76.6–93.6) | 170.7 (159.9–182.5) | 90.4 (86.5–94.4) | 81 (73–89) | 182 (171–194) | 101 (97–106) | 5.0 (4.7–5.3) | 10.0 (9.5–10.7) | 5.3 (5.1–5.5) |
| Lower‐middle income | 737 | 1266 | 8.5 (8.4–8.6) | 4.4 (4.2–4.6) | 1.2 (1.2–1.3) | 51.1 (46.7–55.9) | 168.1 (156.0–181) | 83.0 (79.0–87.5) | 67 (61–72) | 236 (219–254) | 120 (114–126) | 4.0 (3.8–4.3) | 13.3 (12.5–14.1) | 6.6 (6.3–6.8) |
| Low income | 180 | 142 | 8.6 (8.4–8.7) | 4.2 (4.1–4.3) | 1.0 (1.0–1.1) | 5.7 (5.2–6.2) | 20.4 (19.6–21.2) | 6.2 (5.7–6.7) | 30 (28–32) | 112 (107–116) | 34 (32–37) | 4.0 (3.8–4.2) | 14.4 (13.8–14.9) | 4.4 (3.9–4.8) |
| Regional | ||||||||||||||
| Australasia | 13 | 42 | 13.5 (13.4–13.7) | 5.1 (5.0–5.2) | 1.3 (1.2–1.3) | 2.5 (2.2–2.8) | 4.8 (4.2–5.5) | 2.7 (2.4–3.0) | 256 (216–295) | 498 (421–573) | 275 (246–304) | 5.9 (5.4–6.5) | 11.5 (10.4–12.5) | 6.4 (5.9–6.8) |
| Canada and United States | 176 | 703 | 12.3 (12.1–12.4) | 6.0 (5.9–6.1) | 2.9 (2.8–3.0) | 27.8 (22.7–33.1) | 70.2 (58.8–81.5) | 125.6 (112.3–139.9) | 186 (150–223) | 473 (394–555) | 846 (755–942) | 4.0 (3.5–4.4) | 10.0 (9.1–10.9) | 17.9 (16.8–18.9) |
| East/Central Eurasia | 249 | 1302 | 14.4 (14.0–14.8) | 6.6 (6.4–6.8) | 0.9 (0.9–1.0) | 91.4 (82.7–101.4) | 124.6 (114.5–135.7) | 54.0 (51.0–57.3) | 425 (381–475) | 588 (539–644) | 253 (237–270) | 7.0 (6.6–7.5) | 9.6 (9.1–10.1) | 4.1 (4.0–4.3) |
| East/Southeast Asia | 901 | 788 | 9.7 (9.6–9.8) | 6.0 (5.9–6.1) | 0.9 (0.9–0.9) | 39.0 (36.7–41.2) | 90.6 (84.0–97.4) | 31.3 (28.9–33.6) | 54 (51–57) | 125 (115–134) | 42 (39–45) | 4.9 (4.7–5.2) | 11.5 (10.8–12.2) | 4.0 (3.8–4.2) |
| Latin America/Caribbean | 193 | 306 | 7.8 (7.7–7.9) | 5.6 (5.5–5.7) | 1.7 (1.7–1.8) | 4.2 (3.9–4.6) | 35.3 (33.5–37.1) | 29.6 (28.1–31.1) | 26 (24–28) | 216 (203–228) | 187 (177–197) | 1.4 (1.3–1.5) | 11.5 (11.0–12.1) | 9.7 (9.2–10.1) |
| North Africa/Middle East | 116 | 251 | 10.4 (10.2–10.6) | 5.5 (5.4–5.7) | 2.0 (2.0–2.1) | 5.5 (5.1–5.9) | 30.4 (28.8–32.1) | 33.1 (31.4–35.1) | 50 (47–54) | 274 (259–289) | 296 (281–314) | 2.2 (2.1–2.3) | 12.1 (11.6–12.6) | 13.2 (12.7–13.7) |
| South Asia | 463 | 672 | 3.8 (3.7–3.9) | 4.5 (4.2–4.8) | 1.3 (1.3–1.4) | 9.4 (7.5–11.7) | 96.6 (85.9–108.5) | 45.0 (41.4–48.6) | 21 (17–27) | 222 (197–249) | 103 (95–111) | 1.4 (1.1–1.7) | 14.4 (13.1–15.7) | 6.7 (6.3–7.1) |
| Sub‐Saharan Africa | 183 | 136 | 10.8 (10.5–11.0) | 4.1 (4.0–4.2) | 0.8 (0.8–0.9) | 4.7 (4.4–5.0) | 20.5 (19.7–21.3) | 5.5 (5.2–5.8) | 26 (24–27) | 113 (108–117) | 30 (29–32) | 3.5 (3.3–3.7) | 15.1 (14.6–15.6) | 4.0 (3.8–4.3) |
| Western Europe | 253 | 928 | 12.9 (12.8–13.0) | 5.1 (5.0–5.2) | 1.2 (1.2–1.2) | 50.2 (46.1–54.5) | 106.3 (98.8–114.1) | 56.6 (53.6–59.8) | 231 (212–250) | 501 (466–539) | 261 (247–276) | 5.4 (5.2–5.7) | 11.5 (11.0–11.9) | 6.1 (5.9–6.3) |
Each region includes countries as follows: Australasia: Australia, New Zealand; Canada and United States: Canada, United States; East/Central Eurasia: Albania, Armenia, Azerbaijan, Bulgaria, Bosnia and Herzegovina, Belarus, Czech Republic, Estonia, Georgia, Croatia, Hungary, Kazakhstan, Kyrgyzstan, Lithuania, Latvia, Moldova, Macedonia, Montenegro, Mongolia, Poland, Romania, Russian Federation, Serbia, Slovakia, Slovenia, Tajikistan, Turkmenistan, Ukraine, Uzbekistan; East/Southeast Asia: Darussalam, China, Fiji, Micronesia, Indonesia, Cambodia, Kiribati, Republic of Korea, Lao People's Democratic Republic, Sri Lanka, Maldives, Marshall Islands, Myanmar, Malaysia, Philippines, Papua New Guinea, Solomon Islands, Thailand, Timor‐Leste, Tonga, Taiwan, Vietnam, Vanuatu, Samoa, Brunei, Japan, Democratic People's Republic of Korea, Singapore; Latin America/Caribbean: Argentina, Antigua and Barbuda, Bahamas, Belize, Bolivia, Brazil, Barbados, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, Grenada, Guatemala, Guyana, Honduras, Haiti, Jamaica, Saint Lucia, Mexico, Nicaragua, Panama, Peru, Paraguay, El Salvador, Suriname, Trinidad and Tobago, Uruguay, Saint Vincent and the Grenadines, Venezuela; North Africa/Middle East: United Arab Emirates, Bahrain, Algeria, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libyan Arab Jamahiriya, Morocco, Oman, Occupied Palestinian Territory, Qatar, Saudi Arabia, Syrian Arab Republic, Tunisia, Turkey, Yemen; South Asia: Bangladesh, Bhutan, India, Nepal, Pakistan; sub‐Saharan Africa: Angola, Burundi, Benin, Burkina Faso, Botswana, Central African Republic, Côte d'Ivoire, Cameroon, Democratic republic of the Congo, Congo, Comoros, Cape Verde, Djibouti, Eritrea, Ethiopia, Gabon, Ghana, Guinea, Gambia, Guinea‐Bissau, Equatorial Guinea, Kenya, Liberia, Lesotho, Madagascar, Mali, Mozambique, Mauritania, Mauritius, Malawi, Namibia, Niger, Nigeria, Rwanda, Sudan, Senegal, Sierra Leone, Somalia, São Tomé and Príncipe, Swaziland, Seychelles, Chad, Togo, United Republic of Tanzania, Uganda, South Africa, Zambia, Zimbabwe; Western Europe: Andorra, Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Ireland, Iceland, Israel, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, United Kingdom. %E indicates percentage of total energy intake; CHD, ischemic heart disease; n‐6 PUFA, ω‐6 polyunsaturated fat; SFA, saturated fat; TFA, trans fat; UI, uncertainly interval.
Mean intake level (95% UI) was weighted based on the population in each country‐, age‐, and sex‐specific stratum in 2010.
Model decreases SFA to minimally 10%E but only if it can be isocalorically replaced with PUFA to maximally 12%E.
Model increases n‐6 PUFA to 12%E and replaces it isocalorically with either carbohydrates or SFA.
