Abstract
Objectives
Hypertension is a risk factor for the development of cardiovascular and kidney disease, but treatment can substantially reduce risks. Many patients avoid antihypertensive medications due to fear of side effects. While associations between antihypertensives and sexual dysfunction in men have been documented, it remains unclear whether antihypertensives are associated with sexual dysfunction in women. We conducted a cross-sectional analysis of baseline data from women in the Systolic Blood Pressure Intervention Trial (SPRINT) to evaluate the relations among class of antihypertensive medication and the outcomes (a) sexual activity and (b) sexual function.
Methods
SPRINT enrolled individuals 50 and older with hypertension at high risk for cardiovascular disease. A subset of participants completed questionnaires regarding quality of life (QoL), including sexual function. Antihypertensive class was determined by medications taken at baseline.
Results
Of 690 women in the QoL subset of SPRINT, 183 (26.5%) were sexually active. There were no significant differences in sexual activity among women taking one or more antihypertensives and women not taking any. Women taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACEI/ARB) had higher odds of sexual activity [OR 1.66 (1.12-4.27), p=0.011]. Among sexually active women, the prevalence of sexual dysfunction was high (52.5%). No class of medication was associated with sexual dysfunction in the multivariable model.
Conclusions
ACEI/ARB use was associated with higher odds of sexual activity. While prevalence of sexual dysfunction was high, no single class of antihypertensive medication was associated with sexual dysfunction.
Keywords: hypertension, antihypertensive agents, female sexual function, sexual activity
Introduction
Hypertension an important risk factor for the development of cardiovascular disease (1), the leading cause of death in the United States (2). Additionally, hypertension is associated with stroke (3) and kidney failure (4, 5). Adequate treatment of hypertension can result in substantial reductions in morbidity and mortality; however, many patients avoid antihypertensive medications due to the fear of side effects. Seventy percent of patients with hypertension who experience side effects are non-adherent with medications (6) and individuals whose antihypertensive agents have an adverse effect on quality of life (QoL) have 40-60% higher rates of discontinuation than those who are not affected (7).
One important aspect of QoL that may be affected by antihypertensive medications is sexual function. While several studies have documented associations among certain antihypertensive medications—particularly beta-blockers (8, 9) and diuretics (10-14)—with sexual dysfunction in men, it is unclear whether antihypertensive medications are associated with sexual dysfunction in women. The issue is complicated by the fact that hypertension itself is associated with female sexual dysfunction (15-18), and hypertension often develops in tandem with other factors that may influence female sexual function such as aging, menopause, and medical comorbidities. Additionally, female sexual function is a complex phenomenon, influenced by numerous biologic and psychosocial factors.
There are biologically plausible pathways by which antihypertensive medications may affect female sexual function. Many antihypertensive agents act by relaxing the smooth muscle of the tunica media resulting in vasodilation; the increased blood flow to vaginal tissues during arousal is also due to relaxation of smooth muscle (19). Animal models have shown that nitric oxide is involved in female sexual arousal (19); several antihypertensive medications – beta blockers (20) (21), angiotensin-converting enzyme inhibitors (ACEIs) (22, 23), angiotensin receptor blockers (ARBs) (22, 24), and calcium channel blockers (CCBs) (25) – affect the nitric oxide pathway, and may therefore actually improve female sexual function. In contrast, beta-blockers have been found to decrease serum testosterone levels (26-28) in men; their effects on serum testosterone in women are unknown. Serum testosterone has been associated with female sexual dysfunction in some studies (29-34), although this remains an area of controversy (35). If beta-blockers also lower testosterone in women, they may have negative effects on female sexual function.
Our aim was to conduct a cross sectional evaluation of the associations among different classes of antihypertensive medications and (a) sexual activity and (b) sexual function among female participants in Systolic Blood Pressure Intervention Trial (SPRINT). We hypothesized a priori that beta adrenergic blockers would be correlated with poorer sexual function in women, given their potential effects on sexual function in men and based on prior studies (7, 9), while other classes of antihypertensive medications would not.
Methods
Participants
SPRINT is a multicenter randomized controlled trial to test the effects of different blood pressure control targets (<140 mm Hg versus <120 mm Hg) on a variety of outcomes, including cardiovascular events, kidney function, and cognitive function. SPRINT recruited participants aged 50 years and older (including persons with cardiovascular disease, chronic kidney disease (CKD), age ≥75 years, or a 10-year Framingham cardiovascular risk of ≥15%). Persons with >1 g/day proteinuria, diabetes mellitus, polycystic kidney disease, or a history of stroke were excluded. Full details on the design of SPRINT, including definitions for inclusion and exclusion criteria, have been previously published (36, 37). Baseline data were collected in 2010-2013. A random sample of participants (1987/9361, 21.2%) completed questionnaires regarding QoL. Women in the QoL subset are basis for this analysis. All participants provided signed informed consent. The study was approved by the Institutional Review Board at each study site and was registered with clinicaltrials.gov (NCT01206062).
Primary measures
Sexual activity included any activity with or without a partner in the prior 4 weeks. Sexual function was assessed using the Female Sexual Function Index (FSFI)(38), a highly validated19-item questionnaire that assesses sexual function over six domains: desire, arousal, lubrication, orgasm, pain, and satisfaction. Higher scores indicate better sexual function, and a score of <27 has been established as a cutoff for sexual dysfunction (39).