Model decreases TFA to 0.5%E and replaces it isocalorically with n‐6 PUFA or monounsaturated fats or SFA.
Global and Regional Attributable CHD Mortality
In 2010, 711 800 (95% UI 680 700–745 000) CHD deaths per year worldwide were estimated to be attributable to insufficient n‐6 PUFA consumption in place of carbohydrate or SFA, accounting for 10.3% (95% UI 9.9%–10.6%) of total global CHD mortality and for 187 (95% UI 179–196) CHD deaths per year per 1 million adults (Table 3). Of these, 45% (316 400, 95% UI 296 900–337 900) occurred prematurely (aged <70 years), and 43% (306 000, 95% UI 284 300–329 300) occurred among women. As expected based on underlying CHD rates, absolute attributable mortality was higher at older than younger ages. Conversely, attributable proportional CHD mortality was higher at younger versus older ages, consistent with larger proportional effects of diet on CHD at younger ages. Eastern Europe had the most absolute n‐6 PUFA–attributable CHD deaths per year (547 per 1 million adults, 95% UI 464–640) (Figure 1), and Oceania had the highest proportion of n‐6 PUFA–attributable CHD deaths (18.6%, 95% UI 16.9%–20.2%). In comparison, East Asia had both fewest absolute (74 per 1 million adults, 95% UI 63–87) and lowest proportion (6.7%, 95% UI 5.9%–7.5%) of n‐6 PUFA–attributable CHD mortality.
Figure 1.
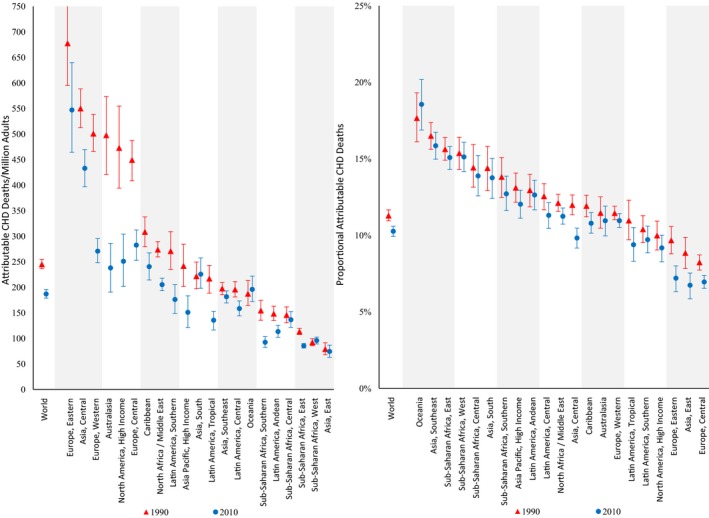
Regional CHD mortality attributable to insufficient n‐6 PUFA intake in 1990 and 2010. The y‐axis represents the CHD deaths per 1 million adults (on the left) or the proportion of CHD deaths (on the right) attributable to insufficient n‐6 PUFA intake. The x‐axis includes the world estimates and estimates for the 21 regions. Red triangles indicate estimates in 1990, whereas blue circles indicate estimates in 2010. The error bars represent the 95% uncertainty level of each estimate. CHD indicates coronary heart disease; n‐6 PUFA, ω‐6 polyunsaturated fat.
When we evaluated the impact of excess SFA intake in place of n‐6 PUFA, an estimated 250 900 (95% UI 236 900–265 800) attributable CHD deaths per year worldwide in 2010 were identified and accounted for 3.6% (95% UI 3.5%–3.7%) of global CHD deaths and 66 (95% UI 62–70) CHD deaths per year per 1 million adults (Table 3). Globally, CHD mortality attributable to higher SFA was only one‐third of that attributable to insufficient n‐6 PUFA, with much of this difference seen in south Asia.
Excess TFA consumption was estimated to cause 537 200 (95% UI 517 600–557 000) CHD deaths per year worldwide in 2010, representing 7.7% (95% UI 7.6%–7.9%) of global CHD mortality and 141 (95% UI 136–146) CHD deaths per year per 1 million adults (Table 3). Of these, women accounted for 44% and premature deaths for 45%. High‐income nations generally had higher TFA‐attributable CHD mortality than lower‐income nations. Younger adults generally experienced higher proportional TFA‐attributable CHD mortality related to both higher consumption and, more so, higher proportional effects of diet on CHD at younger ages. Highest absolute TFA‐attributable CHD mortality was in North America (488 per 1 million adults, 95% UI 428–557) (Figure 2), accounting for 18% of CHD deaths in this region. Sub‐Saharan Africa and the Caribbean had the lowest estimated TFA‐attributable CHD mortality, accounting for <5% of CHD mortality in these regions.
Figure 2.
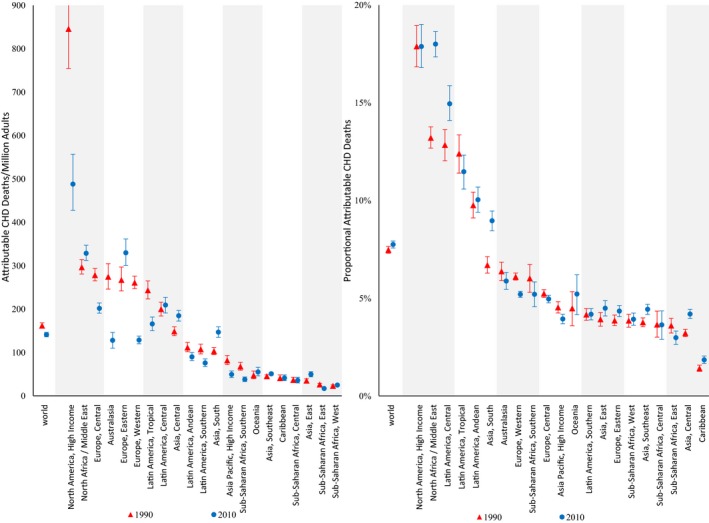
Regional CHD mortality attributable to higher TFA intake in 1990 and 2010. The y‐axis represents the CHD deaths per 1 million adults (on the left) or the proportion of CHD deaths (on the right) attributable to higher TFA consumption. The x‐axis includes the world estimates and the estimates for the 21 regions. Red triangles indicate estimates in 1990, whereas blue circles indicate estimates in 2010. The error bars represent the 95% uncertainty level of each estimate. CHD indicates coronary heart disease; TFA, trans fat.
In sensitivity analyses, allowing higher SFA intake to be replaced by both n‐6 PUFA and MUFA resulted in an estimated 255 900 (95% UI 238 600–276 200) SFA‐attributable CHD deaths per year in 2010 (Table 5), whereas lowering the optimal level of SFA consumption from 10%E to 7%E produced an estimated 376 900 (95% UI 358 600–396 100) SFA‐attributable CHD deaths per year. Evaluating both assumptions simultaneously, global annual SFA‐attributable CHD deaths per year were 459 300 (95% UI 435 300–485 800), accounting for 8.7% (95% UI 8.4%–8.9%) of global CHD deaths.
Table 5.