Antihypertensive class was defined by the medications participants were taking at the baseline visit prior to randomization. Some participants were taking multiple and some were taking no antihypertensive medications. In addition to counting the number of medications used by each participant, we created indicator variables to capture use of any antihypertensive medication, use of ACEI or ARB, use of diuretics, use of beta-blockers, use of CCBs, and the use of any other antihypertensive agents (alpha blockers, alpha-beta blockers, direct vasodilators, direct renin inhibitors, or central alpha-2 agonists and other centrally acting drugs). Each medication class was considered as a dichotomous variable. Classifications were not mutually exclusive for women who were on more than one antihypertensive medication.
Covariates
All covariates were selected a priori based on factors related to sexual function in prior literature. Covariates included blood pressure, demographic variables (age, race and ethnicity, education level, living situation), other medications which may affect sexual function [selective serotonin reuptake inhibitors (SSRIs) or hormone therapy (HT)], and the presence of certain co-morbidities (chronic kidney disease, hyperlipidemia, and cardiovascular disease).
Blood pressure measurements were the average of 3 measurements taken at one-minute intervals after the participant had been sitting quietly in a room for 5 minutes. SPRINT did not assess whether participants were in a romantic relationship, but participants did indicate whether they lived with others or alone. Use of SSRIs or HT was assessed by self-report. Presence of CKD was defined as an estimated GFR of 20-59 ml/min/1.73m2. Hyperlipidemia was defined by serum total cholesterol >200 mg/dL. Cardiovascular disease was defined by previous clinical or subclinical cardiovascular disease other than stroke (individuals with stroke were excluded from SPRINT).
Analysis
Categorical variables are reported as N(%) and continuous variables as mean (SD). Total and domain specific FSFI scores were calculated only for sexually active women, with missing responses replaced with mean imputation (40).
Sexual activity
Among women in the QoL sample, we constructed separate univariable logistic regression models to examine the relation between (a) any versus no antihypertensive medication use and sexual activity; and (b) class of antihypertensive medication; and the outcome, sexual activity. Multivariable models were constructed to examine the relations among the same independent variables and the outcome while adjusting for above covariates. We report odds ratios (OR) and 95% confidence intervals (95% CI).
Sexual function
Among sexually active women in the QoL sample, we fit separate univariable linear regression models to examine the correlation between (a) any antihypertensive medication at baseline versus no antihypertensive medication; and (b) class of antihypertensive medication; and the outcome, total FSFI score. Multivariable models were constructed to examine the relations among the same independent variables and the outcome while adjusting for above covariates. Results are reported as beta coefficients and 95% confidence intervals.
Standard regression diagnostics were used to evaluate assumptions for both logistic and linear models, and no important violations were found. As sensitivity analyses, we also fit multivariable logistic and multivariable linear models where the dichotomous variable for any antihypertensive medication use was replaced by the number of medication classes used (0 to 4). We considered 2-tailed p-values <0.05 statistically significant. In these analyses, we did not adjust for multiple comparisons. All analyses were conducted with SAS 9.4 (Cary, NC, USA).
Results
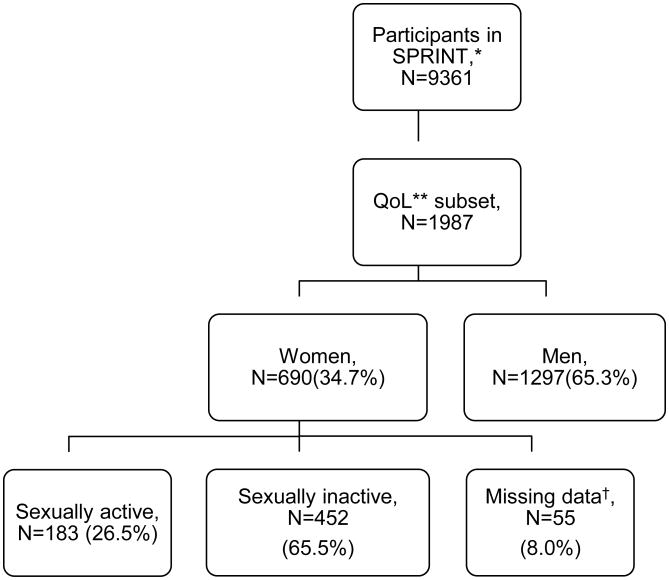
Of the 3332 randomized women in SPRINT, 1102 (33%) were on a beta-blocker, 1173 (35%) were on a calcium channel blocker, 1723 (52%) were on a diuretic, 1902 (57%) were on a RAS blocker, and 255 (8%) were on another antihypertensive medication. There were 690 women in the QoL subset of SPRINT. Of these, 183 (26.5%) were sexually active, 452 (65.5%) were not, and 55 (8.0%) had missing data regarding sexual function (Figure). All women in the QoL subset were included in the analysis of sexual activity. Only sexually active women were included in the analysis of sexual function. Demographic characteristics are summarized in Table 1. In the QoL subset, mean age was 67.6 (SD 10) years and 43.6% were white. The median number of medications used by sexually active women was 2. Sixty-one percent of sexually active women in the QoL subset were taking more than one category of antihypertensive medication. The most common combinations were a diuretic with an ACEI or ARB (18.6%), a beta-blocker with a diuretic and an ACEI or ARB (7.1%), and a calcium channel blocker with a diuretic and an ACEI or ARB (7.1%).
Figure.
*SPRINT=Systolic Blood Pressure Intervention Trial, **QoL=Quality of Life †Includes one woman indicating sexual activity but with missing data on sexual function
Table 1. Baseline characteristics of Women in SPRINT*.
| Baseline characteristic | All women in SPRINT (N=3333), N(%) | Women in the QoL† subset (N=690), N(%) | Sexually active women in the QoL subset (N=183), N(%) | Sexually active women taking at least one anti-hypertensive (N=164), N(%) | Sexually active women taking no anti-hypertensives (N=19), N(%) |
|---|---|---|---|---|---|
| Mean age, years (SD) | 68.5 (9.5) | 67.6 (10) | 63.4 (9.1) | 63.9 (9.2) | 58.6 (7) |
| Race | |||||
| White | 1555 (46.7) | 301 (43.6) | 76 (41.5) | 68 (41.5) | 8 (42.1) |
| African-American | 1270 (38.1) | 276 (40) | 72 (39.3) | 63 (38.4) | 9 (47.4) |
| Hispanic | 453 (13.6) | 103 (14.9) | 29 (15.8) | 28 (17.1) | 1 (5.3) |
| Other | 55 (1.7) | 10 (1.4) | 6 (3.3) | 5 (3) | 1 (5.3) |
| Education status | |||||
| Did not attend college | 986 (29.6) | 216 (31.3) | 39 (21.3) | 35 (21.3) | 4 (21.2) |
| Attended college | 1249 (37.5) | 237 (34.3) | 63 (34.4) | 58 (35.4) | 5 (26.3) |
| College graduate | 436 (13.1) | 103 (14.9) | 34 (18.6) | 29 (17.7) | 5 (26.3) |
| Graduate or prof. school | 651 (19.5) | 132 (19.1) | 47 (25.7) | 42 (25.6) | 5 (26.3) |
| Lives with others (vs. alone) | 2007 (60.4) | 444 (64.5) | 137 (74.9) | 137 (74.9) | 15 (78.9) |
| Mean SBP‡ (mm Hg) | 141.2 (16.9) | 142.3 (17.2) | 140.9 (16.6) | 140.6 (16.6) | 143.1 (17.5) |
| Mean DBP§ (mm Hg) | 77.6 (12.2) | 78.8 (12.3) | 81.4 (13.1) | 80.8 (12.8) | 87.4 (14.5) |
| SSRI‖ user (versus no) | 321 (9.6) | 72 (10.4) | 16 (8.7) | 16 (9.8) | 0 (0) |
| Hormone therapy user | 279 (8.4) | 55 (8) | 15 (8.2) | 15 (9.3) | 1 (5.3) |
| CKD¶ | 1321 (38.6) | 240 (34.8) | 42 (23) | 40 (24.4) | 2 (10.5) |
| Hyperlipidemia | 1895 (56.9) | 390 (56.5) | 99 (54.1) | 91 (55.5) | 9 (47.4) |
| Cardiovascular disease | 512 (15.4) | 106 (15.4) | 23 (12.6) | 20 (12.2) | 3 (15.8) |
Systolic Blood Pressure Intervention Trial,
quality of life,
systolic blood pressure,
diastolic blood pressure,
selective serotonin reuptake inhibitor,
chronic kidney disease
Sexual activity
Results of multivariable models with sexual activity as the dependent variable are presented in Table 2. For the model incorporating use of any antihypertensive medication (Model 1), younger women (p<0.001), more highly educated women (p=0.002), and those living with others (p=0.018) had higher odds of being sexually active. Odds of sexual activity differed among race/ethnic groups (p=0.003), with white, black, and Hispanic women having lower odds than women of other races (including multi-racial women); however, there were only 10 women in this group, so these results should be interpreted with caution. Similar results were observed for the model incorporating use of specific antihypertensive medication classes. Prevalence of sexual activity did not differ for women on any antihypertensive medication(s) versus women on no antihypertensive medication (p=0.81, Model 1).
Table 2. Multivariable factors associated with sexual activity among women in the quality of life subset of SPRINT*, N=635.