Global and Regional CHD Mortality Attributable to SFA, n‐6PUFA and TFA in 2010 With Alternative Models
| Population (Million) | Total CHD Deaths (Thousands) | Mean Intake Level (95% UI)a | CHD Deaths (Thousand) Due to (95% UI) | CHD Deaths/1 Million Population Due to (95% UI) | Proportion of CHD Deaths (%) Due to (95% UI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFA (%E) | n‐6 PUFA (%E) | Higher SFA Replacing n‐6 PUFA or MUFAb (>10.0%E) | Higher SFA Replacing n‐6 PUFAc (>7.0%E) | Higher SFA Replacing n‐6 PUFA or MUFAd (>7.0%E) | Higher SFA Replacing n‐6 PUFA or MUFAb (>10.0%E) | Higher SFA Replacing n‐6 PUFAc (>7.0%E) | Higher SFA Replacing n‐6 PUFA or MUFAd (>7.0%E) | Higher SFA Replacing N‐6 PUFA or MUFAb (>10.0%E) | Higher SFA Replacing N‐6 PUFAc (>7.0%E) | Higher SFA Replacing N‐6 PUFA or MUFAd (>7.0%E) | |||
| Global | |||||||||||||
| Both sexes | |||||||||||||
| Age 25–69, y | 3460 | 2367 | 9.3 (9.3–9.4) | 6.0 (5.9–6) | 98.5 (91.9–106.0) | 146.2 (139.7–153.1) | 176.0 (167.4–185.7) | 28 (27–31) | 42 (40–44) | 51 (48–54) | 4.9 (4.7–5.1) | 7.8 (7.6–8.0) | 9.0 (8.7–9.2) |
| Age ≥70, y | 348 | 4566 | 9.9 (9.8–10.1) | 5.9 (5.8–6) | 157.4 (142.3–175.0) | 230.7 (213.7–247.8) | 283.3 (260.7–307.3) | 453 (410–504) | 664 (615–713) | 815 (750–884) | 3.0 (2.8–3.2) | 4.9 (4.7–5.2) | 5.7 (5.4–6.0) |
| All ages, y | 3808 | 6935 | 9.4 (9.0–9.7) | 6.0 (5.6–6.3) | 255.9 (238.6–276.2) | 376.9 (358.6–396.1) | 459.3 (435.3–485.8) | 67 (63–73) | 99 (94–104) | 121 (114–128) | 4.7 (4.6–4.9) | 7.6 (7.4–7.8) | 8.7 (8.4–8.9) |
| Female | |||||||||||||
| Age 25–69, y | 1723 | 729 | 9.5 (9.4–9.6) | 6.0 (5.9–6.1) | 29.6 (26.9–32.4) | 43.7 (41.3–46.6) | 52.9 (49.2–56.5) | 17 (16–19) | 25 (24–27) | 31 (29–33) | 5.2 (4.9–5.4) | 8.0 (7.7–8.3) | 9.3 (8.9–9.7) |
| Age ≥70, y | 200 | 2513 | 10.2 (10.1–10.4) | 6.0 (5.8–6.1) | 93.3 (79.5–109.6) | 131.3 (116.8–147.3) | 165.2 (145.5–186.9) | 467 (398–549) | 657 (584–737) | 826 (728–935) | 3.2 (3.0–3.5) | 5.1 (4.8–5.5) | 6.0 (5.5–6.4) |
| All ages, y | 1923 | 3244 | 9.6 (9.1–10.1) | 6.0 (5.5–6.5) | 122.9 (109.4–139.8) | 175.0 (160.2–190.7) | 218.1 (198.9–240.5) | 64 (57–73) | 91 (83–99) | 113 (103–125) | 5.0 (4.7–5.2) | 7.7 (7.5–8.0) | 9.0 (8.6–9.3) |
| Male | |||||||||||||
| Age 25–69, y | 1737 | 1638 | 9.2 (9.1–9.2) | 5.9 (5.8–6.0) | 68.9 (63.0–75.4) | 102.5 (96.8–109.1) | 123.1 (115.1–131.2) | 40 (36–43) | 59 (56–63) | 71 (66–76) | 4.6 (4.4–4.9) | 7.6 (7.3–7.9) | 8.6 (8.3–9.0) |
| Age ≥70, y | 148 | 2050 | 9.6 (9.5–9.7) | 5.8 (5.7–6.0) | 64.0 (57.4–71.3) | 99.4 (91.4–107.7) | 118.2 (108.1–128.6) | 434 (389–483) | 673 (619–729) | 800 (732–871) | 2.7 (2.5–3.0) | 4.7 (4.4–5.0) | 5.3 (5.0–5.7) |
| All ages, y | 1884 | 3684 | 9.2 (8.7–9.7) | 5.9 (5.4–6.4) | 133.0 (123.7–143.6) | 201.9 (191.7–212.0) | 241.3 (227.6–255.6) | 71 (66–76) | 107 (102–112) | 128 (121–136) | 4.5 (4.3–4.7) | 7.4 (7.1–7.7) | 8.4 (8.0–8.7) |
| Income level | |||||||||||||
| High income | 755 | 1795 | 11.7 (11.7–11.8) | 5.5 (5.5–5.6) | 81.7 (74.8–88.7) | 137.0 (125.5–148.6) | 151.8 (139.8–164.1) | 108 (99–117) | 181 (166–197) | 201 (185–217) | 6.7 (6.4–7.0) | 11.4 (11.0–11.8) | 12.6 (12.1–13.1) |
| Upper‐middle income | 1528 | 2699 | 9.0 (8.9–9.1) | 7.6 (7.5–7.7) | 97.6 (83.7–114.2) | 129.2 (117.4–142.4) | 183.2 (164.4–203.6) | 64 (55–75) | 85 (77–93) | 120 (108–133) | 2.8 (2.6–3.0) | 5.5 (5.2–5.9) | 6.8 (6.4–7.2) |
| Lower‐middle income | 1212 | 2184 | 8.6 (8.5–8.7) | 4.5 (4.4–4.7) | 67.9 (61.2–76) | 96.0 (89.2–103.0) | 108.4 (99.3–118.9) | 56 (50–63) | 79 (74–85) | 89 (82–98) | 5.8 (5.4–6.2) | 7.5 (7.1–7.9) | 8.4 (8.0–8.9) |
| Low income | 313 | 256 | 8.7 (8.6–8.9) | 4.5 (4.3–4.6) | 8.6 (7.8–9.6) | 14.7 (13.9–15.6) | 15.9 (14.8–17.1) | 28 (25–31) | 47 (44–50) | 51 (47–55) | 5.1 (4.7–5.5) | 8.5 (8.2–8.9) | 9.2 (8.7–9.7) |
| Regional | |||||||||||||
| Australasia | 17 | 38 | 13.6 (13.4–13.8) | 5.0 (5.0–5.1) | 2.4 (1.9–3.0) | 3.8 (3.1–4.6) | 4.0 (3.2–4.8) | 140 (112–170) | 222 (178–266) | 230 (185–277) | 10.5 (9.6–11.6) | 16.1 (14.7–17.5) | 16.9 (15.4–18.6) |
| Canada and United States | 226 | 617 | 11.7 (11.6–11.9) | 6.5 (6.4–6.6) | 25.0 (19.6–31.1) | 48.1 (38.3–58.9) | 50.4 (40.2–61.7) | 111 (86–137) | 213 (169–260) | 223 (177–272) | 6.4 (5.7–7.2) | 12.1 (10.9–13.3) | 12.7 (11.4–14.0) |
| East/Central Eurasia | 273 | 1642 | 13.3 (13.0–13.7) | 7.8 (7.5–8.0) | 106.0 (91.5–124.4) | 113.3 (100.8–127.3) | 176.0 (156.5–198.6) | 388 (335–456) | 415 (369–466) | 645 (574–727) | 10.2 (9.3–11) | 10.1 (9.4–10.8) | 16.4 (15.4–17.5) |
| East/Southeast Asia | 1354 | 1532 | 10.1 (10.0–10.2) | 6.8 (6.7–6.9) | 60.0 (55.6–64.7) | 83.6 (78.5–88.9) | 99.4 (93.0–106) | 44 (41–48) | 62 (58–66) | 73 (69–78) | 5.6 (5.2–6.0) | 8.0 (7.6–8.5) | 9.5 (9.0–10.0) |
| Latin America/Caribbean | 316 | 466 | 8.2 (8.1–8.3) | 6.1 (6.0–6.1) | 3.1 (2.7–3.7) | 13.3 (12.1–14.4) | 15.3 (14.0–16.8) | 10 (9–12) | 42 (38–45) | 49 (44–53) | 1.1 (1.0–1.2) | 4.5 (4.2–4.8) | 5.2 (4.8–5.6) |
| North Africa/Middle East | 225 | 410 | 10.3 (10.1–10.5) | 5.9 (5.8–6.1) | 11.2 (9.9–12.6) | 25.4 (23.6–27.1) | 27.4 (25.3–29.7) | 50 (44–56) | 113 (105–121) | 122 (113–132) | 4.4 (4.0–4.9) | 9.5 (8.9–10.1) | 10.3 (9.6–11) |
| South Asia | 776 | 1274 | 4.2 (4.1–4.2) | 4.8 (4.5–5.0) | 0.0 (0.0–0.0) | 7.9 (6.2–10.1) | 0.2 (0.1–0.3) | 0.0 (0.0–0.0) | 10 (8–13) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.9 (0.7–1.1) | 0.0 (0.0–0.0) |
| Sub‐Saharan Africa | 320 | 209 | 11.3 (11.1–11.6) | 4.2 (4.1–4.3) | 9.5 (8.7–10.3) | 17.2 (16.3–18.0) | 18.3 (17.2–19.5) | 30 (27–32) | 54 (51–56) | 57 (54–61) | 7.0 (6.3–7.6) | 12.2 (11.7–12.8) | 13.1 (12.3–13.9) |