| Model 1 – Any antihypertensive(s) versus none | Model 2 – Class of antihypertensive | |||
|---|---|---|---|---|
|
|
||||
| Variable | OR for being sexually active (95% CI) | P value | OR for being sexually active (95% CI) | P value |
| Antihypertensive class | ||||
| Any antihypertensive use (v. none) | 1.09 (0.55,2.15) | 0.81 | - | - |
| Beta-blocker use (v. no) | - | - | 0.73 (0.47,1.13) | 0.159 |
| ACEI/ARB† use (v. no) | - | - | 1.66 (1.12,2.47) | 0.011 |
| Calcium channel blocker use (v. no) | - | - | 0.83 (0.55,1.27) | 0.40 |
| Diuretic use (v. no) | - | - | 1.06 (0.71,1.56) | 0.78 |
| Other antihypertensive use (v. no) | - | - | 1.04 (0.52,2.12) | 0.90 |
| Age (per 10 years increase) | 0.52 (0.4,0.69) | <0.001 | 0.49 (0.37,0.65) | <0.001 |
| Race/Ethnicity | ||||
| White | Ref | 0.033 | Ref | 0.023 |
| African-American | 0.72 (0.46,1.14) | 0.68 (0.42,1.08) | ||
| Hispanic | 0.66 (0.37,1.19) | 0.59 (0.33,1.08) | ||
| Other | 7.21 (1.33,39.2) | 6.88 (1.21,38.93) | ||
| Education | ||||
| Did not attend college | Ref | 0.002 | Ref | 0.001 |
| Some college | 1.59 (0.97,2.6) | 1.69 (1.03,2.8) | ||
| College graduate | 2.39 (1.31,4.37) | 2.59 (1.4,4.79) | ||
| Graduate or professional school | 2.77 (1.59,4.85) | 2.98 (1.68,5.29) | ||
| Lives with others (v. no) | 1.66 (1.09,2.54) | 0.018 | 1.72 (1.12,2.66) | 0.014 |
| SBP‡ (per 10 mm Hg increase) | 0.99 (0.85,1.14) | 0.86 | 1.01 (0.87,1.17) | 0.90 |
| DBP§ (per 10 mm Hg increase) | 1.02 (0.82,1.28) | 0.85 | 0.99 (0.79,1.25) | 0.96 |
| Use of SSRI‖ | 0.82 (0.43,1.55) | 0.53 | 0.81 (0.43,1.55) | 0.53 |
| Use of hormone therapy | 0.82 (0.41,1.61) | 0.56 | 0.87 (0.44,1.69) | 0.67 |
| Medical comorbidities | ||||
| CKD¶ | 0.74 (0.48,1.13) | 0.165 | 0.75 (0.48,1.17) | 0.20 |
| Hyperlipidemia | 0.89 (0.61,1.3) | 0.55 | 0.94 (0.64,1.39) | 0.77 |
| Cardiovascular disease | 0.78 (0.45,1.34) | 0.37 | 0.75 (0.42,1.31) | 0.31 |
Each estimate is adjusted for all other variables with estimates shown; in Model 2, results for each class of antihypertensive use are adjusted for use of other classes of antihypertensives.
Systolic Blood Pressure Intervention Trial,
angiotensin-converting enzyme inhibitor / angiotensin receptor blocker,
systolic blood pressure,
diastolic blood pressure,
selective serotonin reuptake inhibitor,
chronic kidney disease
However, in the model examining specific antihypertensive classes (Model 2), women taking an ACEI or ARB had increased odds of sexual activity compared to women not taking these medications [OR 1.66 (1.12, 2.47), p=0.011]. No other class of antihypertensive medication was significantly associated with sexual activity. In sensitivity analyses, number of antihypertensive classes used was not significantly related to sexual activity (p=0.99).
Sexual function
Among sexually active women, the mean FSFI score was 25.3 (SD 6) (Table 3). Ninety-six (52.5%) sexually active women were below the cutoff for sexual dysfunction. FSFI domain scores were similar for women taking different classes of antihypertensive medications. In univariable analyses, no class of medication was significantly associated with FSFI score (results not shown). Using the sample standard deviation (6.0), these analyses had 82% (85%, 92%, and 90%) power to detect a difference of 3 units in FSFI scores between beta-blocker (CCB, diuretic, and ACEI/ARB respectively) users and non-users.
Table 3. FSFI* scores among sexually active women in SPRINT.
| All sexually active women (N=183), mean (SD) | Beta-blocker users (N=45), mean (SD) | Calcium channel blocker users (N=50), mean (SD) | Diuretic users (N=95), mean (SD) | ACEI/ARB† users (N=115), mean (SD) | |
|---|---|---|---|---|---|
| Prevalence of sexual dysfunction, N (%) | 96 (52.5) | 22 (48.9) | 27 (54.0) | 51 (53.4) | 66 (57.4) |
| Total FSFI score (range 2.0-36.0) | 25.3 (6) | 24.6 (6.7) | 25.4 (6.4) | 24.8 (6.5) | 24.9 (5.7) |
| Desire (range 1.2-6.0) | 3.6 (1.2) | 3.4 (1.2) | 3.5 (1.3) | 3.6 (1.2) | 3.6 (1.2) |
| Arousal (range 0-6.0) | 4 (1.2) | 3.8 (1.4) | 3.9 (1.4) | 3.9 (1.3) | 3.9 (1.2) |
| Lubrication (range 0-6.0) | 4.5 (1.2) | 4.2 (1.4) | 4.5 (1.2) | 4.5 (1.3) | 4.3 (1.2) |
| Orgasm (range 0-6.0) | 4.4 (1.3) | 4.4 (1.4) | 4.3 (1.5) | 4.4 (1.4) | 4.3 (1.3) |
| Satisfaction (range 0.8-6.0) | 4.2 (1.3) | 4.2 (1.3) | 4.3 (1.4) | 4.1 (1.3) | 4.1 (1.3) |
| Pain (range 0-6.0) | 5.2 (1.1) | 5.1 (1.2) | 5.1 (1.1) | 5.2 (1.0) | 5.1 (1.1) |
Female Sexual Function Index,
Angiotensin converting enzyme inhibitor / angiotensin receptor blocker
In multivariable analyses, there is a trend towards better sexual function among women on no antihypertensive agents that did not reach statistical significance [beta 2.73 (-0.22, 5.68), p=0.069], (Model 1, Table 4). There were no significant associations found between any class of antihypertensive medication and sexual function (Model 2, Table 4). After controlling for specific classes of antihypertensive medications, HT use was associated with poorer sexual function scores [beta -3.42 (-6.74, -0.10), p=0.044]. In sensitivity analyses, the number of antihypertensive medications used was not significantly related to sexual function (p=0.85).