| Western Europe | 301 | 744 | 12.6 (12.5–12.7) | 5.2 (5.1–5.3) | 38.7 (34.7–42.9) | 64.3 (58.5–70.4) | 68.2 (61.9–74.5) | 128 (115–142) | 214 (194–234) | 226 (205–247) | 8.2 (7.8–8.6) | 13.3 (12.7–13.8) | 14.3 (13.7–14.8) |
Each region includes countries as follows: Australasia: Australia, New Zealand; Canada and United States: Canada, United States of America; East/Central Eurasia: Albania, Armenia, Azerbaijan, Bulgaria, Bosnia and Herzegovina, Belarus, Czech Republic, Estonia, Georgia, Croatia, Hungary, Kazakhstan, Kyrgyzstan, Lithuania, Latvia, Moldova, Macedonia, Montenegro, Mongolia, Poland, Romania, Russian Federation, Serbia, Slovakia, Slovenia, Tajikistan, Turkmenistan, Ukraine, Uzbekistan; East/Southeast Asia: Darussalam, China, Fiji, Micronesia, Indonesia, Cambodia, Kiribati, Republic of Korea, Lao People's Democratic Republic, Sri Lanka, Maldives, Marshall Islands, Myanmar, Malaysia, Philippines, Papua New Guinea, Solomon Islands, Thailand, Timor‐Leste, Tonga, Taiwan, Vietnam, Vanuatu, Samoa, Brunei, Japan, Democratic People's Republic of Korea, Singapore; Latin America/Caribbean: Argentina, Antigua and Barbuda, Bahamas, Belize, Bolivia, Brazil, Barbados, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, Grenada, Guatemala, Guyana, Honduras, Haiti, Jamaica, Saint Lucia, Mexico, Nicaragua, Panama, Peru, Paraguay, El Salvador, Suriname, Trinidad and Tobago, Uruguay, Saint Vincent and the Grenadines, Venezuela; North Africa/Middle East: United Arab Emirates, Bahrain, Algeria, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libyan Arab Jamahiriya, Morocco, Oman, Occupied Palestinian Territory, Qatar, Saudi Arabia, Syrian Arab Republic, Tunisia, Turkey, Yemen; South Asia: Bangladesh, Bhutan, India, Nepal, Pakistan; sub‐Saharan Africa: Angola, Burundi, Benin, Burkina Faso, Botswana, Central African Republic, Côte d'Ivoire, Cameroon, Democratic republic of the Congo, Congo, Comoros, Cape Verde, Djibouti, Eritrea, Ethiopia, Gabon, Ghana, Guinea, Gambia, Guinea‐Bissau, Equatorial Guinea, Kenya, Liberia, Lesotho, Madagascar, Mali, Mozambique, Mauritania, Mauritius, Malawi, Namibia, Niger, Nigeria, Rwanda, Sudan, Senegal, Sierra Leone, Somalia, São Tomé and Príncipe, Swaziland, Seychelles, Chad, Togo, United Republic of Tanzania, Uganda, South Africa, Zambia, Zimbabwe; Western Europe: Andorra, Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Ireland, Iceland, Israel, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, United Kingdom. %E indicates percentage of total energy intake; CHD, ischemic heart disease; MUFA, monounsaturated fat; n‐6 PUFA, ω‐6 polyunsaturated fat; SFA, saturated fat; TFA, trans fat; UI, uncertainly interval.
Mean intake level (95% UI) was weighted based on the population in each country‐, age‐, and sex‐specific stratum in 2010.
Model decreases SFA to 10%E and replaces it isocaloriacally with either n‐6 PUFA or MUFA.
Model decreases SFA to 7%E but only if it can be isocalorically replaced with n‐6 PUFA to maximally 12%.
Model decreases SFA to 7%E and replaces it isocaloriacally with either n‐6 PUFA or MUFA.
Nation‐Specific CHD Attributable Mortality
Across 186 individual nations in 2010, the highest number of n‐6 PUFA–attributable absolute CHD deaths were observed in several former Soviet states, in particular Ukraine (647 CHD deaths per year per 1 million adults, 95% UI 505–823) (Figure 3, Table S1). In tropical oil–consuming nations such as Kiribati, Solomon Islands, Philippines, and Malaysia, about 1 in 5 CHD deaths were attributed to insufficient n‐6 PUFA.
Figure 3.
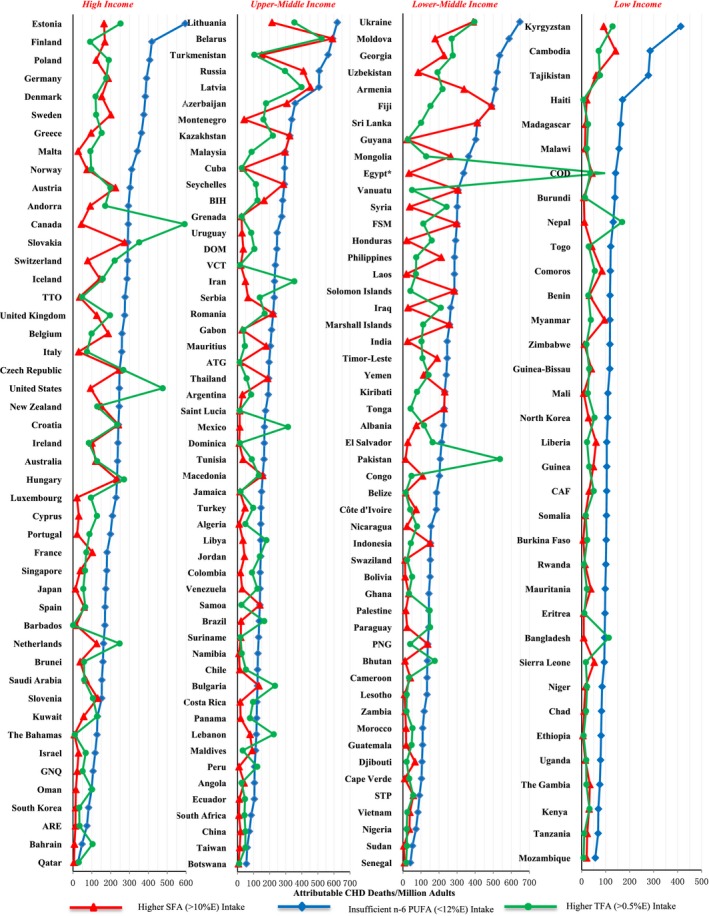
Global absolute CHD mortality attributable to SFA, n‐6PUFA, and TFA in 2010. World Health Organization income levels are as follows: high, ≥$12 616 per capita; upper‐middle, $4086 to $12 615 per capita; lower‐middle, $1036 to $4085 per capita; low, ≤$1035 per capita. Attributable CHD mortality was estimated for (A) higher SFA intake (red triangles), modeled as decreasing consumption to 10%E when isocalorically replaced with PUFA up to 12%E; (B) insufficient n‐6 PUFA (blue diamonds), modeled as increasing consumption to 12%E when isocalorically replaced with either carbohydrates or SFA; and (C) higher TFA (green circles), modeled as decreasing consumption to 0.5%E when isocalorically replacing with other fats. *In Egypt, TFA‐attributable CHD mortality per 1 million adults was 1120, beyond the x‐axis scale. %E indicates percentage of total energy intake; ARE, United Arab Emirates; ATG, Antigua and Barbuda; BIH, Bosnia and Herzegovina; CAF, Central African Republic; CHD, coronary heart disease; COD, Democratic Republic of the Congo; DOM, Dominican Republic; FSM, Federated States of Micronesia; GNQ, Equatorial Guinea; n‐6 PUFA, ω‐6 polyunsaturated fat; PNG, Papua New Guinea; SFA, saturated fat; STP, Sao Tome and Principe; TFA, trans fat; TTO, Trinidad and Tobago; VCT, Saint Vincent and the Grenadines.
In most countries, magnitudes of absolute and proportional SFA‐attributable CHD mortality were smaller than those for n‐6 PUFA (typically ≈60% lower) (Figure 4, Table S1), except in tropical oil–consuming nations with very high SFA intakes. The largest relative differences in n‐6 PUFA– versus SFA‐attributable CHD mortality were found in some South Asian nations, including Pakistan, Bhutan, Nepal, and Bangladesh, as well as Caribbean and sub‐Saharan African nations. In these, CHD mortality attributable to SFA was a fraction (often <10%) of that attributable to insufficient n‐6 PUFA.
Figure 4.