Table 4. Multivariable factors associated with sexual function (FSFI* score) among sexually active women in SPRINT.
| Variable | Model 1 – Any antihypertensive(s) versus no antihypertensives | Model 2 – Class of antihypertensive | ||
|---|---|---|---|---|
|
| ||||
| Beta for FSFI score (95% CI) | P-value | Beta for FSFI score (95% CI) | P-value | |
| Antihypertensive class | ||||
| No antihypertensive use (v. any) | 2.73 (-0.22,5.68) | 0.069 | - | - |
| Beta-blocker use (v. no) | - | - | -0.57 (-2.81,1.66) | 0.61 |
| Calcium channel blocker use (v. no) | - | - | 0.39 (-1.65,2.43) | 0.71 |
| Diuretic use (v. no) | - | - | -1.42 (-3.35,0.5) | 0.146 |
| ACEI/ARB† use (v. no) | - | - | -1.08 (-2.97,0.8) | 0.26 |
| Other Antihypertensive med use (v. no) | - | - | 0.37 (-3.19,3.92) | 0.84 |
| Age (per 10 years increase) | 0.75 (-0.71,2.2) | 0.31 | 0.54 (-0.93,2.01) | 0.47 |
| Race/Ethnicity | ||||
| White | Ref | 0.12 | Ref | 0.159 |
| African-American | 2.37 (0.14,4.59) | 2.33 (0.07,4.6) | ||
| Hispanic | 2.53 (-0.27,5.32) | 2.07 (-0.77,4.92) | ||
| Other | -1.02 (-6.05,4.01) | -1.14 (-6.29,4.01) | ||
| Education | ||||
| Did not attend college | Ref | 0.14 | Ref | 0.114 |
| Some college | 2.36 (-0.2,4.92) | 2.57 (-0.04,5.18) | ||
| College graduate | 3.24 (0.31,6.16) | 3.4 (0.44,6.36) | ||
| Graduate or professional school | 2.43 (-0.34,5.2) | 2.58 (-0.21,5.38) | ||
| Lives with others (v. no) | 1.27 (-0.86,3.39) | 0.24 | 0.99 (-1.19,3.17) | 0.37 |
| SBP‡ (per 10 mm Hg increase) | 0.03 (-0.76,0.82) | 0.93 | 0.01 (-0.81,0.82) | 0.99 |
| DBP§ (per 10 mm Hg increase) | 0.78 (-0.34,1.91) | 0.17 | 0.69 (-0.46,1.84) | 0.24 |
| Use of SSRI‖ | -1.04 (-4.27,2.2) | 0.53 | -1.31 (-4.65,2.04) | 0.44 |
| Use of hormone therapy | -3.14 (-6.4,0.12) | 0.059 | -3.42 (-6.74,-0.1) | 0.044 |
| Medical comorbidities | ||||
| CKD¶ | 0.55 (-1.72,2.83) | 0.63 | -0.05 (-2.51,2.41) | 0.97 |
| Hyperlipidemia | 0.96 (-0.85,2.76) | 0.30 | 0.93 (-0.91,2.77) | 0.32 |
| Cardiovascular disease | 0.14 (-2.58,2.86) | 0.92 | 0.28 (-2.55,3.1) | 0.85 |
Each estimate is adjusted for all other variables with estimates shown; in Model 2, results for each class of antihypertensive use are adjusted for use of other classes of antihypertensives.
Female Sexual Function Index--higher scores indicate better sexual function,
Angiotensin converting enzyme inhibitor/angiotensin receptor blocker,
systolic blood pressure,
diastolic blood pressure,
selective serotonin reuptake inhibitor,
chronic kidney disease
Discussion
In this cross-sectional analysis of sexually active women 50 years and older at high risk for cardiovascular disease, we found that approximately one quarter of women were sexually active, and of these, over half were classified as having sexual dysfunction. Women taking ACEI/ARBs were more likely to be sexually active compared to women not on these medications. No single class of antihypertensive medication was correlated with sexual function.
Other authors have found similar prevalence of sexual activity among older women with medical problems (41). In caring for these women, providers should be aware that some are still sexually active. It could be hypothesized that some of these women became sexually inactive due to sexual dysfunction; however, one prior study found that older women with poor sexual function are just as likely to remain sexually active as those with better sexual function (42).
The prevalence of sexual dysfunction was high in this sample; other studies using the FSFI in older women have also found similar results (42-44). There are associations among medical comorbidities such as cardiovascular disease (45), chronic kidney disease (46, 47), and hypertension (48) with sexual dysfunction. The combination of older age and medical comorbidities may explain the high prevalence of sexual dysfunction in our sample.
Women in SPRINT who were taking an ACEI or ARB at baseline were more likely to be sexually active than women not taking these medications, even when adjusting for other factors that may affect sexual function. The mechanism through which ACEI/ARBs may affect sexual function is not clear. In animal models, inhibition of NO synthase has been shown to reduce smooth muscle relaxation in clitoral tissue (49), and phosphodiesterase-5 inhibitors, which promote NO accumulation, have been shown to enhance stimulation-induced clitoral blood flow (50). ACEIs and ARBs decrease the breakdown of bradykinin, which enhances the accumulation of NO in the endothelium (22); increased NO may lead to increased clitoral bloodflow and improved arousal. Another mechanism may be through QoL; ACEIs and ARBs are associated with better QoL scores (7, 51), and higher QoL is associated with being sexually active (52, 53). While we did control for education as a proxy for socioeconomic status, there still may be socioeconomic differences among women who take ACEI/ARBs and women who do not, and socioeconomic status may be related to whether women are sexually active (54). Additionally, younger patients may be more likely to receive ACEI/ARBs, and younger patients are more likely to be sexually active. While we did adjust for age in our analyses, there is the possibility for residual confounding. Finally, there is the possibility that this association is spurious, and our findings should be confirmed in future studies.
No single class of antihypertensive medication in SPRINT was associated with sexual function. Prior studies of the effects of antihypertensive medications on female sexual function have yielded mixed results. One study found that beta blockers have an adverse effect (9), while three studies did not (11, 55, 56). Two studies found that CCBs are not associated with female sexual function (13, 55), and one study found no association between alpha-blockers, ACEIs, or thiazides and sexual function (55). A more recent study found no associations between CCBs, beta-blockers, ACEI/ARBs, diuretics, or alpha blockers and female sexual dysfunction (57). However, many of these studies are small, and none use a validated measure of sexual function.
Two studies did assess sexual function more comprehensively. In one study, 120 postmenopausal women were randomized to valsartan or atenolol and sexual function was assessed with a 10-item questionnaire (58). These authors found that beta-blockers had adverse effects and ARBs had positive effects on sexual function. In the other study, 160 women aged 18-60 with hypertension were randomized to felodipine + irbesartan or felodipine + metoprolol and sexual function was assessed with the FSFI (59). In women placed on irbesartan, FSFI scores improved, while in women placed on metoprolol, there were no changes in FSFI scores. The mean improvement in FSFI scores for women on irbesartan was by <1 point, so the clinical significance is questionable.
Many women in SPRINT were taking multiple antihypertensive medications, which may make it more difficult to isolate the effects of any one class of medication; however, we did control for other classes of medications in our multivariable analyses. The numbers of women on monotherapy for each class were too small to include them in multivariable analyses. However, the unadjusted mean FSFI total and domain scores appear comparable for women on monotherapy and women on multiple medications (supplemental table).
Our study has several strengths. It used a well-validated, multidimensional assessment of female sexual function. We also controlled for a number of important potential confounders that have not been assessed in prior studies. SPRINT also gave us the opportunity to assess every major class of antihypertensive medication, as opposed to only one or two medications, in the same study. Finally, this study gave us important information about sexual activity and sexual function in an older cohort of women who are often excluded from studies on sexual function.
There are several limitations to this study. First, the sample size is moderate. However, our study had >80% power to detect differences of at least 3 units in FSFI scores for each antihypertensive medication class. While larger studies may be able to detect smaller differences, these differences are not likely to be clinically significant. In addition, there were only 19 women not taking any antihypertensive medications, so results among this group should be interpreted with caution. Second, our data are cross-sectional. Longitudinal studies that evaluate changes in sexual function with different antihypertensive agents could yield more clinically useful information. For example, women may have previously discontinued a particular antihypertensive medication due to sexual side effects. SPRINT is a 6-year study, so we will have the opportunity to follow these women over time.
Some older women with medical comorbidities are sexually active, but prevalence of sexual dysfunction in this group is high. Women taking an ACEI/ARB may be more likely to be sexually active, but cause and effect cannot be determined in these analyses. These results should be confirmed in subsequent longitudinal studies. No single class of antihypertensive medication was associated with sexual dysfunction. These results should be confirmed in longitudinal studies, but based on the power estimates of this study, it appears that antihypertensives are unlikely to affect sexual function.
Acknowledgments
Acknowledgements / Sources of Funding: The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals Inc. Stephen P. Glasser is partially supported by a grant from AMGEN. No other author has any conflicts of interest to report. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: ClinicalTrials.gov Identifier: NCT01206062.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
This manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract. We presented an abstract of this data as poster at the Society of General Internal Medicine (SGIM) national conference in San Diego, California in April 2014.