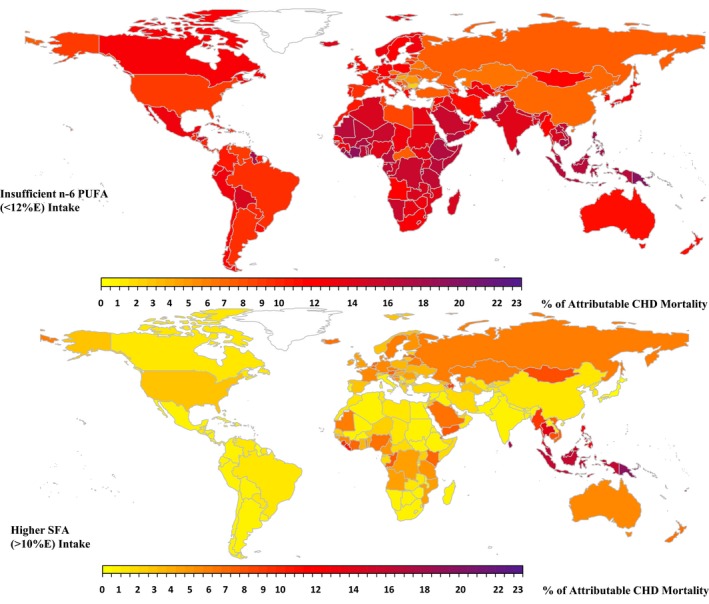
Global proportional CHD mortality attributable to SFA and n‐6 PUFA in 2010. The proportion of CHD mortality attributable to different dietary fats was calculated by dividing the number of attributable CHD deaths by the total number of CHD deaths within each country. The color scale of each map indicates the proportional CHD mortality in 186 countries attributable to the given dietary fat. The optimal level is 10±1%E for SFA and 12±1.2%E for n‐6 PUFA. %E indicates percentage of total energy intake; CHD, coronary heart disease; n‐6 PUFA, ω‐6 polyunsaturated fat; SFA, saturated fat.
Among the 20 most populous countries, Russia, Germany, and Egypt had the highest absolute CHD mortality attributable to insufficient n‐6 PUFA, with >335 CHD deaths per year per 1 million adults in each (Figure 5, Table 4). SFA‐attributable absolute CHD mortality was also highest in Russia as well as in the Philippines and Thailand. In contrast, Iran, Pakistan, and India had few SFA‐attributable CHD deaths but had substantial CHD mortality attributable to insufficient n‐6 PUFA.
Figure 5.
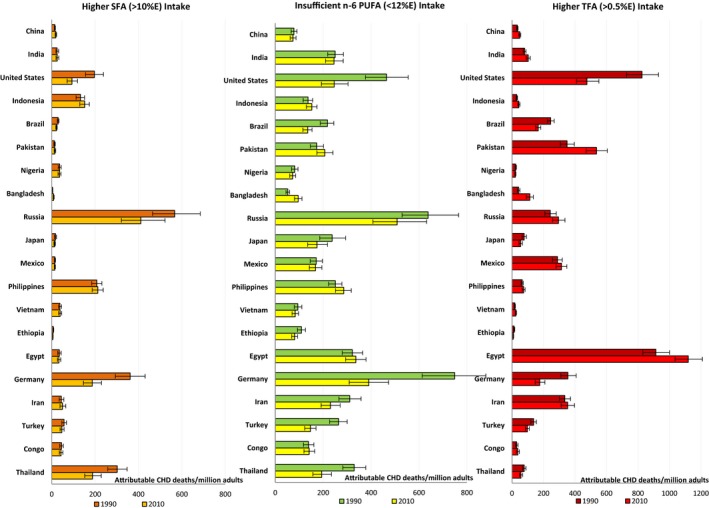
Annual CHD mortality attributable to SFA, n‐6PUFA, and TFA in the world's 20 most populous nations in 1990 and 2010. The x‐axis represents CHD deaths per 1 million adults attributable to different dietary fats, calculated by dividing the number of attributable CHD deaths by the adult population (defined as people aged ≥25 years) of the specific country and then multiplying by 1 million. The y‐axis (from the top to the bottom) shows the 20 most populous countries in 2010. The error bars represent the 95% uncertainty level. The optimal level is 10±1%E for SFA, 12±1.2%E for n‐6 PUFA, and 0.5±0.05%E for TFA. %E indicates percentage of total energy intake; CHD, coronary heart disease; n‐6 PUFA, ω‐6 poly‐unsaturated fat; SFA, saturated fat; TFA, trans fat.
The highest TFA‐attributable absolute CHD mortality was found in Egypt, with 1120 (95% UI 1036–1209) deaths per year per 1 million adults (Figure 3, Table S1). Other countries with substantial TFA‐associated CHD mortality included Canada, Pakistan, and the United States, each with >475 TFA‐attributable CHD deaths per year per 1 million adults. In these countries, excess TFA accounted for >17% of corresponding national CHD mortality (Figure 6). In comparison, 33 of 186 countries had proportional TFA‐attributable mortality <3%.
Figure 6.
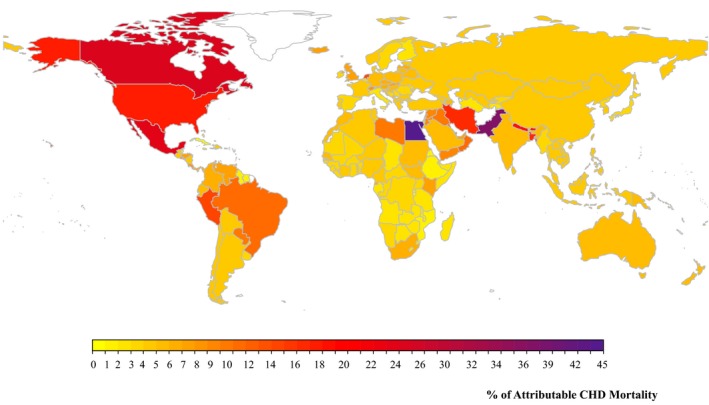
Global proportional CHD mortality attributable to higher TFA intake in 2010. The proportion of CHD mortality attributable to TFA was calculated by dividing the number of attributable CHD deaths by the total number of CHD deaths within each country. The color scale of each map indicates the proportional CHD mortality in 186 countries attributable to TFA. The optimal level is 0.5±0.05%E (percentage of total energy intake). CHD indicates coronary heart disease; TFA, trans fat.
Temporal Trends
From 1990 to 2010, global mean dietary intakes increased by 0.5%E for n‐6 PUFA and 0.1%E for TFA and decreased by 0.2%E for SFA, corresponding to relative changes of +8%, +11%, and −2% (Tables 3 and 4).7 Consistent with these dietary changes, global proportional attributable CHD mortality between 1990 and 2010 decreased by 9% for insufficient n‐6 PUFA and 21% for higher SFA but increased by 4% for higher TFA.
Nearly all world regions experienced stable or declining trends in proportional n‐6 PUFA– and SFA‐attributable CHD mortality over this time period, except for Oceania, which experienced a 5% increase (Figures 1 and 7). For insufficient n‐6 PUFA, Eastern Europe, East Asia, and the Caribbean experienced the most substantial declines in proportional attributable CHD mortality (−26%, −24%, −18%). Conversely, many world regions experienced increases in proportional TFA‐attributable CHD mortality, largest in Asia (+12.5%~33.8%) (Figure 2), Central America (+36.3%), and the Caribbean (+30.7%). In contrast to these developing regions, Western Europe experienced large declines in proportional TFA‐attributable CHD mortality (−14.7%).
Figure 7.
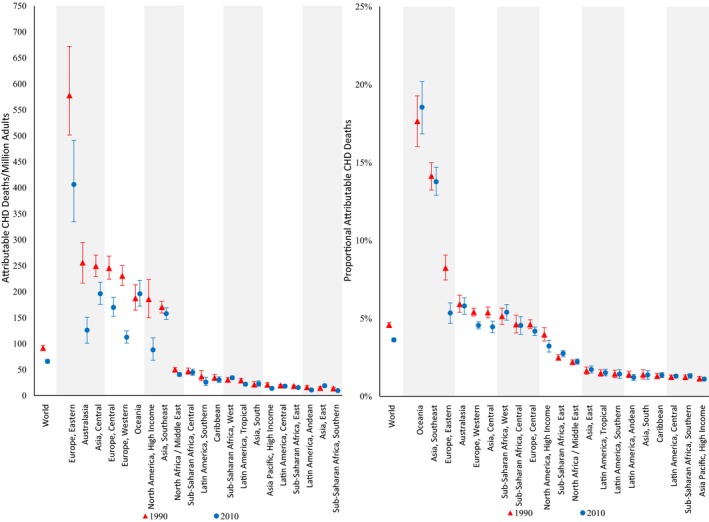
Regional CHD mortality attributable to higher SFA intake in 1990 and 2010. The y‐axis represents the CHD deaths per 1 million adults (on the left) or the proportion of CHD deaths (on the right) attributable to higher SFA intake. The x‐axis includes the world estimates as well as the estimates of the 21 regions. Red triangles indicate estimates in 1990, whereas blue circles indicate estimates in 2010. The error bars represent the 95% uncertainty level of each estimate. CHD indicates coronary heart disease; SFA, saturated fat.
Nation‐specific trends in CHD mortality attributable to different dietary fats from 1990 to 2010 are shown in Tables S1 and S2. Among the 20 most populous nations, the United States, Germany, and Thailand experienced decreases and Bangladesh experienced an increase in age‐standardized CHD mortality per 1 million population that was attributable to all dietary fats (Figure 5).
Discussion
Our new findings, based on best available data on dietary fat consumption; diet‐disease etiologic effects; and country‐, age‐, and sex‐specific CHD mortality, provide estimates of global, regional, and national burdens of CHD mortality attributable to nonoptimal n‐6 PUFA, SFA, and TFA. In 2010, an estimated 711 800, 250 900, and 537 200 CHD deaths worldwide were attributable to nonoptimal n‐6 PUFA, SFA, and TFA, respectively, corresponding to 10.3%, 3.6%, and 7.7% of global CHD mortality. Important heterogeneity was identified across world regions and nations. In addition, between 1990 and 2010, estimated proportional CHD mortality for nonoptimal n‐6 PUFA and SFA decreased by 9% and 21%, respectively, whereas for TFA, it increased 4%. These global trends represented averages of important regional and national differences, such as increases in n‐6 PUFA‐attributable CHD mortality in Oceania but decreases in most other regions and increases in TFA‐attributable CHD mortality in low‐ and middle‐income countries but decreases in Western Europe.
Growing evidence indicates that lowering SFA provides convincing cardiovascular benefits only when replaced by PUFA, whereas cardiovascular benefits of n‐6 PUFA are similar whether replacing SFA or total carbohydrates.4, 6, 10 Our analysis provides, for the first time, a rigorous comparison of global CHD burdens attributable to insufficient n‐6 PUFA versus higher SFA. In 80% of nations, n‐6 PUFA–attributable CHD burdens were at least 2‐fold higher than SFA‐attributable burdens. This suggests that focus on increasing healthful n‐6–rich vegetable oils may provide important public health benefits. In countries such as Ethiopia and Pakistan, n‐6 PUFA–attributable CHD mortality was >15 times that attributable to SFA, suggesting needs to prioritize increases in n‐6 PUFA–rich vegetable oils rather than decreased SFA in these countries. In tropical oil–producing nations in Southeast Asia and Oceania, SFA‐ and n‐6 PUFA–attributable CHD burdens were more similar, consistent with very high consumption of SFA from tropical oils, especially palm oil.
Current evidence on benefits of exchanging SFA with PUFA derives mainly from studies replacing animal fats, especially meats and butter, with soybean and other vegetable oils.4, 5, 26 Cardiovascular effects of SFA from different food sources, or perhaps more relevantly the net cardiovascular effects of different SFA‐rich foods, may differ widely.26, 27 Health effects of tropical oils, for example, may be influenced by triglyceride regioisomerism28 or benefits of trace phytochemicals.29 This remains speculative, and long‐term studies are required to evaluate the health effects of tropical oils. Our results should be considered the best currently available estimates of CHD burdens attributable to average SFA consumption from animal fats, especially meats and butter, when replaced fully with PUFA. Caution should be exercised when interpreting our estimated SFA‐attributable burdens in nations having meaningful SFA intake from other sources, such as cheese, yogurt, or tropical oils. If cardiovascular effects of total SFA are similar for animal fats versus tropical oils, then the identified SFA‐related CHD mortality calls for stronger policy efforts to replace tropical oils with PUFA‐rich vegetable oils in Southeast Asia and Oceania. Current efforts mainly rely on nutrition labeling to reduce SFA, but that may have small effects30 resulting from low public awareness, confusion or misinterpretation of the label, and low access to n‐6–rich alternatives.
In sensitivity analysis, SFA‐attributable burdens would be larger if replacement with either PUFA or MUFA would provide benefits. Using MUFA would also provide a wider, more feasible range of fat/oil alternatives. Unfortunately, evidence for the cardiovascular benefits of total MUFA remains uncertain.4, 10 Based on limited numbers of trials, MUFA from nuts and extra virgin olive oil appears likely to provide cardiometabolic benefits31, 32; however, these represent minor global sources of MUFA and emphasize the need for more research on long‐term health effects of other common sources.
We evaluated 2 potentially optimal levels of SFA: 10%E and 7%E. In 2010, 75 of 186 countries had already achieved the 10%E level, whereas only 18 had achieved 7%E. The latter, however, tended to be poor countries with higher levels of hunger and malnutrition; diets rich in inexpensive, starchy staples; and diets lower in more diverse, healthful foods.12 In these nations, very low SFA consumption is often paired with high consumption of refined grains or starches, which may be more harmful than SFA.4, 5 This highlights the need for caution and monitoring of actual nutrient replacements if SFA is targeted in any given country.
Even at low intake levels, TFA‐attributable mortality remains high globally. This is consistent with unique adverse effects of industrially produced TFA on both lipid and nonlipid pathways.3 We found that between 1990 and 2010, TFA‐attributable CHD mortality decreased in many high‐income countries, consistent with ongoing policy strategies to reduce industrial TFA.33, 34 Nevertheless, we estimated remaining TFA consumption to cause >15% of CHD deaths in countries such as the United States and Canada, exceeding CHD mortality attributable to SFA. Given ongoing industry reformulations and absence of reliable national TFA consumption data, these findings should be interpreted cautiously and updated as more data become available. National reformulations suggest that TFA reduction is slowing in the United States,35 indicating a need for continued surveillance and strong policy efforts.
In contrast to Western nations, we found increased TFA‐attributable burdens in many middle‐ and low‐income countries between 1990 and 2010 (eg, Egypt, Pakistan, Mexico). In these countries, exposure to TFA likely derives not only from industrially packaged foods but also from widespread use of inexpensive partially hydrogenated cooking fats in homes, in small restaurants, and by street‐food vendors. These diverse sources represent a challenge to reducing TFA in developing nations and suggest a need for coordinated national policies including mandatory labeling, direct restrictions, and government‐promoted industry self‐regulation.36, 37
Validity of our estimates is influenced by the validity of the etiologic effects. For n‐6 PUFA and industrial TFA, estimated etiologic effects are similar whether considering predicted effects based on established changes in metabolic risk factors from randomized trials, observed relationships with clinical events in prospective cohorts, or (for PUFA) pooled effects on events in meta‐analysis of clinical trials.4, 6, 20 For SFA replacing PUFA, evidence is similar, although, as noted earlier, such effects appear to vary depending on the food source, making estimated SFA‐attributable burdens more uncertain in nations (and persons) with diverse food sources of SFA. The dietary fats investigated in this study are also 1 component of overall dietary quality. Other cardiometabolic risks, such as other dietary factors, physical activity, smoking, medication, and obesity, influence CHD and contribute to total burdens. Our findings represent estimates of independent contributions of these dietary fats to CHD mortality worldwide, reflecting the average population effect within each age, sex, and country stratum, not the burden for any individual patient. Nevertheless, benefits from other dietary components, such as dietary fiber, plant‐based proteins, and other phytochemicals derived from fruits, vegetables, whole grains, nuts, and legumes, while limiting added sugars and salt, also deserve attention.
Our investigation has several strengths. We used the most valid available global data on dietary consumption based on systematic searches and extensive direct contacts for nationally representative individual‐level dietary surveys, complemented by national food availability and industry data. We evaluated and used evidence on heterogeneity of diet–disease relationships, in particular, by age. Underlying death rates across countries were systematically corrected for differences in data availability and national coding patterns. We incorporated and accounted for sources of uncertainty, including uncertainty in the dietary data and diet–disease etiologic effects. We did not perform ecologic (correlative) analyses of dietary fats and CHD, which could be strongly biased by cross‐national confounders and ecologic fallacy, but rather used comparative risk assessment based on external published evidence on etiologic effects on clinical CHD events.