References
- 1.Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens. 1994;7(7 Pt 2):7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. Leading Causes of Death [Google Scholar]
- 3.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350(9080):757–64. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161(9):1207–16. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165(8):923–8. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 6.Nelson EC, Stason WB, Neutra RR, Solomon HS. Identification of the noncompliant hypertensive patient. Prev Med. 1980;9(4):504–17. doi: 10.1016/0091-7435(80)90045-6. [DOI] [PubMed] [Google Scholar]
- 7.Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, et al. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314(26):1657–64. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- 8.Croog SH, Levine S, Sudilovsky A, Baume RM, Clive J. Sexual symptoms in hypertensive patients. A clinical trial of antihypertensive medications. Arch Intern Med. 1988;148(4):788–94. [PubMed] [Google Scholar]
- 9.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247(12):1707–14. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 10.Kroner BA, Mulligan T, Briggs GC. Effect of frequently prescribed cardiovascular medications on sexual function: a pilot study. Ann Pharmacother. 1993;27(11):1329–32. doi: 10.1177/106002809302701103. [DOI] [PubMed] [Google Scholar]
- 11.Wassertheil-Smoller S, Blaufox MD, Oberman A, Davis BR, Swencionis C, Knerr MO, et al. Effect of antihypertensives on sexual function and quality of life: the TAIM Study. Ann Intern Med. 1991;114(8):613–20. doi: 10.7326/0003-4819-114-8-613. [DOI] [PubMed] [Google Scholar]
- 12.Chang SW, Fine R, Siegel D, Chesney M, Black D, Hulley SB. The impact of diuretic therapy on reported sexual function. Arch Intern Med. 1991;151(12):2402–8. [PubMed] [Google Scholar]
- 13.Ogihara T, Nakagawa M, Ishikawa H, Mikami H, Takeda K, Nonaka H, et al. Effect of manidipine, a novel calcium channel blocker, on quality of life in hypertensive patients. Blood Press Suppl. 1992;3:135–9. [PubMed] [Google Scholar]
- 14.Neaton JD, Grimm RH, Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270(6):713–24. [PubMed] [Google Scholar]
- 15.Manolis A, Doumas M. Sexual dysfunction: the ‘prima ballerina’ of hypertension-related quality-of-life complications. J Hypertens. 2008;26(11):2074–84. doi: 10.1097/HJH.0b013e32830dd0c6. [DOI] [PubMed] [Google Scholar]
- 16.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163(2):460–3. [PubMed] [Google Scholar]
- 17.Doumas M, Tsiodras S, Tsakiris A, Douma S, Chounta A, Papadopoulos A, et al. Female sexual dysfunction in essential hypertension: a common problem being uncovered. J Hypertens. 2006;24(12):2387–92. doi: 10.1097/01.hjh.0000251898.40002.5b. [DOI] [PubMed] [Google Scholar]
- 18.Okeahialam BN, Ogbonna C. Impact of hypertension on sexual function in women. West Afr J Med. 2010;29(5):344–8. [PubMed] [Google Scholar]
- 19.Giraldi A, Marson L, Nappi R, Pfaus J, Traish AM, Vardi Y, et al. Physiology of female sexual function: animal models. J Sex Med. 2004;1(3):237–53. doi: 10.1111/j.1743-6109.04037.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107(21):2747–52. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- 21.Taddei S, Virdis A, Ghiadoni L, Magagna A, Pasini AF, Garbin U, et al. Effect of calcium antagonist or beta blockade treatment on nitric oxide-dependent vasodilation and oxidative stress in essential hypertensive patients. J Hypertens. 2001;19(8):1379–86. doi: 10.1097/00004872-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, et al. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001;103(6):799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Xie YW, Nasjletti A, Xu X, Wolin MS, Hintze TH. ACE inhibitors promote nitric oxide accumulation to modulate myocardial oxygen consumption. Circulation. 1997;95(1):176–82. doi: 10.1161/01.cir.95.1.176. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki H, Minamino T, Tsukamoto O, Kim J, Okada K, Myoishi M, et al. Angiotensin II type 1 receptor blocker prevents atrial structural remodeling in rats with hypertension induced by chronic nitric oxide inhibition. Hypertens Res. 2006;29(4):277–84. doi: 10.1291/hypres.29.277. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97(6):576–80. doi: 10.1161/01.cir.97.6.576. [DOI] [PubMed] [Google Scholar]
- 26.Fogari R, Preti P, Derosa G, Marasi G, Zoppi A, Rinaldi A, et al. Effect of antihypertensive treatment with valsartan or atenolol on sexual activity and plasma testosterone in hypertensive men. Eur J Clin Pharmacol. 2002;58(3):177–80. doi: 10.1007/s00228-002-0456-3. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Tominaga T, Kumagai H, Saruta T. Effects of first-line antihypertensive agents on sexual function and sex hormones. J Hypertens Suppl. 1988;6(4):S649–51. doi: 10.1097/00004872-198812040-00204. [DOI] [PubMed] [Google Scholar]
- 28.Andersen P, Seljeflot I, Herzog A, Arnesen H, Hjermann I, Holme I. Effects of doxazosin and atenolol on atherothrombogenic risk profile in hypertensive middle-aged men. J Cardiovasc Pharmacol. 1998;31(5):677–83. doi: 10.1097/00005344-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(1):129–42. doi: 10.1080/00926230252851258. [DOI] [PubMed] [Google Scholar]
- 30.Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom Med. 1987;49(4):397–409. doi: 10.1097/00006842-198707000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann G, Bancroft J, Braunstein G, Burger H, Davis S, Dennerstein L, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77(4):660–5. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 32.Alexander GM, Sherwin BB, Bancroft J, Davidson DW. Testosterone and sexual behavior in oral contraceptive users and nonusers: a prospective study. Horm Behav. 1990;24(3):388–402. doi: 10.1016/0018-506x(90)90017-r. [DOI] [PubMed] [Google Scholar]