Potential limitations should be considered. Due to less available data, our estimates were more uncertain in some regions, inflating uncertainty of estimated disease burdens. Few national surveys assessed TFA, which we evaluated based on available dietary surveys, blood TFA levels, and industry sales data on partially hydrogenated oils and packaged foods. These findings highlight the need for expanded surveillance of TFA in both developed and developing countries to help inform public policy. Our TFA‐attributable burdens are based on average effects of TFA from partially hydrogenated oils, and certain isomers (eg, 18:2 isomers) may have more harmful effects. Most cohorts included in meta‐analyses of diet–disease relationships did not correct for dietary variation over time, resulting in underestimation of true etiologic effects and attributable mortality. Except for age, modification effects of other cardiometabolic risk factors were not identified; such effects can be incorporated in future analyses if such evidence emerges. We evaluated CHD mortality, and attributable burdens owing to nonfatal CHD events would be higher.
In conclusion, we estimated that insufficient n‐6 PUFA, excess TFA, and, to a lesser extent, excess SFA are leading to significant CHD mortality globally. These findings will help inform global, regional, and national policy priorities and public health programs to reduce burdens of chronic disease.
Appendix
This study was a collaborative effort of the Nutrition and Chronic Diseases Expert Group (NutriCoDE) as part of the 2010 Global Burden of Diseases, Injuries, and Risk Factors (GBD) study. NutriCoDE Core group: Dariush Mozaffarian, Renata Micha, Peilin Shi, Friedman School of Nutrition Science & Policy, Tufts University, Boston; Majid Ezzati, Imperial College London, London, UK; Saman Fahimi, University of Cambridge, Cambridge, UK; Shahab Khatibzadeh, Harvard T.H. Chan School of Public Health, Boston; John Powles, University of Cambridge, Cambridge, UK.
Other members: Ibrahim Elmadfa, Institute of Nutritional Sciences, University of Vienna, Austria; Mayuree Rao, Warren Alpert Medical School of Brown University, Providence, RI; Pattra Wirojratana, Harvard T.H. Chan School of Public Health.
Dietary exposure imputation: Stephen S. Lim, Rebecca E. Engell, Institute for Health Metrics and Evaluation, University of Washington, Seattle; Kathryn G. Andrews, African Leaders Malaria Alliance, Dar es Salaam, Tanzania.
Dietary exposures—corresponding members: Pamela A. Abbott, University of Aberdeen, UK; Morteza Abdollahi, National Nutrition and Food Technology Research Institute, Iran; Enrique O. Abeyá Gilardon, Ministerio de Salud, Argentina; Habibul Ahsan, University of Chicago; Mohannad Abed Alfattah Al Nsour, Eastern Mediterranean Public Health Network (EMPHNET), Jordan; Suad N. Al‐Hooti, Kuwait Institute for Scientific Research, Kuwait; Carukshi Arambepola, Faculty of Medicine, University of Colombo, Sri Lanka; Hubert Barennes, Institut Francophone pour la Médecine Tropicale, Lao PDR; Simon Barquera, Instituto Nacional de Salud Publica (INSP), Mexico; Ana Baylin, University of Michigan, US; Wulf Becker, National Food Agency, Sweden; Peter Bjerregaard, National Institute of Public Health, University of Southern Denmark, Denmark; Lesley T. Bourne, Environment and Health Research Unit, Medical Research Council, South Africa; Neville Calleja, Department of Health Information & Research, Malta; Mario V. Capanzana, Food and Nutrition Research Institute, Philippines; Katia Castetbon, Institut de veille sanitaire, France; Hsing‐Yi Chang, National Health Research Institutes, Taiwan; Yu Chen, New York University School of Medicine; Melanie J. Cowan, WHO, Switzerland; Stefaan De Henauw, Ghent University, Department of Public Health, Belgium; Eric L. Ding, Harvard Medical School and Harvard School of Public Health; Charmaine A. Duante, Food and Nutrition Research Institute‐Department of Science and Technology, Philippines; Pablo Duran, Dirección Nacional de Maternidad e Infancia, Ministerio de Salud de la Nación, Argentina; Ibrahim Elmadfa, Institute of Nutritional Sciences, University of Vienna, Austria; Heléne Enghardt Barbieri; Farshad Farzadfar, Tehran University of Medical Sciences, Iran; Dulitha N. Fernando, Faculty of Medicine, University of Colombo, Sri Lanka; Aida Filipovic Hadziomeragic, Institute of Public Health of Federation of Bosnia and Herzegovina, Bosnia and Herzegovina; Regina M. Fisberg, Faculty of Public Health—University of São Paulo, Brazil; Simon Forsyth; Didier Garriguet, Statistics Canada, Canada; Jean‐Michel Gaspoz, Geneva University Hospitals and Faculty of Medicine of Geneva, Switzerland; Dorothy Gauci, Department of Health Information and Research, Malta; Brahmam N. V. Ginnela, National Institute of Nutrition, Indian Council of Medical Research, India; Idris Guessous, Geneva University Hospitals, Switzerland; Martin C Gulliford, King's College London, UK; Wilbur Hadden; Christian Haerpfer, University of Aberdeen, UK; Daniel J. Hoffman, Rutgers, State University of New Jersey; Anahita Houshiar‐Rad, National Nutrition and Food Technology Research Institute Shahid Beheshti University of Medical Sciences Tehran, Iran, IRIran; Inge Huybrechts, International Agency for Research on Cancer, Lyon, France; Nahla C. Hwalla, American University of Beirut, Lebanon; Hajah Masni Ibrahim, Ministry of Health, Brunei; Manami Inoue, Epidemiology and Prevention Division, Research Center for Cancer Prevention and Screening, National Cancer Center, Japan; Maria D. Jackson, University of the West Indies, Jamaica; Lars Johansson, Norwegian Directorate of Health, Norway; Lital Keinan‐Boker, Ministry of Health, Israel; Cho‐il Kim, Korea Health Industry Development Institute, Republic of Korea; Eda Koksal, Gazi University, Turkey; Hae‐Jeung Lee; Yanping Li, Harvard School of Public Health; Nur Indrawaty Lipoeto, Andalas University, Indonesia; Guansheng Ma, National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, China; Guadalupe L. Mangialavori, Ministerio de Salud de la Nación (National Health Ministry), Argentina; Yasuhiro Matsumura, Bunkyo University, Japan; Stephen T. McGarvey, Brown University; Chan Mei Fen; Robert Koch Institute, Germany; Rafael A. Monge‐Rojas, Costa Rican Institute for Research and Education and Nutrition and Health (INCIENSA), Costa Rica; Abdulrahman Obaid Musaiger, Arab Center for Nutrition, Bahrain; Balakrishna Nagalla, National Institute of Nuyrition, Hyderabad India; Androniki Naska, Department of Hygiene, Epidemiology and Medical Statistics, University of Athens Medical School, Greece; Marga C. Ocke, National Institute for Public Health and the Environment, Netherlands; Maciej Oltarzewski, National Food and Nutrition Institute, Poland; Philippos Orfanos, Department of Hygiene, Epidemiology and Medical Statistics, University of Athens Medical School, Greece; Marja‐Leena Ovaskainen, National Institute for Health and Welfare, Finland; Wen‐Harn Pan, Division of Preventive Medicine and Health Services Research, Institute of Population Health Sciences, National Health Reserch Institutes, Taiwan; Demosthenes B. Panagiotakos, Harokopio University, Greece; Gulden Ayla Pekcan, Hacettepe University Department of Nutrition and Dietetics, Turkey; Stefka Petrova, National Center of Public Health and Analyses, Bulgaria; Noppawan Piaseu, Mahidol University, Thailand; Christos Pitsavos, Athens University Medical School, Greece; Luz Gladys Posada, Universidad de Antioquia, Colombia; Leanne M. Riley, WHO, Switzerland; Luz Maria Sánchez‐Romero, National Institute of Public Health, Mexico; Rusidah B. T. Selamat, Nutrition Division, Ministry of Health Malaysia, Putrajaya, Malaysia; Sangita Sharma; Abla Mehio Sibai, American University of Beirut‐ Faculty of Health Sciences, Lebanon; Rosely Sichieri, State University of Rio de Janeiro, Brazil; Chansimaly Simmala, Institut of Tropical Medecin, Laos; Laufey Steingrimsdottir, Iceland; Gillian Swan; Elżbieta Halina Sygnowska, National Institute of Cardiology, Poland; Lucjan Szponar, National Food and Nutrition Institute, Poland; Heli Tapanainen, National Institute for Health and Welfare, Finland; Robert Templeton; Anastasia Thanopoulou, Diabetes Center, 2nd Department of Internal Medicine, National University of Athens, Hippokration General Hospital, Greece; Holmfridur Thorgeirsdóttir, Directorate of Health, Iceland; Inga Thorsdottir; Antonia Trichopoulou, Hellenic Health Foundation, Greece; Shoichiro Tsugane, National Cancer Center, Japan; Aida Turrini, National Research Institute on Food and Nutrition, Italy; Sirje Vaask, Tallinn University of Technology, Estonia; Coline van Oosterhout, National Institute for Public Health and the Environment, Netherlands; J Lennert Veerman, University of Queensland, Australia; Nowak Verena; Anna Waskiewicz, Institute of Cardiology, Department of Cardiovascular Diseases Epidemiology, Prevention and Health Promotion, Poland; Sahar Zaghloul, National Nutrition Institute, Egypt; Gábor Zajkás, National Institute of Food and Nutrition Sciences, Hungary.
Sources of Funding
This work was undertaken as part of the 2010 Global Burden of Diseases, Injuries, and Risk Factors Study, supported in part by the Bill and Melinda Gates Foundation; and by the National Institutes of Health (R01 HL115189). The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosures
Dr Mozaffarian reports ad hoc honoraria/consulting from Bunge, Haas Avocado Board, Nutrition Impact, Life Sciences Research Organization, Astra Zeneca, and Boston Heart Diagnostics; membership, scientific advisory board, Unilever North America. Other authors declare no conflict of interest.
Supporting information
Table S1. Coronary Heart Disease Mortality Attributable to Saturated Fat, ω‐6 Polyunsaturated Fat, and Trans Fat in 2010 by Country, Age, and Sex
Table S2. Coronary Heart Disease Mortality Attributable to Saturated Fat, ω‐6 Polyunsaturated Fat, and Trans Fat in 1990 by Country, Age, and Sex
Acknowledgments
Author contributions: All authors were responsible for study concept and design, data interpretation, critical manuscript revision, and final manuscript approval. Micha, Khatibzadeh, and Mozaffarian conducted the systematic searches and data collection. Wang performed data analysis and manuscript drafting. Wang and Mozaffarian are study guarantors. All authors had access to all data sources and have responsibility for contents of the report and decision to submit for publication.
(J Am Heart Assoc. 2016;5:e002891 doi: 10.1161/JAHA.115.002891)
Accompanying Tables S1 and S2 are available at http://jaha.ahajournals.org/content/5/1/e002891/suppl/DC1
Individual members of the Nutrition and Chronic Diseases Expert Group (NutriCoDE) are listed in the Appendix.
References
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker‐Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez‐Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez‐Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui‐Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Willett WC. Trans fatty acids and cardiovascular risk: a unique cardiometabolic imprint? Curr Atheroscler Rep. 2007;9:486–493. [DOI] [PubMed] [Google Scholar]
- 4. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta‐analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta‐analysis of prospective cohort studies. Circulation. 2014;130:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D; Global Burden of Diseases N, Chronic Diseases Expert Group NutriCo DE . Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country‐specific nutrition surveys. BMJ. 2014;348:g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ; Comparative Risk Assessment Collaborating G . Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 9. Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. Alpha‐linolenic acid and risk of cardiovascular disease: a systematic review and meta‐analysis. Am J Clin Nutr. 2012;96:1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scientific report of the 2015 dietary guidelines advisory committee. USDA; 2015. [Google Scholar]
- 11. Micha R, Kalantarian S, Wirojratana P, Byers T, Danaei G, Elmadfa I, Ding E, Giovannucci E, Powles J, Smith‐Warner S, Ezzati M, Mozaffarian D; Global Burden of Diseases N, Chronic Disease Expert G . Estimating the global and regional burden of suboptimal nutrition on chronic disease: methods and inputs to the analysis. Eur J Clin Nutr. 2012;66:119–129. [DOI] [PubMed] [Google Scholar]
- 12. Imamura F, Micha R, Khatibzadeh S, Fahimi S, Shi P, Powles J, Mozaffarian D; Global Burden of Diseases N, Chronic Diseases Expert G . Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health. 2015;3:e132–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khatibzadeh SM, Micha R, Afshin A, Rao M, Yakoob MY, Mozaffarian D. Major dietary risk factors for chronic diseases: a systematic review of the current evidence for causal effects and effect sizes. Circulation. 2012;125:AP060. [Google Scholar]
- 14. Simopoulos AP. The importance of the omega‐6/omega‐3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. [DOI] [PubMed] [Google Scholar]
- 15. Simopoulos AP, Leaf A, Salem N Jr. Essentiality of and recommended dietary intakes for omega‐6 and omega‐3 fatty acids. Ann Nutr Metab. 1999;43:127–130. [DOI] [PubMed] [Google Scholar]
- 16. Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak‐Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta‐analysis. BMJ. 2013;346:e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–1041, 1041.e1021‐1015. [DOI] [PubMed] [Google Scholar]
- 18. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta‐analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta‐analysis. Ann Intern Med. 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 20. Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2009;63(suppl 2):S22–S33. [DOI] [PubMed] [Google Scholar]
- 21. Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu F, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation. 2001;103:856–863. [DOI] [PubMed] [Google Scholar]
- 22. Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, Becker W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. 2014;58: eCollection 2014 doi:10.3402/fnr.v58.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating G, Asia‐Pacific Cohort Studies C, Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in E, Emerging Risk Factor C, Prospective Studies C . The age‐specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishida C, Uauy R. Who scientific update on health consequences of trans fatty acids: introduction. Eur J Clin Nutr. 2009;63(suppl 2):S1–S4. [DOI] [PubMed] [Google Scholar]
- 25. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. [DOI] [PubMed] [Google Scholar]
- 26. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibai TA, Jacobs DR Jr, Nettleton J. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunter JE. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids. 2001;36:655–668. [DOI] [PubMed] [Google Scholar]
- 29. Edem DO. Palm oil: biochemical, physiological, nutritional, hematological, and toxicological aspects: a review. Plant Foods Hum Nutr. 2002;57:319–341. [DOI] [PubMed] [Google Scholar]
- 30. Shangguan S, Smith J, Ma W, Tanz L, Afshin A, Mozaffarian D. Effectiveness of point‐of‐purchase labeling on dietary consumption and nutrient contents of foods: a systemic review and meta‐analysis (abstract). Circulation. 2015;131(suppl 1):AP323. [Google Scholar]
- 31. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM; OmniHeart Collaborative Research G . Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. [DOI] [PubMed] [Google Scholar]
- 32. Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, Gomez‐Gracia E, Ruiz‐Gutierrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez‐Gonzalez MA; Investigators PS . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 33. Department of Health and Human Services FaDA . Tentative determination regarding partially hydrogenated oils; request for comments and for scientific data and information. Friday, November 08, 2013. Available at: https://federalregister.gov/a/2013-26854. Accessed December 30, 2015.
- 34. Canada H. Regulations amending the food and drug regulations (nutrition labelling, nutrient content claims and health claims). Canada Gazette 137, Part II, January 1, 2003. Available at: http://canadagazette.gc.ca/partII/2003/20030101/html/sor110e.htm. Accessed October 30, 2008.
- 35. Otite FO, Jacobson MF, Dahmubed A, Mozaffarian D. Trends in trans fatty acids reformulations of us supermarket and brand‐name foods from 2007 through 2011. Prev Chronic Dis. 2013;10:E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Downs SM, Thow AM, Leeder SR. The effectiveness of policies for reducing dietary trans fat: a systematic review of the evidence. Bull World Health Organ. 2013;91:262–269H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Downs SM, Thow AM, Ghosh‐Jerath S, McNab J, Reddy KS, Leeder SR. From Denmark to Delhi: the multisectoral challenge of regulating trans fats in India. Public Health Nutr. 2013;16:2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Coronary Heart Disease Mortality Attributable to Saturated Fat, ω‐6 Polyunsaturated Fat, and Trans Fat in 2010 by Country, Age, and Sex
Table S2. Coronary Heart Disease Mortality Attributable to Saturated Fat, ω‐6 Polyunsaturated Fat, and Trans Fat in 1990 by Country, Age, and Sex