- 33.Riley A, Riley E. Controlled studies on women presenting with sexual drive disorder: I. Endocrine status. J Sex Marital Ther. 2000;26(3):269–83. doi: 10.1080/00926230050084669. [DOI] [PubMed] [Google Scholar]
- 34.Nappi RE, Abbiati I, Luisi S, Ferdeghini F, Polatti F, Genazzani AR. Serum allopregnanolone levels relate to FSFI score during the menstrual cycle. J Sex Marital Ther. 2003;29(1):95–102. doi: 10.1080/713847135. [DOI] [PubMed] [Google Scholar]
- 35.Cawood EH, Bancroft J. Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med. 1996;26(5):925–36. doi: 10.1017/s0033291700035261. [DOI] [PubMed] [Google Scholar]
- 36.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 39.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Bahlburg HF, Dolezal C. The female sexual function index: a methodological critique and suggestions for improvement. J Sex Marital Ther. 2007;33(3):217–24. doi: 10.1080/00926230701267852. [DOI] [PubMed] [Google Scholar]
- 41.Gass ML, Cochrane BB, Larson JC, Manson JE, Barnabei VM, Brzyski RG, et al. Patterns and predictors of sexual activity among women in the Hormone Therapy trials of the Women's Health Initiative. Menopause. 2011;18(11):1160–71. doi: 10.1097/gme.0b013e3182227ebd. [DOI] [PubMed] [Google Scholar]
- 42.Thomas HN, Chang CC, Dillon S, Hess R. Sexual activity in midlife women: importance of sex matters. JAMA Intern Med. 2014;174(4):631–3. doi: 10.1001/jamainternmed.2013.14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chedraui P, Perez-Lopez FR, San Miguel G, Avila C. Assessment of sexuality among middle-aged women using the Female Sexual Function Index. Climacteric. 2009;12(3):213–21. doi: 10.1080/13697130802607727. [DOI] [PubMed] [Google Scholar]
- 44.Dargis LT, G, Cadieux J, Villeneuve L, Preville M, Boyer R. Validation of the Female Sexual Function Index (FSFI) and presentation of norms in older women. Sexologies. 2012;21:126–31. [Google Scholar]
- 45.Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, et al. Sexual problems among women and men aged 40-80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17(1):39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 46.Toorians AW, Janssen E, Laan E, Gooren LJ, Giltay EJ, Oe PL, et al. Chronic renal failure and sexual functioning: clinical status versus objectively assessed sexual response. Nephrol Dial Transplant. 1997;12(12):2654–63. doi: 10.1093/ndt/12.12.2654. [DOI] [PubMed] [Google Scholar]
- 47.Hanon O, Mounier-Vehier C, Fauvel JP, Marquand A, Jaboureck O, Justin EP, et al. Sexual dysfunction in treated hypertensive patients. Results of a national survey. Arch Mal Coeur Vaiss. 2002;95(7-8):673–7. [PubMed] [Google Scholar]
- 48.Duncan LE, Lewis C, Jenkins P, Pearson TA. Does hypertension and its pharmacotherapy affect the quality of sexual function in women? Am J Hypertens. 2000;13(6 Pt 1):640–7. doi: 10.1016/s0895-7061(99)00288-5. [DOI] [PubMed] [Google Scholar]
- 49.Cellek S, Moncada S. Nitrergic neurotransmission mediates the non-adrenergic non-cholinergic responses in the clitoral corpus cavernosum of the rabbit. Br J Pharmacol. 1998;125(8):1627–9. doi: 10.1038/sj.bjp.0702278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vemulapalli S, Kurowski S. Sildenafil relaxes rabbit clitoral corpus cavernosum. Life Sci. 2000;67(1):23–9. doi: 10.1016/s0024-3205(00)00596-8. [DOI] [PubMed] [Google Scholar]
- 51.Hill JF, Bulpitt CJ, Fletcher AE. Angiotensin converting enzyme inhibitors and quality of life: the European trial. J Hypertens Suppl. 1985;3(2):S91–4. [PubMed] [Google Scholar]
- 52.Lindau ST, Gavrilova N. Sex, health, and years of sexually active life gained due to good health: evidence from two US population based cross sectional surveys of ageing. BMJ. 2010;340:c810. doi: 10.1136/bmj.c810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762–74. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Addis IB, Van Den Eeden SK, Wassel-Fyr CL, Vittinghoff E, Brown JS, Thom DH. Sexual activity and function in middle-aged and older women. Obstet Gynecol. 2006;107(4):755–64. doi: 10.1097/01.AOG.0000202398.27428.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimm RH, Jr, Grandits GA, Prineas RJ, McDonald RH, Lewis CE, Flack JM, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of Mild Hypertension Study (TOMHS) Hypertension. 1997;29(1 Pt 1):8–14. doi: 10.1161/01.hyp.29.1.8. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Stable EJ, Halliday R, Gardiner PS, Baron RB, Hauck WW, Acree M, et al. The effects of propranolol on cognitive function and quality of life: a randomized trial among patients with diastolic hypertension. Am J Med. 2000;108(5):359–65. doi: 10.1016/s0002-9343(00)00304-1. [DOI] [PubMed] [Google Scholar]
- 57.Spatz ES, Canavan ME, Desai MM, Krumholz HM, Lindau ST. Sexual activity and function among middle-aged and older men and women with hypertension. J Hypertens. 2013;31(6):1096–105. doi: 10.1097/HJH.0b013e32835fdefa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fogari R, Preti P, Zoppi A, Corradi L, Pasotti C, Rinaldi A, et al. Effect of valsartan and atenolol on sexual behavior in hypertensive postmenopausal women. Am J Hypertens. 2004;17(1):77–81. doi: 10.1016/j.amjhyper.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Ma R, Yu J, Xu D, Yang L, Lin X, Zhao F, et al. Effect of felodipine with irbesartan or metoprolol on sexual function and oxidative stress in women with essential hypertension. J Hypertens. 2012;30(1):210–6. doi: 10.1097/HJH.0b013e32834e1e2e. [DOI] [PubMed] [Google Scholar]